Abstract
A major focus in the field of tissue engineering is the regulation of essential cell behaviors through biophysical and biochemical cues from the local extracellular environment. The impact of nanotopographic cues on human corneal epithelial cell (HCEC) contact guidance, proliferation, migration and adhesion have previously been demonstrated. In the current report, we have expanded our study of HCEC response to include both biophysical and controlled biochemical extracellular cues. By exploiting methods for the layer-by-layer coating of substrates with reactive poly(ethylene imine) and poly(2-vinyl-4,4-dimethylazlactone) (PEI/PVDMA)-based multilayer thin films, we have incorporated a single adhesion peptide motif, Arg-Gly-Asp (RGD), onto topographically patterned substrates. This strategy eliminates protein adsorption onto the surface, thus decoupling the effects of the HCEC response to topographic cues from adsorbed proteins and the soluble media proteins. The direction of cell alignment was dependent on the scale of the topographic cues, and, to less of an extent, the culture medium. In EpiLife® medium, cell alignment to unmodified-NOA81 topographic features, which allowed for protein adsorption, differed significantly from cell alignment on RGD-modified features. These results demonstrate that the surface chemical composition affects significantly how HCECs respond to topographic cues. In summary, we demonstrate the modulation of the HCEC response to environmental cues through critical substrate and soluble parameters.
Keywords: cornea, epithelial cell, RGD peptide, nanotopography
1. Introduction
The extracellular matrix (ECM) serves as a natural scaffold for cells, acting as a mechanical support as well as creating a distinct microenvironment to which cells can respond. Within our tissue of interest, the human cornea, as well as other epithelial tissues, lies a specialized matrix referred to as the basement membrane (BM). The BM is a thin and highly specialized extracellular matrix component found between the epithelium and stromal layers and plays a critical role in the organization, maintenance and integrity of the overlying corneal epithelium [1-3]. This specialized matrix is highly complex with both biophysical and biochemical components [4, 5]. Cells integrate these external cues to trigger a cascade of intercellular mechanistic pathways that ultimately result in the modulation of specific cell phenotypes including proliferation, migration, adhesion, differentiation and apoptosis [6]. Each of these properties are essential for the formation and maintenance of an epithelial tissue. Current tissue engineering approaches seek to take advantage of these biophysical and biochemical features through the fabrication of substrates that mimic specific aspects of the native in vivo microenvironment derived from the ECM in order to promote or inhibit epithelial behaviors and improve the likelihood of the success of the engineered tissue. Currently available corneal prosthetics have focused mainly on the stromal and not the epithelial component of the tissue which may explain the poor re-epithelialization and ultimate failure of the replacement. With this approach, we believe that incorporation of ECM elements, specifically nano- and micron- scale topographic cues, as well as biochemical components in the form of adherent peptides from the basement membrane, will provide for missing elements in the current design and improve the future design of tissue replacements, specifically for the human cornea.
The complexity of the corneal BM presents a significant problem in regenerative medicine due to the limited information available regarding the biophysical and biochemical factors that dictate complex biological processes, such as the formation of epithelial tissue. The specific biophysical and biochemical elements from the BM that are critical to promote the creation and maintenance of the epithelial component within an engineered corneal tissue have yet to be clearly defined. Therefore, our strategy over the course of the last decade has focused on the systematic characterization and quantitation of biophysical cues from the native basement membrane of several different species and tissues, including the human cornea with the goal of incorporating some of these components back into a corneal prosthetic [7-11]. We have successfully quantified several biophysical characteristics including the size range of complex topographic features including intertwining fibers and pores of varying nanoscale and submicron dimensions and the compliance of the basement membrane on which human corneal epithelial cells (HCECs) reside [5, 12]. These characterized properties have been utilized as a guide for substrate design to establish the impact of specific basement membrane biophysical cues on essential HCEC behaviors. We have successfully demonstrated and confirmed the scale-dependent impact that nano- to micron-sized cues have on influencing adhesion [13], proliferation [14], migration [15] and contact guidance of HCEC cells [16-18].
In addition to topography, the basement membrane also presents bioactive adhesive molecules as well as soluble growth factors that are sequestered by the matrix. Systematic control of each set of cellular cues is critical in order to decouple the biophysical and biochemical cues and elucidate how each signal impacts HCEC behavior. For example, the HCEC response to topographic cues is influenced by several external environmental factors that include soluble biochemical factors within the tissue culture media. Specifically, the extent and preferred orientation of HCEC alignment is dependent on whether the cells are cultured in serum-containing or serum-free media. In previous experiments, cells were found to interact with the topographic surface through an irreversible adsorbed layer of protein that was deposited upon immersion of the silicon [16-20] or polyurethane-based [14, 15] substrates into the culture medium. HCECs plated onto biomimetic nano scale ridge/groove structures align either parallel or perpendicular to the underlying substrate topography depending on the cell culture medium [16-18, 20, 21].
More recent studies have investigated the combined impact of biochemical responses that modulate HCEC contact guidance and response to topographic cues through the exposure of HCEC cells to varying soluble factors using different growth media [17]. Although it is a reasonable assumption that the surface chemistry is homogeneous, the chemical composition of the adsorbed protein layer depends on several conditions, which include the substrate material, the components within the culture medium and the scale of the substrate topography [22-24]. Previous work using topographic features that allow for protein adsorption have been unable to separate the HCEC response to topographic cues due to surface chemical heterogeneities.
The objective of the current study was to incorporate ECM peptides onto topographically patterned substrates to control both topographic and adherent biochemical cues and demonstrate the influence on essential HCEC behaviors. The peptide we selected for incorporation onto topographically patterned substrates is Arg-Gly-Asp (RGD). The RGD peptide sequence is located within major ECM components including the proteins laminin and fibronectin, serves as an adhesive ligand to integrins, and has been demonstrated to impact HCEC behavior [25-30]. We have recently demonstrated successful control of the adherent biochemical environment through the immobilization of RGD on surfaces coated with reactive polymer multilayers fabricated by the layer-by-layer deposition of poly(ethylene imine) and poly(2-vinyl-4,4-dimethylazlactone) (PEI/PVDMA) [31-33]. These resulting PEI/PVDMA films allow for the stable and covalent integration of our peptide of interest (RGD), resulting in the control of cell-substrate attachment and the inhibition of non-specific protein absorption from the tissue culture media to the nano and micron scale substrates without disruption of the underlying topographic features [33]. HCEC cells will be exposed to varying soluble factors through two distinct culture media including serum-free EpiLife® or serum growth factor rich Epithelial medium. HCEC contact guidance to the RGD and control d-glucamine-modified topographic features provides a measurable endpoint to demonstrate that topography, surface chemistry and soluble components within the media cue cell behaviors both independently and in combination. Contact guidance of cells is highly correlated with the direction of cell migration [15], and provides an efficient endpoint to determine if the cells are integrating the physical and chemical cues of the material. The methods developed here also provide a means for high-throughput analysis of the cellular response to interactions with controlled surface chemistry, topography and soluble factors.
2. Materials and Methods
2.1 Materials
Branched poly (ethylene imine) (PEI, Mn = 10,000 g/mol, Mw = 25,000 g/mol) and solvents were purchased from Sigma-Aldrich (Milwaukee, WI). 2-Vinyl-4, 4-dimethylazlactone (VDMA) monomer was a kind gift from Steve Heilmann at 3M (Minneapolis, MN). Poly(2-vinyl-4,4-dimethylazlactone) (PVDMA, Mn = 18,162, PDI = 3.1) was synthesized according to methods described in Buck et al. [34]. The peptides GGGRGDSP (“RGD”) and GGGRDGSP (“RDG”) were synthesized at the University of Wisconsin Biotechnology Center (Madison, WI). d-Glucamine was purchased from TCI America (Portland, OR). Silgard-184 silicone elastomer base and curing agents were purchased from Dow Corning (Midland, MI). NOA81 optical adhesive was purchased from Norland Products (Cranbury, NJ). Cell culture and staining reagents were purchased from Invitrogen (Carlsbad, CA) unless otherwise noted.
2.2 Fabrication of micro and nanoscale ridge/groove features
Silicon chips containing six regions of ridge/groove features (400, 800, 1200, 1600, 2000, and 4000 nm in pitch) and flat control areas were fabricated using X-ray lithography as described previously [13, 16]. The silicon chips were used as masters for soft-lithography reproduction into NOA81 optical adhesive. PDMS stamps were generated as previously described [14, 35]. The resulting composite stamp consisted of a “hard” poly(dimethylsiloxane) (PDMS) to retain the topographic features and a pliable PDMS layer for easy removal and handling of the stamp. A dime-sized amount of NOA81 optical adhesive was deposited onto an oxygen-plasma-treated glass slide. Slides were placed in a spin coater for 40 seconds at 4000 rmp. The topographically patterned PDMS stamp was placed onto the NOA81 coated slides and cured in a XL-1500 UV cross-linker under 365 nm light for 100 minutes after which the stamp was carefully removed.
