SUMMARY
IL-33 is elevated in afflicted tissues of patients with mast cell-dependent chronic allergic diseases. Based on its acute effects on mouse mast cells (MCs), IL-33 is thought to play a role in the pathogenesis of allergic disease through MC activation. However, the manifestations of prolonged IL-33 exposure on human MC function, which best reflect the conditions associated with chronic allergic disease, are unknown. We now find that long-term exposure of human and mouse MCs to IL-33 results in a substantial reduction of MC activation in response to antigen. This reduction required >72 h exposure to IL-33 for onset and 1–2 wk for reversion following IL-33 removal. This hypo-responsive phenotype was determined to be a consequence of MyD88-dependent attenuation of signaling processes necessary for MC activation including antigen-mediated calcium mobilization and cytoskeletal reorganization; potentially as a consequence of down-regulation of the expression of PLCγ1 and Hck. These findings suggest that IL-33 may play a protective, rather than a causative role in MC activation under chronic conditions and, furthermore, reveal regulated plasticity in the MC activation phenotype. The ability to down-regulate MC activation in this manner may provide alternative approaches for treatment of MC-driven disease.
Keywords: IL-33, phenotype plasticity, mast cells, phospholipase Cγ1, Hck, degranulation, cytokines
INTRODUCTION
Mast cells (MCs) are tissue–resident cells of hematopoietic origin that participate in innate and acquired immune defense mechanisms through the release of an array of inflammatory mediators following receptor-dependent activation (1). These mediators, however, are also responsible for initiating the cellular and pathological manifestations of allergic disorders including anaphylaxis, asthma, rhinitis, and atopic dermatitis (2). MCs are predominantly activated through antigen/IgE-mediated aggregation of the high affinity IgE receptor (FcεRI) (3). However, it is apparent that cytokines and other molecules present in the surrounding milieu can acutely amplify MC signaling and responses to antigen (4).
IL-33 levels are reported to be elevated in lungs of patients with chronic asthma (5) and, based on the reports that IL-33 enhances MC activation (6–9), it has been proposed that IL-33 may play a role in the pathogenesis of allergic disorders. The effects of IL-33 on MC activation have, however, been controversial. Although it is recognized that IL-33 induces cytokine release from MCs in culture (7, 10–12) and markedly enhances the ability of antigen to elicit this response (6–9), some report that IL-33 also stimulates or enhances degranulation and production of prostaglandin D2 (9, 12) whereas others report that IL-33 neither induces nor enhances FcεRI-mediated degranulation and eicosanoid production (7, 8, 10, 11).
The amplification of cytokine release by IL-33 in antigen-stimulated MCs in culture was noted after acute exposure to IL-33, conditions that may not mimic the long-term effects of IL-33 on MC function associated with pathologic conditions of atopic or rheumatoid arthritic disease. We thus investigated the long-term effects of IL-33 on the ability of antigen to activate human (Hu) and mouse MCs.
Here we report that, following prolonged IL-33 exposure, MCs become refractory to stimulation via FcεRI, with respect to degranulation, as a consequence of the attenuation of critical signaling processes required for MC activation. These findings provide the first evidence that HuMCs can be reprogrammed to a hypo-responsive phenotype in this manner and thus may have important implications for how MC activation may be regulated in health and disease. The ability to manipulate MC activation in this manner may also provide a basis for the developmental of novel approaches for therapeutic intervention of mast cell-driven disease.
MATERIALS AND METHODS
Cell culture
Primary human (Hu)MCs were prepared from CD34+ peripheral blood progenitors isolated from healthy volunteers following informed consent under a protocol (NCT00001756) approved by the NIH internal review board. HuMCs were prepared and cultured as described (13) with or without recombinant IL-33 (10 ng/ml), and used for studies between 7–9 weeks of age.
All mouse studies, with the exception of those involving the use of ST2−/− mice, were conducted under a protocol approved by the NIAID Institutional Animal Care and Use Committee at NIH. Studies involving the use of ST2−/− mice were approved by East Carolina University’s Institutional Animal Care and Use Committee. These studies were conducted in accordance with the National Institutes of Health Guidelines for the care and use of laboratory animals.
Mouse bone marrow-derived MCs (BMMCs) were prepared from wild type (WT; C57BL/6 background, Jackson Laboratory), ST2−/−, and MyD88−/− mice. ST2−/− mice on a C57BL/6 genetic background were obtained from Dr. Robert B. Fick at Merck Research Labs, Division of Biologics, Palo Alto, CA. Homozygous MyD88−/− mice on a C57BL/6 genetic background (14, 15) were obtained from Dr. Shizuo Akira (Osaka University, Osaka, Japan) by way of Dr. Helene Rosenberg (NIAID, NIH). WT mice on an identical genetic background were sex and age matched. BMMCs were developed and cultured as described (16, 17) with or without recombinant IL-33 (10 ng/ml), and used for studies between 4–6 weeks of culture. The endotoxin content of the IL-33 was < 0.1 ng/μg of IL-33; well below (>105 fold less than) that required to substantially influence mast cell activation (100 ng/ml) (18).
Cell activation, degranulation
For degranulation and cytokine release, HuMCs and BMMCs were incubated overnight in cytokine-free media containing biotinylated myeloma human IgE (100 ng/ml) (19) or mouse monoclonal DNP-IgE (Sigma-Aldrich, St. Louis, MO; 100 ng/ml), respectively. The following day, the cells were rinsed with HEPES buffer (HEPES (10 mM), NaCl (137 mM), KCl (2.7 mM), Na2HPO4.7H2O (0.4 mM), glucose (5.6 mM, pH 7.4) CaCl2·2H2O (1.8 mM), MgSO4·7H2O (1.3 mM, pH7.4)) containing 0.04% BSA (Sigma-Aldrich) then treated as described (19) and in the figure legends. Degranulation was calculated as the percentage of total β-hexosaminidase recovered from the supernatant (19, 20).
