Abstract
Background
The goal of breast cancer screening is to reduce breast cancer mortality. Mammography is the standard screening method for detecting breast cancer early. Breast magnetic resonance imaging (MRI) is recommended to be used in conjunction with mammography for screening subsets of women at high risk for breast cancer. We offer the first study to provide national estimates of breast MRI use among women in the United States.
Methods
We analyzed data from women who responded to questions about having a breast MRI on the 2010 National Health Interview Survey. We assessed report of having a breast MRI and reasons for it by sociodemographic characteristics and access to health care and computed 5-year and lifetime breast cancer risk using the Gail model.
Results
Among 11,222 women who responded, almost 5% reported ever having a breast MRI and 2% reported having an MRI within the 2 years preceding the survey. Less than half of the women who reported having a breast MRI were at increased risk. Approximately 60% of women reported having the breast MRI for diagnostic reasons. Women who ever had a breast MRI were more likely to be older, black, and insured and to report a usual source of health care compared to women who reported no MRI.
Conclusions
Breast MRI use may be underused or overused in certain subgroups of women.
Impact
As access to health care improves, the use of breast MRI and the appropriateness of its use for breast cancer detection will be important to monitor.
Introduction
The goal of screening for breast cancer is to reduce the number of women who die from breast cancer while providing the least adverse impact on women who do not have breast cancer, but this can only occur with early detection of clinically relevant breast cancer and appropriate treatment. Currently, mammography is the best way to identify breast cancer before it is clinically detectable (1, 2). However, mammography will only detect 65% to 90% of breast cancers, and sensitivity is lower among women with dense breast tissue. Further, mammography has a false-positive rate of 2% to 11% (3, 4). In addition to its high false-positive rates, screening mammography is associated with over-diagnosis of ductal carcinoma in situ and invasive breast cancer (5). Thus, research continues on the development of imaging tools with higher sensitivity and specificity for detecting clinically relevant breast cancer.
Breast magnetic resonance imaging (MRI), which uses magnetic fields instead of x-rays, is a tool that has received great interest in its use for breast cancer detection. Breast MRI sensitivity ranges from 80% to 95% for detecting breast cancer. However, it has a higher false-positive rate of 20% to 80%, compared with mammography (6–8, 9). Breast MRI has been primarily used as a diagnostic tool to assess abnormalities identified by mammography and the extent of known breast cancer (10, 11). In more recent years, breast MRI has shown some benefit in the detection of breast cancer, especially for women with multifocal disease, who have dense breasts, or at high risk of breast cancer secondary to inherited genetic mutations (9, 12, 13).
In 2007, the American Cancer Society (ACS) published recommendations for annual breast MRI along with annual mammography screening for women who have ≥20% lifetime risk for developing breast cancer, who carry the BRCA mutation, or have a first-degree relative with BRCA mutation (14). However, the extent to which breast MRI has been used in the United States is not well known. To estimate the use of breast MRI, the Centers for Disease Control and Prevention and the National Cancer Institute cosponsored the inclusion of several new questions on use of breast MRI in the Cancer Control Supplement to the 2010 U.S. National Health Interview Survey (NHIS). In this paper, we describe the proportion of women in the United States who have had breast MRI, their reasons for having it, and factors associated with breast MRI use.
Materials and Methods
We used data from the 2010 NHIS, a nationally representative sample of the civilian, non-institutionalized, and household population of the United States (15). The NHIS is an annual, multipurpose health survey administered by the National Center for Health Statistics (NCHS) with data collected through in-person interviews. Basic health, demographic, and cancer history information is available from the Sample Adult Core of the Basic Module. In 2010, the NHIS Cancer Control Supplement included questions regarding cancer history, cancer screening, family history of cancer, and other health-related behaviors. A total of 27,157 adults were interviewed in the 2010 NHIS, with a final sample adult response rate of 60.8%. Our study sample included women aged 30 and older who responded to the series of questions related to breast MRI utilization.