2.3 Fabrication of PEI/PVDMA films on topographically patterned substrates
Reactive PEI/PVDMA multilayer films were fabricated on glass substrates containing NOA81 molded topographic features using a layer-by-layer approach, as previously described [32]. Substrates were rinsed with acetone, ethanol, methanol, and deionized water, and then 1) submerged in a solution of PEI in acetone (20 mM with respect to molecular weight of the polymer repeat unit) for 30 seconds, 2) submerged for 30 seconds in two consecutive acetone rinse solutions, 3) submerged in a solution of PVDMA in acetone (20 mM with respect to the polymer repeat unit) for 30 seconds, and finally, 4) submerged in two more subsequent acetone rinse baths for 30 seconds each. Each cycle (including steps 1 through 4) allows for the addition of one layer. Two cycles were completed to form the PEI/PVDMA films used for this study. Substrates were immediately dried with compressed nitrogen and stored in a vacuum desiccator prior to use.
2.4 Quantitation of film thickness on topographically patterned substrates
To measure the thickness of the PEI/PVDMA films on our topographically patterned substrates, we used atomic force microscopy (AFM). Samples included for measurements were composed of unfunctionalized topographically patterned NOA81 to provide a baseline and substrates coated with PEI/PVDMA films as described above. The film thickness of the smallest topographic pitch of 400 nm on each sample was measured using atomic force microscopy (AFM). The data were acquired in tapping mode on a Nanoscope Multimode atomic force microscope (Veeco/Digital Instruments, Santa Barbara, CA) using scan rates of 3 mm/s to obtain 256×256 pixel images over an area of 3 mm × 3 mm. Silicon cantilevers with a spring constant of 40N/m were used (model NSC15/NoAl, MikroMaschUSA, Portland, OR). A minimum of five ridge widths per substrate were measured and averaged using the Nanoscope software (Veeco/Digital Instruments, Santa Barbara, CA).
2.5 Immobilization of RGD on film coated topographic substrates
Topographically patterned substrates coated with PEI/PVDMA films were functionalized with the cell attachment promoting peptide RGD or control scrambled peptide RDG using the following protocol. Peptide solutions were composed of 0, 5, 10, 15, and 20 mol% RGD or 20 mol% RDG, with the remaining percent composed of d-glucamine, a chemical motif that prevents the non-specific absorption of proteins (i.e. 10 mol% peptide + 90 mol% d-glucamine). The total sum concentration of the mixture components was held constant at 110 mM. Individual topographically patterned substrates coated with PEI/PVDMA films were coated with one of the peptide/d-glucamine solutions, allowed to react for 1 hour, followed by rinsing with DMSO and ethanol. Next, substrates were dried with compressed nitrogen and stored up to three days in a vacuum desiccator prior to use in cell culture. The peptide-modified films will be referred to by the percent peptide within the reaction solution used for fabrication. (e.g., 20% RGD/d-glucamine refers to films that result from reaction of PEI/PVDMA coated substrates with a solution containing 22 mM RGD and 88 mM d-glucamine). Individual peptide functionalized topographic substrates were placed into each well of a 12-well tissue culture plate (BD Falcon, CA). In preparation for tissue culture, all substrates were exposed to UV light for 15 min in a sterile laminar flow hood.
2.6 Primary human corneal epithelial cell harvest and culture
Human cadaver corneas were graciously donated by the Lions Eye Bank of Wisconsin (Madison, WI) or the Missouri Lions Eye Bank (Columbia, MO). Primary human corneal epithelial cells (HCEC) were harvested as previously described [16]. Following disaggregation of HCECs with a dispase solution (1.2 units/ml at 37° C for 4 hours, Boehringer Mannheim, Germany) cells from 2-4 corneas were centrifuged and resuspended in either Epithelial medium or in EpiLife® medium. Epithelial medium contained a 3:2 ratio of Ham's F12:Dulbelco's Modified Eagles medium (DMEM) supplemented with 2.5 % (v/v) fetal bovine serum (FBS), 0.4 μg/ml hydrocortisone, 8.4 ng/ml cholera toxin, 5 μg/ml insulin, 24 μg/ml adenine, 10 ng/ml epidermal growth factor, 100 units penicillin, and 100 μg/ml streptomycin [36, 37]. HCECs in epithelial medium were plated into 100 mm tissue culture plates containing a mitomycinc treated Swiss 3T3 fibroblast layer. EpiLife® medium was supplemented with a proprietary combination of bovine serum albumin, bovine transferrin, hydrocortisone, recombinant human insulin-like growth factor type-1, prostaglandin, and recombinant human epidermal growth factor (EpiLife® Defined Growth Supplement). Cells in EpiLife® medium were plated into 100 mm tissue culture plates coated with fibronectin and collagen (FNC coating mix, AthenaES, Baltimore, MD). All HCECs were incubated at 37°C and 5% CO2 until they reached approximately 70% confluence. Cells were used between passages 1 and 3 and were plated at a density of 10,000 cells per cm2 (for cells in epithelial medium) or 25,000 cells per cm2 (for cells in EpiLife® medium). The plating efficiency for cells in EpiLife® was decreased compared to those in regular epithelial medium, thus the higher plating density to allow for the same density of cells after plating. All cells were incubated for 24 hours after plating to allow for attachment and spreading.
2.7 Immunocytochemistry
To analyze our experimental endpoints, cells were fixed and stained to allow for visualization of the actin cytoskeleton and the nucleus. Following HCEC plating and 24 hours of incubation, the surfaces were rinsed with phosphate-buffered saline (PBS, pH 7.2). Cell morphology was preserved through fixation with 4% paraformaldehyde-PBS (Electron Microscopy Sciences, PA) at room temperature for 20 minutes. Following a PBS wash, the cells were permeabilized with 0.1% (v/v) Triton X –100 (Sigma-Aldrich, MO) in PBS for 7 minutes, washed in PBS for 10 minutes, and then immersed in 1% (w/w) bovine serum albumin (Sigma-Aldrich, St. Louis, MO) in PBS for 20 minutes to block non-specific binding. Cells were rinsed with PBS for 10 minutes, followed by incubation with 5 g/ml of FITC-phalloidin (Sigma-Aldrich, St. Louis, MO) containing 0.1 g/ml 4′,6-diamidino-2-phenylindole (DAPI) in PBS for 40 minutes, to label both filamentous actin (green or red) and the nucleus (blue). Following a final rinse with PBS, each substrate was stored in PBS until imaged.
2.8 Quantification of cell shape and orientation
Samples were imaged using a Zeiss Axiovert 200M fluorescence microscope (Carl Zeiss, Jena, Germany). Images of fluorescent cells on each of the substrates were obtained using a 10X objective lens. At least four images were taken of cells within each topographically patterned feature size as well as control flat areas within the same sample. Image analysis was performed using AxioVision software (Carl Zeiss, Jena, Germany). The total cell number, area, angle of alignment and elongation factor measurements were collected from cells within each image as previously described [16, 38]. Cell elongation is defined as the ratio between the length and breadth of the cell. A cell was deemed elongated if this factor was greater than 1.3 [16]. Alignment angle was defined as the angle between the long axis of the cell and the long axis of the underlying topographic pattern. A cell was considered aligned parallel if the angle was less than 10° and perpendicular if the angle was between 80° and 90°. For more detailed analysis, cells were sorted into 10 degree increments (0-10, 10-20, 20-30, 30-40, 40-50, 50-60, 60-70, 70-80, and 80-90 degrees). Results represent the arithmetic mean percent of the total single cell population.
2.9 Competitive RGD binding assay with soluble peptide
HCECs were seeded onto topographically patterned substrates that consisted of control unfunctionalized NOA81 alone or coated with 10% RGD/d-glucamine films. Cells were allowed to attach and spread for 24 hours. The 100 mM stock solutions of RGD and RDG were prepared by dissolution in sterile deionized water. The control diluent solution of sterile water alone or one of the prepared 100 mM peptide solutions was added to the HCEC culture medium resulting in a final concentrations of 1 mM peptide. Cells were incubated with diluent or soluble RGD or RDG peptide and images were acquired at time 0 and 24 hours following incubation. A minimum of seven phase images for each topographic substrate were taken using a Zeiss Axiovert 100M microscope at a magnification of 10X. Each image was analyzed for the total cell number. For each topographic area, the average cell number at 24 hours was normalized to the average cell number obtained at hour 0.
2.10 Statistical analysis
Experiments were analyzed using analysis of variance (ANOVA). When variability was determined to be significant (P < 0.05), the t-test was used to determine significance (P < 0.05) between groups. Significance was further divided into “statistically significant” (0.01 ≤ P < 0.05), “very significant” (0.001 ≤ P < 0.01), and “extremely significant” (P < 0.001).
3. Results
3.1 Thickness of PEI/PVDMA films on topographic features
To determine the impact of controlled surface chemistry on HCEC cell morphology and contact guidance, we fabricated peptide-modified (RGD or RDG) topographically patterned substrates. Ridge/groove patterned substrates with pitches of 400 to 4000 nm were coated with PEI/PVDMA films composed of two bilayers and then treated with 110 mM (peptide + d-glucamine) solutions containing RGD and d-glucamine (0 to 20 mol% RGD) or the control peptide RDG, a non-cell adhesive scrambled RGD peptide [39-43], and d-glucamine to immobilize the molecules and create surfaces that elicit specific cell-substrate interactions. A schematic demonstrating our fabrication process is illustrated in Figure 1.
