Abstract
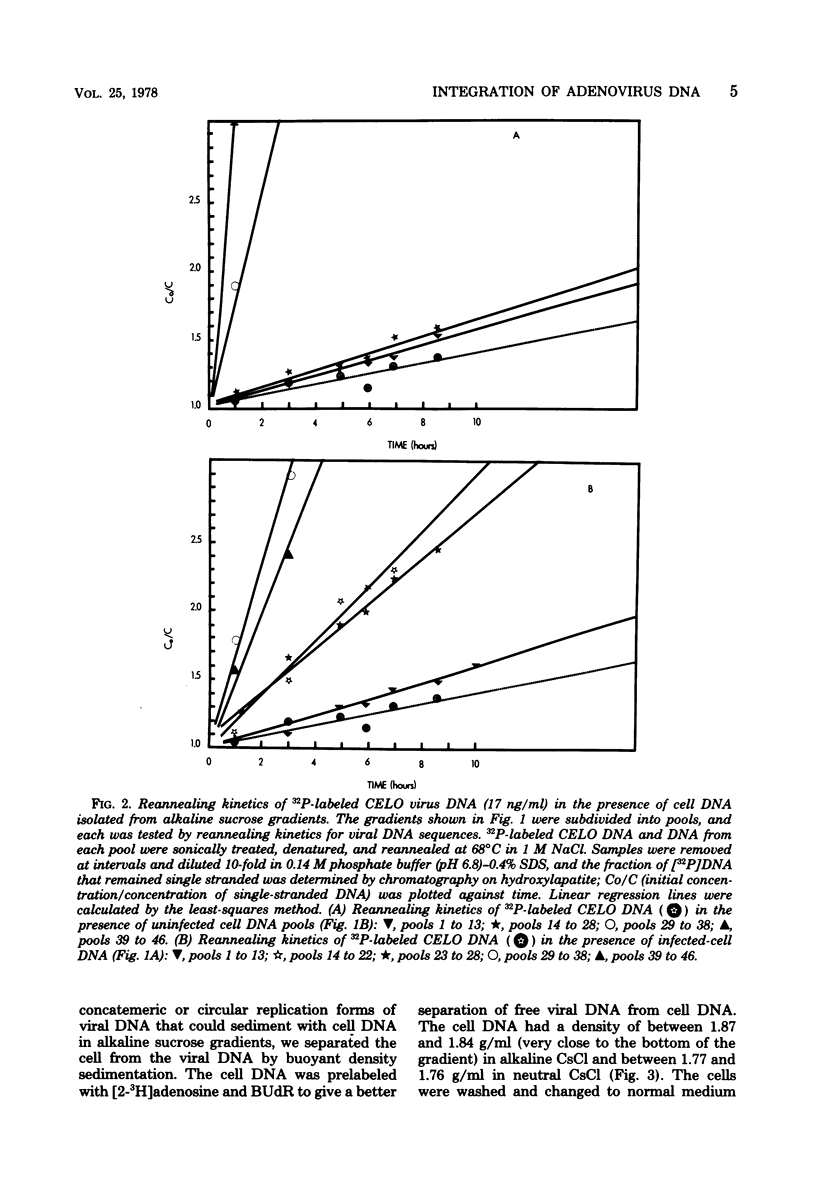
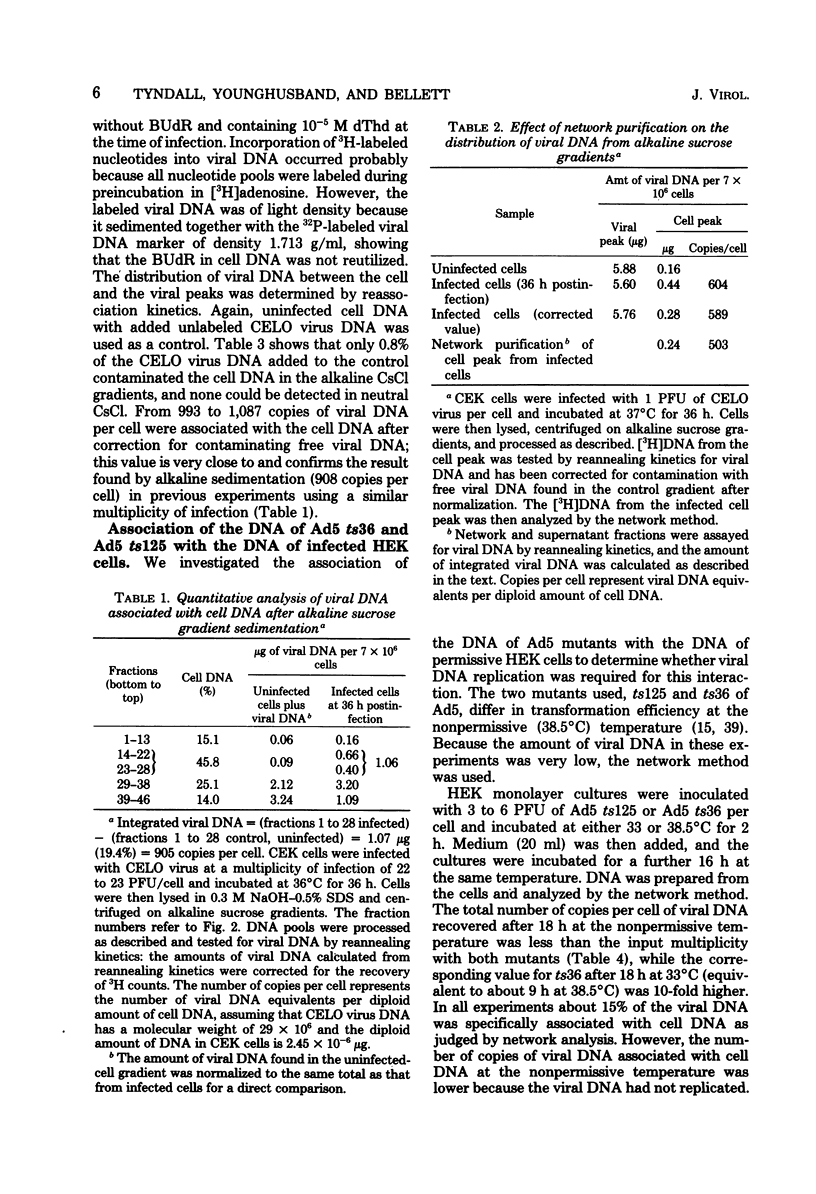
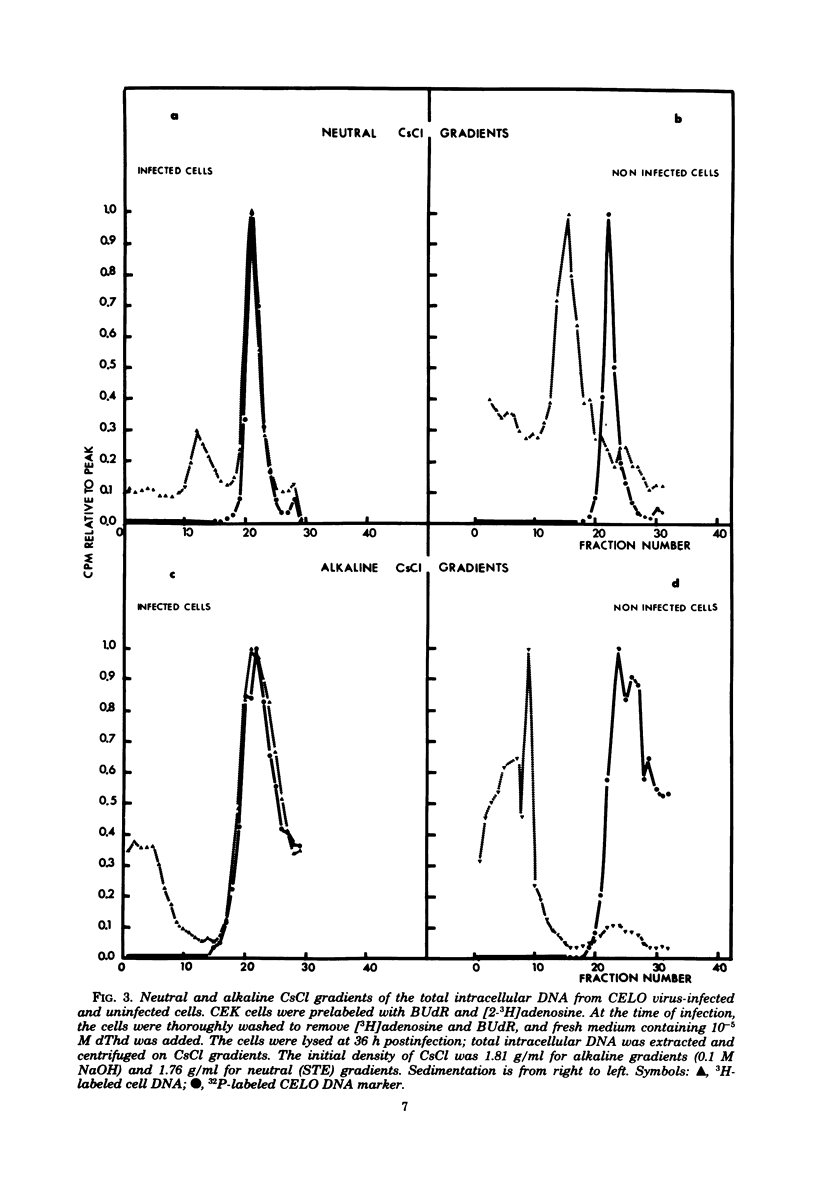
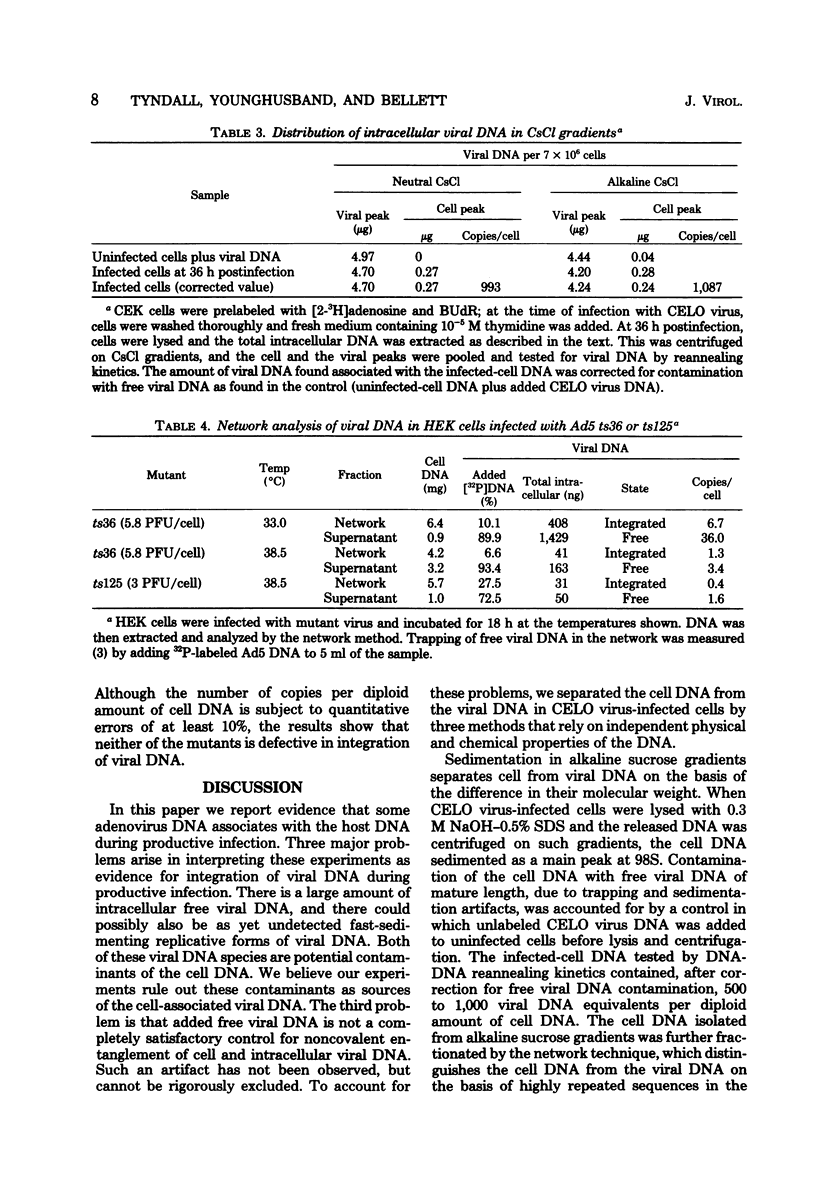
We have investigated the association of viral DNA with cell DNA in chicken embryo kidney (CEK) cells productively infected with chicken embryo lethal orphan (CELO) virus and in human (HEK) cells infected with mutants ts36 and ts125 of human adenovirus type 5 under permissive and restrictive conditions. Cell and viral DNA molecules were separated after CELO virus infection of CEK cells by alkaline sucrose gradient centrifugation, network formation, and CsCl density gradient centrifugation, methods that rely on different properties of the DNA. The cell DNA was then tested for viral sequences by DNA reannealing kinetics. Between 500 and 1,000 viral genome equivalents per cell were found at 36 h postinfection associated with cell DNA purified by each method. These values greatly exceeded the amount of free viral DNA found contaminating cell DNA prepared by the same methods from uninfected cells to which CELO virus DNA had been added. Quantitative agreement in the amounts of viral DNA found associated with cell DNA purified by these different methods suggests that CELO virus DNA is integrated into chick cell DNA during lytic infection. Similar experiments in HEK cells using mutants ts36 and ts125 of adenovirus type 5 at both restrictive and permissive temperatures showed that the same proportion of viral DNA is associated with cell DNA in the absence of viral DNA replication, and this suggests that the difference in the frequency with which cells are transformed by these mutants is not due to a difference in the frequency integration.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Yates V. J., Jasty V., Mancini L. O. The in vitro transformation by an avian adenovirus (CELO). 3. Human amnion cellcultures. J Natl Cancer Inst. 1969 Sep;43(3):575–580. [PubMed] [Google Scholar]