In vivo studies and isolation of peritoneal MCs
The acute effects of IL-33 (IL-33-induced anaphylaxis) were examined in 6 wk old C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME). The mice were sensitized with anti-DNP IgE mAb (21) (3 μg i.v.) (a generous gift from Juan Rivera, NIAMS, NIH and isolated from ascites supplied by Dr. Fu-Tong Liu, Davis School of Medicine, University of California, Sacramento, CA). After 24 h, the mice were injected with recombinant IL-33 (2 μg in 200 μl) or PBS retro-orbitally (i.v), and the anaphylactic response was monitored by recording changes in core body temperature every 5 min for 2 h using an implantable electronic transponder (IPTT-300, Bio Medic Data Systems, Seaford, DE). As a positive control, mice were challenged with antigen (200 μg of DNP-human serum albumin (HSA), Sigma-Aldrich, St. Louis, MO).
To examine the consequences of prolonged exposure to IL-33 on the mast cell compartment in vivo, mice were injected i.p. with 1 μg IL-33 (500 μl) or PBS every second day for a total of 12 days. The 5th and 6th injections were supplemented with 1 μg anti-DNP-IgE to sensitize the mice. Two days after the 6th injection, blood-free peritoneal cells were recovered by lavage with HEPES buffer containing 0.2% (w/v) BSA. Peritoneal cells were immediately labeled with 10 μM Fluo-4 AM (Invitrogen, Carlsbad, CA) and APC-labeled KIT specific Ab (BD Biosciences, San Jose, CA) in the presence of 5 mM probenecid (Sigma-Aldrich) for 30 min at room temperature in the HEPES/BSA (0.2%). The cells were then washed with HEPES/BSA (0.2%)/2.5 mM probenecid. Before measurement, the cells were sedimented, resuspended in pre-warmed (37 °C) HEPES/BSA (0.2%) and analyzed at 37 °C by FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) to obtain a time-course of Fluo-4 AM fluorescence of the gated KIT+ cell population. A 2 s averaged geometric mean of the recorded Fluo-4 AM fluorescence (MFI) was subsequently analyzed. To evaluate the data, the difference in the averaged MFI within the interval 30–50 s post (maximal fluorescence) and 20-0 s prior (basal fluorescence) each challenge (delta MFI) was determined. The delta MFI for control (vehicle-challenged cells) was subtracted from delta MFI for antigen-challenged cells and the resultant values normalized to maximal response.
Immunoblotting and intracellular Ca2+ determination
For immunoblotting, cell lysates from BMMCs and HuMCs were prepared as described (22). The following antibodies were used: p-PLCγ1 (Tyr783) (Biosource, San Diego, CA), Hck, LAT, PLCγ1, Syk (Santa Cruz Biotechnology, Santa Cruz, CA), β-actin (Sigma-Aldrich), p-LAT (Tyr191) (Millipore, MA), p-Btk (Tyr511), Btk (BD Biosciences). All other antibodies [p-p38 (Thr180/Tyr182), p38, p-JNK (Thr183/Tyr185), JNK, p-ERK (Thr202/Tyr204), ERK, p-AKT (Ser473), p-AKT (Thr308), AKT] were from Cell Signaling Technology (Danvers, MA). Intracellular Ca2+ was determined utilizing Fura-2 as described (23).
Real-time PCR
Total RNA was isolated from 3×106 cells using RNeasy Plus Mini kit (Qiagen, Valencia, CA). One μg of RNA was used for reverse transcription reaction using random hexamers and Superscript III reverse transcriptase (Invitrogen) in a 20 μl reaction. One μl of the resulting cDNA was used for Real Time PCR using Taqman Gene Expression Assay for PLCγ1 (Mm 01247293_m1) or Hck (Mm01241463_m1) according to the manufacturer’s instructions.
Actin polymerization
After treating the cells as described in the figure legends, cells were fixed for 15 min at RT using 4% PFA in PBS containing 5 mM EDTA and 5 mM EGTA, washed with 2% BSA in PBS containing EGTA, and attached to glass slides. Cells were stained with FITC-labeled phalloidin (Sigma-Aldrich) (1:100) in 0.1% Saponin, 2% BSA, in PBS/5 mM EGTA for 1 h at RT. After washing, slides were mounted using mounting solution containing DAPI (Molecular Probes); imaging was obtained using a Zeiss LSM-510-UV Confocal Microscope.
Affymatrix Gene Chip analysis
These studies were conducted as part of a three way analysis where non-treated BMMCs were compared to one group in which the cells were treated with SCF (100 ng/ml) and to one group where the cells were treated with IL-33. The SCF-control group comparison data are included in the supplemental information to (24). The methods are described in this reference. We report here the comparison data between the control and IL-33 groups. The data are deposited in Gene Expression Omnibus (GEO; GSE39382) (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=rlunfyaaiiyguhk&acc=GSE39382)
Statistical analysis
Data are represented as the means ± S.E. from experiments conducted on separate animals or cell preparations (n values are indicated in figure legends). The statistical analyses between two sets of data were performed by unpaired or paired Student’s t-test, or between multiple sets by ANOVA with a post-test as indicated. Differences were considered significant when P<0.05.
