Abstract
This review summarizes the emerging role of AMP-activated protein kinase (AMPK) in mediating endocrine regulation of metabolic fluxes in the liver. There are a number of hormones which, when acting on the liver, alter AMPK activation. Here we describe those hormones associated with activation and de-activation of AMPK and the potential mechanisms for changes in AMPK activation state. The actions of these hormones, in many cases, are consistent with downstream effects of AMPK signaling thus strengthening the circumstantial case for AMPK-mediated hormone action. In recent years, genetic mouse models have also been used in an attempt to establish the role of AMPK in hormone-stimulated metabolism in the liver. Few experiments have, however, firmly established a causal relationship between hormone action at the liver and AMPK signaling.
Keywords: AMPK, Endocrine, Glucagon, Adiponectin, Energy Charge, Metabolism
1. Introduction
Feeding, fasting, physical exertion, and metabolic diseases such as obesity and diabetes require a complex neuroendocrine response that activates pathways for fuel storage and energy production (Wasserman, 2009). The hypothesis that AMP-activated protein kinase (AMPK) serves as a transducer, sensing energy state and modulating metabolic pathways in accordance with metabolic needs (Hardie and Hawley, 2001), has driven extensive research in recent years. Endocrine responses can occur in response to acute stimuli or can act to provide a chronic “metabolic” tone to the liver by altering expression of enzymes involved in macronutrient carbon flux. The physiological role and regulation of AMPK in mediating hormone and pharmacological action is contentious (Foretz et al., 2010; Holland et al., 2011; Miller et al., 2011). However, there is ample evidence that AMPK activity is altered under physiological conditions characterized by changes in hormone concentrations (Berglund et al., 2011, 2010, 2009; Camacho et al., 2006; Carlson and Winder, 1999; Kawaguchi et al., 2002; Munday et al., 1991; Park et al., 2002; Witters et al., 1994).
Two major topics will be addressed in this review: (1) candidate hormones that may be involved in the regulation of AMPK and (2) how endocrine control of AMPK may be important in the control of metabolic flux. The second point will be addressed in light of the AMPK paradox, which denotes the discrepancy between the metabolic effects of AMPK transgenesis from those associated physiologically with changes in AMPK activation state. Experiments in vitro regarding the metabolic role of AMPK signaling in hormone action have been highly informative but have limitations in translation to physiological conditions. The study of how AMPK works to modulate hepatic fuel fluxes in vivo has posed a considerable challenge to the field, considering it requires well-controlled experiments free of confounding variables (e.g. changes in blood glucose).
The following sections are structured around putative hormonal regulators of AMPK and the possible impact of AMPK signaling on liver metabolism. Next, the synergistic, antagonistic, and parallel signaling mechanisms involving AMPK and insulin are discussed. The review also covers current research that challenges canonical roles of hepatic AMPK activity. The objective of this review is to focus on direct endocrine regulation of AMPK signaling and hepatic metabolism. A complete evaluation of inter-organ communication exceeds the objective of this review. However, it is important to recognize that changes in endocrine, neurohumoral, and nutrient signaling have a substantial impact on AMPK activity in the brain (Ronnett et al., 2010). This is important because regulatory mechanisms in the brain including nutrient sensing in the hypothalamus regulate AMPK activity and hepatic glucose flux (Yang et al., 2010, Lam et al., 2011). It is also noteworthy that some signals discussed in the present review that directly impact hepatic metabolism and AMPK activity also play a role in the central response to changes in cellular and organismal nutrient status (Ronnett et al., 2010).
2. Endocrine Action
2.1 Glucagon
Glucagon was one of the first hormones implicated to act through AMPK. Elevations in cAMP and glucagon led to the deactivation of HMG-CoA reductase (Beg et al., 1973) and acetyl-coA carboxylase (ACC) (Holland et al., 1984; Sim et al., 1988), respectively. Both enzymes are targets of AMPK action (Carling et al., 1987). Glucagon stimulation of rat hepatocytes results in the phosphorylation and deactivation of ACC, which was initially thought to result from direct inhibition by cAMP-dependent protein kinase (PKA) (Holland et al., 1984). Further study revealed that the inhibitory phosphorylation of ACC activity results from AMPK, not PKA (Sim et al., 1988). Accordingly, increased glucagon or conditions associated with increased glucagon (e.g. exercise, fasting) causes AMPK activation in the liver (Berglund et al., 2011, 2009; Carlson and Winder, 1999; Kimball et al., 2004; Longuet et al., 2008; Munday et al., 1991; Rivera et al., 2010). It is likely that some of the diverse effects attributed to PKA are mediated by the activation of AMPK.
To understand the physiological role of glucagon, one must appreciate that this hormone is secreted from the pancreas into the hepatic portal vein, which supplies most of the blood flow to the liver. This anatomical arrangement permits efficient channeling of glucagon to the liver (Wasserman et al., 1993). The experimental difficulty is that important changes in portal venous glucagon can be undetected in systemic blood since the liver extracts a high percentage of glucagon (Jaspan et al., 1984; Wasserman et al., 1993). Exercise, for example, is associated with a large increase in portal venous glucagon. Glucagon increases hepatic glucose production (Hirsch et al., 1991; Lavoie et al., 1997; Wasserman et al., 1989, 1985, 1984), fat oxidation (Wasserman et al., 1995), amino acid metabolism (Halseth et al., 1998; Wasserman et al., 1988), and ureagenesis (Exton and Park, 1966; Heimberg et al., 1969; Kimmig et al., 1983; Krishna et al., 2000). More recently we showed that elevated glucagon increases the AMP/ATP ratio and activates AMPK in vivo (Berglund et al., 2011, 2009). Moreover, glucagon receptor signaling is essential to AMPK activation during fasting and exercise (Berglund et al., 2011, 2009).
Hepatic energy charge is regulated by glucagons
Glucagon is elevated and AMPK is activated under conditions uptake (Kimmig et al., 1983) and fat oxidation (Heimberg et al., 1969) are elevated. The coupled relationship in which O2 between TCA cycling and gluconeogenesis is paralleled by a coupling of ATP formation and breakdown. The net effect of increased TCA cycling and gluconeogenesis would appear to be a discharge of liver ATP, as exercise and fasting increase the AMP/ATP ratio (Berglund et al., 2009). Activating fatty acids for oxidation (Kawaguchi et al., 2002) and anaplerosis from amino acids requires ATP. As a result of anaplerosis, gluconeogenesis and ureagenesis expend 6 and 4 moles of ATP, respectively, to produce a mole of glucose (Hems et al., 1966). The movement of lactate, alanine, pyruvate, and oleate carbons into the TCA cycle increases AMP levels (Williamson et al., 1969) and supplies substrate for gluconeogenesis. Indeed, glucagon signaling increases hepatic AMP concentrations and activates AMPK (Berglund et al., 2011, 2009).
AMPK phosphorylation (pThr172) increases with elevations in AMP (Berglund et al., 2009), presumably as a consequence of phosphorylation by LKB1 (Hawley et al., 2003; Shaw et al., 2004; Woods et al., 2003). Since glucagon increases cytosolic Ca2+ (Charest et al., 1983), it is possible that glucagon-mediated increases in AMPK phosphorylation result from the activation of CaMKKα/β. However, this seems rather unlikely given their trace expression in the liver (Anderson et al., 1998). WT mice fasted for 18hr or exercised until exhaustion exhibit a ~5 and ~10-fold increase in the AMP/ATP ratio, respectively—an effect not present in glucagon receptor null mice (Berglund et al., 2009). By using a novel glucose clamp technique that elevates circulating glucagon without hyperglycemia or hyperinsulinemia in vivo, it has been shown that flux through PEPCK is necessary for the glucagon-mediated increase in the AMP/ATP ratio and activation of AMPK (Berglund et al., 2009).
