SUMMARY
Aldosterone is increased in diabetes and contributes to the development of diabetic nephropathy. We hypothesized that reduction in aldosterone production in diabetes by amlodipine or aliskiren improves diabetic kidney disease by attenuating renal oxidative stress and fibrosis. Normoglycemic and streptozotocin-induced diabetes Sprague-Dawley rats were given vehicle, amlodipine or aliskiren individually and combined for six weeks. At the end of study, we evaluated BP, 24h urinary sodium (UNaV) and aldosterone excretion rates, renal interstitial fluid (RIF) levels of nitric oxide (NO), cGMP and 8-isoprostane, and renal morphology. BP was not significantly different between any of experimental groups. UNaV increased in diabetic animals and was not affected by different treatments. Urinary aldosterone excretion increased in diabetic rats receiving vehicle and decreased with amlodipine and aliskiren individually or combined. RIF NO and cGMP levels were reduced in vehicle treated diabetic rats and increased with amlodipine or aliskiren given individually and combined. RIF 8-isoprostane levels and renal immunostaining for PAS and fibronectin were increased in vehicle treated diabetic rats and decreased with aliskiren individually or combined with amlodipine. We conclude that inhibition of aldosterone by amlodipine or aliskiren ameliorates diabetes induced renal injury via improvement of NO-cGMP pathway, and reduction in oxidative stress and fibrosis, independent of BP changes.
Keywords: Diabetes, kidney, aldosterone, oxidative stress, amlodipine, renal fibrosis
INTRODUCTION
Diabetes is associated with an increase of the renin-angiotensin system (RAS) activity including aldosterone production (1,2). In addition to its physiological role in regulating renal sodium and water reabsorption and potassium secretion in the distal nephron, aldosterone promotes tissue inflammation, remodeling and fibrosis (3,4).
Aldosterone also plays a direct role in the production and activity of reactive oxygen species, an important component for the development of tissue injury. Aldosterone was demonstrated to inhibit the production of nitric oxide (NO) (5) and its second messenger cyclic guanosine 3′,5′-monophosphate (cGMP) (6), and to enhance the renal production of 8-isoprostane, a marker for endogenous superoxide activity (7,8). In contrast, the inhibition of aldosterone with mineralocorticoid-receptor antagonists suppressed oxidative stress and inflammation (9,10). NO acts as an endogenous antioxidant that helps to prevent the development of fibrosis. However, the relation between NO-cGMP pathway, oxidative stress and the profibrotic actions of aldosterone in diabetic kidney is not well elucidated.
The blockade of RAS with angiotensin converting enzyme inhibitors or angiotensin receptor blockers is widely used to prevent or delay the development of diabetic nephropathy. The therapeutic effects of these agents are attributed to the reduction in Ang II generation or activity. However, it is not clear if other pharmacologic agents such as drugs used in management of hypertension and reduce aldosterone production could reduce renal injury in diabetes. In the present study, we compared the effects of the direct renin inhibitor aliskiren that decreases angiotensin II and aldosterone production (15), and the dihydropyridine-type calcium channel blocker (CCB) amlodipine that directly reduces aldosterone (13,14), individually and combined, independent of changes in blood pressure, on NO-cGMP pathway, oxidative stress and renal fibrosis. Recent studies suggested that the combination of CCBs and RAS inhibitors have further beneficial effects on reduction of blood pressure and proteinuria and improvement of endothelial function by enhancing NO production (16,18). Amlodipine was demonstrated to reduce inflammation and fibrosis in different rat models (19,20). However, the effects of amlodipine in slowing the progression of albuminuria and its role on aldosterone production in diabetes are unclear.
In the present study, we evaluated the hypothesis that reduction of aldosterone production in diabetes, induced by amlodipine or aliskiren treatment, directly enhances the renal production of NO-cGMP and reduces diabetes-induced renal oxidative stress and fibrosis, independent of changes in BP.
