Abstract
Malignant gliomas are lethal brain tumors for which novel therapies are urgently needed. In animal models, vaccination with tumor-associated antigens efficiently primes T cells to clear gliomas. In clinical trials cancer vaccines have been less effective at priming T cells and extending survival. Generalized immune suppression in the tumor draining lymph nodes has been documented in multiple cancers. However, a systematic analysis of how vaccination at various distances from the tumor (closest to farthest) has not been reported. We investigated how the injection site chosen for vaccination dictates CD8 T cell priming and survival in an ovalbumin (OVA) transfected murine glioma model. Glioma-bearing mice were vaccinated with Poly:ICLC plus OVA protein in the neck, hind leg, or foreleg for drainage into the cervical, inguinal, or axillary lymph nodes respectively. OVA-specific CD8 T cell number, TCR affinity, effector function, and infiltration into the brain decreased as the vaccination site approached the tumor. These effects were dependent on the presence of the tumor, as injection site did not appreciably affect CD8 T cell priming in tumor-free mice. Our data suggest the site of vaccination can greatly impact the effectiveness of cancer vaccines. Considering that previous and ongoing clinical trials have used a variety of injection sites, vaccination site is potentially a critical aspect of study design that is being overlooked.
Introduction
Active tumor immunotherapy shows great promise in animal models but has yet to achieve widespread success in the clinic. Vaccines have been extensively tested in clinical trials for the treatment of glioma. Glioma patients have been vaccinated in multiple sites including the scapula draining into the axilla (1), the anterior upper thigh (2), the upper arm (3), and the cervical regions (4, 5). Data are insufficient to correlate response rates with vaccination site. There have been few basic studies examining priming following vaccination as it relates to anatomic location.
The sentinel lymph nodes (draining lymph node, DLN, nearest to the tumor) are in direct lymphatic drainage from the primary tumor (6, 7) and are the DLN most prone to immune suppression (8-10). In breast cancer and melanoma patients, T cells isolated from the sentinel lymph nodes have suppressed activation and proliferation in response to various mitogens compared to T cells isolated from the more distal lymph nodes (11-14). Multiple mechanisms contribute to this local suppression. Tumor-elaborated soluble factors, such as TGFγ and prostaglandin E2 (15-18), can act at the tumor site or DLN to dampen T cell reactivity. In experimental systems, additional documented mechanisms of local immune suppression at the DLN include regulatory T cell-mediated killing of tumor antigen presenting DCs (19) and T cell receptor nitration by myeloid derived suppressor cells (20).
With respect to brain tumors, the immunologically specialized nature of the brain and its draining cervical lymph nodes must also be considered. Initial experiments revealed that vaccination with antigen into the brain can trigger higher antibody titers compared to vaccination in the periphery (21). In contrast, Th1-mediated delayed type hypersensitivity responses are absent or blunted when the same antigen is delivered to the brain (reviewed in (22)). These findings support a model whereby the cervical lymph nodes have an intrinsic Th2 bias in steady state conditions. Additional experiments showed that CD8 T cells undergo initial expansion following intracerebral tumor cell challenge, but fail to differentiate into cytotoxic T lymphocytes (CTLs) (23). However, it was unclear if this was due to tumor-induced immune suppression, a lack of co-stimulation, or an intrinsic bias against CTL development in the cervical lymph nodes. Normal mouse cerebral spinal fluid can suppress CD8 T cell activation in ex vivo assays, which is restored by a TGFγ blocking antibody (24), implicating brain-derived TGFγ as one soluble mediator of CTL suppression in the cervical lymph nodes.