Breast MRI
Respondents were asked whether they had ever had a breast MRI and if so, when they had their most recent one. From these responses, we defined women as having had a recent breast MRI if they reported having one within the 2 years preceding survey. Although the ACS guidelines for breast MRI screening recommends annual MRI for high-risk women, there were too few women who reported having breast MRIs to examine its use within one year. Respondents were also asked to select, from a series of reasons, their main reason for having this breast MRI: a follow-up of an abnormal mammogram; because of a breast problem; because my healthcare provider told me I was high risk; I have a family history of breast cancer; part of a routine exam; I requested it; and other. From these responses, we defined the breast MRI as a screening exam if they indicated that they requested it, it was part of a routine exam, because they were told they were at high risk, or had a family history of breast cancer. We defined the breast MRI as a diagnostic exam if they reported it was a follow up of an abnormal mammogram or because of a breast problem.
Breast cancer risk
We examined various risk factors for breast cancer since they would influence whether a woman reported having a recent breast MRI, and whether a woman reported the reasons for the breast MRI as screening or diagnostic. These factors included results of the most recent mammogram (normal, abnormal), number of prior breast biopsies (0, ≥1), family history of breast cancer (yes, no), and personal history of breast cancer (yes, no). We also examined whether a woman had a mammogram within the past 2 years (yes, no) and a personal history of any other cancer (yes, no).
Additionally, we computed 5-year and lifetime Gail risk scores for the women in our study population who reported no prior history of breast cancer. We determined their composite risk for developing breast cancer (16–18) using the National Cancer Institute Breast Cancer Risk Assessment Tool model (19). Data from the 2000 and 2005 NHIS has been used previously to estimate the U.S. population 5-year and lifetime risk of developing breast cancer (20–22). These studies computed Gail scores using age (<50 years, ≥50 years), age at menarche (<12 years, 12–13 years, ≥14 years), age at first live birth (<20 years, 20–24 years, 25–29 years or nulliparous, ≥30 years), number of benign breast biopsies (0, 1, ≥2), and number of first-degree relatives with breast carcinoma (0, 1, ≥2). Women with absolute 5-year risk of ≥1.66% or lifetime risk ≥20% were considered to be at increased risk for developing breast cancer, and those with lower scores were considered average risk.
Correlates of breast MRI utilization
We also examined various sociodemographic characteristics and access to health care factors for associations with reported breast MRI utilization. The sociodemographic characteristics included age (30–39 years, 40–49 years, 50–64 years, 65–74 years, and ≥75 years), race/ethnicity (white non-Hispanic, black non-Hispanic, Asian non-Hispanic, other non-Hispanic, and Hispanic), education (less than high school graduate, high school graduate, some college, college graduate), income (<100% Federal Poverty Level [FPL], 100%–199% FPL, 200%–299% FPL, 300%–399% FPL, ≥400% FPL), and region (Northeast, Midwest, South, West). Poverty threshold data were taken from the multiply imputed income files (23). Access to health care was measured by insurance coverage (privately insured, publicly insured, and uninsured) and usual source of health care (yes, no).
Statistical analyses
Descriptive statistics are presented as weighted percentages with 95% confidence intervals based on a logit transformation. Statistical testing for differences in weighted percentages was performed using the Rao-Scott Pearson chi-square test. We used SAS-callable SUDAAN and SAS survey procedures (version 9.2; SAS, Cary, NC) to account for the complex, multistage sampling design and to obtain results weighted to reflect the civilian, non-institutionalized population of the United States. For all analyses, significance was determined at P <0.05.
Results
Our final sample included 11,222 women who responded “yes” or “no” to having had a breast MRI. Almost 5% of women reported ever receiving a breast MRI, with almost half of these women reporting that they had a recent MRI (Table 1). Women who reported ever having an MRI were more likely to be older, black, and have a usual source of health care and less likely to be uninsured compared with women who reported never having a breast MRI. Breast cancer risk factors and mammography use also differed for women who reported ever having a breast MRI. These women were more likely to report having had a recent mammogram, abnormal results from that recent mammogram, prior breast biopsies, a personal history of breast and non-breast cancer, a family history of breast cancer, and to have an increased 5-year and lifetime breast cancer risk, compared with women who reported not having a breast MRI. Based on the Gail model risk score for breast cancer, 18% of the entire sample of women was determined to have an increased 5-year risk for breast cancer and 1% had an increased lifetime risk. Among the women who reported ever having a breast MRI, only 30% had an increased 5-year risk and 3% had an increased lifetime risk. Among women who reported a recent breast MRI, only 26% had an increased 5-year risk and 1% an increased lifetime risk. Approximately 6% of women with increased 5-year risk for breast cancer and 10% with increased lifetime risk reported ever having had an MRI (data not shown). In comparison, among women at average 5-year and lifetime risk, 3% and 4%, respectively, reported ever having had an MRI.