- Bellett A. J. Covalent integration of viral DNA into cell DNA in hamster cells transformed by an avian adenovirus. Virology. 1975 Jun;65(2):427–435. doi: 10.1016/0042-6822(75)90048-3. [DOI] [PubMed] [Google Scholar]
- Bellett A. J., Younghusband H. B. Replication of the DNA of chick embryo lethal orphan virus. J Mol Biol. 1972 Dec 30;72(3):691–709. doi: 10.1016/0022-2836(72)90185-4. [DOI] [PubMed] [Google Scholar]
- Burger H., Doerfler W. Intracellular forms of adenovirus DNA. 3. Integration of the DNA of adenovirus type 2 into host DNA in productively infected cells. J Virol. 1974 May;13(5):975–992. doi: 10.1128/jvi.13.5.975-992.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W. Three size-classes of intracellular adenovirus deoxyribonucleic acid. J Virol. 1971 Jun;7(6):707–719. doi: 10.1128/jvi.7.6.707-719.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Gallimore P. H., McDougall J. K. Correlation between tumor induction and the large external transformation sensitive protein on the cell surface. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3570–3574. doi: 10.1073/pnas.73.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Burger H., Ortin J., Fanning E., Brown D. T., Mestphal M., Winterhoff U., Weiser B., Schick J. Integration of adenovirus DNA into the cellular genome. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):505–521. doi: 10.1101/sqb.1974.039.01.063. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970 Nov;6(5):652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Doerfler W. Intracellular forms of adenovirus DNA. V. Viral DNA sequences in hamster cells abortively infected and transformed with human adenovirus type 12. J Virol. 1976 Nov;20(2):373–383. doi: 10.1128/jvi.20.2.373-383.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Sambrook J., Williams J. F., Sharp P. A. Viral nucleic acid sequences in transformed cells. IV. A study of the sequences of adenovirus 5 DNA and RNA in four lines of adenovirus 5-transformed rodent cells using specific fragments of the viral genome. Virology. 1976 Jul 15;72(2):456–470. doi: 10.1016/0042-6822(76)90174-4. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Ensinger M. J., Kauffman R. S., Mayer A. J., Lundholm U. Cell transformation: a study of regulation with types 5 and 12 adenovirus temperature-sensitive mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):419–426. doi: 10.1101/sqb.1974.039.01.054. [DOI] [PubMed] [Google Scholar]
- Green M. R., Chinnadurai G., Mackey J. K., Green M. A unique pattern of integrated viral genes in hamster cells transformed by highly oncogenic human adenovirus 12. Cell. 1976 Mar;7(3):419–428. doi: 10.1016/0092-8674(76)90172-0. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Intermediates in the synthesis of type 2 adenovirus deoxyribonucleic acid. J Virol. 1971 Nov;8(5):675–683. doi: 10.1128/jvi.8.5.675-683.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini L. O., Anderson J., Jasty V., Yates V. J. Tumor induction in hamsters inoculated with an avian adenovirus (CELO). Brief report. Arch Gesamte Virusforsch. 1970;30(2):261–262. doi: 10.1007/BF01250198. [DOI] [PubMed] [Google Scholar]