METHODS
Animal preparation
The University of Virginia Animal Care and Use Committee approved all study protocols. 24-h urine and renal interstitial fluid (RIF) collections, and kidneys tissues (21) were used in the present study. In brief, male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 230 to 260 g were randomly allocatedinto groups (n = 10 each group) of control (normal) and diabetes groups receiving vehicle, amlodipine or aliskiren individually and combined. Diabetes was induced by intraperitoneal injection of 65 mg/kg of STZ (Sigma-Aldrich, Saint Louis, MO). Normoglycemic control rats were injected with an equal volume of sterile saline. Treatments were initiated the day after STZ injection and given for a period of 6 weeks. Amlodipine (Novartis, East Hanover, NJ, USA) was administered by oral gavage at a dose of 10 mg/kg/day. Aliskiren (Novartis, East Hanover, NJ, USA) was infused at a rate of 10 mg/kg/day via osmotic minipump (models 2ML2 and 2ML4; Alzet, Cupertino, CA). The control and DM rats treated with amlodipine were implanted with a sham osmotic minipump containing saline. For minipump implantation, one day after STZ or vehicle injection, rats were anesthetized with the combination of ketamine (80 mg/kg; I.P.) and xylazine (8 mg/kg; I.P.). The osmotic minipumps were surgically implanted subcutaneously in the subscapular region of all rats.
Body weight, blood glucose, 24-h urinary sodium (UNaV) and aldosterone excretions, and systolic blood pressure (SBP) monitoring
Body weight, blood glucose, 24-h urine collections, and SBP were obtained at baseline and at the end of study. For blood glucose determinations, blood was collected from tail vein after overnight fasting and glucose was measured usinga glucometer (Bayer HealthCare, Mishawaka, IN). For urine collections, rats were placed in individual metabolic cages for a period of 24-h. The volume of urine collected was determined gravimetrically and a urine sample was kept at −80°C until assayed. Urinary sodium concentration of each sample was measured using flame photometer IL 943 (Instrumentation Laboratory, Bedford, MA). Urinary aldosterone excretion was determined by an aldosterone enzyme immunoassay (EIA) kit-monoclonal (Cayman Chemical, Ann Harbor, MI). SBP was measured in non-anesthetized rats using a tail-cuff non-invasive multi channel blood pressure system (IITC Life Sciences, Woodland Hills, CA). The mean values of the recorded SBP were calculated.
In vivo renal interstitial fluid (RIF) NO, cGMP, and 8-isoprostane collections
To determine the RIF levels of NO, cGMP, and 8-isoprostane, we constructed a microdialysis probe as described elsewhere (20,22). At the end of study, RIF collections were performed in each animal under sodium pentobarbital anesthesia (50 mg/kg I.P.; Sigma) and a dialysis probe was placed in the renal cortex as previously described (21,22).
RIF storage and assays
The RIF collections were immediately stored at −80°C until assayed. RIF NO recovery levels were measured using its stable metabolic products nitrate/nitrite fluorometric assay kit (Cayman) and presented as μmol/min. RIF cGMP recovery levels were measured using a cyclic GMP EIA kit (Cayman). RIF 8-isoprostane recovery levels were measured using a 8-isoprostane EIA kit (Cayman). Both RIF cGMP and RIF 8-isoprostane are presented as fmol/min.
Renal immunohistochemical staining
At the end of each experiment, animals were euthanized and kidneys were harvested. For histological analyses, kidney was immersed in Bouin’s fixative solution (Sigma). The kidney tissue blocks were embedded in paraffin and cut into 3-μm slices. After being deparaffinized using xylene and ethanol dilutions and rehydration, the sections were processed with Periodic acid-Schiff (PAS; Sigma) to evaluate renal fibrosis. For immunohistochemical staining, kidney sections were incubated overnight at 4°C with primary antibodies directed against mouse fibronectin monoclonal antibody (1:100 dilution; sc-18825, Santa Cruz Biotechnology, Santa Cruz, CA). In the next day, sections were incubated for 1-h with secondary antibody at room temperature. Negative controls were included by omitting the primary antibody. The immunostaining images were captured by light microscopy using a Qimaging Micropublisher 5.0 RTV camera coupled to a Zeiss Axiophot microscopy (Carl Zeiss, Jena, Germany). Immunostaining quantification was performed according to the previously published protocol (17).
Statistical analysis
Comparisons among different treatment groups were assessed by ANOVA followed by a Tukey test for post-hoc comparisons. Data are expressed as mean ± SE. P<0.05 isconsidered statistically significant.