Table 1.
Characteristics of women aged 30 and older who reported having or not having had a breast MRI — National Health Interview Survey, United States, 2010
| All | Ever MRI | Recent MRIa | No MRI | ||
|---|---|---|---|---|---|
| (N=11222) | (n=545) | (n=253) | (n=10677) | ||
| Wtd% (95% CI) | Wtd % (95% CI) | Wtd % (95% CI) | Wtd % (95% CI) | P-valueb | |
| Total | 100% | 4.7 (4.3–5.2) | 2.1 (1.8–2.5) | 95.3 (94.8–95.7) | |
| Age | N=11222 | N=545 | N=253 | N=10677 | <0.0001 |
| 30–39 | 21.6 (20.7–22.5) | 7.0 (4.8–10.1) | 9.0 (5.5–14.4) | 22.3 (21.4–23.3) | |
| 40–49 | 23.2 (22.3–24.2) | 21.2 (17.1–26.0) | 26.0 (19.6–33.7) | 23.3 (22.4–24.4) | |
| 50–64 | 31.9 (30.8–32.9) | 43.5 (38.8–48.2) | 40.6 (33.6–48.1) | 31.3 (30.2–32.4) | |
| 65–74 | 12.3 (11.6–13.1) | 13.7 (10.7–17.4) | 14.1 (9.8–19.8) | 12.3 (11.6–13.0) | |
| 75+ | 10.9 (10.3–11.6) | 14.6 (11.5–18.4) | 10.2 (6.8–15.1) | 10.7 (10.1–11.5) | |
| Race | N=11222 | N=545 | N=253 | N=10677 | 0.0160 |
| White,non-Hispanic | 70.5 (69.3–71.6) | 68.5 (64.0–72.6) | 56.9 (49.5–63.9) | 70.6 (69.3–71.8) | |
| Black,non-Hispanic | 11.9 (11.1–12.7) | 16.8 (13.3–21.1) | 24.3 (18.2–31.6) | 11.6 (10.8–12.5) | |
| Asian,non-Hispanic | 4.8 (4.3–5.3) | 3.3 (2.1–5.2) | 5.1 (2.9–8.9) | 4.8 (4.3–5.4) | |
| Other, non-Hispanic | 1.0 (0.8–1.3) | 1.0 (0.3–3.1) | 1.1 (0.2–5.0) | 1.0 (0.8–1.3) | |
| Hispanic | 11.9 (11.2–12.7) | 10.4 (8.1–13.1) | 12.6 (9.0–17.4) | 12.0 (11.2–12.7) | |
| Education | N=11184 | N=540 | N=251 | N=10644 | 0.8967 |
| < high school graduate | 13.8 (13.0–14.6) | 12.5 (9.9–15.8) | 14.0 (9.7–19.7) | 13.8 (13.0–14.7) | |
| High school graduate | 26.9 (25.9–27.9) | 27.8 (23.6–32.5) | 25.6 (19.8–32.5) | 26.9 (25.9–27.9) | |
| Some college | 30.3 (29.3–31.4) | 30.6 (25.9–35.8) | 29.4 (22.9–36.8) | 30.3 (29.3–31.4) | |
| ≥College graduate | 29.0 (27.8–30.3) | 29.0 (24.5–34.1) | 31.0 (24.8–38.0) | 29.0 (27.8–30.3) | |
| Income status, %FPL | N=11222 | N=545 | N=253 | N=10677 | 0.2476 |
| <100 | 12.0 (11.3–12.8) | 12.0 (9.4–15.3) | 15.5 (11.2–21.0) | 12.0 (11.3–12.8) | |
| 100–199 | 19.0 (18.0–19.9) | 15.1 (11.6–19.4) | 15.9 (11.0–22.5) | 19.1 (18.2–20.2) | |