RESULTS
IL-33 promotes a hypo-responsive HuMC phenotype
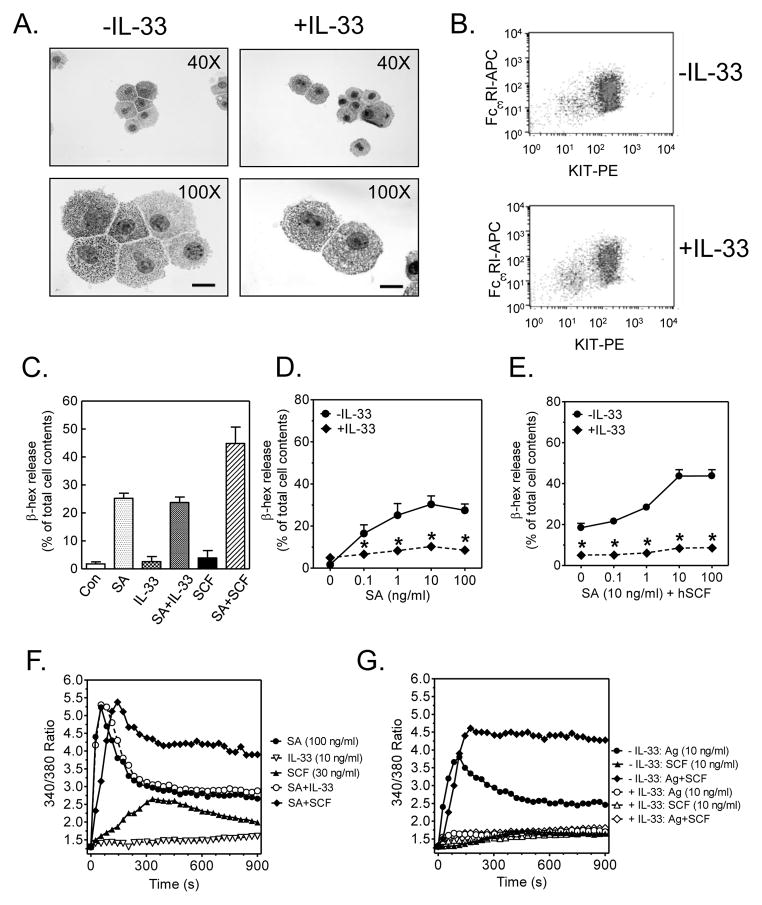
HuMCs were generated in the presence or absence of IL-33 for the duration of the culture period (7–9 wk). There was little difference in the morphological characteristics and purity (>99%) between the two cell populations (Fig. 1A), and no detectable difference in surface expression of FcεRI or KIT (Fig. 1B). In HuMCs cultured in the absence of IL-33, acutely added IL-33 neither induced degranulation nor enhanced FcεRI-mediated degranulation IL-33 (Fig. 1C). In contrast and consistent with previous studies (23), antigen-induced degranulation was enhanced by the KIT-ligand, SCF, in these cells. However, HuMCs exposed to IL-33 for the duration of the culture exhibited markedly reduced FcεRI-mediated degranulation in the absence (Fig. 1D) or presence of SCF (Fig. 1E) when compared to cells not exposed to IL-33. MC degranulation is dependent on an increase in cytosolic calcium regardless of the type of stimulant (4, 25). Consistent with this requirement, and a previous study (8), IL-33 did not induce an increase in cytosolic calcium nor enhanced that elicited by antigen whereas SCF could do so in IL-33-naive HuMCs (Fig. 1F). Nevertheless, the antigen-mediated calcium signal and the ability of SCF to enhance this signal were significantly impaired in cells chronically exposed to IL-33 (Fig. 1G).
Fig. 1. The effect of IL-33 exposure on HuMCs.
(A.) HuMCs were cultured with or without IL-33 for 7–9 wk and cytospins were stained with toluidine blue and analyzed by light microscopy. The scale bar is 10 μm. (B.) FcεRI and KIT expression in cells from A. was analyzed by flow cytometry. (C.) Naïve HuMCs were sensitized overnight with biotinylated IgE (100 ng/ml) and challenged with various stimulants individually, or in the indicated combinations: SA (streptavidin, 100 ng/ml), IL-33 (10 ng/ml), or SCF (30 ng/ml), and the release of β-hexosaminidase was determined. (D., E.) Cells in A. were sensitized with biotinylated Hu IgE overnight, then stimulated with antigen (streptavidin) alone (D.) or in the presence of SCF (E.) and degranulation after 30 min (β-hex release) (F.) Cells were sensitized and treated as in C. and the calcium signal was determined. (G.) The calcium signal in the cells sensitized and triggered as in D. and E. was similarly assessed. Data in A., B., F., and G. are representative of 3 experiments conducted on separate cell preparations. In C., the results are means ± S.E. of two separate experiments conducted in duplicate or triplicate. Data in D. and E. are represented as the means ± S.E. (n=6 to 14; *: P<0.05, Student’s t-test).
Taken together, these data revealed that prolonged exposure of HuMCs to IL-33 down-regulated FcεRI-mediated and SCF/KIT-enhanced degranulation, and that this appeared to be a manifestation of IL-33-mediated attenuation of signaling events downstream of the FcεRI or KIT.
Onset and reversibility of IL-33 suppressive actions
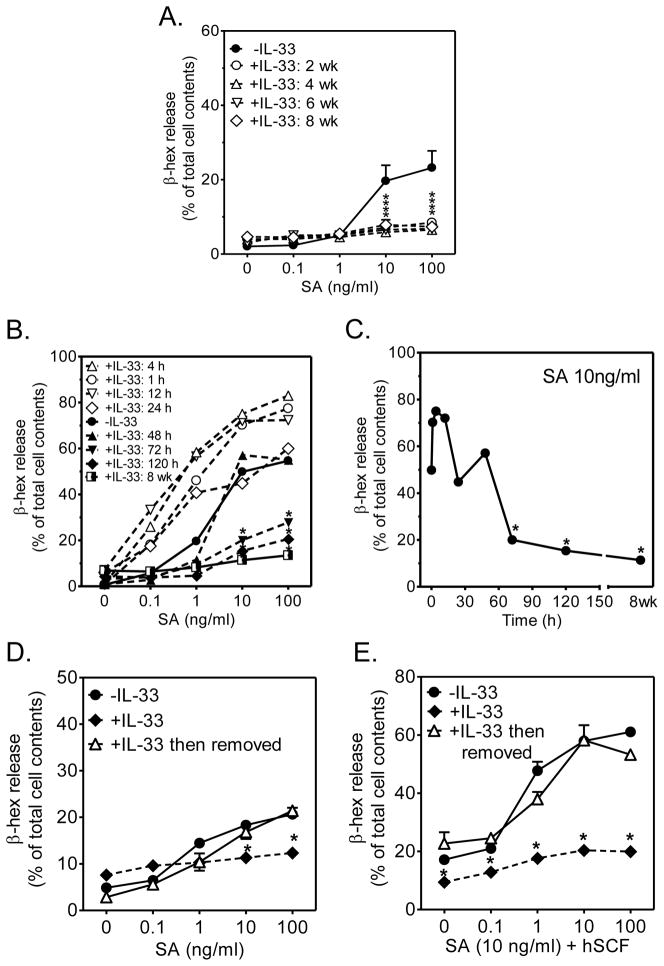
During maturation of HuMCs, we observed that IL-33 induced an identical deficiency in degranulation in HuMCs whether IL-33 was added 2, 4, or 6 wk prior to examining degranulation in mature (8 wk) cells (Fig. 2A). When cells were incubated with IL-33 for periods of 4 to 12 h before the addition of SA, degranulation was moderately enhanced (Fig. 2B, C). However, with more sustained exposure to IL-33, the extent of degranulation progressively declined to levels well below those observed before addition of IL-33 (Fig. 2B, C).