A role for AMPK in glucagon-mediated changes in hepatic lipid metabolism
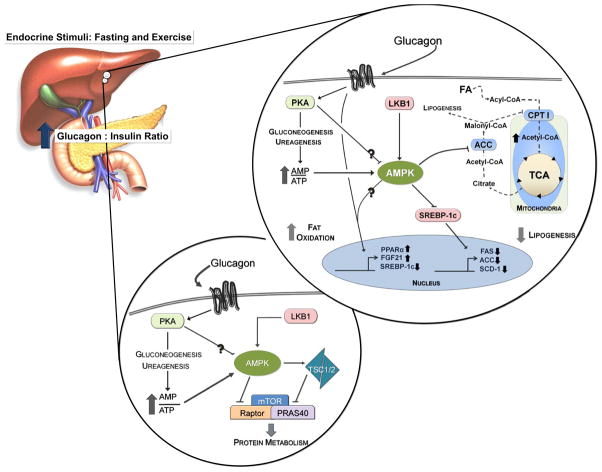
Fasting activates AMPK in the liver, which inversely corresponds to ACC activity (Munday et al., 1991). The inverse relationship between AMPK and ACC activities may be related and explain aspects of metabolic zonation in the liver. Periportal parenchyma have elevated capacity for gluconeogenesis, ureagenesis, β-oxidation and ketogenesis; these functions correspond to hormonal (e.g. glucagon) and substrate gradients in the liver (Jungermann and Kietzmann, 1996). In the fasted state, AMPK activity is elevated predominantly in the periportal zone and corresponds to reduced ACC activity (Witters et al., 1994). This is consistent with glucagon concentrations being highest in the periportal region of the liver. As noted above, glucagon elevates fat oxidation in the liver (Heimberg et al., 1969). High intensity exercise also results in reduced ACC activity, lowered malonyl-CoA concentrations, and increased 3-hydroxybutyrate and AMPK activation (Carlson and Winder, 1999). Glucagon-mediated activation of AMPK may acutely suppress lipid biogenesis and promote fat oxidation in the liver by attenuating the ability of ACC to generate malonyl-CoA, thus lessening carbon substrate for lipogenesis and CPT1 suppression (Figure 1). Likewise, glucagon represses expression of SREBP-1c (Foretz et al., 1999), a controller of lipogenesis in the liver. Elegant work with AMPK activators demonstrates that AMPK phosphorylates and reduces SREBP-1c activity (Li et al., 2011). In accordance, hepatocytes lacking AMPKβ1 have elevated triglyceride synthesis and impaired fatty acid oxidation (Dzamko et al., 2010). It is thus possible that glucagon, through AMPK activation, inhibits SREBP-1c activity (Figure 1).
Figure 1. AMPK activation may mediate or potentiate several glucagon-mediated hepatic adaptations to fasting and exercise.
Glucagon-signaling promotes ATP consuming processes which increases the AMP:ATP ratio and activates AMPK. Glucagon-mediated increases in fat oxidation, inhibition of lipogenesis, and decreased protein synthesis may be mediated through AMPK. Signaling pathways are represented by solid lines. Dotted lines denote substrates.
Work in mice either lacking (Andreelli et al., 2006) or overexpressing (Foretz et al., 2005) the AMPKα2 subunit in the liver support the premise that AMPK activation suppresses lipogenesis and promotes fat oxidation. Liver deletion of AMPKα2 results in elevated plasma free fatty acid, triglycerides, and decreases in β-hydroxybutyrate following a 5hr fast (Andreelli et al., 2006). Interestingly, glucagon-receptor null mice exhibit a similar fasting (16hr) plasma lipid profile with elevations in triglycerides and free fatty acids (Longuet et al., 2008). Conversely, adenoviral-mediated overexpression of constitutively active AMPKα2 in the liver (AMPKα2-CA) decreases plasma triglycerides and increases β-hydroxybutyrate following a 24Hr fast (Foretz et al., 2005). AMPKα2-CA mice also display elevated liver protein lipase transcript levels, hepatic triglycerides, and cholesterol levels in the liver during fasting—suggesting AMPK activation promotes fatty-acid uptake and attenuates triglyceride/cholesterol release from the liver (Foretz et al., 2005). Acutely, glucagon phenocopies the effects of elevated AMPK activation, as it also prevents the release of triglycerides into the plasma and promotes their accumulation in the liver while increasing fat oxidation (Heimberg et al., 1969). There is a clear functional overlap between glucagon and AMPK signaling with respect to fat oxidation in the liver.
Elevations in glucagon action and AMPK activation during long-term voluntary and forced exercise correspond to the amelioration of fatty liver in mice on a high-fat diet (Berglund et al., 2011). As previously mentioned, acute and chronic exercise cause increases in the AMP/ATP ratio and AMPK activation in the liver that are dependent on glucagon receptor signaling and flux through PEPCK (Berglund et al., 2009). Elevations in the AMP/ATP ratio, AMPK activation, and transcripts encoding PPARα and FGF21 accompany the reversal of fatty liver (Berglund et al., 2011). In adipocytes, FGF21 increases AMPK activation, the NAD+/NADH ratio, and oxygen consumption; these effects of FGF21 on mitochondrial metabolism are dependent on PGC-1α, LKB1, AMPK, and SIRT1 (Chau et al., 2010). FGF21 overexpression in the liver increases the mRNA encoding PGC-1α as well as the expression of its target genes involved in hepatic glucose production and mitochondrial oxidative phosphorylation. FGF21 promotes fed and fasted ketogenesis, fed O2 consumption, β-oxidation, and gluconeogenesis (Potthoff et al., 2009). One can speculate that the actions of FGF21 mediate some of the persistent effects of glucagon on the liver. Regardless, an intact glucagon-AMPK signaling network may be essential to diminish obesity-related pathology in the liver. From these data, one can surmise a role for AMPK in potentiating the effects of glucagon on hepatic lipid anabolism and catabolism.
AMPK and hepatic glucose production
“a mystery inside an enigma.” The role of AMPK signaling in control of glucose release from the liver can be paraphrased by the words of Sir Winston Churchill. It is a mystery inside an enigma. The results obtained in transgenic and mutant mice are contrary to what one might expect from physiological observations. Hepatic LKB1 deletion causes hyperglycemia and glucose intolerance (Shaw et al., 2005). Likewise, liver-specific AMPKα2 deletion results in glucose intolerance, albeit to a lesser degree than LKB1−/− mice (Andreelli et al., 2006). moreover, overexpression of AMPKα2 in the liver decrease fasting and fed glucose levels and the transcripts encoding the PEPCK and G6Pase enzymes (Foretz et al., 2005). The phenotype of these genetic models would suggest that endocrine mediated activation of AMPK (e.g. glucagon) would result in reduced hepatic glucose production. The enigma is that AMPK is activated in physiology at times when glucose production is high.
Mechanistically, glucagon stimulates the gluconeogenic program in a PKA dependent manner. PKA phosphorylates CREB (Altarejos and Montminy, 2011; Gonzalez and Montminy, 1989) which upregulates PGC-1α, in turn, acts to increase the expression of gluconeogenic enzymes (Herzig et al., 2001; Yoon et al., 2001) and glucose production in hepatocytes (Yoon et al., 2001). PGC-1α’s ability to co-activate gluconeogenic gene expression is lost in the absence of hepatocyte nuclear factor 4α (HNF4α) (Rhee et al., 2003). Likewise PGC-1α co-activates FOXO1 which arguments gluconeogenic gene expression in FAO cells (Puigserver et al., 2003). AMPK disrupts HNF4α activity through phosphorylation (Hong et al., 2003) and AICAR-mediated activation of AMPK coincides with reductions in HNF4α protein levels and target gene expression (Figure 2) (Leclerc et al., 2001). Liver-specific knockout of PGC-1α attenuates fasting-mediated increases in gene expression for the PEPCK and G6Pase enzymes (Handschin et al., 2005). However, livers from PGC-1α-null mice have impaired gluconeogenic glucose production from the TCA cycle, despite normal fasting levels of the aforementioned transcripts (Burgess et al., 2006). This is further explored in a subsequent section.