| 200–299 | 16.5 (15.6–17.5) | 15.2 (11.7–19.4) | 12.2 (8.1–18.0) | 16.6 (15.6–17.6) | |
| 300–399 | 14.2 (13.3–15.1) | 15.3 (11.1–20.8) | 17.5 (12.3–24.4) | 14.1 (13.3–15.0) | |
| 400+ | 38.3 (36.9–39.8) | 42.4 (37.2–47.8) | 38.8 (31.8–46.3) | 38.1 (36.7–39.6) | |
| Region | N=11222 | N=545 | N=253 | N=10677 | 0.3408 |
| Northeast | 17.7 (16.5–18.8) | 19.0 (15.2–23.4) | 21.4 (14.6–30.3) | 17.6 (16.5–18.8) | |
| Midwest | 23.1 (21.9–24.3) | 19.4 (16.1–23.2) | 20.2 (15.0–26.5) | 23.3 (22.1–24.5) | |
| South | 35.8 (34.3–37.4) | 38.1 (33.1–43.3) | 37.3 (30.2–45.0) | 35.7 (34.1–37.3) | |
| West | 23.4 (22.0–24.9) | 23.6 (19.4–28.4) | 21.1 (15.6–27.9) | 23.4 (22.0–24.9) | |
| Insurance coverage | N=11197 | N=543 | N=253 | N=10654 | 0.0178 |
| Private | 68.7 (67.6–69.9) | 72.8 (68.1–77.0) | 68.6 (62.0–74.5) | 68.5 (67.3–69.7) | |
| Public Only | 18.1 (17.3–19.0) | 18.7 (15.3–22.7) | 19.6 (15.0–25.2) | 18.1 (17.2–19.0) | |
| Uninsured | 13.1 (12.4–13.9) | 8.5 (6.2–11.6) | 11.8 (7.7–17.7) | 13.4 (12.6–14.2) | |
| Usual source of healthcare | N=11222 | N=545 | N=253 | N=10677 | 0.0064 |
| Yes | 90.1 (89.4–90.8) | 93.7 (91.1–95.6) | 94.2 (90.2–96.6) | 89.9 (89.2–90.6) | |
| No | 9.9 (9.2–10.6) | 6.3 (4.4–8.9) | 5.8 (3.4–9.8) | 10.1 (9.4–10.8) | |
| Had recent mammograma | N=11126 | N=541 | N=251 | N=10585 | <0.0001 |
| Yes | 55.4 (54.2–56.6) | 80.9 (76.8–84.5) | 90.8 (85.0–94.5) | 54.2 (52.9–55.4) | |
| No | 44.6 (43.4–45.8) | 19.1 (15.5–23.2) | 9.2 (5.5–15.0) | 45.8 (44.6–47.1) | |
| Results of recent mammogram | N=8452 | N=523 | N=243 | N=7929 | <0.0001 |
| Normal | 89.4 (88.5–90.2) | 76.2 (72.0–80.0) | 64.5 (57.8–70.7) | 90.2 (89.3–91.1) | |
| Abnormal | 10.6 (9.8–11.5) | 23.8 (20.0–28.0) | 35.5 (29.3–42.2) | 9.8 (8.9–10.7) | |
| Number of prior breast biopsies | N=8384 | N=495 | N=231 | N=7889 | <0.0001 |
| 0 | 81.2 (80.2–82.2) | 60.8 (55.3–66.0) | 66.4 (58.5–73.5) | 82.5 (81.4–83.5) | |
| ≥1 | 18.8 (17.8–19.8) | 39.2 (34.0–44.7) | 33.6 (26.5–41.5) | 17.5 (16.5–18.6) | |
| Family history of breast cancer | N=10801 | N=527 | N=248 | N=10274 | <0.0001 |
| Yes | 12.1 (11.3–12.9) | 19.6 (15.7–24.2) | 20.9 (15.4–27.8) | 11.7 (11.0–12.5) | |
| No | 87.9 (87.1–88.7) | 80.4 (75.8–84.3) | 79.1 (72.2–84.6) | 88.3 (87.5–89.0) | |