Figure 2. Kinetics of onset of inhibition of degranulation by IL-33 and reversibility of IL-33-induced hypo-responsive phenotype.
(A.) Developing HuMC cultures were exposed to IL-33 (10 ng/ml) for indicated times prior to examining FcεRI-dependent degranulation in mature (8 wk) HuMCs. (B.) Mature HuMCs were incubated with IL-33 (10 ng/ml) for 1, 4, 12, 24, 48, 72 h and 120 h, then degranulation (β-hex release) of IgE-biotin sensitized cells was determined. (C.) Data from B. re-plotted as a time course using the 10 ng/ml antigen concentration. (D., E.) HuMCs were cultured with IL-33 for 7 weeks, IL-33 removed for an additional 2 weeks, then degranulation (β-hex release) of IgE-biotin sensitized cells determined. Data are represented as the means ± S.E. (n=3; *: P<0.05 for comparison with cells cultured in the absence of IL-33, Student’s t-test).
To examine whether the changes induced by IL-33 were reversible, HuMCs were maintained in media containing IL-33 for 8 wk then IL-33 was removed from the media and degranulation assessed weekly. The hypo-responsive phenotype gradually reverted to normal and, within 2 weeks, the cells previously exposed to IL-33 showed similar FcεRI-mediated degranulation to HuMCs not exposed to IL-33 (Fig. 2D, E).
These data suggest that the ability of IL-33 to promote a hypo-responsive MC phenotype occurs irrespective of the stage of MC development, requires at least 72 h exposure and is slowly reversible.
Elevated IL-33 levels in vivo suppress antigen-induced MC activation
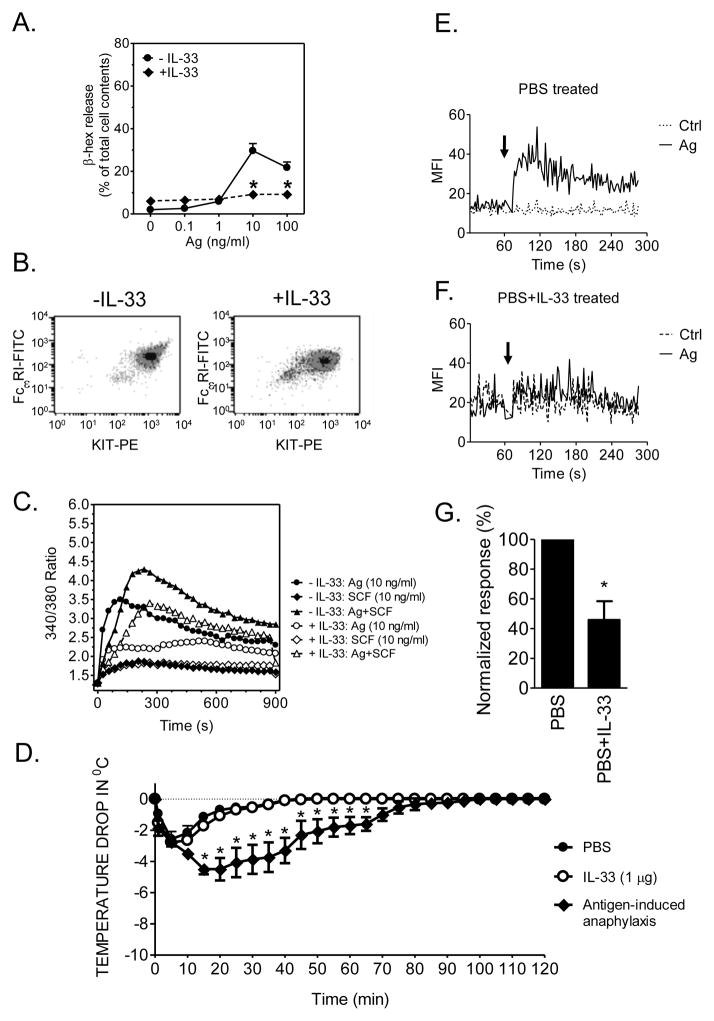
We next examined whether elevated IL-33 levels in vivo similarly promotes a hypoactive MC compartment. We first established that degranulation in mouse BMMCs grown in the presence of IL-33 were also refractory to antigen challenge (Fig. 3A), and that this phenotype was also not associated with reduced KIT and FcεRI expression (Fig. 3B) but was accompanied by reduced cell activation as assessed by a decrease in the antigen-mediated calcium signal (Fig. 3C).
Fig. 3. The effect of prolonged IL-33 exposure on mouse MCs.
(A.) BMMCs were cultured with or without IL-33 during the 4–6 wk development, sensitized with DNP-specific mouse IgE overnight, then stimulated with the indicated concentrations of DNP-HSA (antigen) (B.) BMMCs were cultured with or without IL-33 for 4–6 wk and FcεRI and KIT expression was analyzed by flow cytometry. (C.) Cells were sensitized and cultured as in A. then stimulated with DNP-HSA (Ag) or SCF alone or in combination and calcium response determined. (D.) IgE-sensitized mice were injected with of IL-33 (2 μg), DNP-HSA (200 μg) or PBS retro-orbitally, and the change in core body temperature was monitored. (E., F.) Peritoneal cells from PBS treated (E.) and from mice treated for 12 days with IL-33 (6 doses of 1 μg on alternative days) and sensitized with DNP-specific mouse IgE (2 doses of 1 μg on alternative days prior to stimulation) (F.) were labeled with a KIT-specific antibody and Fluo-4 AM. The calcium responses of the KIT-positive population of these cells to vehicle alone (Ctrl) or antigen (Ag, DNP-HSA; 100 ng/ml) were determined by acquisition of their Fluo-4 AM fluorescence using flow cytometry and two-second averaged geometric mean fluorescence (MFI) was analyzed. (G.) Delta MFI values (see methods) for PBS- (E.) and IL-33- (F.) treated cells were calculated and normalized responses evaluated. Data in A. are the means ± S.E. (n=14) and in B. and C. are representative of 3 experiments conducted on separate cell preparations. In D., data are presented as the means ± S.E. of n=4 and the difference among the groups of mice is indicated (*: P<0.05, two-way ANOVA with Bonferroni’s posttest). Data in G. are presented as the means ± S.E. of n=4. The arrows in E. and F. indicate the time of cell challenge. *: P<0.05 for comparison with cells not exposed to IL-33, Student’s t-test)
Consistent with the lack of effect on degranulation (Fig. 1), a single injection of IL-33 failed to induce an anaphylactic response in mice (Fig. 3D). To examine chronic effects of IL-33 on the mast cell compartment, mice were injected i.p. with IL-33 (1 μg) or PBS every two days for a total of 12 days. Two days after the last injection and sensitization with IgE, peritoneal cells from the control and IL-33-treated mice were collected and the activation capacity of KIT-positive MCs to antigen was determined by examining the calcium signal by flow cytometry. As shown in Figure 3E-G, MCs collected from the control mice responded as expected to antigen whereas cells collected from IL-33-treated mice were refractory to such stimulation. Thus, as was observed for BMMCs, long term exposure of MCs to IL-33 in situ resulted in a marked decrease in the ability of antigen to induce MC activation.