Despite support for Th2 immune deviation in the brain DLN, there is evidence that CD8 T cell responses play a tumoricidal role in human gliomas. Infiltration of CD8 T cells is a positive prognostic factor in glioma patients (25). Furthermore, immunological synapses between CD8 T cells and glioma cells have been documented in humans (26). Interestingly, glioblastoma patients receiving autologous tumor lysate-pulsed dendritic cell vaccines had superior survival when their gene expression was of mesenchymal rather than the proneural molecular signature; the mesenchymal signature is inflammatory and was correlated with significantly more infiltrating CD8 T cells at the tumor site compared to proneural tumors (27). Regardless of these spontaneous or vaccine-induced T cell responses, global immune suppression has been widely accepted to occur in glioma patients. Many studies done to establish this conclusion were conducted with leukocytes harvested after treatment with glucocorticoids or chemotherapy, clouding the contribution of the tumor versus the treatment on dampened immunity. More recent data suggest that treatment with glucocorticoids and alkylating chemotherapy plays a significant role in inducing global immune suppression, because both drugs are associated with rapid post-treatment lymphopenia, and elevation in regulatory T cell or myeloid derived suppressor cell frequency (28, 29). The severity of lymphopenia following the standard of care (steroids, chemotherapy, and radiation) negatively correlates with overall survival in glioblastoma patients (30).
There is stronger experimental evidence for profound local immune suppression at the tumor site in gliomas. Studies in spontaneous murine models suggest that gliomas accrue immune suppressive cell populations even at early, asymptomatic stages (19). As gliomas develop, recruitment of myeloid derived suppressor cells is mediated in part through a cyclooxygenase-2 and prostaglandin E2 axis (31), decreasing CTL recruitment to the tumor bed. Local immune suppression is further enforced by hypoxia, leading to reduced effector function of T cells via increased production of immunosuppressive factors (32). Moreover, glioma cells express inhibitory ligands that can trigger T cell tolerance including HLA-E and B7-H1 (33, 34). However, little is known about how these mechanisms of suppression at the tumor site impact priming responses in the cervical lymph nodes. One purpose of this study was to shed insight on this poorly understood area.
Based on evidence that much of the tumor-derived immune suppression in other malignancies is anatomically graded rather than global (8-10, 35, 36), we reasoned that vaccination distal to gliomas could be exploited to improve active immunotherapy. We focused our investigation on the relationship between vaccination sites and the ensuing CD8 T cell response. Vaccination in close proximity to the tumor DLN (cervical or axillary regions) resulted in no survival benefit. In contrast, vaccinations in DLN further from the brain (in the inguinal region) increased survival of glioma-bearing mice. Mice vaccinated in the cervical region had CD8 T cells with lower TCR affinity and weakened effector functions compared to priming at the inguinal region. Considering that previous clinical trials have used a variety of injection sites, these data reveal a neglected yet potentially critical variable for trial design. Injection site is a crucial aspect of active tumor immunotherapy in this murine model that demands investigation in glioma patients.
Material and Methods
Animal models and cell lines
Tumors were implanted into female C57BL/6 mice (6-8 weeks old) that were purchased from Jackson Laboratory and maintained in a specific pathogen-free facility according to the guidelines of the University of Minnesota Animal Care and Use Committee. The GL261 orthotopic transplant model was established in C57BL/6 (B6) or B6 Nur77GFP transgenic mice (37) (kind gift from Dr. Kristin Hogquist, University of Minnesota Center for Immunology) by inoculation with 15,000 GL261 cells or OVA-transfected GL261 cells (GL261-OVA hereafter) in 1 μL phosphate-buffered saline. Tumors were implanted stereotactically into the right striatum; coordinates were 2.5-mm lateral, 0.5-mm anterior of bregma, and 3-mm deep from the cortical surface of the brain (38).