| Personal history of breast cancer | N=11202 | N=543 | N=253 | N=10659 | <0.0001 |
| Yes | 3.3 (2.9–3.7) | 23.2 (19.1–27.8) | 24.6 (18.5–31.8) | 2.3 (2.0–2.6) | |
| No | 96.7 (96.3–97.1) | 76.8 (72.2–80.9) | 75.4 (68.2–81.5) | 97.7 (97.4–98.0) | |
| Personal history of other cancer | N=11209 | N=545 | N=253 | N=10664 | 0.0015 |
| Yes | 8.3 (7.8–9.0) | 13.0 (9.9–17.0) | 13.5 (8.9–19.8) | 8.1 (7.5–8.8) | |
| No | 91.7 (91.0–92.2) | 87.0 (83.0–90.1) | 86.5 (80.2–91.1) | 91.9 (91.2–92.5) | |
| Gail5-year risk | N=10822 | N=417 | N=190 | N=10405 | <0.0001 |
| Average risk | 82.1 (81.2–82.9) | 70.1 (64.1–75.5) | 73.9 (65.8–80.6) | 82.5 (81.6–83.4) | |
| Increased risk (≥1.66%) | 17.9 (17.1–18.8) | 29.9 (24.5–35.9) | 26.1 (19.4–34.2) | 17.5 (16.6–18.4) | |
| Gail Lifetimerisk | N=10822 | N=417 | N=190 | N=10405 | 0.0041 |
| <20% | 98.9 (98.7–99.1) | 97.0 (93.9–98.6) | 98.7 (94.5–99.7) | 99.0 (98.8–99.2) | |
| ≥20% | 1.1 (0.9–1.3) | 3.0 (1.4–6.1) | 1.3 (0.3–5.5) | 1.0 (0.8–1.2) |
Recent defined as having within past two years.
P-value based on Rao-Scott Pearson chi-square test testing for differences in ever and no MRI for all variables except income status. Income status p-value based on Wald F test from unadjusted logistic regression model (due to multiply-imputed data).
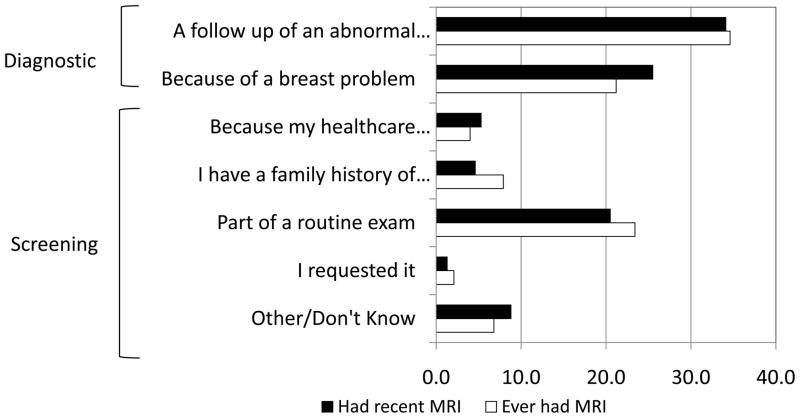
Among women who reported ever having or having had recent breast MRI, the most common reason for having the MRI was “follow-up of an abnormal mammogram,” followed by “because of a breast problem” and “part of a routine exam.” (Figure 1) Among women who reported having a recent breast MRI, 60% reported diagnostic reasons for this MRI (Table 2). There were statistically significant differences in education and insurance coverage between these two groups. Women reporting a screening MRI were more likely to report a normal recent mammogram and to have an increased 5-year risk for breast cancer and were less likely to report a prior breast biopsy or a personal history of breast cancer.
Figure 1.
Self-Reported Reasons for having a breast MRI among women aged 30 and older — National Health Interview Survey, United States, 2010.