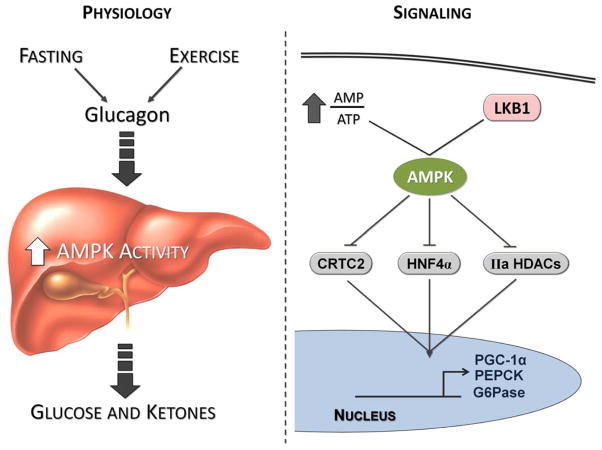
Figure 2. Juxtaposition of the physiological conditions that activate AMPK with the AMPK-mediated signaling mechanisms implicated in the regulation of gluconeogenesis.
As discussed in the text, AMPK activation is highest during conditions (fasting and exercise) when glucagon action, glucose production, and ketogenesis are elevated. Paradoxically, AMPK is implicated in the restraint of gluconeogenesis through the phosphorylation of CRTC2, HNF-4α, and Class IIa HDACs (IIa HDACs).
CRTC2 (TORC2), a transcriptional co-activator of CREB, could also function as a regulatory point between glucagon, insulin, LKB1, and AMPK signaling in gluconeogenesis (Dentin et al., 2007; Koo et al., 2005). While glucagon and forskolin promote CREB-CRTC2 mediated upregulation of gluconeogenic gene transcription, AMPK phosphorylation sequesters CRTC2 in the cytoplasm which reduces its activity (Figure 2) (Koo et al., 2005). Hepatic deletion of LKB1 and AMPK decreases CRTC2 phosphorylation (Foretz et al. 2010; Shaw et al., 2005). The absence of hepatic CRTC2 results in reductions in gluconeogenic gene expression during fasting. CRTC2-null hepatocytes have impaired glucagon-stimulated glucose production (Le Lay et al., 2009).
Recent studies explore a role for Class IIa HDACs and FOXO in glucagon-stimulated gluconeogenesis (Mihaylova et al., 2011, Wang et al., 2011). In response to glucagon, Class IIa HDACs translocate into the nucleus, recruit HDAC3, and promote FOXO-dependent gene transcription of gluconeogenic targets. LKB1-dependent kinases (e.g. AMPK) phosphorylate and inactivate Class IIa HDACs (HDACs 4/5/7). Interestingly, treatment of hepatocytes with glucagon reduces phosphorylation of HDACs4/5/7 at the sites of LKB1-dependent kinase action. shRNAs targeting these HDACs improve glucose tolerance in db/db, ob/ob, and high fat fed mice. By increasing the nuclear entry of these HDACS, glucagon could promote, at least in part, FOXO dependent-gluconeogenic gene transcription another avenue of intervention for glucagon and potentially AMPK in hepatic glucose production (Mihaylova et al., 2011).
These genetic models and mechanistic data support a regulatory role for AMPK in hepatic glucose production. However, the results obtained with these genetic models do not, in fact, uniformly predict the metabolic response of the liver to the hormones or conditions that regulate the activity of AMPK under physiological conditions. As described earlier, glucagon plays an integral role in maintaining glucose homeostasis by stimulating glucose production during the adaptive response to fasting and exercise. Paradoxically, a genetic increase in AMPK activity in the liver results in an inhibition, and not an increase, in glucose release from the liver (Foretz et al., 2005).
Recent work in liver AMPKα1α2/LKB1 knockout models reveal that the glucose lowering effects of metformin and adiponectin might be AMPK independent (Foretz et al., 2010; Holland et al., 2011; Miller et al., 2011; Miller and Birnbaum, 2010). Interestingly, mice lacking liver AMPKα1α2 have similar fasting and fed blood glucose levels as WT mice (Foretz et al., 2010). Germline removal of the AMPKβ1 subunits results in significant impairments in hepatic AMPKα1α2 activity (Dzamko et al., 2010). Despite elevations in basal gluconeogenic gene transcription, hepatocytes isolated from these mice exhibit normal basal glucose production and similar reductions in glucose output versus wild-type mice when stimulated with the AMPK activators A769662 and AICAR (Scott et al., 2008). The potential overlap in signaling and function between AMPK and other LKB1-dependent kinases further complicates data interpretation from in vivo and in vitro models of AMPK activation or deletion (Shackelford and Shaw, 2009). For example, overexpression of salt-inducible kinases (SIK) 1 and 2 reduces fasting glucose levels and gluconeogenic gene expression (Koo et al., 2005). Moreover, both impair CRTC2 activity by promoting its phosphorylation at S171 and sequestration in the cytoplasm (Dentin et al., 2007, Koo et al., 2005, Screaton et al., 2004).
At present, the results in the literature do not produce sufficient consensus to assign a definitive role for hepatic AMPK in glucose production. Investigators have often made the assumption that changes in gene expression are equivalent to changes in hepatic glucose flux. This is not necessarily true. This is best illustrated by experiments using perfused livers from 24hr fasted mice with graded reductions in PEPCK content (Burgess et al., 2007). Isotopic tracers were used to determine hepatic glucose and TCA cycle fluxes. Metabolic flux analysis of this data indicates that PEPCK has a rather low control coefficient (0.18) for gluconeogenesis from the TCA cycle (Burgess et al., 2007). Although these studies are confined to a very specific set of circumstances (i.e. perfused livers from long-term fasted mice), they do emphasize the need to be cautious when ascribing changes in gene and protein expression to changes in flux.
Inhibition of protein synthesis and AMPK
The inhibitory effect of glucagon on liver protein synthesis (Woodside et al., 1974) may also result from elevated AMPK activity (Kimball et al., 2004). This view is consistent with the ability of AMPK to decrease protein anabolism. Glucagon markedly increases AMPK activation while decreasing mTORC1 signaling to 4E-BP1 and S6K1 (Kimball et al., 2004). During periods of elevated glucagon, insulin (an mTORC1 activator) is unable to deactivate AMPK or fully restore mTORC1 signaling (Baum et al., 2009). However, exercise-mediated activation of AMPK reduces the translation of 5′-terminal oligopyrimidine tract mRNAs not total protein synthesis (Reiter et al., 2008). It has been shown that PKA mediates the phosphorylation of LKB1 and supports its ability to repress cell growth (Sapkota et al., 2001). Other factors that increase the intrahepatic AMP/ATP ratio or activate AMPK have a similar impact on mTORC1 signaling and protein synthesis (Dubbelhuis and Meijer, 2002). Extensive signaling work has identified at least two sites where AMPK intercedes in the mTORC1 signaling pathway: TSC2 (Inoki et al., 2003) and raptor (Gwinn et al., 2008). In both cases, phosphorylation by AMPK leads to a reduction in mTORC1 signaling (Figure 1). The effects of glucagon on protein synthesis are very much in line with the effects of AMPK activation. The possibility clearly exists that these actions of glucagon are mediated by AMPK.
2.2 Adiponectin
Suppression of hepatic glucose production
The effects of adipokines on whole body and tissue-specific metabolism have been studied extensively in recent years. The adipokine, adiponectin, acts as an insulin-sensitizer that suppresses hepatic glucose production (Berg et al., 2001). The ability of adiponectin to sensitize the liver to insulin is accompanied by reduced gluconeogenic gene expression (Combs et al., 2001).