To generate GL261-OVA, a minigene encoding four peptides presented by H-2b class I or class II molecules was constructed as a single coding sequence. The nucleotide sequences were codon optimized for expression in mice and were synthesized (GenScript, Piscataway, NJ). The peptides were encoded in the following order: human gp10025-33 (presented by Db), chicken OVA257-264 (presented by Kb), chicken OVA323-339 (presented by I-Ab), and mouse I-Ea52-68 (presented by I-Ab). To facilitate antigen processing, each peptide was flanked on both sides by the six amino acid residues that surrounded the peptide in the corresponding native protein. This construct was inserted into the multiple cloning site of the pIRES-DsRed-Express vector (Clontech, Mountain View, CA) linearized with BamHI and EcoRI. Parental GL261 cells were transfected with this vector and selected using 2 mg/mL G418. The selected cells were sub-cloned by limiting dilution and a clone with a high level of antigen expression (as assessed by DsRed Express fluorescence) was chosen and used for all subsequent experiments.GL261-OVA and parental GL261 were cultured in DMEM media containing 10% FBS, penicillin/streptomycin (100 U/ml), and 0.1 mg/ml Normocin® (Invivogen). 500 μg/mL G418 was added to maintain selection of GL261-OVA cells during routine culture. GL261 cells for tumor lysate vaccine were cultured in neural stem cell media consisting of DMEM/F12 (1:1) with L-glutamine, sodium bicarbonate, penicillin/streptomycin (100 U/ml), B27 and N2 supplements (Gibco), and 0.1-mg/ml Normocin® (Invivogen). Cultures were maintained at 5% O2 and supplemented with 20 ng/mL EGF and FGF semi-weekly (R&D Systems, Minneapolis, MN).
Vaccine preparation and injection
Tumor lysates were prepared by dissociating cells with non-enzymatic dissociation solution (Sigma), washing twice with PBS, resuspending in 500 μL PBS, and freezing initially by placing in −80°C overnight. Cells were further lysed by five cycles of freezing in liquid nitrogen and thawing in a 56°C water bath. Cell debris was pelleted by centrifugation at 14,000 RCF, and the protein concentration of the supernatant was determined using a Bradford assay. Pellets were resuspended, and lysates were stored at −80°C until use. Each vaccine was prepared on the day of vaccination and consisted of 65 μg of protein tumor lysate and 50 μg phosphorothioated type-B CpG ODN 685 (5′-tcgtcgacgtcgttcgttctc-3′; SBI Biotech, Japan) in a final volume of 100 μL, injected intradermally at the indicated site. For survival studies, vaccines were administered weekly starting three days post-tumor implantation for a total of 6 doses. For OVA vaccinations, 100 μg OVA (Fisher Bioreagents) and 10 μg Poly:ICLC (Oncovir, Inc.) in 100 μL were injected intradermally in hind leg, foreleg, or back of the neck. For the GL261-OVA survival study, mice were vaccinated on days 14-17 post inoculation, then on days 21-24. For other CD8 T cell priming experiments, the animals were intradermally vaccinated on four consecutive days with 100 μg OVA and 10 μg Poly:ICLC, and once more on day seven.
T cell receptor (TCR) affinity measurements
Nur77GFP mouse splenocytes were collected and plated at a concentration of 7.5 × 105 cells/well in a 96 well plate. Cells were stimulated with either 10−6, 10−8, 10−10, or 10−12 moles of SIINFEKL peptide. Non-stimulated cells were used as a control. Following 8 hr incubation, cells were harvested and stained with CD8 and the SIINFEKL/Kb dextramer. GFP expression within the CD8+ SIINFEKL/H-2Kb dextramer+ gate was analyzed by flow cytometry.
Measurement of relative TCR affinity by a competitive tetramer fall-off assay was adapted from a previously described method (39). Briefly, 2 × 107 splenocytes from the vaccinated Nur77GFP mice were stained with the SIINFEKL/Kb dextramer and anti-CD8 antibodies as described above. After two washes, cells were incubated with or without 0.5 mg unlabeled competitor anti-SIINFEKL-H-2Kb antibody (clone eBio25-D1.16 eBioscience) at 4°C. At the indicated times, 100-μL aliquots were removed and added to an equal volume of 2% PFA in PBS and run on a Becton Dickinson Canto three-laser flow cytometer. Normalized total fluorescence (NTF) minus background staining was calculated using the following formula: NTFtime i = (% pos. X MFI)timei / (% pos. X MFI)time 0 as in reference (39).