Table 2.
Characteristics of women aged 30 and older who reported having had a recenta breast MRI for screening versus diagnostic reasons — National Health Interview Survey, United States, 2010
| Screeningb | Diagnosticc | ||
|---|---|---|---|
| n=97 | n=141 | ||
| Wtd % (95% CI) | Wtd % (95% CI) | P-valued | |
| Total | 40.2 (33.0–47.8) | 59.8 (52.2–67.0) | |
| Age | N=97 | N=141 | 0.3626 |
| 30–39 | 8.5 (4.0–17.3) | 10.5 (5.6–18.9) | |
| 40–49 | 17.6 (10.6–27.8) | 29.3 (20.2–40.4) | |
| 50–64 | 45.6 (34.2–57.6) | 37.8 (28.8–47.8) | |
| 65–74 | 15.3 (8.0–27.4) | 14.3 (9.2–21.4) | |
| 75+ | 12.9 (7.0–22.5) | 8.1 (4.5–14.0) | |
| Race | N=97 | N=141 | 0.1819 |
| White, non-Hispanic | 54.8 (42.5–66.5) | 58.3 (48.8–67.3) | |
| Black, non-Hispanic | 26.5 (17.7–37.9) | 22.8 (15.4–32.2) | |
| Asian, non-Hispanic | 1.3 (0.4–4.1) | 6.8 (3.5–12.8) | |
| Other, non-Hispanic | 2.2 (0.3–13.9) | 0.0 (0.0–2.6) | |
| Hispanic | 15.2 (8.8–25.2) | 12.1 (7.6–18.8) | |
| Education | N=97 | N=139 | 0.0426 |
| < high school graduate | 19.6 (12.0–30.3) | 11.0 (6.6–17.8) | |
| High school graduate | 25.7 (17.5–36.1) | 25.3 (17.5–35.1) | |
| Some college | 34.6 (24.1–46.8) | 25.4 (17.5–35.3) | |
| ≥College graduate | 20.2 (12.3–31.3) | 38.3 (29.7–47.7) | |
| Income status, %FPL | N=97 | N=141 | 0.2344 |
| <100 | 19.1 (12.1–28.9) | 14.7 (9.4–22.2) | |
| 100–199 | 16.7 (9.7–27.1) | 15.6 (9.0–25.5) | |
| 200–299 | 13.3 (7.0–23.8) | 10.1 (5.4–18.1) | |
| 300–399 | 21.8 (12.8–34.5) | 12.9 (7.6–21.0) | |
| 400+ | 29.1 (19.4–41.2) | 46.8 (37.4–56.4) | |
| Region | N=97 | N=141 | 0.7080 |
| Northeast | 18.6 (10.4–31.2) | 23.1 (13.7–36.3) | |
| Midwest | 21.0 (13.0–32.1) | 20.6 (13.7–29.7) | |
| South | 34.9 (25.0–46.4) | 37.9 (28.4–48.5) | |
| West | 25.4 (16.8–36.6) | 18.4 (12.3–26.7) | |
| Insurance coverage | N=97 | N=141 | 0.0457 |
| Private | 62.2 (50.9–72.4) | 71.7 (62.6–79.3) | |
| Public Only | 27.9 (18.9–39.1) | 13.7 (9.0–20.3) | |
| Uninsured | 9.8 (4.7–19.4) | 14.6 (8.6–23.6) | |
| Usual source of healthcare | N=97 | N=141 | 0.6538 |
| Yes | 92.8 (84.2–96.9) | 94.4 (89.2–97.1) | |
| No | 7.2 (3.1–15.8) | 5.6 (2.9–10.8) | |
| Had recent mammograma | N=97 | N=139 | 0.7128 |
| Yes | 89.1 (81.3–93.9) | 90.9 (80.8–95.9) | |
| No | 10.9 (6.1–18.7) | 9.1(4.1–19.2) | |
| Results of recent mammogram | N=89 | N=139 | 0.0001 |
| Normal | 80.7 (69.3–88.6) | 49.3 (40.0–58.7) | |
| Abnormal | 19.3 (11.4–30.7) | 50.7 (41.3–60.0) | |
| Number of prior breast biopsies | N=87 | N=130 | 0.0472 |
| 0 | 73.5 (61.3–83.0) | 58.7 (48.4–68.3) | |
| ≥1 | 26.5 (17.0–38.7) | 41.3 (31.7–51.6) | |
| Family history of breast cancer | N=96 | N=137 | 0.1403 |
| Yes | 27.6 (17.2–41.1) | 17.3 (11.0–26.0) | |
| No | 72.4 (58.9–82.8) | 82.7 (74.0–89.0) | |
| Personal history of breast cancer | N=97 | N=141 | 0.0081 |
| Yes | 14.4 (8.0–24.6) | 32.7 (23.6–43.3) | |
| No | 85.6 (75.4–92.0) | 67.3 (56.7–76.4) | |
| Personal history of other cancer | N=97 | N=141 | 0.3773 |
| Yes | 13.9 (7.2–25.2) | 9.8 (5.7–16.6) | |
| No | 86.1 (74.8–92.8) | 90.2 (83.4–94.3) | |
| Gail 5-year risk | N=82 | N=95 | 0.0396 |
| Average risk | 64.0 (51.0–75.2) | 80.3 (69.2–88.1) | |
| Increased risk (≥1.66%) | 36.0 (24.8–49.0) | 19.7 (11.9–30.8) | |
| Gail Lifetime risk | N=82 | N=95 | 0.6270 |
| <20% | 98.0 (87.3–99.7) | 99.0 (93.1–99.9) | |