Adiponectin stimulates processes that provoke an increase in AMP (Figure 3) (Miller et al., 2011) and mice treated with adiponectin have enhanced AMPK activation in both skeletal muscle and liver (Yamauchi et al., 2007). In muscle cells, adiponectin has been shown to work through an adapter protein (APPL1) to signal to AMPK and promote fatty acid oxidation and glucose uptake (Mao et al., 2006). APPL1 promotes the cytosolic translocation of LKB1 and, consequently, activation of AMPK during adiponectin or metformin stimulation (Zhou et al., 2009). Adiponectin also provokes an influx of extracellular Ca2+, which may activate CaMKKβ in skeletal muscle (Iwabu et al., 2010)—another mechanism for AMPK activation that might be relevant in some cell types, though unlikely in hepatocytes.
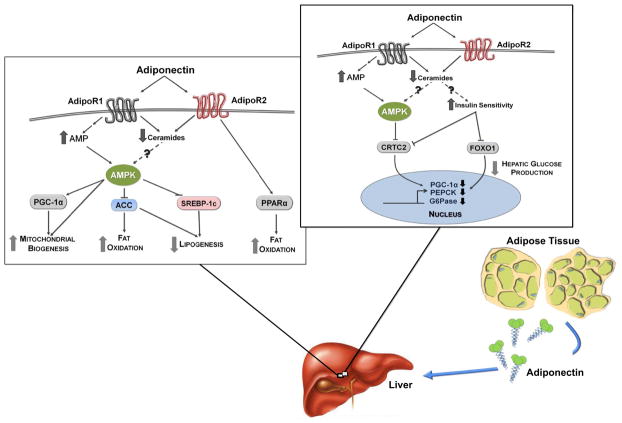
Figure 3. Hypothetical role for AMPK signaling in adiponectin’s action on liver metabolism.
Adiponectin works at the liver to reduce hepatic lipid accumulation (increased fat oxidation and reduced lipogenesis) and glucose production through mechanisms that may involve AMPK. Signaling pathways are denoted by solid lines. Dotted lines denote a recently proposed yet undeveloped mechanism for adiponectin signaling in the liver.
Interestingly, overexpression of a dominant-negative AMPKα1 mutant in the liver prevents adiponectin-mediated reductions in fasting glucose and transcripts encoding the PEPCK and G6Pase enzymes (Yamauchi et al., 2002). Whole-body knockout of AdipoR1, one of two adiponectin receptors in the liver, also results in an increase in glucose production and indices of gluconeogenesis (Yamauchi et al., 2007; Yamauchi et al., 2003). Accordingly, adiponectin knockout mice have impairments in insulin sensitivity and elevated hepatic glucose production (Nawrocki et al., 2006). Conversely, small increases in AdipoR1 result in elevated AMPK activation, attenuated transcript levels for the PEPCK and G6Pase enzymes (Figure 3), and lowered endogenous glucose production in db/db mice (Yamauchi et al., 2007). It is reasonable to hypothesize that adiponectin exerts a tonic effect on AMPK activity and its downstream targets in the liver, which may work to maintain glucose homeostasis.
Hepatic Lipid Metabolism
Just as glucagon-mediated increases in the AMP/ATP ratio and AMPK activity coincide with elevated fat oxidation (Berglund et al., 2011), adiponectin action stimulates AMPK activation and pro-oxidative, anti-lipogenic pathways in the liver (Figure 3) (Yamauchi et al., 2007). AdipoR1 levels are reduced in db/db mice. Restoration of AdipoR1 to normal levels in db/db mice decreases the mRNA encoding SREBP-1c—a transcriptional regulator of lipid biogenic genes (Yamauchi et al., 2007). Conversely, adenoviral delivery of siAdipoR1 results in a robust increase in the mRNA encoding SREBP-1c in the livers of db/db mice (Awazawa et al., 2009). It is possible that LKB1-AMPK signaling promotes adiponectin mediated decreases in SREBP-1c expression (Figure 3). LKB1 removal in hepatocytes abrogates AMPK activation and upregulates srebf1c transcript levels (Awazawa et al., 2009). Thorough in vivo work is necessary to determine whether endocrine action on the liver (i.e. adiponectin, glucagon) promotes a similar, AMPK-dependent phenotype.
Yamauchi et al. (2007) have suggested that AdipoR1 and R2 have differential roles with respect to AMPK, hepatic glucose production, fat synthesis and oxidation in the liver. As noted, adenoviral-mediated restoration of AdipoR1 appears to activate AMPK and reduce indices of hepatic glucose and lipid production in db/db mice. Whereas, increases in AdipoR2 lower liver triglycerides and fatty acids while upregulating PPARα and its target genes independently of AMPK activation (Yamauchi et al., 2007). This difference in receptor functions suggests the receptors may utilize independent mechanisms to promote a “healthy” liver lipid profile. Thiazolidinedionces (TZDs) are PPARγ agonists that activate AMPK, reduce ACC activity, and decrease malonyl-CoA concentrations in the liver. Further implicating adiponectin-AMPK signaling in hepatic lipid regulation, loss of adiponectin ameliorates TZD-induced AMPK activation while ACC activation and malonyl-CoA levels remain high (Nawrocki et al., 2006).
In addition to regulating gluconeogenic gene transcription, PGC-1α works to increase the expression of nuclear respiratory factors 1 (NRF-1) and 2 (NRF-2). PGC-1α also co-activates NRF-1. Several nuclear and mitochondrial promoters contain NRF-1 and NRF-2 recognition sites which, among others, include genes encoding the respiratory complexes and components of mtDNA transcription/replication (Kelly and Scarpulla, 2004). Moreover, ectopic overexpression of PGC-1 and PPARα in vitro leads to the induction of genes involved in mitochondrial fat oxidation and increases in palmitate oxidation (Vega et al., 2000). PGC-1α might play a significant role in oxidation in the liver. This is highlighted by observations that fasting results in hepatic steatosis in PGC-1α-null mice. Hepatocytes isolated from these mice have impairments in fat oxidation, mitochondrial respiratory function, and increased triglyceride synthesis (Leone et al., 2005). Hepatic gene expression of TCA cycle enzymes and components of the mitochondrial oxidative-phosphorylation system are also attenuated in the livers of PGC-1α-null mice (Burgess et al., 2006). Perfusion studies demonstrate that PGC-1α-null livers have impaired hepatic glucose production—an effect that appears to be secondary to impairments in TCA cycle fluxes and oxidative metabolism (Burgess et al., 2006).
AMPK has been shown to be important for the expression of PGC-1α in the muscle in a model of energy deprivation (Zong et al., 2002). Also, it has been shown that AMPK increases PGC-1α activity through phosphorylation Jäger et al., 2007) and may promote its deacetylation and activity through SIRT1 (Cantó et al., 2010, 2009). Through these mechanisms, AMPK could support mitochondrial biogenesis and fat oxidation through PGC-1α (Figure 3). Livers lacking the AMPKα1α2 subunits have impaired O2 consumption, an attenuated mitochondrial biogenic mRNA profile, and reduced levels of cytochrome C and COX IV, consistent with the notion that AMPK promotes mitochondrial biogenesis through PGC-1α (Guigas et al., 2007). These data are consistent with endocrine activation of AMPK (adiponectin, glucagon) and a pro-oxidative condition in the liver.
AMPK is one of several signaling mechanisms that converge on PGC-1α to coordinate nvironment with cellular energy status. AMPK may regulate PGC-1α through direct phosphorylation, a decrease in CRTC2 activity, changes in its acetylation status (Cantó et al., 2010, 2009), or an unidentified mechanism. The signals that converge on PGC-1α altered with changes in endocrine, energy and nutritional state—thus, PGC-1α’s activity in the liver likely mirrors the dynamic nature of its regulators. For example, PGC-1α receives signals from Sirt1 (Cantó et al., 2010, 2009; Feige et al., 2008; Gerhart-Hines et al., 2007), and mTORC1 (Lustig et al., 2011), which plays important role in nutrition metabolism. It should be noted that AMPK’s impact on CRTC2 in the liver seems antithetical to its stimulation of PGC-1α activity in the muscle. Certainly, the relationship between AMPK and PCG-1α in the liver might differ from its interaction in other tissues. Thus, the role of AMPK in the regulation of PGC-1α is likely a function of the integration of input from numerous sources.