Figure 1. Schematic of the topographic features following deposition of PEI/PVDMA.

Two bilayers of PEI/PVDMA films were deposited onto topographic features with six different pitches: 400, 800, 1200, 1600, 2000, and 4000 nm. (1:1 ration of groove/ridge width). A film thickness of 20 nm was determined using AFM by quantitating changes in the measurement of ridge width when compared to unmodified control topographic substrates. The addition of the PEI/PVDMA and increase in ridge width is depicted in purple. The chemical addition of RGD or RDG peptide is also represented.
Prior to cell plating experiments, the integrity of the topographic features following deposition of the PEI/PVDMA films was examined using atomic force microscopy (AFM). First, we imaged the control unfunctionalized NOA81 substrates which demonstrated uniform ridge/groove topographic features on each pitch (400-4000 nm) with an average ridge/groove ratio of 1:1 and a depth of 300 nm. Changes to the topographic substrates would be most evident on the smallest pitch; therefore, quantitative measurements on the 400 nm surfaces were obtained to provide a baseline prior to film deposition. Quantitative measurement of the ridge width of the smallest pitch of 400 nm demonstrate the largest variation of the 1:1 ridge/groove ratio with ridge measurements of 220 ±5 nm. Following PEI/PVDMA film deposition, the ridge width of 220 nm increased by an average of 40 nm to 260 nm, corresponding to a film thickness of about 20 nm, which is in agreement with previous measurements of film growth on planar silicon substrates [44]. Despite the change in ratio of ridge to groove width on the smallest 400 nm pitch, a substantial groove (i.e. 180 nm grooves decreased to 140 nm) remained as illustrated in Figure 1. In addition, the small increase in width insignificantly alters the dimensions of the topographic pitch above 400 nm. In summary, our measurements confirm that deposition of the PEI/PVDMA films did not significantly distort or alter the topographic features.
3.2 Confirmation of specific cell-RGD interactions on topographic features
Prior to cell contact guidance experiments, we confirmed that HCEC cells bind to functionalized, topographically patterned substrates through specific binding to the RGD peptide. To demonstrate specificity, we measured several endpoints including HCEC attachment, spreading, and competitive inhibition in the presence of soluble RGD peptide. For each of these endpoints, substrates were prepared by coating the 400 to 4000 nm pitch topographic features with PEI/PVDMA films and then treating the substrates with mixtures of 0 to 20 mol% RGD (remaining % is /d-glucamine). HCECs in epithelial or EpiLife® media were plated onto the substrates and were allowed to attach and spread for 24 hours. Cells were fixed and stained for actin (phalloidin-FITC) and nuclei (DAPI).
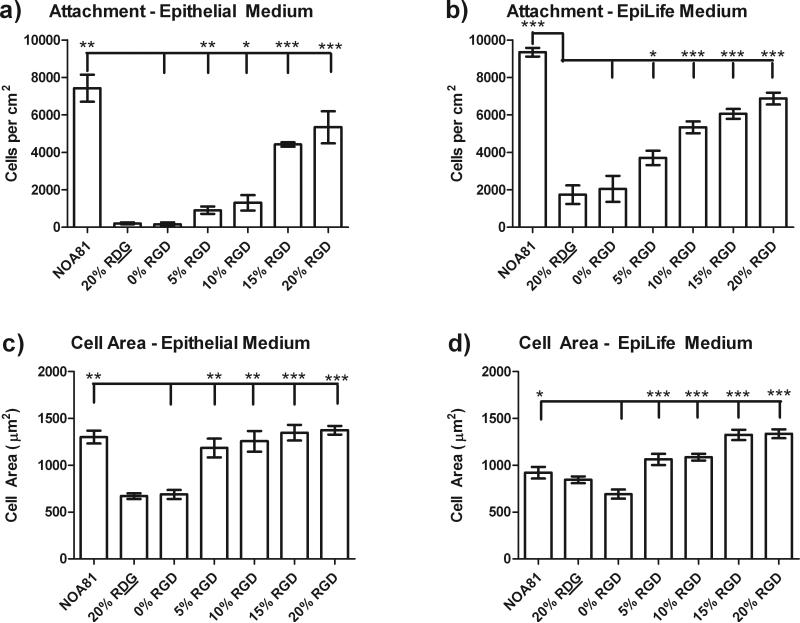
As expected, higher concentrations of RGD peptide on both the flat and topographically patterned substrates allowed for a linear increase in the total number of HCEC cells attached to the substrate regardless of the media type (Figure 2). In epithelial medium, a limited number of cells attached to the 20% RDG/d-glucamine and 0% RGD/d-glucamine controls with 212 +/- 53 and 159 +/- 100 cells/cm2, respectively (see Figure 2A). HCECs that were attached to these substrates did not spread well and retained a rounded morphology. In comparison to cells on the 0% RGD/d-glucamine films, HCECs demonstrated a 6, 8, 28, 34, and 47-fold significant increase in the number of cells attached to the 5%, 10%, 15%, 20% RGD/d-glucamine and unmodified NOA81 substrates, respectively. In addition, compared to 0% RGD/d-glucamine surfaces, the HCEC projected area was significantly greater, 1.5- and 2-fold, on the 5 to 20% RGD/d-glucamine and NOA81 surfaces (Figure 2C).
Figure 2. HCEC attachment and cell area increased with increasing levels of RGD.
HCEC attachment in epithelial medium (a) and EpiLife® medium (b) monotonically increased with increasing %RGD in the RGD/d-glucamine solution used to functionalize topographically patterned and flat substrates. HCEC projected area in Epithelial (c) and EpiLife® (d) media also demonstrated a monotonic increase with increasing %RGD. Since cell attachment and spreading was equal on topographically patterned and flat substrates, the data presented here is representative data over all of the surfaces. NOA81 substrates, which allow for non-specific protein adsorption, were used as a positive control for cell attachment and spreading. *0.01≤P<0.05, **0.001≤P<0.01, ***P<0.001
Similar to cells in epithelial medium, HCECs in EpiLife® medium demonstrated monotonically increasing cell attachment and projected area with increasing %RGD in the RGD/d-glucamine mixture. A number of cells attached to the 0% RGD/d-glucamine and 20% RDG/d-glucamine controls (2048 +/- 697 and 1746 +/- 498 cells/cm2, respectively); however, they retained a rounded morphology. Increasing the amount of RGD in the solution resulted in monotonically increasing cell attachment (Figure 2B). A two to five-fold increased number of cells attached to the 5 to 20% RGD/d-glucamine and unmodified NOA81 surfaces compared to the 0% RGD/d-glucamine controls (Figure 2B). Similarly, HCECs demonstrated a 1.5 to 2 fold increase in projected cell area on the 5 to 20% RGD/d-glucamine surfaces compared to the 0% RGD (Figure 2D). In summary, the relative amount of RGD bound to the surface proved to be the most significant predictor of HCEC attachment and spreading on RGD-modified topographic features and flat controls regardless of the cell culture medium used.
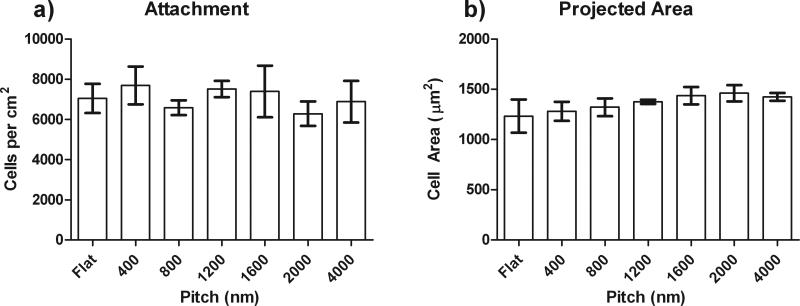
We also investigated whether the addition of functionalized RGD to our topographic substrates in either epithelial medium or Epilife altered the total cell attachment or overall cell area of the cells (Figure 3). Representative data from the quantitative analysis of HCEC cell attachment and area from cells plated on the highest concentration of 20% RGD peptide is shown. The HCECs on the highest density of RGD peptide on each topographic surface maintained an average of 7,000 total cells (A) and an area of 1200 m2 (B) regardless of the media or topographic feature. Together, these data demonstrate that HCEC attachment and spreading to RGD/d-glucamine-modified surfaces is dependent on the relative amount of immobilized RGD and is not inhibited or enhanced by the presence of topographic cues.
Figure 3. HCEC attachment and cell area was not dependent on the topography.