| ≥20% | 2.0 (0.3–12.7) | 1.0 (0.1–6.9) |
Recent defined as having within past two years.
Screening responses include part of a routine exam, my healthcare provider told me I was high-risk, I have a family history of breast cancer, and I requested it.
Diagnostic responses include follow-up of an abnormal mammogram and because of a breast problem.
P-value based on Rao-Scott Pearson chi-square test for all variables except income status. Income status p-value based on Wald F test from unadjusted logistic regression model (due to multiply-imputed data).
Discussion
Although breast MRI has recently been recommended as an adjunct tool for breast cancer screening among women who are at high risk, the number of women in this group is limited. We found that approximately 18% of women in our study had an increased 5-year risk for breast cancer and 1% had a ≥20% lifetime risk representing approximately 14.8 million and 879,000 women, respectively. These findings are consistent with previous studies (20–22). Women who reported having a breast MRI were more likely to report risk factors for breast cancer or to have an estimated increased risk for breast cancer compared to other women. However, reports of having a breast MRI were uncommon among women at increased risk for breast cancer.
Even though this study has a large sample size representative of U.S. women aged 30 and older, there are several limitations. First, NHIS data are self-reported, which are subject to recall bias. Studies have shown that self-report of mammography use results in overestimates of rates of screening (24, 25). There are no comparable data related to the accuracy of breast MRI recall. Second, NHIS data do not capture information regarding BRCA genetic mutations for breast cancer and only captures first-degree relatives with a history of breast cancer that could result in an underestimate of women at increased risk for breast cancer. Third, data on reasons for obtaining a breast MRI may not provide a complete picture. Since only one reason is listed in the data, the NHIS does not delineate whether a single or combined set of factors resulted in a woman having a breast MRI. Last, because this is the first year that breast MRI questions have been asked on a national survey, recent MRI use could not be defined as within one year preceding the survey to be consistent with screening recommendations due to few women reported having a breast MRI.
Our study is the first to provide national estimates of breast MRI use. These data suggest both underuse and overuse among subsets of women. When more data become available, future studies could explore the influence of insurance coverage, health system characteristics, provider behavior and patient preferences on breast MRI use. As access to health care improves and screening guidelines associated with breast MRI become more widely adopted, it will be important to monitor the use of breast MRI and the appropriateness of its use for breast cancer detection.
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure: All authors are federal government employees. The National Health Interview Survey and preparation of this manuscript were entirely funded by the U. S. government. The authors have no financial disclosures or conflict of interest.