Flow cytometry
A Becton Dickinson Canto three-laser flow cytometer was used for data acquisition. Anti-mouse CD8α-Pacific Blue (clone 53-6.7 eBioscience) was purchased from eBioscience. A SIINFEKL/Kb dextramer –PE was used for detection of SIINFEKL/Kb-binding CD8 T cells (Immunodex). For whole blood staining, 50 μL blood was obtained via orbital bleed and placed in 100 μL of a 1:10 dilution of heparin and PBS. Five μL of SIINFEKL/Kb dextramer was added to blood and incubated at room temperature for 10 min, and 1 μL (0.5 mg) of antibodies were added to cells and incubated for an additional 20 min at room temperature. Blood was lysed by adding 1 mL 1:10 dilution lysis buffer (BD Pharmigen), incubated for 10 min at room temperature, centrifuged twice and resuspended in 100 μL PBS and analyzed. For analysis of secondary lymphoid tissues cells were isolated from draining lymph nodes and were suspended at 5 × 105 cells in 100 μL PBS. Five μL SIINFEKL/Kb dextramer was added to cells and incubated at room temperature for 10 min; 1 μL (0.5μg) of CD8 antibody was added to cells and incubated for an additional 20 min at 4°C; cells were then washed twice and analyzed by flow cytometry.
Brain infiltrating lymphocyte isolation
Brain-infiltrating lymphocytes (BILs) were prepared by perfusing four-mice/treatment group with phosphate buffered saline. Brains were minced into a crude suspension in cold RPMI 1640 complete media with 10% FBS. Suspensions were then passed through a 40 μm filter, washed once in cold PBS, and spun on the following percoll gradient: 3 mL 70% (v/v, in PBS) bottom, 3 mL 37% middle, and 4 mL 30% with resuspended pellet. BILs were centrifuged at 800 RCF at 4°C for 20 min and collected from the cell interface and washed in cold RPMI 1640 complete media with 10% FBS.
Cytotoxic T lymphocyte analyses
These assays were conducted as previously described (40). Briefly, DLNs were harvested, dissociated, and lymphocytes were incubated with CFSE-labeled GL261 or GL261-OVA cells for 4 hrs at effector to target ratios (E/T) of 0:1 and 25:1 and analyzed for cytotoxicity according to the manufacturer’s protocol (Immunochemistry, LLC). Following incubation, the percentage of CFSE-labeled target cells that stained with 7-AAD was determined by flow cytometry. Data were reported as specific lysis by correcting for background 7-AAD staining using the following formula: % CFSE+ 7-AAD+ at 25:1 E/T ratio - % CFSE+7-AAD+ at 0:1 E/T ratio.
Interferon gamma detection
Lymphocytes isolated from draining lymph nodes were plated at a concentration of 5 × 105 cells/well and pulsed with 2 μg of SIINFEKL peptide for 12 hrs. Following incubation, 50 μL of culture supernatant was analyzed for IFNγ using a flow cytometric bead array according to the manufacturer’s protocol (BD Biosciences). Cells were then harvested, surface stained with CD8 and SIINFEKL/Kb dextramer, then intracellularly stained for IFNγ according to the manufacturer’s protocol (BD Biosciences). To determine the number of secreted molecules of IFNγ per antigen-specific CD8 T cell, IFNγ concentration was converted to molarity and divided by the number of plated cells per well. In addition, lymphocytes were harvested from the lymph nodes, stained for CD8 then intracellularly stained for IFNγ. Flow cytometry was used to determine the fraction of viable cells that were antigen-specific CD8 T cells, and a hemocytometer was used to determine the number of viable cells plated.
Statistical analysis
Statistical comparisons were made by ANOVA, followed by post hoc comparisons using a 2-tailed t-test. Differences in animal survival were evaluated by log-rank test. All tests were performed with Prism 4 software (Graph Pad Software, Inc). P values <0.05 were considered significant. Column statistics were calculated to validate significance within the values of each treatment group.