RESULTS
Body weight, BP, blood glucose and 24h urine
At baseline, there were no significant differences in body weight, blood glucose, urine output, and SBP between all groups. At the end of study, compared to normoglycemic control group, body weight was significantly reduced in all diabetic groups. Compared to baseline, fasting blood glucose and 24h urine volume of DM rats were significantly increased. There were no significant differences in fasting blood glucose or 24h urine volume between different treatments of the diabetic rats. At the end of study (Table 1), UNaV was significantly increased in DM rats compared to normoglycemic controls. However, there were no significant differences in UNaV between different treatments of DM rats. Levels of SBP in normoglycemic controls at the end of study were not significantly different from those of vehicle treated DM rats. There were no significant differences in SBP levels between different diabetic groups treated with amlodipine (112±1 mmHg) or aliskiren (113±2 mmHg) alone and combined (112±0.3 mmHg).
TABLE 1.
Body weight, blood glucose, 24-h urine output, urinary sodium excretion (UNaV), and systolic blood pressure (SBP) at the end of study in normoglycemic control and diabetic (DM) rats given vehicle or treated with amlodipine (DM+Amlo) or aliskiren (DM+Alisk) individually and combined (DM+Alisk+Amlo). n = 10 each group.
| Control | DM | DM+Amlo | DM+Alisk | DM+Amlo +Alisk | |
|---|---|---|---|---|---|
| Body Weight (g) | 480±11 | 300±20* | 311±13* | 297±11* | 293±11* |
| Blood Glucose (mg/dl) | 88±3 | 499±29* | 488±19* | 473±30* | 459±25* |
| Urine Output (mL/d) | 24±3 | 205±16* | 184±9* | 183±10* | 175±10* |
| UNaV (mmoL/d) | 2.68±0.12 | 4.05±0.25* | 3.93±0.16* | 3.71±0.29* | 3.61±0.22* |
| SBP (mm Hg) | 112±0.4 | 113±0.5 | 112±0.5 | 113±1.6 | 112±0.3 |
P<0.05 vs. normoglycemic control.
Urinary aldosterone excretion in normal and DM rats
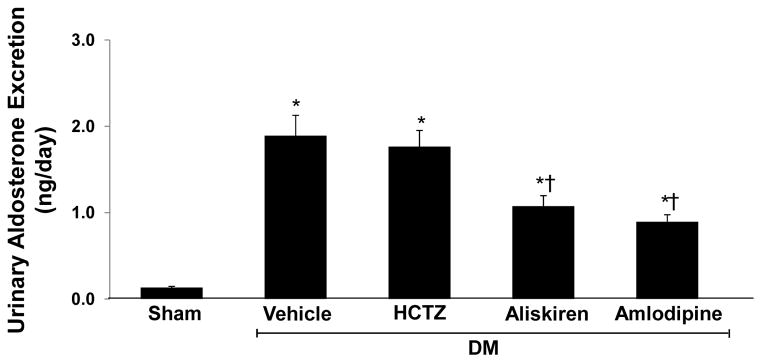
At the end of study, compared to normoglycemic control group, urinary aldosterone excretion rate was significantly higher in vehicle treated DM rats (0.13 ± 0.02 vs. 1.89 ± 0.27 ng/day; P < 0.0001). There was a significant reduction in urinary aldosterone excretion in DM rats treated with amlodipine (−53%, P < 0.001) or aliskiren (−43%, P < 0.01) alone and combined (50%, P< 0.001) (Figure 1).
Figure 1.
Urinary aldosterone excretion at the end of study of sham and streptozotocin-induced diabetic (DM) rats treated with vehicle, amlodipine or aliskiren alone and combined. Data are mean ± SEM. *P<0.05 vs. control; †P<0.05 vs. DM+Vehicle.