HCEC attachment (a) and projected cell area (b) on RGD/d-glucamine modified films were similar on flat and all pitches of ridge/groove topographic features functionalized with RGD/D-glucamine. The data presented here is representative data for surfaces fabricated from solutions of 20% RGD/d-glucamine. *0.01≤P<0.05, **0.001≤P<0.01, ***P<0.001
To further confirm that the presence of topography did not compromise the specificity of the cell-surface interaction, we performed competitive binding experiments with soluble RGD peptide. For simplicity, we tested 10% RGD/d-glucamine-modified surfaces since our previous work has demonstrated competitive inhibition of corneal epithelial cell attachment on 3 to 20% RGD/d-glucamine surfaces in the presence of soluble RGD [45]. HCECs were allowed to attach for 24 hours to unmodified NOA81 topographic features and 10% RGD/d-glucamine-modified features. Cells were then exposed to soluble RGD or RDG peptides at a total concentration of 1 mM or in the absence of peptide. After 24 hours of incubation with soluble RGD, approximately 50% of the HCECs detached from the 10% RGD/d-glucamine films (on all pitches and flat controls, data not shown). HCECs incubated with 1 mM RDG or no peptide remained attached to 10% RGD/d-glucamine-modified substrates. Likewise, HCECs did not significantly detach from unmodified NOA81 surfaces with either RGD or RDG peptides (for all pitches and flat controls).
3.3 HCEC alignment on RGD functionalized topographic cues in Epithelial medium
We have previously demonstrated that HCEC cell orientation to the underlying topography changes depending on the soluble environment provided by the tissue culture media [16, 17, 19, 21, 46-48]. First, we investigated the impact that controlled cell-substrate interactions through the RGD peptide would have on the ability of HCECs to align to topographic cues in epithelial medium. Fluorescently labeled HCECs on topographically patterned functionalized control RDG and varying densities of RGD substrates were imaged and analyzed for alignment.
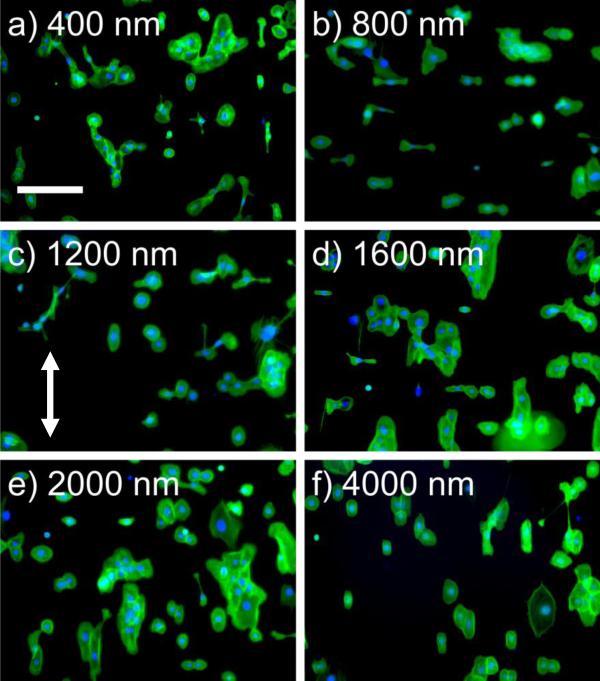
Twenty-four hours after plating, limited cell attachment was observed on control 0% RGD/d-glucamine and scrambled control 20% RDG/d-glucamine films and no alignment was calculated. On unfunctionalized NOA81 and 5-20% RGD/d-glucamine topographically patterned surfaces, HCECs in epithelial medium demonstrated an alignment response that was dependent on the pitch of the ridge/groove topography (Figure 4). For simplicity, only the 20% RGD/d-glucamine data is shown. Qualitative images of the HCECs on unmodified topographic substrates and 20% RGD/d-glucamine topographic features suggest increased parallel alignment on the 400 and 4000 nm pitch and a mixture of perpendicular and parallel alignment on the 800 to 1200 nm pitch as compared to flat controls.
Figure 4. The presence of RGD does not promote a change in the HCEC contact guidance in Epithelial medium.
HCECs in epithelial medium on functionalized (20%RGD/D-glucamine) topographically patterned surfaces demonstrate a similar alignment response. Representative images of HCEC cells on topographic 20% RGD/d-glucamine-modified surfaces stained with FITC-phalloidin (green) and DAPI (blue). The arrow denotes the direction of the ridge/groove structures. Predominantly parallel alignment was observed for HCECs on a) 400 nm and f) 4000 nm pitch ridge/groove features. Strong perpendicular alignment was observed for HCECs on b) 800 nm pitch topography. A mixture of parallel and perpendicular cells were observed on c) 1200 nm, d) 1600 nm and e) 2000 nm pitch topography. Scale bar = 200 μm.
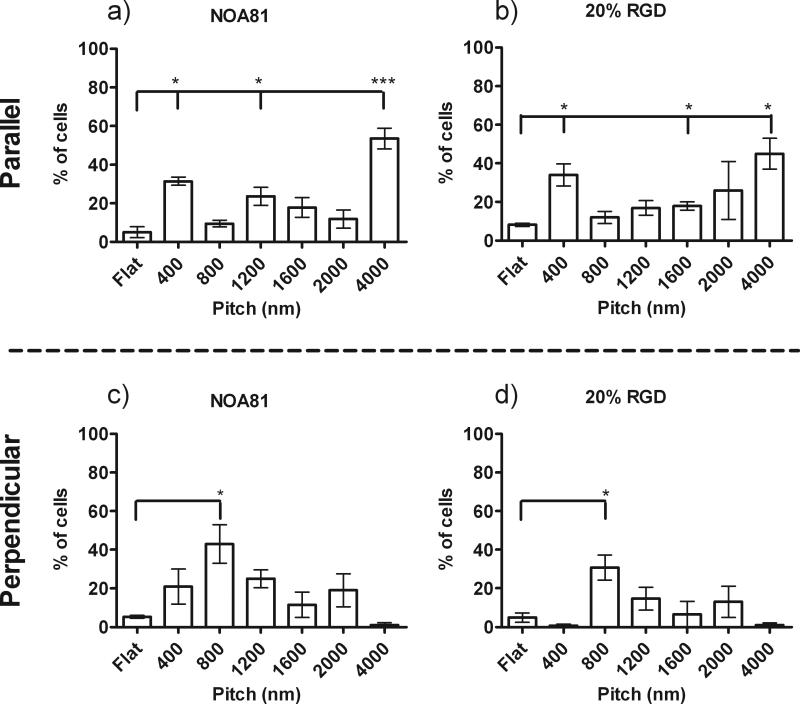
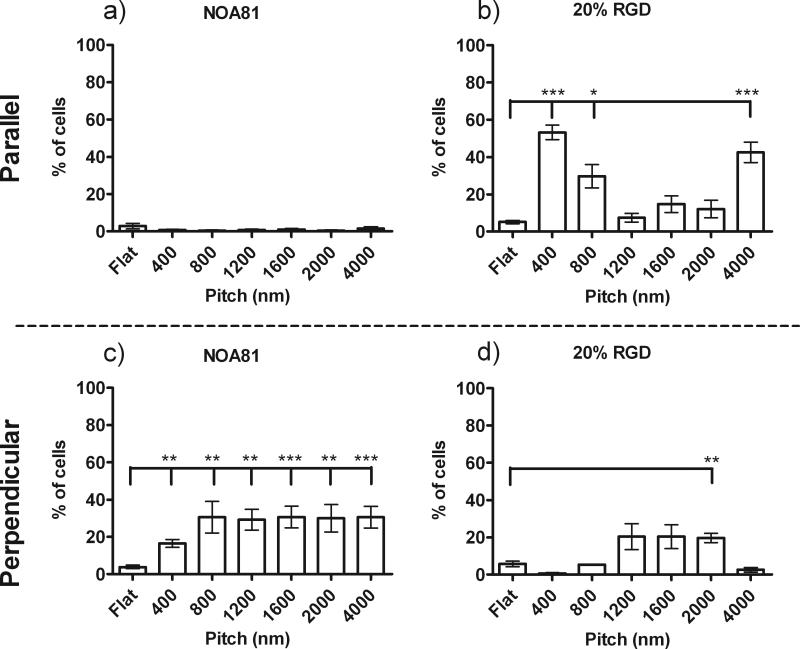
Quantitative analysis demonstrates that the most significant parallel alignment was observed on the 400 and 4000 nm pitch features with 3- to 5-fold more cells elongated and oriented to the topographic cues when compared to flat controls (Figure 5a and b). To a slightly lesser degree, there was an increase in parallel cell alignment of the HCECs to 1200 and 1600 nm pitch surfaces compared to flat controls. HCECs on unmodified NOA81 and 20% RGD/d-glucamine topographic features demonstrated increased perpendicular alignment (i.e. angle of cell major axis between 0 and 10° from topography) on the 800 to 2000 nm pitch features compared to flat controls. HCECs exhibited the most significant perpendicular alignment on the 800 nm pitch with 2- to 4-fold more cells aligned perpendicular to the 800 nm pitch features compared to all other pitches and flat controls, Figure 5c and d). In conclusion, addition of the RGD peptide to the topographic substrates did not alter the HCEC response to topographic cues when cultured in Epithelial medium.
Figure 5. Quantitative Measurement of HCEC orientation on RGD functionalized topographically patterned substrates in Epithelialmedium.