Having established that mouse BMMCs exhibited the same IL-33-induced hypo-responsive phenotype as that observed in the HuMCs, we utilized mouse BMMCs for further mechanistic studies.
Absence of IL-33-induced phenotypic changes in ST2−/− and MyD88−/− BMMCs
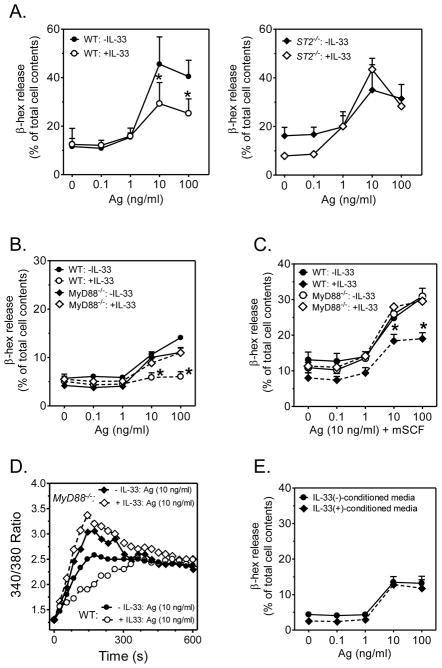
The IL-33 receptor, ST2, signals through the MyD88 adaptor molecule (26). We therefore investigated whether the inhibitory effects of IL-33 were similarly dependent on MyD88, by employing BMMCs developed from MyD88−/− mice. As shown in Fig. 4A, as expected, the ability of chronic IL-33 exposure to down-regulate antigen-mediated degranulation was significantly impaired in ST2-deficent BMMCs. Furthermore, such down-regulation was also not observed in the MyD88−/− BMMCs upon stimulation with antigen in the absence (Fig. 4B) or presence (Fig. 4C) of SCF. It should be noted that in this series of experiments, we observed lower levels of degranulation than observed in Fig. 3, although within the range of variability noted among different experiments with BMMCs. Similarly, the ability of chronic IL-33 exposure to reduce the antigen-mediated calcium signal was not observed in the MyD88−/− BMMCs (Fig. 4D).
Fig. 4. The effect of prolonged IL-33 exposure on degranulation in BMMCs derived from ST2−/− and MyD88−/− mice.
(A.) BMMCs, developed from wild type (WT) or ST2−/− mice, cultured in the absence or presence of IL-33 (10 ng/ml) for 4 wk were sensitized then challenged with DNP-HSA (Ag) for 30 min and degranulation (β-hex release) determined. (B., C.) BMMCs, developed from wild type (WT) or MyD88−/− mice, cultured in the absence or presence of IL-33 (10 ng/ml) for 4 wk, were sensitized then challenged with DNP-HSA (Ag) (B.) or Ag with SCF (C.) for 30 min and degranulation (β-hex release) determined. (D.) The calcium signal in WT and MyD88−/− BMMCs, sensitized and treated as described in B. and C. was determined by Fura-2. (E.) Conditioned media from WT cells treated or not with IL-33 was added to the MyD88−/− BMMCs for 2 wk then degranulation (β-hex release) determined. Data are represented as the means ± S.E. of n=3 (A., E.) or (n=6 (B., C.) or are representative of n=3 (D.); *: P<0.05, Paired Student’s t-test.
The MyD88−/− cells were then used to exclude the possibility that the effects of IL-33 were dependent on the secondary release of other cytokines. Conditioned medium was taken from WT BMMCs incubated for 2 weeks in IL-33 then its ability to reduce degranulation examined; the MyD88−/− BMMCs were thus used to avoid any potential effects of IL-33 carried-over in the conditioned media. As shown in Fig. 4E, the conditioned media failed to recapitulate the hypo-responsive phenotype. From these data we conclude that the ability of IL-33 to induce a hypo-responsive MC phenotype was due to a direct action of IL-33 mediated through MyD88.
Diminished phospholipase (PL)Cγ1 and Hck expression in IL-33-cultured MCs
Multiple early events contribute to the signaling pathways leading to FcεRI-mediated degranulation of MCs (27). These events culminate in the calcium signal and cytoskeletal reorganization that are required for exocytosis. As shown in Figures 1G and 3C, the IL-33-induced hypo-responsive phenotype was associated with a significantly reduced calcium signal. To identify underlying defect(s) that could account for this attenuated calcium signal, two approaches were adopted: gene arrays to examine changes in expression of potentially responsible signaling molecules at the mRNA level, and immunoblot analysis to determine IL-33-dependent alterations not only in expression but also activation of relevant signaling molecules.