RIF NO, cGMP, and 8-isoprostane in normal and DM rats
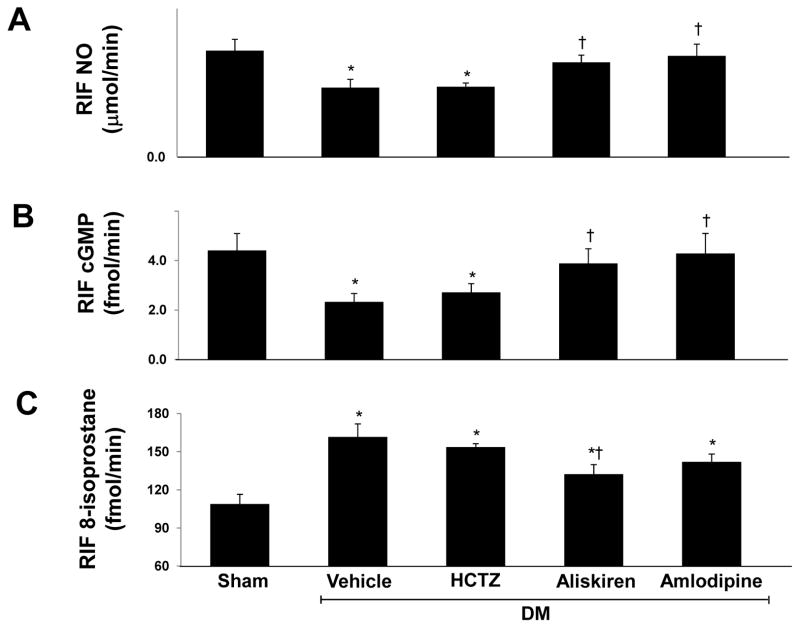
RIF NO (Figure 2A) and RIF cGMP (Figure 2B) levels were significantly reduced (−35% and −37% respectively, P < 0.04) in vehicle treated DM rats compared to normoglycemic controls. In DM rats, RIF NO and RIF cGMP levels were significantly increased in response to amlodipine (46% and 67%, P < 0.05), aliskiren (36% and 57% respectively, P < 0.04) or their combination (72% and 115%, P < 0.01). Compared to aliskiren alone, combined aliskiren and amlodipine caused further increase in RIF NO and RIF cGMP (26% and 37%, P < 0.05 respectively). Compared to normoglycemic controls, RIF 8-isoprostane levels (Figure 2C) were increased (49%, P < 0.01) in vehicle treated DM rats. 8-isoprostane levels did not show significant changes with amlodipine but were significantly decreased with aliskiren (−18%, P < 0.05) alone or combined with amlodipine (−22%, P < 0.02).
Figure 2.
Renal interstitial fluid (RIF) levels of nitric oxide (NO; A), cGMP (B), and 8-isoprostane (C) at the end of study in sham and streptozotocin-induced diabetic (DM) rats treated with vehicle, amlodipine or aliskiren alone and combined. Data are mean ± SEM. *P<0.05 vs. control; †P<0.05 vs. DM+Vehicle; ‡ P<0.05 vs. DM+Aliskiren.
PAS and fibronectin immunostaining in normal and DM rat kidneys
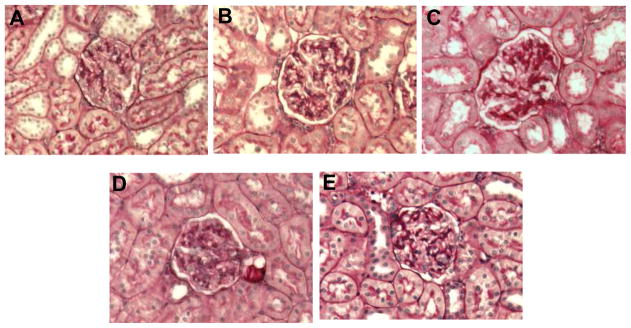
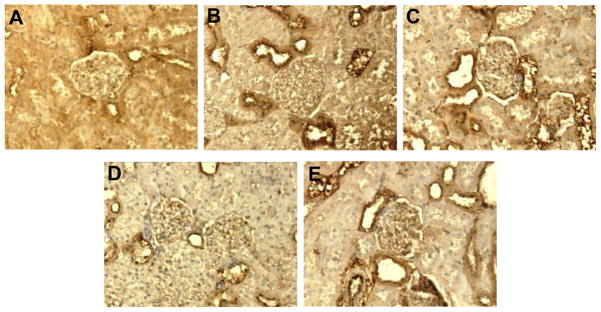
Renal PAS and fibronectin immunostaining were mainly localized to renal glomeruli and tubules, respectively. Compared to normoglycemic controls (Figure 3A), renal PAS staining increased in kidney sections of vehicle treated DM rats (Figure 3B). Renal PAS staining was reduced in DM rats treated with aliskiren alone (Figure 3C) or combined with amlodipine (Figure 3E). Amlodipine alone did not influence PAS staining (Figure 3D). Compared to normoglycemic controls (Figure 4A), renal fibronectin immunostaining, increased in vehicle treated DM rats (Figure 4B). Renal fibronectin was reduced in DM rats treated with aliskiren (Figure 4C) alone or combined with amlodipine (Figure 4E) but was not influenced by treatment with amlodipine (Figure 4D).