- Mancini L. O., Yates V. J., Jasty V., Anderson J. Ependymomas induced in hamsters inoculated with an avian adenovirus (CELO). Nature. 1969 Apr 12;222(5189):190–191. doi: 10.1038/222190a0. [DOI] [PubMed] [Google Scholar]
- May J. T., Robinson A. J., Eaton B. T. A study of the sequences of chicken embryo lethal orphan (CELO) virus DNA present in a transformed hamster cell line with use of specific fragments of the virus genome. Virology. 1975 Dec;68(2):483–489. doi: 10.1016/0042-6822(75)90288-3. [DOI] [PubMed] [Google Scholar]
- Mayer A. J., Ginsberg H. S. Persistence of type 5 adenovirus DNA in cells transformed by temperature-sensitive mutant, H5ts125. Proc Natl Acad Sci U S A. 1977 Feb;74(2):785–788. doi: 10.1073/pnas.74.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall J. K. Adenoviruses--interaction with the host cell genome. Prog Med Virol. 1975;21:118–132. [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Hanawalt P. C. Isolation of DNA replication complexes from uninfected and adenovirus-infected HeLa cells. J Mol Biol. 1971 Nov 28;62(1):65–80. doi: 10.1016/0022-2836(71)90131-8. [DOI] [PubMed] [Google Scholar]
- Pearson G. D. Intermediate in adenovirus type 2 replication. J Virol. 1975 Jul;16(1):17–26. doi: 10.1128/jvi.16.1.17-26.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendola R. G., Yates V. J. In vitro neoplastic transformation of hamster embryonic skin and subcutaneous tissue cells with small-plaque and large-plaque variants of an avian adenovirus (chicken embryo lethal orphan). Cancer Res. 1973 Feb;33(2):351–355. [PubMed] [Google Scholar]