AMPK-independent role of adiponectin in hepatic fuel fluxes
The ability of endocrine signals to mediate decreases in hepatic energy charge provides evidence for the importance of AMP as an intracellular indicator and regulator of energy state. Interestingly, adiponectin’s efficacy at reducing glucose production in vivo is partially compromised in the absence of hepatic LKB1 (Miller et al., 2011). The ability of adiponectin to diminish gene transcripts for proteins involved in anabolism—PGC-1α, PEPCK, G6Pase, SREBP-1c, FAS, ACC is still intact even in the absence of LKB1 or AMPK activation (Miller et al., 2011). Moreover, complete removal of AMPKα1α2 in hepatocytes does not impair adiponectin’s diminution of gluconeogenic gene transcripts or glucose production during Bt2-cAMP stimulation (Miller et al., 2011). Treating hepatocytes from CRTC2 knockout mice with adiponectin obviates the glucagon-mediated increase in mRNA encoding PGC-1α (Miller et al., 2011).
Adiponectin’s positive metabolic impact on the liver may be mediated through decreases in ceramide levels (Holland et al., 2011). Mice on a high-fat diet or lacking adiponectin have large increases in hepatic ceramide levels. Adiponectin administration or overexpression reduces liver ceramides and improves indices of hepatic insulin sensitivity, potentially through the intrinsic ceramidase activity of AdipoR1 and R2 (Holland et al., 2011). Consistent with the work of Miller et al., 2011, adiponectin substantially lowers blood glucose levels in liver-specific LKB1−/− mice. Work performed in non-hepatocyte cell lines suggest that adiponectin promotes the conversion of ceramide to S1P—which has been demonstrated to activate AMPK; however, this mechanism needs to be thoroughly tested, given that export and degradation keep S1P in trace amounts. A new model proposes that adiponectin works by stimulating the intrinsic ceramidase activity of its receptors, lowering ceramide content and improving Akt/PKB signaling (Holland et al., 2011).
2.3. Resistin
Resistin, another adipokine, reduces AMPK activity (Muse et al., 2004) in the liver and may contribute to hepatic insulin resistance (Steppan et al., 2001). Resistin levels rise during obesity and promote hyperglycemia and insulin resistance in high fat-fed, wild-type mice (Steppan et al., 2001). Reducing plasma resistin with a resistin-specific antisense oligonucleotide (ASO) normalizes fasting plasma glucose levels in high fat-fed mice (Muse et al., 2004). Likewise, endogenous glucose production is normalized in high fat-fed ASO treated mice during a hyperinsulinemic-euglycemic clamp. Notably, this is accompanied by an increase in hepatic AMPK activation and normalization of transcript levels encoding the G6Pase enzyme (Muse et al., 2004). Resistin-specific ASO treatment also ameliorates impairments in liver Akt signaling in high fat fed mice (Muse et al., 2004). High fat-fed, resistin knockout mice display improvements in indices of hepatic glucose production and elevated AMPK activation (Banerjee et al., 2004).
Consistent with the metabolic profile described above is the demonstration that insulin sensitivity in skeletal muscle, adipose tissue, and the liver, as assessed using a hyperinsulinemic-euglycemic clamp, is improved in high fat-fed, resistin-knockout mice (Qi et al., 2006). ob/ob mice lacking resistin have reductions in liver triglycerides, plasma glucose, and suppressed lipogenic transcript and protein levels (Singhal et al., 2008). The role of AMPK, if any, in this improved metabolic profile remains to be determined.
2.4 Leptin
Leptin acts through AMPK in a number of tissues to maintain energy balance (Steinberg et al., 2009). For example, leptin elevates AMPKα2 activity through the sympathetic nervous system in skeletal muscle (Minokoshi et al., 2002). Leptin or leptin receptor deficiencies in rodents result in impaired hepatic AMPK activity and correspond to elevations in malonyl-CoA and ACC activity (Yu et al., 2004). However, the lipid lowering effects of leptin the liver require PPARα and may occur independently of change in AMPK activation (Lee et al., 2002). Nevertheless, a recent study in AML12 cells shows an increase in AMPK activation in the presence of leptin (Moon et al., 2012). It has also been reported that central infusion of leptin has no impact on AMPK activation in the liver in ewes (Laker et al., 2011).
Liver-specific disruption of leptin receptor signaling results in a significant increase in liver triglycerides and cholesterol in mice (Huynh et al., 2010). Plasma glucose and gluconeogenic gene transcript levels are unaffected by the disruption. However, male mice with disrupted leptin receptor signaling in the liver have better glucose tolerance following an OGTT (Huynh et al., 2010). Whether AMPK has a role in any of these effects requires further inquiry.
2.5 Ghrelin
Ghrelin is an appetite stimulating gut peptide. Ghrelin administration in rats results in reductions in AMPK activation (Barazzoni et al., 2005; Kola et al., 2005) and increases in transcript levels for the ACC, FAS, and G6Pase enzymes in the liver. Increases in liver triglycerides accompany the transcriptional changes (Barazzoni et al., 2005). One could posit a role for ghrelin in attenuating AMPK-mediated reductions in hepatic lipid metabolism and glucose production; however, this is highly speculative.
3. Convergence of Insulin and AMPK Signaling Pathways in the Liver
The balance between anabolism and catabolism must be tightly regulated in an organ as dynamic as the liver. Insulin stimulates the synthesis of protein, lipids, and glycogen. By reducing hepatic glucose output, insulin maintains euglycemia during feeding. Insulin and AMPK signaling pathways overlap at key signaling loci to maintain organ homeostasis. An imbalance in insulin and AMPK signaling compromises fuel fluxes and is observable in several metabolic stresses (diabetes, obesity, under-nutrition). Components of the insulin signaling pathway and AMPK signaling will be briefly juxtaposed to provide a broad perspective of how endocrine inputs may regulate central metabolic processes in the liver.
3.1 Direct regulation
Insulin signaling has been shown to reduce AMPK activity in the liver (Witters and Kemp, 1992). There is evidence that Akt/PKB-1 meditated phosphorlation of AMPK (α1Ser485/α2Ser491) results in a decrease in AMPK-Thr172 phosphorylation following insulin pre-treatment (Horman et al., 2006). Interestingly, AMPK Ser485 phosphorylation is elevated while AMPK-Thr172 phosphorylation remains unaltered during hyperinsulinemic clamps in the dog—an effect reversed by glucagon, hypoglycemia, or both (Rivera et al., 2010). Whether this phosphorylation has a physiological impact on hepatic metabolism requires further inquiry.
3.2 mTORC1
mTORC1, a central controller of growth, is activated by insulin, growth factors, and nutrients with the result that translation is increased. Insulin promotes mTORC1 signaling by inhibiting TSC1/TSC2 complex activity, an endogenous mTORC1 repressor (Inoki et al., 2002). During low nutrient availability, however, increases in AMPK activity reduce mTORC1 signaling by promoting TSC1/TSC2 activity and raptor dissociation from mTOR—as previously described (Gwinn et al., 2008; Inoki et al., 2003). The reciprocity of insulin and AMPK signaling creates sensitive regulation of protein anabolism/catabolism in the liver. As noted, glucagon increases AMPK activation and has been shown to dominantly repress mTORC1 in hepatocytes exposed to both hormones (Baum et al., 2009).
3.3 Glucose and Lipid Metabolism
There has been considerable research on the acute and chronic action of insulin and AMPK on hepatic glucose production. Insulin and AMPK share mutual and also use exclusive signaling pathways to reduce the expression of gluconeogenic enzymes. AMPK and related kinases have been suggested to regulate hepatic glucose production through the inhibition of CRTC2 (Koo et al., 2005), regulation of SHP (Kim et al., 2008), and, more recently, inhibition of Class IIa HDACs (Mihaylova et al., 2011). Insulin has been shown to also obstruct a functional CREB-CRTC2-CBP/p300 complex, thus decreasing gluconeogenic gene upregulation (Altarejos and Montminy, 2011). Insulin inhibits CRTC2 activity through the activation of SIK2—which phosphorylates and promotes CRTC2 degradation (Dentin et al., 2007). Insulin also inhibits gluconeogenic gene expression (Granner et al., 1983) by excluding FOXO1 from the nucleus (Biggs et al., 1999; Nakae et al., 1999) and reducing PGC-1α’s impact on gluconeogenic genes (Lee et al., 2007; Puigserver et al., 2003).