Figure 3.
Representative images of PAS staining in renal glomeruli (dark red). Sham (A) and streptozotocin-induced diabetic rats treated with vehicle (B), amlodipine (C) or aliskiren (D) alone and combined (E). PAS staining was increased in glomeruli of vehicle treated diabetic rats, reduced with aliskiren alone or combined with amlodipine and not affected by amlodipine alone treatment. x200 magnification.
Figure 4.
Representative images of fibronectin (FN) immunostaining in renal tubular area (stained dark brown). Sham (A) and streptozotocin-induced diabetic rats treated with vehicle (B), amlodipine (C) or aliskiren (D) individually and combined (E). FN immunostaining was increased in vehicle treated diabetic rats, reduced with aliskiren alone or combined with amlodipine and not affected by amlodipine alone treatment. x200 magnification.
DISCUSSION
Diabetes is associated with an increase of the RAS activity, including the production of Ang II and aldosterone (1). In the kidney, aldosterone is a proinflammatory factor that induces inflammation, fibrosis, mesangial cell proliferation and podocyte injury (4,23,24). In the course leading to development of diabetic nephropathy, a major diabetes complication, aldosterone plays a pivotal role in the pathophysiology of renal inflammation and fibrosis (23,24). However, the influence of commonly used antihypertensive agents such as the CCB amlodipine on the mechanisms involving aldosterone and lead to development of diabetic nephropathy is not fully elucidated. In the present study, we compared the effects of the calcium channel blocker amlodipine and the direct renin inhibitor on aldosterone production, renal production of NO-cGMP, 8-isoprostane and fibrosis in STZ-induced diabetes rat model. This rat model is characterized by normotensive levels of BP that was not influenced by different treatments employed in this study. Absence of changes in BP throughout this study eliminated the contribution of the antihypertensive effects of these drugs on diabetes induced renal injury. Our results demonstrated that increased aldosterone production in STZ-induced diabetic rats was associated with reduced NO-cGMP and increased 8-isoprostane levels and fibrosis in diabetic kidney. Reduction of aldosterone production in diabetes induced by amlodipine or aliskiren alone and combined improved renal interstitial levels of NO and cGMP. In addition, reduction of aldosterone with aliskiren alone or combined with amlodipine reduced renal levels of 8-isoprostane and fibrosis.
CCBs, including amlodipine, lower BP by reducing intracellular calcium concentration in vascular smooth muscle cells. In addition, these drugs have several pleiotropic effects as inhibition of oxidative stress and aldosterone production (13–14). In animal models, CCBs affected oxidative stress by increasing endothelial NO production and the superoxide scavenging activity (25–26). They also attenuated plasma levels of isoprostanes and restored NO availability in essential hypertensive patients (27) and improved NO-mediated endothelial function in coronary artery disease patients (28). However, the effects of amlodipine on diabetic kidney pathology are not well established.
Our current results demonstrated that increased aldosterone levels in diabetes were associated with worsening of renal oxidative stress. In contrast, reduction in levels of aldosterone, induced by amlodipine or aliskiren treatments, led to increased renal levels of NO and cGMP. These observed changes in aldosterone production were independent of variations in blood pressure or sodium concentration considering that UNaV, although elevated in diabetic rats, was not affected by either amlodipine or aliskiren treatments. The role of aldosterone in modulating the renal production of NO and cGMP was previously demonstrated in studies using vascular smooth muscle cells (5,6). In addition, both amlodipine and aliskiren were previously reported to improve impaired NO production (29,30). We also demonstrated that inhibition of renin activity reduced renal levels of 8-isoprostane. However, its production was not affected by treatment with amlodipine. 8-isoprostane is widely used as a marker for endogenous superoxide activity and its production is directly stimulated by multiple mechanisms including Ang II, inflammatory cytokines, and aldosterone (8). The differences between amlodipine and aliskiren treatments on renal production of 8-isoprostane could be related to their different effects on Ang II production. The effects of aliskiren are mainly mediated by reduction in angiotensin II formation and AT1 receptor stimulation. Amlodipine, in addition to reducing aldosterone production, it has direct effects on inhibiting tissue oxidative stress and inflammation (20,25,26).