References
- 1.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727–37. W237–42. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA. 2005;293:1245–56. doi: 10.1001/jama.293.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sardanelli F, Giuseppetti GM, Panizza P, Bazzocchi M, Fausto A, Simonetti G, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in fatty and dense breasts using the whole-breast pathologic examination as a gold standard. Am J Roentgenol. 2004;183:1149–57. doi: 10.2214/ajr.183.4.1831149. [DOI] [PubMed] [Google Scholar]
- 4.Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–49. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 5.Jorgensen KJ, Zahl PH, Gotzsche PC. Overdiagnosis in organised mammography screening in Denmark. A comparative study. BMC Womens Health. 2009;9:36. doi: 10.1186/1472-6874-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lord SJ, Lei W, Craft P, Cawson JN, Morris I, Walleser S, et al. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer. 2007;43:1905–17. doi: 10.1016/j.ejca.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Bluemke DA, Gatsonis CA, Chen MH, DeAngelis GA, DeBruhl N, Harms S, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA. 2004;292:2735–42. doi: 10.1001/jama.292.22.2735. [DOI] [PubMed] [Google Scholar]
- 8.Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246:116–24. doi: 10.1148/radiol.2461061298. [DOI] [PubMed] [Google Scholar]
- 9.Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening mri to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehman CD, Gatsonis C, Kuhl CK, Hendrick RE, Pisano ED, Hanna L, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 11.Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Cancer Network. 2009;7:193–201. doi: 10.6004/jnccn.2009.0013. [DOI] [PubMed] [Google Scholar]
- 12.Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–37. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 13.Kriege M, Brekelmans CT, Obdeijn IM, Boetes C, Zonderland HM, Muller SH, et al. Factors affecting sensitivity and specificity of screening mammography and MRI in women with an inherited risk for breast cancer. Breast Cancer Res Treat. 2006;100:109–19. doi: 10.1007/s10549-006-9230-z. [DOI] [PubMed] [Google Scholar]
- 14.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. Data File Documentation, National Health Interview Survey, 2010 (machine readable data file and documentation) National Center for Health Statistics, Centers for Disease Control and Prevention; Hyattsville, Maryland: 2011. [Google Scholar]
- 16.Gail M, Rimer B. Risk-based recommendations for mammographic screening for women in their forties. J Clin Oncol. 1998;16:3105–14. doi: 10.1200/JCO.1998.16.9.3105. [DOI] [PubMed] [Google Scholar]
- 17.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 18.Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91:1541–8. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. [Accessed on April 25, 2012];Breast Cancer Risk Assessment Macro. Available at http://dcegcancergov/bb/tools/bcrasasmacro.
- 20.Sabatino SA, Burns RB, Davis RB, Phillips RS, Chen YH, McCarthy EP. Breast carcinoma screening and risk perception among women at increased risk for breast carcinoma: results from a national survey. Cancer. 2004;100:2338–46. doi: 10.1002/cncr.20274. [DOI] [PubMed] [Google Scholar]
- 21.Graubard BI, Freedman AN, Gail MH. Five-year and lifetime risk of breast cancer among U.S. subpopulations: implications for magnetic resonance imaging screening. Cancer Epidemiol Biomarkers Prev. 2010;19:2430–6. doi: 10.1158/1055-9965.EPI-10-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526–32. doi: 10.1093/jnci/95.7.526. [DOI] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics. [Accessed April 24, 2012];Multiple imputation of family income and personal earnings in National Health Interview Survey: methods and examples. Available at http://wwwcdcgov/nchs/data/nhis/tecdoc_2010pdf2011.
- 24.Njai R, Siegel PZ, Miller JW, Liao Y. Misclassification of survey responses and black-white disparity in mammography use, Behavioral Risk Factor Surveillance System, 1995–2006. Prev Chronic Dis. 2011;8(3) [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon NP, Hiatt RA, Lampert DI. Concordance of self-reported data and medical record audit for six cancer screening procedures. J Natl Cancer Ins. 1993;85:56. doi: 10.1093/jnci/85.7.566. [DOI] [PubMed] [Google Scholar]