HCECs in epithelial medium demonstrate similar alignment responses to topographic features on unmodified NOA81 (a and c) and all RGD/d-glucamine -modified substrates, including 20% RGD/d-glucamine (b and d). Significantly more cells aligned parallel (long axis of cell <10° from long axis of ridges) to the underlying ridge/groove features with pitches of 400 nm and 4000 nm compared to flat controls. In addition, HCEC alignment was predominantly perpendicular (80°< long axis of cell <90° from long axis of ridges) to 800 nm pitch topography. Some parallel cell alignment was also measured on the 1200 nm, 1600 nm and 2000 nm pitch topography. *0.01≤P<0.05, **0.001≤P<0.01, ***P<0.001. Graphs are representative of experiments completed in triplicate.
3.4 HCECs alignment in EpiLife® medium
Next, we investigated how the addition of controlled surface chemistry through RGD in the presence of topographic cues in a different culture medium, EpiLife® Basal Medium, would impact HCEC contact guidance. HCECs in EpiLife® medium were stained, imaged and analyzed for elongation and alignment 24 hours after plating onto control unfunctionalized and RGD modified topographic substrates (Figure 6). Similar to HCEC contact guidance in epithelial medium, control surfaces that did not support significant cell attachment and spreading including the 0% RGD/d-glucamine and 20% RDG/d-glucamine film did not demonstrate preferential alignment of cells to any topographic features and flat controls (data not shown).
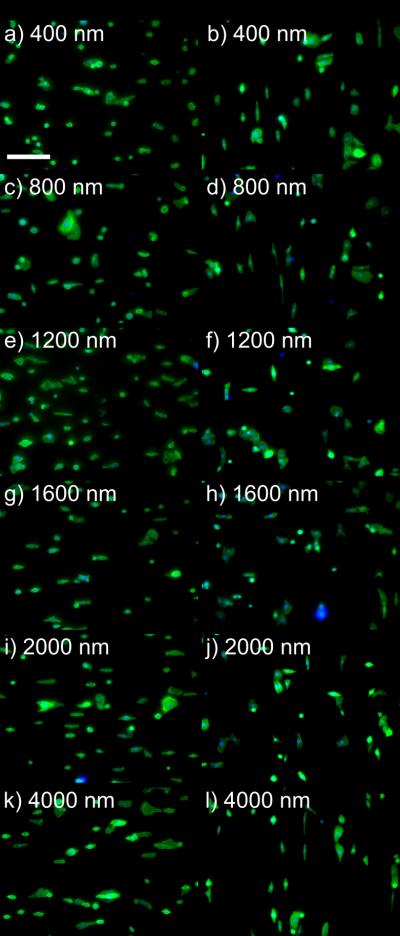
Figure 6. The presence of RGD to HCECs in EpiLife® alters the cell orientation to topographic cues.
HCECs in EpiLife® medium demonstrated different alignment responses to topographic features when comparing cell orientation on unfunctionalized topography to RGD/d-glucamine-modified topographic substrates. Representative images were taken of HCEC cells on each substrate. Twenty-four hours post-plating, cells were stained with FITC-phalloidin (green) and DAPI (blue). Perpendicular alignment was observed for HCECs on all unmodified NOA81 pitch ridge/groove substrates (a, c, e, g, i, k). On topography functionalized with 20% RGD/d-glucamine, predominantly parallel alignment was observed for HCECs on b) 400 nm, d) 800 nm and l) 4000 nm pitch ridge/groove features, while a mixture of parallel and perpendicular cells were observed on c) 1200 nm, d) 1600 nm and e) 2000 nm pitch topography. Scale bar = 200 μm.
Initial observations of HCEC cells in EpiLife® demonstrate the differences in the contact guided response of the cells on unfunctionalized NOA81 or RGD/ d-glucamine modified substrates. HCEC cells on NOA81 exhibited a perpendicular alignment to the topography for the 400 nm through 4000 nm pitch (Figure 6a,c,e,g,i,k) whereas the 20% RGD modified substrates exhibited preferential alignment in the direction of the topography on the 400 nm, 800 nm and 4000 nm pitch (Figure 6b, d, l) and a mixture of perpendicular and parallel alignment on the 1200 through 2000 nm pitch (Figure 6f, h, and j).
Quantitative analysis of the HCEC response to alignment verified the results from our qualitative observations (Figure 7). HCECs on unmodified NOA81 topographic features did not demonstrate significant parallel alignment to any ridge/groove features and flat controls (Figure 7a). However, HCECs on the unmodified NOA81, exhibited a 2-fold increase in perpendicular alignment on the 400 nm pitch features, and a 4-fold increase on the 800 nm to 4000 nm pitch features compared to flat controls (Figure 7b).
Figure 7. Quantitation of HCEC orientation and alignment on RGD functionalized topographic substrates in EpiLife®.
Primary HCEC cell cultured in EpiLife® medium demonstrate altered contact guidance to unfunctionalized topography (a and c) and all RGD-functionalized substrates, including 20% RGD (b and d). HCEC cells plated onto unmodified topography aligned perpendicular to all pitch ridge/groove features. On RGD modified topography, significantly more cells aligned parallel (long axis of cell <10° from long axis of ridges) to underlying ridge/groove features with pitches of 400, 800 and 4000 nm compared to flat controls. A significant increase in perpendicular alignment was observed on the 2000 nm pitch topography. Some parallel or perpendicular cell alignment was measured on the 1200 nm and 1600 nm pitch topography. *0.01≤P<0.05, **0.001≤P<0.01, ***P<0.001
In contrast to the perpendicular alignment of HCECs on unmodified NOA81, HCEC cell alignment in EpiLife® medium on RGD/d-glucamine-modified topographic features exhibited parallel and/or perpendicular alignment response, depending on the pitch of the topography. On 5 to 20% RGD/d-glucamine-modified features, HCEC parallel and perpendicular alignment profiles were similar, demonstrating no dependency on the relative amount of RGD immobilized on the surface. For simplicity, only the cell data for 20% RGD/d-glucamine surfaces is reported since these surfaces allowed for the highest numbers of cells to attach, thus providing the most robust cell analysis. HCECs aligned predominately parallel to 400, 800 and 4000 nm ridge/groove features, demonstrating a 7 to 10-fold increase in parallel cell alignment to these three pitches compared to flat controls (Figure 7b). HCEC demonstrated both parallel and perpendicular alignment to pitches of 1200, 1600 and 2000 nm. Specifically, between 1.5- and 2-fold more cells aligned parallel, and between 2 and 3-fold more cells aligned perpendicular to 1200, 1600 and 2000 nm topographic features compared to flat controls (Figure 7b and d). In summary, our results demonstrate the impact of soluble cues from the media and RGD on the contact guidance of HCEC cells.
4. Discussion
Due to the ability to control the surface chemistry presented to the HCEC cells, the RGD/d-glucamine-functionalized substrates enable the investigation of topographic cueing of HCECs under conditions in which the biophysical cue is presented independently from either the surface chemical composition or soluble factors. This is different from past studies that have used substrates that allow for the adsorption of proteins from the cell culture medium. Although these past studies have reported differences in HCEC behavior due to soluble factors within the media, they have been unable to decouple the effects of surface adsorbed proteins from the effects of soluble culture medium components on the HCEC response to topographic cues [17]. In order to elucidate which particular biochemical cue or binding motif is responsible for alterations in response to topographic cues and begin to explore the internal mechanistic pathways regulating cell response to biophysical cues it is essential to fabricate substrates that present a single cell-binding peptide.
This study explores the effects of a single cell-binding domain on HCEC response to topographic cues by coating ridge/groove features with reactive PEI/PVDMA films and subsequently immobilizing an RGD-containing peptide and the non-fouling molecule d-glucamine on the surface. We chose RGD because it is a well-studied cell-binding motif that binds with transmembrane integrins and is found in extracellular matrix proteins including laminin and fibronectin. In addition, recent studies have demonstrated that cell attachment via RGD-integrin binding and subsequent focal adhesion formation directly impact actin cytoskeleton formation and contraction [49-51] and this has been proposed as a main mechanism through which cells are cued by biophysical properties of their extracellular environment [52-54].
Previous studies have demonstrated that PEI/PVDMA films can coat a range of materials with complex geometries which would allow for the addition of biochemical cues to a wide range of materials available for tissue engineering [34, 44]. In addition, a recent study has confirmed using confocal microscopy that PEI/PVDMA films coat uniformly the side walls and the bottoms of micron-scale wells [55]. In this report, NOA81 nano- and micron-scale ridge/groove features were coated with PEI/PVDMA multilayers. We confirmed that the reactive PEI/PVDMA multilayers coated the topographic features with a conformal film through AFM measurements. AFM measurements demonstrated that the smallest features (400 nm pitch) coated with films two bilayers thick (20 nm films) retained the grooves between ridge tops and that the larger pitch feature dimensions were insignificantly affected. These data demonstrate that PEI/PVDMA films can be successfully deposited onto the topographic features of these patterned culture substrates, thus providing a rapid method for creating a reactive surface for further chemical modification for tissue engineering as well as other applications.