Increased oxidative stress is directly related to the development of renal fibrosis (31). In diabetic kidney, increased levels of reactive oxygen species activate the production of the transcription factor nuclear factor kappa B (NF-κB) that in turn stimulates the production of the inflammatory cytokines leading to fibrosis (32). Recently, we demonstrated that enhanced levels of renal Ang II in STZ-induced diabetic rats were associated with increased renal expression of the transcription factor NF-κB and the renal levels of the inflammatory cytokines tumor necrosis factor-α and interleukin 6. These changes were accompanied by increased urinary albumin excretion (21). The influence of aldosterone on oxidative stress was previously demonstrated in vivo. In these studies, aldosterone infusion increased the production of reactive oxygen species in rat kidney and activated NF-κB and renal fibrosis in Ang II infused rats (7,33). In contrast, administration of antioxidant drugs to aldosterone-treated animals reduced oxidative stress and attenuated inflammation and collagen accumulation in the kidney (7). In addition, the blockade of aldosterone action with mineralocorticoid receptor antagonists (34,35) or aldosterone synthase inhibitors (36,37) prevented the development of inflammation and fibrosis in different tissues, including the kidney.
The main mechanisms by which aldosterone induces inflammation include promotion of inflammatory cell infiltration and adhesion molecules and stimulation of reactive oxygen species formation (4,37). The involvement of aldosterone in modulating inflammation leading to fibrosis and remodeling was demonstrated in different tissues, including the heart, vasculature, and kidney (4,10,24). Fibrosis is a major contributor to development of end stage renal disease in diabetes. In our current study, elevated aldosterone levels were associated with increased PAS and fibronectin immunostaining in diabetic kidneys. These factors were reduced in diabetic rats treated with aliskiren alone or combined with amlodipine. The lack of effects of amlodipine alone on preventing increases in renal 8-isoprostane in this diabetic animal model may have contributed to the absence of beneficial effects of this drug on renal fibrosis.
CONCLUSIONS
We demonstrated that in diabetic kidney, reduction in the production of aldosterone induced by the antihypertensive agents amlodipine or aliskiren, improved renal oxidative stress by increasing NO-cGMP production. Combination therapy of amlodipine and aliskiren further improved renal NO-cGMP levels. In addition, aliskiren alone or combined with amlodipine reduced renal 8-isoprostane levels and fibrosis. These findings were independent of BP changes. Our results suggest that reduction of aldosterone by amlodipine or aliskiren is an important beneficial effect of these drugs to prevent the development of diabetic kidney disease.
Acknowledgments
Sources of Funding: This study was supported by grants from Novartis Institutes for Biomedical Research, Inc. and NIH NIDDK-078757 and NIH HL091535 to Helmy M. Siragy.
References
- 1.Hollenberg NK, Stevanovic R, Agarwal A, Lansang MC, Price DA, Laffel LMB, Williams GH, Fisher NDL. Plasma aldosterone concentration in the patient with diabetes mellitus. Kidney Int. 2004;65:1435–1439. doi: 10.1111/j.1523-1755.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- 2.Siragy HM, Awad A, Abadir P, Webb R. The angiotensin II type 1 receptor mediates renal interstitial content of tumor necrosis factor-alpha in diabetic rats. Endocrinology. 2003;144:2229–2233. doi: 10.1210/en.2003-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert KC, Brown NJ. Aldosterone and inflammation. Curr Opin Endocrinol Diabetes Obes. 2010;17:199–204. doi: 10.1097/med.0b013e3283391989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda U, Kanbe T, Nakayama I, Kawahara Y, Yokoyama M, Shimada K. Aldosterone inhibits nitric oxide synthesis in rat vascular smooth muscle cells induced by interleukin-1B. Eur J Pharmacol. 1995;290:69–73. doi: 10.1016/0922-4106(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 6.Maron BA, Zhang YY, Handy DE, Beuve A, Tang SS, Loscalzo J, Leopold JA. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem. 2009;284:7665–7672. doi: 10.1074/jbc.M809460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iglarz M, Touyz RM, Viel EC, Amiri F, Schiffrin EL. Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction with the renin-angiotensin system. Am J Hypertens. 2004;17:597–603. [PubMed] [Google Scholar]