- Pettersson U. Letter: Some unusual properties of replicating adenovirus type 2 DNA. J Mol Biol. 1973 Dec 25;81(4):521–527. doi: 10.1016/0022-2836(73)90521-4. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Bellett A. J. Complementary strands of CELO virus DNA. J Virol. 1975 Mar;15(3):458–465. doi: 10.1128/jvi.15.3.458-465.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Huebner R. J., Lane W. T. Induction of tumors in hamsters with an avian adenovirus (CELO). Science. 1965 Sep 3;149(3688):1108–1108. doi: 10.1126/science.149.3688.1108. [DOI] [PubMed] [Google Scholar]
- Schick J., Baczko K., Fanning E., Groneberg J., Burger H., Doerfler W. Intracellular forms of adenovirus DNA: integrated form of adenovirus DNA appears early in productive infection. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1043–1047. doi: 10.1073/pnas.73.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Bellett A. J., Robinson A. J. Replication of linear adenovirus DNA is not hairpin-primed. Nature. 1977 Oct 20;269(5630):723–725. doi: 10.1038/269723a0. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C. Viral DNA synthesis in isolated nuclei from adenovirus-infected KB cells. FEBS Lett. 1972 Mar;21(1):7–10. doi: 10.1016/0014-5793(72)80149-2. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willians J. F., Young C. S., Austin P. E. Genetic analysis of human adenovirus type 5 in permissive and nonpermissive cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):427–437. doi: 10.1101/sqb.1974.039.01.055. [DOI] [PubMed] [Google Scholar]
- Younghusband H. B., Bellett A. J. Mature form of the deoxyribonucleic acid from chick embryo lethal orphan virus. J Virol. 1971 Sep;8(3):265–274. doi: 10.1128/jvi.8.3.265-274.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Hausen H., Sokol F. Fate of adenovirus type 12 genomes in nonpermissive cells. J Virol. 1969 Sep;4(3):256–263. doi: 10.1128/jvi.4.3.256-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