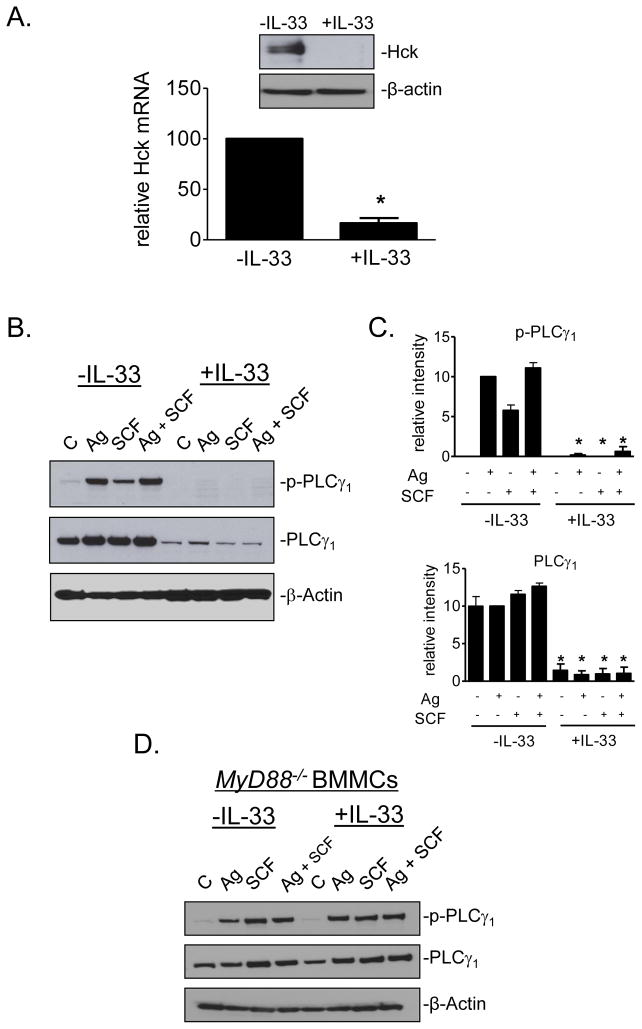
The microarray analysis was conducted on BMMCs cultured in the presence or absence of IL-33 for 4 wk. As expected, an extensive number of genes were either upregulated or downregulated following IL-33 treatment (Supplemental Table 1). However, there were little obvious changes in ST2 (also by immunoblot analysis; data not shown) or any of the genes encoding signaling molecules that would be predicted to impact calcium mobilization (27). Nevertheless, the array analysis did reveal a significant downregulation of Hck, a Src kinase which has been demonstrated to be critical for antigen-mediated cytoskeletal rearrangement through the regulation of actin/tubulin depolymerization/polymerization as well as degranulation (28). The downregulation of Hck expression was confirmed by quantitative PCR and at the protein level (Fig. 5A).
Fig. 5. The expression of Hck and PLCγ1 in IL-33-cultured cells.
(A.) The Hck mRNA and protein expression in BMMCs cultured with or without IL-33 for 2 wk were evaluated by real-time PCR and immunoblotting. (B.) Sensitized BMMCs, cultured in the absence or presence of IL-33 (10 ng/ml) for the duration of the culture were challenged with DNP-HSA (Ag; 10 ng/ml) in the presence or absence of SCF (10 ng/ml) for 2 min then phospho- and total proteins detected by immunoblotting. (C.) Immunoblots from B. were normalized to loading control (Syk; see Fig. S1 and S2) and evaluated. (D.) PLCγ1 expression in MyD88−/− BMMCs, cultured in the absence or presence of IL-33 (10 ng/ml) as described for B. In A., B., and D., the immunoblots are representative of 3 experiments conducted on separate cell preparations. Data in A. and C. are represented as the means ± S.E. (n=3–8; *: P<0.05, Student’s t-test).
To further investigate the underlying cause for the attenuated calcium signal in IL-33-treated cells, we explored whether PLCγ activation, and other signals required for calcium mobilization (27), were similarly affected at the protein level. IL-33 indeed markedly decreased the inducible phosphorylation of PLCγ1 and this reflected a similar decrease in PLCγ1 expression (Fig. 5B, C). Similar reductions were observed in phosphorylation of LAT and LAT2 (Fig. S1 and S2), but without apparent reduction in their expression. In contrast to PLCγ1, the expression of Btk, AKT, Syk and the MAP kinases p38, JNK, and ERK1/2 were relatively unaffected (Fig. S1 and S2). Correlating with the lack of a calcium response, the IL-33-induced reduction in PLCγ1 expression and phosphorylation was not observed in the MyD88−/− BMMCs (Fig. 5D).
Inefficient actin depolymerization/polymerization in MCs following prolonged IL-33 exposure
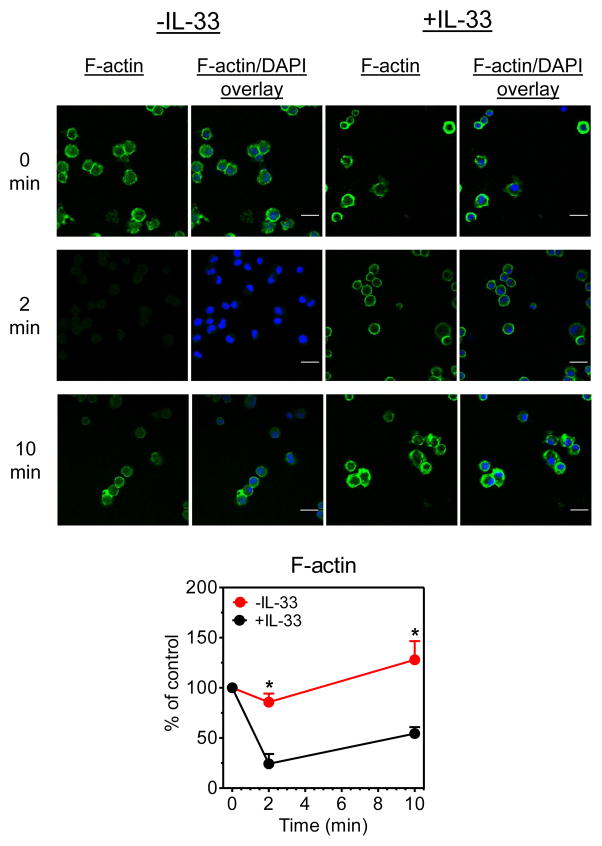
Due to the recognized role of Hck in cytoskeletal rearrangement (29) required for mast cell degranulation, we examined whether the diminished Hck expression in IL-33-treated MCs also resulted in attenuated antigen-mediated actin polymerization/depolymerization. As expected, antigen challenge of untreated BMMCs resulted in initial actin depolymerization followed by re-polymerization (Fig. 6). However, as for calcium signaling, actin, and hence, cytoskeletal rearrangement was substantially attenuated in the cells pre-exposed to IL-33.
Fig. 6. The effect of prolonged IL-33 exposure on actin polymerization.