The topographically patterned substrates coated with reactive PEI/PVDMA multilayers and treated with RGD and d-glucamine promoted well-defined cell-RGD interactions. Control experiments demonstrated that cell attachment and spreading was minimal on topographic and flat substrates coated with PEI/PVDMA films and treated with a mixture of a non-adhesive peptide RDG, a scrambled sequence of RGD, and d-glucamine. In contrast, a 2- to 4-fold increases in the concentration of RGD (thus, increases in the surface density of RGD) promoted a monotonic increase in the number of cells attaching to topographically patterned and flat substrates. This observation is in agreement with a previous study that demonstrated a similar monotonic increase in hTCEpi (an immortalized human corneal epithelial cell line) attachment to flat PEI/PVDMA films functionalized with 0 to 20% RGD/d-glucamine [33]. It is interesting to note that the number of attached HCECs were similar on both the flat and topographically patterned areas for a given %RGD/d-glucamine. A cell on a flat surface is exposed to approximately twice the surface area, and thus approximately twice the RGD-motifs, than a cell on topographically pattered substrates [13, 16]. The observation of similar HCEC attachment on both the flat and topographic surfaces suggests that the adhesive quality of the available surface area may be more influential for cell attachment than the overall number of RGD-motifs to which a cell is exposed, a concept that has been proposed by others [56, 57]. In a final assay, HCECs rounded and detached from the RGD/d-glucamine-modified topographic features at the same rate as cells on RGD-modified flat films when exposed to soluble RGD. This observation demonstrates the competitive inhibition of integrin binding to surface-bound RGD motifs, which is indicative of specific integrin-RGD interactions and consistent with previous studies [33, 58-62]. Together, these observations demonstrate that culture substrates with topographic features can be coated with amine-reactive PEI/PVDMA films and treated with RGD/d-glucamine mixtures to provide chemically functionalized topographically patterned substrates that promote specific cell-substrate interactions.
HCEC contact guidance to RGD/d-glucamine-modified topographic cues was dependent on a combination of multiple factors: the scale of topographic cues, the surface chemical composition and the soluble cell culture medium. Contact guidance of cells has at least two significant implications: (1) this method can be a predictive model for the direction of migration, as we have previously demonstrated [15, 63], and (2) it is an efficient method to determine if the cells are integrating the physical cues of the underlying topographic features. Previous studies have demonstrated that changes in the scale of topography and culture medium can affect HCEC contact guidance individually and in combination [16-18, 21]. In these previous investigations it was not possible to decouple the effects of the culture medium components in solution and adsorbed onto the surface on the HCEC response to topographic cues. In addition, there may be differences in the levels of protein adsorption due to the scale of the topographic features that would affect the cell response [22-24]. In this report, we were able to decouple these effects and demonstrate that both the surface chemical composition and soluble composition individually affect how cells respond to nano and micron scale topography. The determination of the biophysical and biochemical cues will allow for the integration of the appropriate topographic cues into the design of biomaterials. For example, these cues could promote the directionality and speed of migration, as well as proliferation and differentiation to eventually populate a biomaterial. Ultimately this process may encourage faster tissue regeneration as well as the integrity and strength of the epithelial tissue.
HCEC contact guidance to nano and micron scale ridge/grove features in serum-containing medium was similar on both the RGD/d-glucamine-modified and unfunctionalized control surfaces. HCECs in epithelial medium aligned to all topographic cues (400 to 4000 nm pitches) and the direction of alignment, either parallel or perpendicular, was dependent on the pitch of the topography. Similar HCEC alignment responses were observed on topographic cues that allowed for protein adsorption (i.e. unmodified NOA81) and those with controlled cell-RGD interactions. These results demonstrate that (1) the scale of the topography, not differences in the adsorbed proteins, is regulating the cell response to topographic cues, (2) RGD-integrin binding is one mechanism through which cells internalize and communicate biophysical cues, and (3) the surface chemistries of both the adsorbed protein layer on NOA81 and RGD/d-glucamine-modified films cue cells in a similar fashion. One theory as to why the NOA81 surface cues cells similarly to the RGD-modified films is that the adsorbed proteins, from the epithelial medium, present several RGD motifs to which the cells bind. This idea is supported by the fact that epithelial medium contains 2.5% fetal bovine serum, which is an undefined complex mixture of proteins that contain extracellular matrix proteins with RGD-binding domains including fibronectin and vitronectin [64, 65].
When the cell culture medium does not contain serum, the response of HCECs to topographic cues is different between surfaces that are modified with RGD and surfaces that promote protein adsorption. HCEC alignment in EpiLife® Basal Medium exhibited perpendicular alignment to all topographic pitches on unmodified NOA81 surfaces that allowed for non-specific protein adsorption. In contrast, on substrates that promoted specific RGD-cell interactions, cells demonstrated parallel alignment to 400, 800 and 4000 nm pitches, and combined parallel and perpendicular alignment on 1200, 1600 and 2000 nm pitches. The observation that HCECs elongate and align to all RGD-modified topographic pitches further demonstrates that the topography is cuing the cells through RGD-integrin mechanisms. In addition, the switch in contact guidance from perpendicular to parallel between cells on protein adsorbing surfaces and RGD-modified surfaces, respectively, indicate that the surface chemical composition is affecting how cells respond to topographic cues. Potentially, the medium components in EpiLife® that adsorb to the unmodified NOA81 surface are not similar to RGD and/or do not activate the same intercellular pathways that the RGD-integrin interaction activates. Unlike epithelial medium that contains serum, EpiLife® contains a more defined set of soluble factors, such as growth factors. The limited factors that are in EpiLife® may result in fewer RGD motifs on the surface compared to those in the Epithelial medium providing an explanation as to why the NOA81 surfaces in EpiLife® cue the HCECs in a manner that is significantly different from the RGD-modified surfaces.
Under conditions where the surface chemical composition and topographic features are identical (i.e. RGD-modified topographic features), HCEC contact guidance demonstrates some dependency on the soluble medium components. On most topographic features, HCEC contact guidance to RGD-modified topography in both EpiLife® and Epithelial medium was similar, suggesting that the two main factors that dictate HCEC contact guidance are the scale of the topographic features and the surface chemical composition. In both media, HCECs aligned predominantly parallel to 400 and 4000 nm pitch surfaces and demonstrated mixed parallel and perpendicular alignments to 1200, 1600, and 2000 nm pitch surfaces. However, there was one notable exception that demonstrates that the soluble environment also plays a critical role: HCECs in EpiLife® aligned parallel to 800 nm pitch ridge/groove features, while HCECs in epithelial medium aligned perpendicular to 800 nm pitch features. These results demonstrate that HCEC contact guidance is not only modulated by the scale of topographic cues and the surface chemical composition, but it is also modulated by the soluble environment, as previous studies have also demonstrated [17, 21].
Future studies that examine how individual and combinations of cell-binding motifs and biophysical cues affect intercellular mechanisms can be achieved by using the fabrication method presented here. Surface composition can be modulated by functionalizing the free azlactone-motifs of the PEI/PVDMA films with extracellular matrix peptides, including RGD, AG73 [66, 67], YIGSR [68, 69], and IKVAV [69, 70], and combinations thereof. These studies may help elucidate the mechanistic pathways associated with the cellular response to topographic cues by probing one pathway at a time. Such studies may help explain, for example, why HECE's preferentially aligned parallel to 400 and 4000 nm pitch surfaces in higher percentages than on 1200 to 2000 nm pitch surfaces. Understanding how HCECs respond to these changes in biophysical and biochemical cues can be used to advance the field of biomaterials design for improved in vitro cell culture, prosthetics and many other biotechnology applications.
5. Conclusions
We examined the effects of well-defined topographic features with controlled cell-substrate interactions on HCEC contact guidance in two different culture media. PEI/PVDMA reactive multilayer films were used to coat substrates with six different ridge/groove topographically patterned areas ranging from 400 to 4000 nm pitch. The surfaces of the films were functionalized with RGD and d-glucamine to promote specific cell-RGD interactions. HCECs demonstrated similar RGD-mediated attachment and spreading on both topographically patterned and flat surfaces. HCEC aligned to RGD/d-glucaminefunctionalized topographic features in both culture mediums. Results obtained from HCECs cultured in two distinct media demonstrate that HCEC contact guidance is dependent on the combination of the following three cues: topography, the chemical composition of the cell substrate, and the soluble medium components. Specifically, the HCECs plated in the presence of Epilife exhibited a significant increase in parallel alignment in the presence of RGD peptide on the surface as opposed to the preferential perpendicular alignment observed on substrates that allow for non-specific adsorption of proteins. Together, the data presented here highlight that the scale of topographic cues, the surface chemical composition and the cell culture medium independently and in combination cue HCEC behavior.
Supplementary Material
Supplementary Figure 1. AFM images demonstrate no disruption of the topographic features. Atomic force microscopy (AFM) data were acquired in tapping mode on a Nanoscope Multimode atomic force microscope (Veeco/Digital Instruments, Santa Barbara,CA) using scan rates of 3μm/s to obtain 256 × 256 pixel images over an area of 3 μm × 3 μm. Silicon cantilevers with a spring constant of 40N/m were used (modelNSC15/NoAl,MikroMaschUSA,Portland,OR). The ridge width was calculated from images using the Nanoscope software (Veeco/Digital Instruments, Santa Barbara, CA) for both the control acetone treated and substrate with two bilayers.