- 8.Practico D, Lawson JA, Roakach J, Fitzgerald GA. The isoprostanes in biology and medicine. Trends Endocrinol Metab. 2001;12:243–247. doi: 10.1016/s1043-2760(01)00411-8. [DOI] [PubMed] [Google Scholar]
- 9.Onozato ML, Tojo A, Kobayashi N, Goto A, Matsuoka H, Fujita T. Dual blockade of aldosterone and angiotensin II additively suppresses TGF-beta and NADPH oxidase in the hypertensive kidney. Nephrol Dial Transplant. 2007;22:1314–1322. doi: 10.1093/ndt/gfl780. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, Zhang A, Ding G, Chen R. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. Am J Physiol Renal Physiol. 2009;296:F1323–F1333. doi: 10.1152/ajprenal.90428.2008. [DOI] [PubMed] [Google Scholar]
- 11.Staessen J, Lijnen P, Fagard R, Verschueren LJ, Amery A. Rise in plasma concentration of aldosterone during long-term angiotensin II suppression. J Endocrinol. 1981;91:457–465. doi: 10.1677/joe.0.0910457. [DOI] [PubMed] [Google Scholar]
- 12.Schjoedt KJ, Andersen S, Rossing P, Tarnow L, Parving HH. Aldosterone escape during blockade of the renin-angiotensin system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia. 2004;47:1936–1939. doi: 10.1007/s00125-004-1542-0. [DOI] [PubMed] [Google Scholar]
- 13.Nadler JL, Hsueh W, Horton R. Therapeutic effect of calcium channel blockade in primary aldosteronism. J Clin Endocrinol Metab. 1985;60:896–899. doi: 10.1210/jcem-60-5-896. [DOI] [PubMed] [Google Scholar]
- 14.Aguilera G, Catt KJ. Participation of voltage-dependent calcium channels in the regulation of adrenal glomerulosa function by angiotensin II and potassium. Endocrinology. 1986;118:112–118. doi: 10.1210/endo-118-1-112. [DOI] [PubMed] [Google Scholar]
- 15.Persson F, Lewis JB, Lewis EJ, Rossing P, Hollenberg NK, Hans-Henrik P. Impact of aliskiren treatment on urinary aldosterone levels in patients with type 2 diabetes and nephropathy: an AVOID substudy. J Renin Angiotensin Aldosterone Syst. 2012;13:118–121. doi: 10.1177/1470320311417272. [DOI] [PubMed] [Google Scholar]
- 16.Zhou MS, Jaimes EA, Raij L. Benazepril combined with either amlodipine or hydrochlorothiazide is more effective than monotherapy for blood pressure control and prevention of end-organ injury in hypertensive Dahl rats. J Cardiovasc Pharmacol. 2006;48:857–861. doi: 10.1097/01.fjc.0000238598.09152.30. [DOI] [PubMed] [Google Scholar]
- 17.Kokolakis G, Panagis L, Stathopoulos E, Giannikaki E, Tosca A, Krüger-Krasagakis S. From the protein to the graph: how to quantify immunohistochemistry staining of the skin using digital imaging. J Immunol Methods. 2008;331:140–6. doi: 10.1016/j.jim.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Mohler ER, 3rd, Herrington D, Ouyang P, Mangano C, Ritter S, Davis P, Purkayastha D, Gatlin M, Vogel RA EXPLORE Investigators. A randomized, double-blind trial comparing the effects of amlodipine besylate/benazepril HCl vs amlodipine on endothelial function and blood pressure. J Clin Hypertens (Greenwich) 2006;8:692–698. doi: 10.1111/j.1524-6175.2006.05690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toblli JE, Ferder L, Angerosa M, Inserra F. Effects of amlodipine on tubulointerstitial lesions in normotensive hyperoxaluric rats. Hypertension. 1999;34:284–258. doi: 10.1161/01.hyp.34.4.854. [DOI] [PubMed] [Google Scholar]