Lipid metabolism and synthesis highlight another juxtaposition of insulin and AMPK action in the liver. As previously discussed, several lines of evidence suggest that AMPK acutely and chronically suppresses lipid biogenesis while promoting fat oxidation. Through increases in cAMP, glucagon and epinephrine activate AMPK, which rapidly inactivates ACC in the liver. On the other hand, insulin decreases AMPK activity (Witters and Kemp, 1992) while increasing ACC activity (Mabrouk et al., 1990; Witters and Kemp, 1992). The precise mechanism for insulin-mediated activation of ACC remains unresolved (Brownsey et al., 2006) but could be due to both covalent and allosteric modifications.
Transcriptional regulation of pro-lipogenic genes by AMPK and insulin converge on SREBP-1c. As mentioned, glucagon (Foretz et al., 1999) and AMPK (Li et al., 2011) work to reduce SREBP-1c expression and activity. Thus, intermittent glucagon-stimulated activation of AMPK such as that seen during regular exercise (Berglund et al., 2011) could generate a pro-oxidative, anti-lipogenic tone in the liver through the activation of PPARα and the inhibition of mTORC1 and SREBP-1c (Figure 1). In contrast, insulin plus glucose increases the transcripts encoding SREBP-1c and the expression of target genes (Foretz et al., 1999; Li et al., 2010). Insulin-mediated control of hepatic lipogenesis has focused on SREBP-1c in the normal and insulin-resistant states (Brown and Goldstein, 2008). Recent work demonstrates that insulin may work through mTORC1 dependent and independent mechanisms to promote hepatic lipogenesis (Li et al., 2010; Wan et al., 2011; Yecies et al., 2011).
4. Conclusion
The growing body of work implicating or directly demonstrating that AMPK has a regulatory role in the control of hepatic metabolic fluxes suggests that the enzyme may be a functional mediator of endocrine action in normal and pathological states (Table 1). It is important to recognize that restricting AMPK conceptually to a binary role will inadequately describe how it mediates hormone action in the liver, which is the site of convergence of so many metabolic pathways and regulatory signals. The integration of sophisticated methods to study metabolic flux, well controlled physiological model systems, and tools to interrupt or promote cell signaling in vivo are important in advancing our knowledge of the physiological role of the endocrine-AMPK axis in the liver.
Table 1. Hormone action on AMPK activity and related metabolism in the liver.
The table above summarizes the putative endocrine effectors of AMPK activation in the liver and condenses their downstream effects on signaling and substrate utilization into central metabolic processes.
| Hormone | AMPK Activity | Hormone Effect on Hepatic Metabolism |
|---|---|---|
| Glucagon |
|
↑ Fatty Acid Oxidation ↓ Lipid Biogenesis ↑ Glucose Production ↓ Protein Synthesis |
| Adiponectin |
|
↑ Fatty Acid Oxidation ↓ Lipid Biogenesis ↓ Glucose Production |
| Resistin |
|
↑ Glucose Production |
| Leptin |
|
↑ Fatty Acid Oxidation |
| Ghrelin |
|
↑ Fat Accumulation |
| Insulin |
|
↓ Fatty Acid Oxidation ↑ Lipid Biogenesis ↓ Glucose Production ↑ Protein Synthesis |
A number of hormones with well-defined metabolic regulatory roles activate or de-activate AMPK in the liver.
Glucagon receptor signaling causes an increase in AMP/ATP and AMPK activity under physiological conditions that requires flux through the gluconeogenic enzyme, PEPCK.
The glucagon receptor is essential for effects of long-term physical activity on the increase in AMPK activation and the concomitant reduction in liver fat in mice fed a high fat diet.
Adiponectin sensitizes the liver to the actions of insulin, activates AMPK, and promotes an anti-lipogenic, pro-oxidative program in the liver.
Precise balance between the opposing effects of insulin and AMPK signaling on macronutrient flux is essential to liver metabolic function.
AMPK signaling is a hub that integrates intrahepatic intracellular signals generated by hormone action, as well as intrinsic signals generated by the metabolic state of the liver.
Acknowledgments
This work was supported by grants, NIH R37 DK050277 and U24 DK059637.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altarejos JY, Montminy M. CREB and the CRTC coactivators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–51. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biol Chem. 1998;273:31880–9. doi: 10.1074/jbc.273.48.31880. [DOI] [PubMed] [Google Scholar]
- Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B. Liver adenosine monophosphate-activated protein kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology. 2006;147:2432–41. doi: 10.1210/en.2005-0898. [DOI] [PubMed] [Google Scholar]
- Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun. 2009;382:51–6. doi: 10.1016/j.bbrc.2009.02.131. [DOI] [PubMed] [Google Scholar]
- Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, Steppan CM, Ahima RS, Obici S, Rossetti L, Lazar MA. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–8. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G. Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E228–35. doi: 10.1152/ajpendo.00115.2004. [DOI] [PubMed] [Google Scholar]
- Baum JI, Kimball SR, Jefferson LS. Glucagon acts in a dominant manner to repress insulin-induced mammalian target of rapamycin complex 1 signaling in perfused rat liver. Am J Physiol Endocrinol Metab. 2009;297:E410–5. doi: 10.1152/ajpendo.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg ZH, Allmann DW, Gibson DM. Modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity with cAMP and with protein fractions of rat liver cytosol. Biochem Biophys Res Commun. 1973;54:1362–9. doi: 10.1016/0006-291x(73)91137-6. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Kang L, Lee-Young RS, Hasenour CM, Lustig DG, Lynes SE, Donahue EP, Swift LL, Charron MJ, Wasserman DH. Glucagon and lipid interactions in the regulation of hepatic AMPK signaling and expression of PPARalpha and FGF21 transcripts in vivo. Am J Physiol Endocrinol Metab. 2010;299:E607–14. doi: 10.1152/ajpendo.00263.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Berglund ED, Lee-Young RS, Lustig DG, Lynes SE, Donahue EP, Camacho RC, Meredith ME, Magnuson MA, Charron MJ, Wasserman DH. Hepatic energy state is regulated by glucagon receptor signaling in mice. J Clin Invest. 2009;119:2412–22. doi: 10.1172/JCI38650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Lustig DG, Baheza RA, Hasenour CM, Lee-Young RS, Donahue EP, Lynes SE, Swift LL, Charron MJ, Damon BM, Wasserman DH. Hepatic glucagon action is essential for exercise-induced reversal of mouse fatty liver. Diabetes. 2011;60:2720–2729. doi: 10.2337/db11-0455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA. 1999;96:7421–6. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–6. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Brownsey RW, Boone AN, Elliot JE, Kulpa JE, Lee WM. Regulation of acetyl-CoA carboxylase. Biochem Soc Trans. 2006;34:223–7. doi: 10.1042/BST20060223. [DOI] [PubMed] [Google Scholar]
- Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in intact mouse liver. Cell Metab. 2007;5:313–20. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α)-deficient mice. J Biol Chem. 2006;281:19000–19008. doi: 10.1074/jbc.M600050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho RC, Donahue EP, James FD, Berglund ED, Wasserman DH. Energy state of the liver during short-term and exhaustive exercise in C57BL/6J mice. Am J Physiol Endocrinol Metab. 2006;290:E405–8. doi: 10.1152/ajpendo.00385.2005. [DOI] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliot PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–9. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–22. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- Carlson CL, Winder WW. Liver AMP-activated protein kinase and acetyl-CoA carboxylase during and after exercise. J Appl Physiol. 1999;86:669–74. doi: 10.1152/jappl.1999.86.2.669. [DOI] [PubMed] [Google Scholar]
- Charest R, Blackmore PF, Berthon B, Exton JH. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983;258:8769–73. [PubMed] [Google Scholar]
- Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC1alpha pathway. Proc Natl Acad Sci USA. 2010;107:12553–8. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–81. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentin R, Liu Y, Koo SH, Hedrick S, Vargas T, Heredia J, Yates J, 3rd, Montminy M. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–9. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- Dubbelhuis PH, Meijer AJ. Hepatic amino acid-dependent signaling is under the control of AMP-dependent protein kinase. FEBS Lett. 2002;521:39–42. doi: 10.1016/s0014-5793(02)02815-6. [DOI] [PubMed] [Google Scholar]
- Dzamko N, van Denderen BJ, Hevener AL, Jørgensen SB, Honeyman J, Galic S, Chen ZP, Watt MJ, Campbell DJ, Steinberg GR, Kemp BE. AMPK beta1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem. 2010;285:115–22. doi: 10.1074/jbc.M109.056762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton JH, Park CR. The stimulation of gluconeogenesis from lactate by epinephrine, gulcagon, cyclic 3′,5′-adenylate in the perfused rat liver. Pharmacol Rev. 1966;18:181–8. [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Cantó C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliot PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–58. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54:1331–9. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–69. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Pacot C, Dugail I, Lemarchand P, Guichard C, Le Liépvre X, Berthelier-Lubrano C, Spiegelman B, Kim JB, Ferré P, Foufelle F. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol Cell Biol. 1999;19:3760–8. doi: 10.1128/mcb.19.5.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–23. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–80. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Granner D, Andreone T, Sasaki K, Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature. 1983;305:549–51. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- Guigas B, Taleux N, Foretz M, Detaille D, Andreelli F, Viollet B, Hue L. AMP-activated protein kinase-independent inhibition of hepatic mitochondrial oxidative phosphorylation by AICA riboside. Biochem J. 2007;404:499–507. doi: 10.1042/BJ20070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halseth AE, Rhéaume N, Messina AB, Reed EK, Krishna MG, Flakoll PJ, Lacy DB, Wasserman DH. Regulation of hepatic glutamine metabolism during exercise in the dog. Am J Physiol. 1998;275:E655–64. doi: 10.1152/ajpendo.1998.275.4.E655. [DOI] [PubMed] [Google Scholar]
- Handschin C, Lin J, Rhee J, Pever AK, Chin S, Wu PH, Meyer UA, Spiegelman BM. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell. 2005;122:505–15. doi: 10.1016/j.cell.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–9. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg M, Weinstein I, Kohout M. The effects of gulcagon, dibutyryl cyclic adenosine 3′,5′-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J Biol Chem. 1969;244:5131–9. [PubMed] [Google Scholar]
- Hems R, Ross BD, Berry MN, Krebs HA. Gluconeogenesis in the perfused rat liver. Biochem J. 1966;101:284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–83. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Hirsch IB, Marker JC, Smith LJ, Spina RJ, Parvin CA, Holloszy JO, Cryer PE. Insulin and glucagon in prevention of hypoglycemia during exercise in humans. Am J Physiol. 1991;260:E695–704. doi: 10.1152/ajpendo.1991.260.5.E695. [DOI] [PubMed] [Google Scholar]
- Holland R, Witters LA, Hardie DG. Glucagon inhibits fatty acid synthesis in isolated hepatocytes via phosphorylation of acetyl-CoA carboxylase by cyclic-AMP-dependent protein kinase. Eur J Biochem. 1984;140:325–33. doi: 10.1111/j.1432-1033.1984.tb08105.x. [DOI] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YH, Varanasi US, Yang W, Leff T. AMP-activated protein kinase regulates HNF4alpha transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem. 2003;278:495–501. doi: 10.1074/jbc.M304112200. [DOI] [PubMed] [Google Scholar]
- Horman S, Vertommen E, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, Rider MH. Insulin-antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–40. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- Huynh FK, Levi J, Denroche HC, Gray SL, Voshol PJ, Neumann UH, Speck M, Chua SC, Covey SD, Kieffer TJ. Disruption of hepatic leptin signaling protects mice from age- and diet-related glucose intolerance. Diabetes. 2010;59:3032–40. doi: 10.2337/db10-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signaling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–9. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspan JB, Ruddick J, Rayfield E. Transhepatic glucagon gradients in man: evidence for glucagon extraction by human liver. J Clin Endocrinol Metab. 1984;58:287–92. doi: 10.1210/jcem-58-2-287. [DOI] [PubMed] [Google Scholar]
- Jungermann K, Kietzmann T. Zonation of parenchymal and nonparenchymal metabolism in liver. Annu Rev Nutr. 1996;16:179–203. doi: 10.1146/annurev.nu.16.070196.001143. [DOI] [PubMed] [Google Scholar]
- Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–68. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B, Jang WG, Cho WJ, Ha J, Lee IK, Lee CH, Choi HS. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes. 2008;57:306–14. doi: 10.2337/db07-0381. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Siegfried BA, Jefferson LS. Glucagon represses signaling through the mammalian target of rapamycin in rat liver by activating AMP-activated protein kinase. J Biol Chem. 2004;279:54103–9. doi: 10.1074/jbc.M410755200. [DOI] [PubMed] [Google Scholar]
- Kimmig R, Mauch TJ, Scholz R. Actions of glucagon on flux rates in perfused rat liver. 2 Relationship between inhibition of glycolysis and stimulation of respiration by glucagon. Eur J Biochem. 1983;136:617–20. doi: 10.1111/j.1432-1033.1983.tb07785.x. [DOI] [PubMed] [Google Scholar]
- Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–11. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Krishna MG, Coker RH, Lacy DB, Zinker BA, Halseth AE, Wasserman DH. Glucagon response to exercise is critical for accelerated hepatic glutamine metabolism and nitrogen disposal. Am J Physiol Endocrinol Metab. 2000;279:E638–45. doi: 10.1152/ajpendo.2000.279.3.E638. [DOI] [PubMed] [Google Scholar]
- Laker RC, Henry BA, Wadley GD, Clarke IJ, Canny BJ, McConell GK. Central infusion of leptin does not increase AMPK signaling in skeletal muscle of sheep. Am J Physiol Regul Integ Comp Physiol. 2011;300:R511–8. doi: 10.1152/ajpregu.00079.2010. [DOI] [PubMed] [Google Scholar]
- Lam CK, Chari M, Rutter GA, Lam TK. Hypothalamic nutrient sensing activates a forebrain-hindbrain neuronal circuit to regulate glucose production in vivo. Diabetes. 2011;60:107–13. doi: 10.2337/db10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie C, Ducros F, Bourque J, Langelier H, Chiasson JL. Glucose metabolism during exercise in man: the role of insulin and glucagon in the regulation of hepatic glucose production and gluconeogenesis. Can J Physiol Pharmacol. 1997;75:26–35. doi: 10.1139/cjpp-75-1-26. [DOI] [PubMed] [Google Scholar]
- Leclerc I, Lenzner C, Gourdon L, Vaulont S, Kahn A, Viollet B. Hepatocyte nuclear factor-4alpha involved in type 1 maturity-onset diabetes of the young is a novel target of AMP-activated protein kinase. Diabetes. 2001;50:1515–21. doi: 10.2337/diabetes.50.7.1515. [DOI] [PubMed] [Google Scholar]