Sensitized BMMCs, incubated or not in IL-33 (10 ng/ml) for the duration of the culture, were challenged with DNP-HSA (10 ng/ml) for 2 min or 10 min. Cells were stained for F-actin (green) and nuclei (blue). The degree of polymerization was evaluated from the normalized fluorescence intensities. The scale bar is 20 μm and the data are represented as the means ± S.E. (n=3; *: P<0.05 for comparison with cells cultured in the absence of IL-33 by Student’s t-test).
We conclude from the above data that IL-33 promotes a hypo-responsive mast cell phenotype and that this is at least in part a consequence of the parallel attenuation of the calcium signal, as a consequence of decreased PLCγ1 expression, and cytoskeletal reorganization, as a consequence of down-regulation of Hck expression (25, 27–29).
DISCUSSION
The ability of IL-33 to modify MC activation has recently been the topic of active investigation due to reports of elevated IL-33 levels in the lungs of asthmatics and affected tissues of patients with rheumatoid arthritis, Crohn’s disease, atopic dermatitis, and psoriasis; diseases that are associated with MC activation (5, 30–32). A role for IL-33 in MC-driven allergic reactions had also been suggested by the report that IL-33 alone induces anaphylaxis in a mouse model, and degranulation of MCs in culture (33) (now retracted). However, as described by others in mouse BMMCs (8, 11, 12), we observed that IL-33 itself failed to induce calcium mobilization and thus was unable to induce degranulation (Fig. 1). Furthermore, we observed no anaphylactic response in the mouse following direct challenge with IL-33 (Fig. 3). From these data, we conclude that, under acute conditions, IL-33 does not influence MC degranulation. Nevertheless, our data clearly demonstrate that prolonged exposure to IL-33 (Fig. 2) markedly reduces FcεRI-mediated MC degranulation and that this was associated with MyD88-dependent (Fig. 4 and 5) IL-33-induced attenuation of the FcεRI-induced calcium signal (Fig. 1) and cytoskeletal rearrangement which are required for effective degranulation (Fig. 6).
The observed IL-33-dependent down-regulation of PLCγ1 and Hck likely accounted for, or at least contributed to, the impaired calcium signal and cytoskeletal rearrangement in the hypo-responsive phenotype. Of note, Hck-deficient BMMCs display impaired degranulation and associated actin reorganization (28) and we have recently documented that a hypo-responsive mouse BMMC phenotype induced by prolonged exposure to SCF is also associated with down-regulation of Hck (24). In contrast to the effects of IL-3, however, the SCF-induced hypo-responsive phenotype was not associated with similar defects in PLCγ1 expression and calcium signaling (24). This difference may be related to the distinctly different signaling pathways through which IL-33 and SCF operate (4).
PLCγ1 is required for induction of the calcium signal (25) and, as for Hck (28), the associated actin reorganization (34). Both of these signals are necessary formast cell degranulation. Thus, the IL-33-dependent down-regulation of Hck and PLCγ1 likely accounted for the impaired cytoskeletal rearrangement and marked attenuation of the FcεRI-mediated calcium signal in the hypo-responsive phenotype. In addition, as the antigen-induced PLCγ-dependent calcium signal interacts synergistically with weak calcium signals generated by SCF (23) to further enhance antigen-mediated degranulation, this could explain why IL-33 also reduced the ability of SCF and PGE2 to enhance degranulation.
It is possible that IL-33 may act upon other essential signaling events even though we observed little change in the expression of other critical signaling proteins for mast cell activation. For example, antigen-induced LAT and LAT2 phosphorylation, but not protein levels, was substantially reduced in the IL-33-treated MCs. It has been proposed that the positive effects of Hck may in part be mediated through suppression of the inhibitory actions of Lyn (28), and such actions would likely be diminished as a consequence of the decrease in Hck expression by IL-33. Certainly, the diminished levels of Hck in IL-33-treated cells would be expected to share similar phenotypic characteristics as the Hck−/− MCs.
We propose that the prolonged effects of IL-33 on MC activation through modification of the expression of critical signaling molecules can be viewed as phenotypic reprogramming. The extent to which other factors can modulate the HuMC activation phenotype in the manner described in this paper is currently unknown. Nevertheless, as noted above, we have recently shown that prolonged exposure to SCF also induces a hypo-responsive phenotype in the mouse (24). Similarly, we observed that prolonged exposure to IL-1 which signals in a similar manner as IL-33 also down-regulates antigen-mediated degranulation in BMMCs (Fig. S3). In contrast to the IL-33 receptor, ST2, which signals through a MyD88 and the interleukin-1 receptor accessory protein (IL-1RAcP) complex (4, 31), it has recently been shown that prolonged exposure to agonists of TLRs which require MyD88, but not IL-1RAcP, for signaling, enhance antigen-mediated degranulation (35). This implies that although MyD88 is essential, signals conferred by IL-1AcP are also required for IL-33 induction of the hypo-responsive phenotype.
Overall, our results suggest that the secretory response of MCs to antigen in a pathophysiological setting may not only be finely regulated in an acute manner (36), but that persistent increases in factors in the surrounding milieu might conceivably dampen pro-inflammatory responses of MCs following an innate immune reaction and assist resolution of the immune response. In the case of IL-33, the elevated level of IL-33 in asthmatic lungs rather than contributing to the disease may actually protect by tempering further MC-dependent inflammation. This, and other studies (37), and our recent finding that BMMCs are rendered similarly quiescent by long-term exposure to a physiologic factor, SCF (24), point perhaps to a broad array of conditioning factors to which MCs might be exposed in health and disease states. The possible implication is that, if the downregulation of signaling proteins by IL-33 or SCF is a mechanism for long-term prevention of inappropriate activation of MCs in a pathologic situation, then any disruption of such a process would render MCs hyperactive leading to MC-driven disease. Our observations may thus have implications for the treatment of allergic disorders if, for example, receptor ligands with more restricted actions than IL-33 or SCF were found to shift MCs to a quiescent state.