- 20.Siragy HM, Xue C, Webb RL. Beneficial effects of combined benazepril-amlodipine on cardiac nitric oxide, cGMP, and TNF-alpha production after cardiac ischemia. J Cardiovasc Pharmacol. 2006;47:636–642. doi: 10.1097/01.fjc.0000211750.01326.b3. [DOI] [PubMed] [Google Scholar]
- 21.Matavelli LC, Huang J, Siragy HM. Combined aliskiren and amlodipine reduce albuminuria via reduction in renal inflammation in diabetic rats. J Cardiovasc Pharmacol. 2012;59:281–287. doi: 10.1097/FJC.0b013e31823fc3f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6:261–273. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- 24.Siragy HM, Xue C. Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp Physiol. 2008;93:817–824. doi: 10.1113/expphysiol.2008.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brovkovych V, Kalinowski L, Muller-Peddinghaus R, Malinski T. Synergistic antihypertensive effects of nifedipine on endothelium: concurrent release of NO and scavenging of superoxide. Hypertension. 2001;37:34–39. doi: 10.1161/01.hyp.37.1.34. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Hintze TH. Amlodipine releases nitric oxide from canine coronary microvessels: an unexpected mechanism of action of a calcium channel-blocking agent. Circulation. 1998;97:576–580. doi: 10.1161/01.cir.97.6.576. [DOI] [PubMed] [Google Scholar]
- 27.Taddei S, Virdis A, Ghiadoni L, Magagna A, Favilla S, Pompella A, Salvetti A. Restoration of nitric oxide availability after calcium antagonist treatment in essential hypertension. Hypertension. 2001;37:943–948. doi: 10.1161/01.hyp.37.3.943. [DOI] [PubMed] [Google Scholar]
- 28.ENCORE Investigators. Effect of nifedipine and cerivastatin on coronary endothelial function in patients with coronary artery disease: ENCORE I study (Evaluation of Nifedipine and Cerivastatin On Recovery of coronary Endothelial function) Circulation. 2003;107:422–428. doi: 10.1161/01.cir.0000046488.52939.bf. [DOI] [PubMed] [Google Scholar]
- 29.Imanishi T, Tsujioka H, Ikejima H, Kuroi A, Takarada S, Kitabata H, Tanimoto T, Murahaki Y, Mochizuki S, Goto M, Yoshida K, Akasaka T. Renin inhibitor aliskiren inproves impaired nitric oxide bioavailability and protects against atherosclerotic changes. Hypertension. 2008;52:563–572. doi: 10.1161/HYPERTENSIONAHA.108.111120. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda U, Shimpo M, Ohki R, Takahashi M, Yamamoto K, Ikeda M, Minota S, Shimada K. Amlodipine increases nitric oxide synthesis in cytokine-stimulated cultured vascular smooth muscle cells. J Hypertens. 2000;18:1597–1604. doi: 10.1097/00004872-200018110-00010. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009;297:F461–F470. doi: 10.1152/ajprenal.90735.2008. [DOI] [PubMed] [Google Scholar]
- 32.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 33.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–848. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 34.Neves MF, Amiri F, Virdis A, Diep QN, Schiffrin EL CIHR Multidisciplinary Research Group on Hypertension. Role of aldosterone in angiotensin II-induced cardiac and aortic inflammation, fibrosis, and hyperthrophy. Can J Physiol Pharmacol. 2005;83:999–1006. doi: 10.1139/y05-068. [DOI] [PubMed] [Google Scholar]
- 35.Susic D, Varagic J, Ahn S, Matavelli LC, Frohlich ED. Long-term mineralocorticoid receptor blockade reduces fibrosis and improves cardiac perfomance and coronary hemodynamics in elderly SHR. Am J Physiol Heart Circ Physiol. 2007;292:H175–H179. doi: 10.1152/ajpheart.00660.2006. [DOI] [PubMed] [Google Scholar]
- 36.Lea WB, Kwak ES, Luther JM, Fowler SM, Wang Z, Ma J, Fogo AB, Brown NJ. Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int. 2009;75:936–944. doi: 10.1038/ki.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiebeler A, Nussberger J, Shagdarsuren E, Rong S, Hilfenhaus G, Al-Saadi N, Dechend R, Wellner M, Meiners S, Maser-Gluth C, Jeng AY, Webb RL, Luft FC, Muller DN. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation. 2005;111:3087–3094. doi: 10.1161/CIRCULATIONAHA.104.521625. [DOI] [PubMed] [Google Scholar]