- Lee Y, Yu X, Gonzales F, Mangelsdorf DJ, Wang MY, Richardson C, Witters LA, Unger RH. PPAR alpha is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue. Proc Natl Acad Sci USA. 2002;99:11848–53. doi: 10.1073/pnas.182420899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Lay J, Tuteja G, White P, Dhir R, Ahima R, Kaestner KH. CRTC2 (TORC2) contributes to the transcriptional response to fasting in the liver but is not required for the maintenance of glucose homeostasis. Cell Metab. 2009;10:55–62. doi: 10.1016/j.cmet.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Nat Acad Sci USA. 2010;107:3441–6. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Monks B, Qingyuan G, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1α transcription coactivator. Nature. 2007;447:1012–6. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–88. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longuet C, Sinclair EM, Maida A, Baggio LL, Maziarz M, Charron MJ, Drucker DJ. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 2008;8:359–71. doi: 10.1016/j.cmet.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig Y, Ruas JL, Estall JL, Lo JC, Devarakonda S, Laznik D, Choi JH, Ono H, Olsen JV, Spiegelman BM. Separation of the gluconeogenic and mitochondrial functions of PGC-1{alpha} through S6 kinase. Genes Dev. 2011;25:1232–44. doi: 10.1101/gad.2054711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrouk GM, Helmy IM, Thampy KG, Wakil SJ. Acute hormonal control of acetyl-CoA carboxylase. The roles of insulin, glucagon, and epinephrine. J Biol Chem. 1990;265:6330–8. [PubMed] [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–23. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechuad PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–21. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Birnbaum MJ. An energetic tale of AMPK-independent effects of metformin. J Clin Invest. 2010;120:2267–70. doi: 10.1172/JCI43661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Chu Q, Le Lay J, Scherer PE, Ahima RS, Kaestner KH, Foretz M, Viollet B, Birnbaum MJ. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J Clin Invest. 2011;121:2518–28. doi: 10.1172/JCI45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HS, Chamberland JP, Mantzoros CS. Amylin and leptin activate overlapping signaling pathways in an additive manner in mouse GT1–7 hypothalamic, C2C12 muscle and AML12 liver cell lines. Diabetologia. 2012;55:215–25. doi: 10.1007/s00125-011-2332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday MR, Milic MR, Takhar S, Holness MJ, Sugden MC. The short-term regulation of hepatic acetyl-CoA carboxylase during starvation and re-feeding in the rat. Biochem J. 1991;280:733–7. doi: 10.1042/bj2800733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muse ED, Obici S, Bhanot S, Monia BP, McKay RA, Rajala MW, Scherer PE, Rossetti L. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232–9. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–5. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–60. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA. 2009;106:10853–8. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–5. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Qi Y, Nie Z, Lee YS, Singhal NS, Scherer PE, Lazar MA, Ahima RS. Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes. 2006;55:3083–90. doi: 10.2337/db05-0615. [DOI] [PubMed] [Google Scholar]
- Reiter AK, Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMPK represses TOP mRNA translation but not global protein synthesis in liver. Biochem Biophys Res Commun. 2008;374:345–50. doi: 10.1016/j.bbrc.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc Natl Acad Sci USA. 2003;100:4012–7. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera N, Ramnanan CJ, An Z, Farmer T, Smith M, Farmer B, Irimia JM, Snead W, Lautz M, Roach PJ, Cherrington AD. Insulin-induced hypoglycemia increases hepatic sensitivity to glucagon in dogs. J Clin Invest. 2010;120:4425–35. doi: 10.1172/JCI40919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem. 2009;109:17–23. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JS, Williams MR, Morrice N, Deak M, Alessi DR. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell growth. J Biol Chem. 2001;276:19469–82. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- Scott JW, van Denderen BJ, Jorgensen SB, Honeyman JE, Steinberg GR, Oakhill JS, Iseli TJ, Koay A, Gooley PR, Stapleton D, Kemp BE. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol. 2008;15:1220–30. doi: 10.1016/j.chembiol.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, 3rd, Takemori H, Okamoto M, Montminy M. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–75. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated protein kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–6. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim AT, Hardie DG. The low activity of acetyl-CoA carboxylase in basal and glucagon stimulated hepatocytes is due to phosphorylation by AMP-activated protein kinase and not cyclic AMP-dependent protein kinase. FEBS Lett. 1988;233:294–8. doi: 10.1016/0014-5793(88)80445-9. [DOI] [PubMed] [Google Scholar]
- Singhal NS, Patel RT, Qi Y, Lee YS, Ahima RS. Loss of resistin ameliorates hyperlipidemia and hepatic steatosis in leptin-deficient mice. Am J Physiol Endocrinol Metab. 2008;295:E331–8. doi: 10.1152/ajpendo.00577.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Watt MJ, Febbraio MA. Cytokine regulation of AMPK signaling. Front Biosci. 2009;14:1902–16. doi: 10.2741/3350. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–76. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, Leavens KF, Saleh D, Easton RM, Guertin DA, Peterson TR, Kaestner KH, Sabatini DM, Birnbaum MJ. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP-1c. Cell Metab. 2011;14:516–27. doi: 10.1016/j.cmet.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Moya N, Niessen S, Hoover H, Mihaylova MM, Shaw RJ, Yates JR, 3rd, Fischer WH, Thomas JB, Montminy M. A hormone-dependent module regulating energy balance. Cell. 2011;145:596–606. doi: 10.1016/j.cell.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab. 2009;296:E11–21. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman DH, Lacy DB, Bracy DP. Relationship between arterial and portal vein immunoreactive glucagon during exercise. J Appl Physiol. 1993;75:724–9. doi: 10.1152/jappl.1993.75.2.724. [DOI] [PubMed] [Google Scholar]
- Wasserman DH, Lickley HL, Vranic M. Interactions between glucagon and other counterregulatory hormones during normoglycemic exercise in dogs. J Clin Invest. 1984;74:1404–13. doi: 10.1172/JCI111551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman DH, Lickley HL, Vranic M. Important role of glucagon during exercise in diabetic dogs. J Appl Physiol. 1985;59:1272–81. doi: 10.1152/jappl.1985.59.4.1272. [DOI] [PubMed] [Google Scholar]
- Wasserman DH, O’Doherty RM, Zinker BA. Role of the endocrine pancreas in control of fuel metabolism by the liver during exercise. Int J Obes Relat Metab Disord. 1995;19:S22–30. [PubMed] [Google Scholar]
- Wasserman DH, Spalding JA, Lacy DB, Colburn CA, Goldstein RE, Cherrington AD. Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. Am J Physiol. 1989;257:E108–17. doi: 10.1152/ajpendo.1989.257.1.E108. [DOI] [PubMed] [Google Scholar]
- Wasserman DH, Williams PE, Lacy DB, Green DR, Cherrington AD. Importance of intrahepatic mechanisms to gluconeogenesis from alanine during exercise and recovery. Am J Physiol. 1988;254:E518–25. doi: 10.1152/ajpendo.1988.254.4.E518. [DOI] [PubMed] [Google Scholar]
- Williamson JR, Scholz R, Browning ET. Control mechanisms of gluconeogenesis and ketogenesis. II Interactions between fatty acid oxidation and the citric acid cycle in perfused rat liver. J Biol Chem. 1969;244:4617–27. [PubMed] [Google Scholar]
- Witters LA, Gao G, Kemp BE, Quistorff B. Hepatic 5′-AMP-activated protein kinase: zonal distribution and relationship to acetyl-coA carboxylase activity in varying nutritional states. Arch Biochem Biophys. 1994;308:413–9. doi: 10.1006/abbi.1994.1058. [DOI] [PubMed] [Google Scholar]
- Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of 5′-AMP-activated protein kinase. J Biol Chem. 1992;267:2864–7. [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Woodside KH, Ward WF, Mortimore GE. Effects of glucagon on general protein degradation and synthesis in perfused rat liver. J Biol Chem. 1974;249:5458–63. [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- Yang CS, Lam CK, Chari M, Cheung GW, Kokorovic A, Gao S, Leclerc I, Rutter GA, Lam TK. Hypothalamic AMP-activated protein kinase regulates glucose production. Diabetes. 2010;59:2435–43. doi: 10.2337/db10-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, Manning BD. Akt stimulates hepatic Srebp1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–8. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47:2012–21. doi: 10.1007/s00125-004-1570-9. [DOI] [PubMed] [Google Scholar]
- Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, Liu DD, Torres JM, Jia W, Lechleiter JD, Liu F, Dong LQ. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem. 2009;284:22426–35. doi: 10.1074/jbc.M109.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA. 2002;99:15983–7. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]