Acknowledgements
The authors would like to thank Natasha Kalkhof and Rachel Conti for their technical support. The authors would also like to thank Dr. Christopher Thode, Bernardo Yanez-Soto, Dr. Maren Buck, Prof. William Murphy, and Michelle Wilson for their helpful discussions. Support to P. F. N. and C. J. M. was provided by the NIH-National Eye Institute (1RO1EY017367-01A and 1RO1EY0161134-01A2) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Eye Institute or the NIH. Support to D. M. L. was provided by the NSF (DMR-0520527) through a grant to the Materials Research Science and Engineering Center (MRSEC) at the University of Wisconsin. A. H. B. is a NSF Graduate Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
None
References
- 1.Kolega J, Manabe M, Sun TT. Basement membrane heterogeneity and variation in corneal epithelial differentiation. Differentiation. 1989;42:54–63. doi: 10.1111/j.1432-0436.1989.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 2.Nishida T. The cornea: stasis and dynamics. Nippon Ganka Gakkai Zasshi. 2008;112:179–212. [PubMed] [Google Scholar]
- 3.Zieske JD, Mason VS, Wasson ME, Meunier SF, Nolte CJM, Fukai N, et al. Basement membrane assembly and differentiation of cultured corneal cells: importance of culture environment and endothelial cell interaction. Experimental Cell Research. 1994;214:621–33. doi: 10.1006/excr.1994.1300. [DOI] [PubMed] [Google Scholar]
- 4.Timpl R, Aumailley M. Biochemistry of basement membranes. Advances in Nephrology from the Necker Hospital. 1989;18:59–76. [PubMed] [Google Scholar]
- 5.Last JA, Liliensiek SJ, Nealey PF, Murphy CJ. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. Journal of Structural Biology. 2009;167:19–24. doi: 10.1016/j.jsb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeBleu VS, MacDonald B, Kalluri R. Structure and Function of Basement Membranes. Experimental Biology and Medicine. 2007;232:1121–9. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- 7.Abrams GA, Schaus SS, Goodman SL, Nealey PF, Murphy CJ. Nanoscale topography of the corneal epithelial basement membrane and descemet's membrane of the human. Cornea. 2000;19:57–64. doi: 10.1097/00003226-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Abrams GA, Bentley E, Nealey PF, Murphy CJ. Electron microscopy of the canine corneal basement membranes. Cells Tissues Organs. 2002;170:251–7. doi: 10.1159/000047929. [DOI] [PubMed] [Google Scholar]
- 9.Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell and Tissue Research. 2000;299:39–46. doi: 10.1007/s004419900074. [DOI] [PubMed] [Google Scholar]
- 10.Liliensiek SJ, Nealey P, Murphy CJ. Characterization of endothelial basement membrane nanotopography in rhesus macaque as a guide for vessel tissue engineering. Tissue Engineering Part A. 2009;15:2643–51. doi: 10.1089/ten.tea.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brody S, Anilkumar T, Liliensiek S, Last JA, Murphy CJ, Pandit A. Characterizing nanoscale topography of the aortic heart valve basement membrane for tissue engineering heart valve scaffold design. Tissue Engineering. 2006;12:413–21. doi: 10.1089/ten.2006.12.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soofi SS, Last JA, Liliensiek SJ, Nealey PF, Murphy CJ. The elastic modulus of Matrigel as determined by atomic force microscopy. Journal of Structural Biology. 2009;167:216–9. doi: 10.1016/j.jsb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karuri NW, Liliensiek S, Teixeira AI, Abrams G, Campbell S, Nealey PF, et al. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. Journal of Cell Science. 2004;117:3153–64. doi: 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liliensiek SJ, Campbell S, Nealey PF, Murphy CJ. The scale of substratum topographic features modulates proliferation of corneal epithelial cells and corneal fibroblasts. Journal of Biomedical Materials Research Part A. 2006;79:185–92. doi: 10.1002/jbm.a.30744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diehl KA, Foley JD, Nealey PF, Murphy CJ. Nanoscale topography modulates corneal epithelial cell migration. Journal of Biomedical Materials Research Part A. 2005;75:603–11. doi: 10.1002/jbm.a.30467. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro- and nanostructured substrates. Journal of Cell Science. 2003;116:1881–92. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira AI, McKie GA, Foley JD, Bertics PJ, Nealey PF, Murphy CJ. The effect of environmental factors on the response of human corneal epithelial cells to nanoscale substrate topography. Biomaterials. 2006;27:3945–54. doi: 10.1016/j.biomaterials.2006.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser SA, Ting YH, Mallon KS, Wendt AE, Murphy CJ, Nealey PF. Sub-micron and nanoscale feature depth modulates alignment of stromal fibroblasts and corneal epithelial cells in serum-rich and serum-free media. Journal of Biomedical Materials Research Part A. 2008;86:725–35. doi: 10.1002/jbm.a.31519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foley JD, Grunwald EW, Nealey PF, Murphy CJ. Cooperative modulation of neuritogenesis by PC12 cells by topography and nerve growth factor. Biomaterials. 2005;26:3639–44. doi: 10.1016/j.biomaterials.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 20.Teixeira AI, Abrams GA, Murphy CJ, Nealey PF. Cell behavior on lithographically defined nanostructured substrates. Journal of Vacuum Science & Technology B. 2003;21:683–7. [Google Scholar]
- 21.Tocce EJ, Smirnov VK, Kibalov DS, Liliensiek SJ, Murphy CJ, Nealey PF. The ability of corneal epithelial cells to recognize high aspect ratio nanostructures. Biomaterials. 2010;31:4064–72. doi: 10.1016/j.biomaterials.2010.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denis FdrA, Hanarp P, Sutherland DS, Gold J, Mustin C, Rouxhet PG, et al. Protein Adsorption on Model Surfaces with Controlled Nanotopography and Chemistry. Langmuir. 2002;18:819–28. [Google Scholar]
- 23.Galli C, Collaud Coen M, Hauert R, Katanaev VL, Gröning P, Schlapbach L. Creation of nanostructures to study the topographical dependency of protein adsorption. Colloids and Surfaces B: Biointerfaces. 2002;26:255–67. [Google Scholar]
- 24.Roach P, Farrar D, Perry CC. Surface Tailoring for Controlled Protein Adsorption:  Effect of Topography at the Nanometer Scale and Chemistry. Journal of the American Chemical Society. 2006;128:3939–45. doi: 10.1021/ja056278e. [DOI] [PubMed] [Google Scholar]
- 25.Ruoslahti E. RGD and other recognition sequences for integrins. Annual Review of Cell and Developmental Biology. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 26.Imoto Y, Ohguro N, Yoshida A, Tsujikawa M, Inoue Y, Tano Y. Effects of RGD peptides on cells derived from the human eye. Jpn J Ophthalmol. 2003;47:444–53. doi: 10.1016/s0021-5155(03)00135-7. [DOI] [PubMed] [Google Scholar]
- 27.Filenius S, Tervo T, Virtanen I. Production of fibronectin and tenascin isoforms and their role in the adhesion of human immortalized corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:3317–25. doi: 10.1167/iovs.02-1146. [DOI] [PubMed] [Google Scholar]
- 28.Ge H, Cao W, Leng F, Chen W, Liu P. Modified BIGH3 with an RGDRGD motif promotes human corneal epithelial cell adhesion and migration in vitro. Curr Eye Res. 2008;33:215–23. doi: 10.1080/02713680801911218. [DOI] [PubMed] [Google Scholar]
- 29.Fong E, Tirrell DA. Collective cell migration on artificial extracellular matrix proteins containing full-length fibronectin domains. Adv Mater. 2010;22:5271–5. doi: 10.1002/adma.201002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fong E, Tzlil S, Tirrell DA. Boundary crossing in epithelial wound healing. Proc Natl Acad Sci U S A. 2010;107:19302–7. doi: 10.1073/pnas.1008291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tocce EJ, Broderick AH, Murphy KC, Liliensiek SJ, Murphy CJ, Lynn DM, et al. Functionalization of reactive polymer multilayers with RGD and an antifouling motif: RGD density provides control over human corneal epithelial cell-substrate interactions. J Biomed Mater Res A. 2011 doi: 10.1002/jbm.a.33233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buck ME, Breitbach AS, Belgrade SK, Blackwell HE, Lynn DM. Chemical modification of reactive multilayered films fabricated from poly(2-alkenyl azlactone)s: design of surfaces that prevent or promote mammalian cell adhesion and bacterial biofilm growth. Biomacromolecules. 2009;10:1564–74. doi: 10.1021/bm9001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tocce EJ, Broderick AH, Murphy KC, Liliensiek SJ, Murphy CJ, Lynn DM, et al. Functionalization of reactive polymer multilayers with RGD and an antifouling motif: RGD density provides control over human corneal epithelial cell-substrate interactions. J Biomed Mater Res A. 2012;100:84–93. doi: 10.1002/jbm.a.33233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buck ME, Lynn DM. Functionalization of Fibers Using Azlactone-Containing Polymers: Layer-by-Layer Fabrication of Reactive Thin Films on the Surfaces of Hair and Cellulose-Based Materials. ACS Applied Materials & Interfaces. 2010;2:1421–9. doi: 10.1021/am1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odom TW, Love JC, Wolfe DB, Paul KE, Whitesides GM. Improved pattern transfer in soft lithography using composite stamps. Langmuir. 2002;18:5314–20. [Google Scholar]