Supplementary Material
Acknowledgments
We thank Dr. Shizuo Akira (Osaka University) and Dr. Helene Rosenberg (LAD/NIAID/NIH) for the MyD88−/− mice, Dr. Robert B. Fick at Merck Research Labs, Division of Biologics, Palo Alto, CA for the ST2−/− mice, and Dr. Fu-Tong Liu, Davis School of Medicine, University of California, Sacramento, CA and Dr. Juan Rivera, NIAMS, NIH for anti-DNP-IgE mAb. The authors would also like to thank Kishore Kanakabandi, Dan Sturdevant, Kimmo Virtaneva, and Steve Porcella, of Rocky Mountain Laboratories, NIAID, NIH, Montana, for performing the gene arrays and for providing the methods section related to these studies. We finally thank Šárka Smržová for expert technical assistance.
Financial support for this work was provided by the Division of Intramural Research of NIAID, NHLB, I and NCI within the National Institutes of Health, National Institutes of Health grant R01 ES019311 (JMB), National Institutes of Health grant RO1 TW006612 (GRIP) (M.V.A.); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Brazil (M.V.A.).
Abbreviations
- Ag
antigen
- BMMCs
bone marrow-derived mast cells
- DNP
dinitrophenyl
- FcεRI
high affinity receptor for IgE
- Hu
human
- MCs
mast cells
- SA
streptavidin
- SCF
stem cell factor
References
- 1.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 3.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 4.Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Critical reviews in immunology. 2011;31:475–529. doi: 10.1615/critrevimmunol.v31.i6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 6.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Laboratory investigation; a journal of technical methods and pathology. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 8.Andrade MV, Iwaki S, Ropert C, Gazzinelli RT, Cunha-Melo JR, Beaven MA. Amplification of cytokine production through synergistic activation of NFAT and AP-1 following stimulation of mast cells with antigen and IL-33. Eur J Immunol. 2011;41:760–772. doi: 10.1002/eji.201040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver MR, Margulis A, Wood N, Goldman SJ, Kasaian M, Chaudhary D. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflammation research: official journal of the European Histamine Research Society … [et al.] 2010;59:207–218. doi: 10.1007/s00011-009-0088-5. [DOI] [PubMed] [Google Scholar]
- 10.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 11.Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcεRI signals. Journal of leukocyte biology. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 12.Moulin D, Donzé O, Talabot-Ayer D, Mézin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40:216–225. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- 14.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 15.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuehn HS, Beaven MA, Ma HT, Kim MS, Metcalfe DD, Gilfillan AM. Synergistic activation of phospholipases Cγ and Cβ: a novel mechanism for PI3K-independent enhancement of FcεRI-induced mast cell mediator release. Cell Signal. 2008;20:625–636. doi: 10.1016/j.cellsig.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM. The phospholipase Cγ1-dependent pathway of FcεRI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J Biol Chem. 2003;278:48474–48484. doi: 10.1074/jbc.M301350200. [DOI] [PubMed] [Google Scholar]
- 18.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcεR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuehn HS, Rådinger M, Gilfillan AM. Measuring mast cell mediator release. In: Coligan John E, et al., editors. Current protocols in immunology. Unit7. Chapter 7. 2010. p. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi OH, Lee JH, Kassessinoff T, Cunha-Melo JR, Jones SVP, Beaven MA. Antigen and carbachol mobilize calcium by similar mechanisms in a transfected mast cell line (RBL-2H3 cells) that expresses ml muscarinic receptors. J Immunol. 1993;151:5586–5595. [PubMed] [Google Scholar]
- 21.Liu FT, Bohn JW, Ferry EL, Yamamoto H, Molinaro CA, Sherman LA, Klinman NR, Katz DH. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980;124:2728–2737. [PubMed] [Google Scholar]
- 22.Tkaczyk C, Metcalfe DD, Gilfillan AM. Determination of protein phosphorylation in FcεRI-activated human mast cells by immunoblot analysis requires protein extraction under denaturing conditions. J Immunol Methods. 2002;268:239–243. doi: 10.1016/s0022-1759(02)00210-7. [DOI] [PubMed] [Google Scholar]
- 23.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcεRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 24.Ito T, Smrž D, Jung MY, Bandara G, Desai A, Smržová Š, Kuehn HS, Beaven MA, Metcalfe DD, Gilfillan AM. Stem cell factor programs the mast cell activation phenotype. J Immunol. 2012;188:5428–5437. doi: 10.4049/jimmunol.1103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma HT, Beaven MA. Regulation of Ca2+ signaling with particular focus on mast cells. Critical reviews in immunology. 2009;29:155–186. doi: 10.1615/critrevimmunol.v29.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadina M, Jefferies CA. IL-33: a sheep in wolf’s clothing? Science’s STKE: signal transduction knowledge environment. 2007;2007:pe31. doi: 10.1126/stke.3902007pe31. [DOI] [PubMed] [Google Scholar]
- 27.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 28.Hong H, Kitaura J, Xiao W, Horejsi V, Ra C, Lowell CA, Kawakami Y, Kawakami T. The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood. 2007;110:2511–2519. doi: 10.1182/blood-2007-01-066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guiet R, Poincloux R, Castandet J, Marois L, Labrousse A, Le Cabec V, Maridonneau-Parini I. Hematopoietic cell kinase (Hck) isoforms and phagocyte duties - from signaling and actin reorganization to migration and phagocytosis. Eur J Cell Biol. 2008;87:527–542. doi: 10.1016/j.ejcb.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, Kurowska-Stolarska M, McKenzie AN, McInnes IB, Liew FY. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy. 2010;40:200–208. doi: 10.1111/j.1365-2222.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- 32.Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi S, Stavrianeas N, Peterson E, Leeman S, Conti P. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pushparaj PN, Tay HK, H’Ng CS, Pitman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A. 2009;106:9773–9778. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Kuehn HS, Rådinger M, Brown JM, Ali K, Vanhaesebroeck B, Beaven MA, Metcalfe DD, Gilfillan AM. Btk-dependent Rac activation and actin rearrangement following FcεRI aggregation promotes enhanced chemotactic responses of mast cells. J Cell Sci. 2010;123:2576–2585. doi: 10.1242/jcs.071043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saluja R, Delin I, Nilsson GP, Adner M. FcεR(1)-Mediated Mast Cell Reactivity Is Amplified through Prolonged Toll-Like Receptor-Ligand Treatment. PLoS One. 2012;7:e43547. doi: 10.1371/journal.pone.0043547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639–646. doi: 10.1016/j.jaci.2009.08.035. quiz 647–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, Ford J, Conrad D, Watowich S, Moralle MR, Kepley CL, Murray PJ, Ryan JJ. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.