- 36.Allen-Hoffmann BL, Rheinwald JG. Polycyclic aromatic hydrocarbon mutagenesis of human epidermal keratinocytes in culture. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:7802–6. doi: 10.1073/pnas.81.24.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabatini LM, Allen-Hoffmann BL, Warner TF, Azen EA. Serial Cultivation of Epithelial Cells from Human and Macaque Salivary Glands. In Vitro Cellular & Developmental Biology. 1991;27A:939–48. doi: 10.1007/BF02631121. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira AI, Nealey PF, Murphy CJ. Responses of human keratocytes to micro- and nanostructured substrates. Journal of Biomedical Materials Research Part A. 2004;71A:369–76. doi: 10.1002/jbm.a.30089. [DOI] [PubMed] [Google Scholar]
- 39.Feng Y, Mrksich M. The synergy peptide PHSRN and the adhesion peptide RGD mediate cell adhesion through a common mechanism. Biochemistry. 2004;43:15811–21. doi: 10.1021/bi049174+. [DOI] [PubMed] [Google Scholar]
- 40.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. Journal of Biomedical Materials Research. 1998;39:266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 41.Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24:893–900. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 42.Schuler M, Owen GR, Hamilton DW, de Wild M, Textor M, Brunette DM, et al. Biomimetic modification of titanium dental implant model surfaces using the RGDSP-peptide sequence: a cell morphology study. Biomaterials. 2006;27:4003–15. doi: 10.1016/j.biomaterials.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Drumheller PD, Elbert DL, Hubbell JA. Multifunctional poly(ethylene glycol) semi-interpenetrating polymer networks as highly selective adhesive substrates for bioadhesive peptide grafting. Biotechnol Bioeng. 1994;43:772–80. doi: 10.1002/bit.260430812. [DOI] [PubMed] [Google Scholar]
- 44.Buck ME, Zhang J, Lynn DM. Layer-by-Layer Assembly of Reactive Ultrathin Films Mediated by Click-Type Reactions of Poly(2-Alkenyl Azlactone)s. Advanced Materials. 2007;19:3951–5. [Google Scholar]
- 45.Tocce EJ, Broderick A, Murphy KC, Liliensiek SJ, Murphy CJ, Lynn DM, et al. Functionalization of reactive polymer multilayers with RGD and an anti-fouling motif: Control over RGD density provides control over human corneal epithelial cell-substrate interactions. doi: 10.1002/jbm.a.33233. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajnicek AM, Britland S, McCaig CD. Contact guidance of CNS neurites on grooved quartz: influence of groove dimensions, neuronal age and cell type. Journal of Cell Science. 1997;110:2905–13. doi: 10.1242/jcs.110.23.2905. [DOI] [PubMed] [Google Scholar]
- 47.Gerecht S, Bettinger CJ, Zhang Z, Borenstein JT, Vunjak-Novakovic G, Langer R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials. 2007;28:4068–77. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajnicek AM, Foubister LE, McCaig CD. Alignment of corneal and lens epithelial cells by co-operative effects of substratum topography and DC electric fields. Biomaterials. 2008;29:2082–95. doi: 10.1016/j.biomaterials.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Burridge K, Chrzanowska-Wodnicka M, Zhong C. Focal adhesion assembly. Trends in Cell Biology. 1997;7:342–7. doi: 10.1016/S0962-8924(97)01127-6. [DOI] [PubMed] [Google Scholar]
- 50.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 51.von Wichert G, Haimovich B, Feng G-S, Sheetz MP. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 2003;22:5023–35. doi: 10.1093/emboj/cdg492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamada M, Sheetz MP, Sawada Y. Activation of a Signaling Cascade by Cytoskeleton Stretch. Developmental Cell. 2004;7:709–18. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Bruinsma R. Theory of Force Regulation by Nascent Adhesion Sites. Biophysical Journal. 2005;89:87–94. doi: 10.1529/biophysj.104.048280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicolas A, Safran SA. Limitation of Cell Adhesion by the Elasticity of the Extracellular Matrix. Biophysical journal. 2006;91:61–73. doi: 10.1529/biophysj.105.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Broderick AH, Azarin SM, Buck ME, Palecek SP, Lynn DM. Fabrication and selective functionalization of amine-reactive polymer multilayers on topographically patterned microwell cell culture arrays. Biomacromolecules. 2011;12:1998–2007. doi: 10.1021/bm200296a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harbers GM, Healy KE. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. Journal of Biomedical Materials Research Part A. 2005;75:855–69. doi: 10.1002/jbm.a.30482. [DOI] [PubMed] [Google Scholar]
- 57.Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: a combined computational and experimental approach. Biomaterials. 2007;28:4409–17. doi: 10.1016/j.biomaterials.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 58.Massia SP, Hubbell JA. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. Journal of Cell Biology. 1991;114:1089–100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts C, Chen CS, Mrksich M, Martichonok V, Ingber DE, Whitesides GM. Using Mixed Self-Assembled Monolayers Presenting RGD and (EG)3OH Groups To Characterize Long-Term Attachment of Bovine Capillary Endothelial Cells to Surfaces. Journal of the American Chemical Society. 1998;120:6548–55. [Google Scholar]
- 60.Berg MC, Yang SY, Hammond PT, Rubner MF. Controlling mammalian cell interactions on patterned polyelectrolyte multilayer surfaces. Langmuir. 2004;20:1362–8. doi: 10.1021/la0355489. [DOI] [PubMed] [Google Scholar]
- 61.Halstenberg S, Panitch A, Rizzi S, Hall H, Hubbell JA. Biologically engineered protein-graft-poly(ethylene glycol) hydrogels: a cell adhesive and plasmin-degradable biosynthetic material for tissue repair. Biomacromolecules. 2002;3:710–23. doi: 10.1021/bm015629o. [DOI] [PubMed] [Google Scholar]
- 62.Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. Journal of Biomedical Materials Research. 2002;60:217–23. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 63.Liliensiek SJ, Wood JA, Yong J, Auerbach R, Nealey PF, Murphy CJ. Modulation of human vascular endothelial cell behaviors by nanotopographic cues. Biomaterials. 2010;31:5418–26. doi: 10.1016/j.biomaterials.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayman EG, Ruoslahti E. Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. J Cell Biol. 1979;83:255–9. doi: 10.1083/jcb.83.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayman EG, Pierschbacher MD, Suzuki S, Ruoslahti E. Vitronectin--a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res. 1985;160:245–58. doi: 10.1016/0014-4827(85)90173-9. [DOI] [PubMed] [Google Scholar]
- 66.Nomizu M, Kim WH, Yamamura K, Utani A, Song SY, Otaka A, et al. Identification of cell binding sites in the laminin alpha 1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. The Journal of Biological Chemistry. 1995;270:20583–90. doi: 10.1074/jbc.270.35.20583. [DOI] [PubMed] [Google Scholar]
- 67.Hoffman MP, Nomizu M, Roque E, Lee S, Jung DW, Yamada Y, et al. Laminin-1 and laminin-2 G-domain synthetic peptides bind syndecan-1 and are involved in acinar formation of a human submandibular gland cell line. The Journal of Biological Chemistry. 1998;273:28633–41. doi: 10.1074/jbc.273.44.28633. [DOI] [PubMed] [Google Scholar]
- 68.Iwamoto Y, Graf J, Sasaki M, Kleinman HK, Greatorex DR, Martin GR, et al. Synthetic pentapeptide from the B1 chain of laminin promotes B16F10 melanoma cell migration. Journal of Cellular Physiology. 1988;134:287–91. doi: 10.1002/jcp.1041340216. [DOI] [PubMed] [Google Scholar]
- 69.Vukicevic S, Luyten FP, Kleinman HK, Reddi AH. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell. 1990;63:437–45. doi: 10.1016/0092-8674(90)90176-f. [DOI] [PubMed] [Google Scholar]
- 70.Tashiro K, Sephel GC, Weeks B, Sasaki M, Martin GR, Kleinman HK, et al. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. The Journal of Biological Chemistry. 1989;264:16174–82. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. AFM images demonstrate no disruption of the topographic features. Atomic force microscopy (AFM) data were acquired in tapping mode on a Nanoscope Multimode atomic force microscope (Veeco/Digital Instruments, Santa Barbara,CA) using scan rates of 3μm/s to obtain 256 × 256 pixel images over an area of 3 μm × 3 μm. Silicon cantilevers with a spring constant of 40N/m were used (modelNSC15/NoAl,MikroMaschUSA,Portland,OR). The ridge width was calculated from images using the Nanoscope software (Veeco/Digital Instruments, Santa Barbara, CA) for both the control acetone treated and substrate with two bilayers.