Results
Vaccination in the hind leg enhances survival benefit
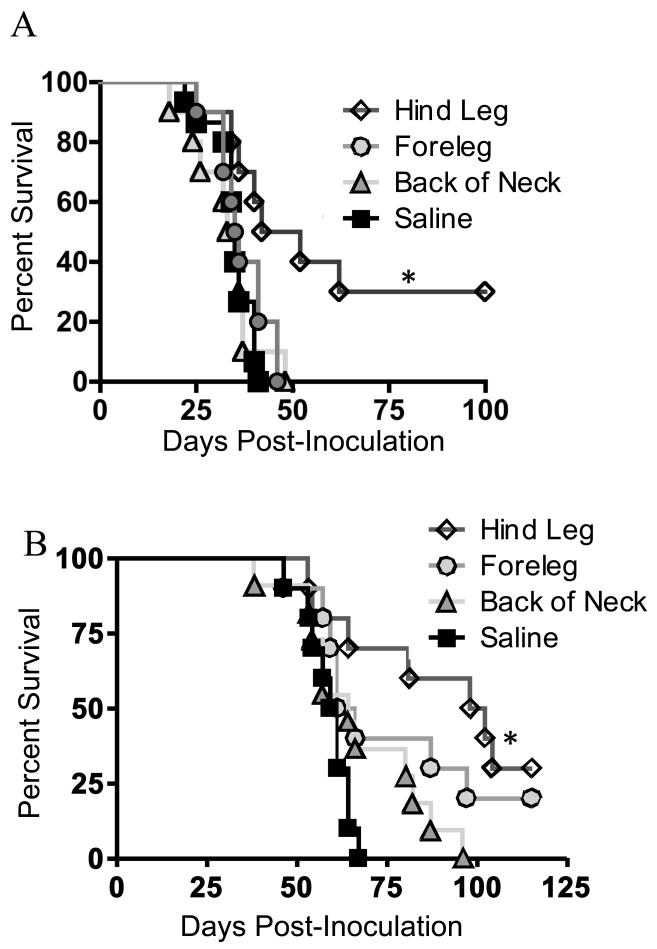
We previously demonstrated that vaccinating glioma-bearing mice with a poly-antigen tumor lysate/CpG vaccine enhanced survival (40). However, these previous studies relied on vaccination in two sites in the same animal: the hind leg and the neck, which is in contrast to most clinical trials that employ single vaccination sites. To investigate potential differences in efficacy of vaccination between three different sites, glioma-bearing mice were vaccinated in the back of the neck, fore leg, or hind leg with GL261 lysate mixed with CpG. Mice primed in the hind leg exhibited significantly increased long-term survival compared to mice vaccinated in the back of the neck, foreleg, or saline control, which had no survival benefit (Fig 1A).
FIGURE 1. Vaccination in the hind leg has superior therapeutic benefit.
A, Vaccination with GL261 tumor lysate and CpG was administered weekly starting three days post-GL261 implantation. B, OVA plus Poly:ICLC vaccination was administered for 4 consecutive days starting 14 days post-OVA-GL261 implantation, then on days 21-24 for a total of 8 doses. Mice were monitored for survival. Kaplan Meier plot illustrating survival (n=10/group; * indicates P<0.01 by Log-rank test. Data are representative of two independent experiments).
Although this experiment was suggestive of differential T cell priming, the use of a poly-antigen lysate vaccine did not permit tracking of antigen-specific T cells. To test an antigen specific response, we developed a model whereby mice bearing intracranial GL261-OVA were vaccinated with OVA plus Poly:ICLC in three different sites. In this experiment, there was an incremental decrease in survival as the vaccination site approached the tumor. Mice vaccinated in the back of the neck showed no survival benefit compared to controls, whereas mice vaccinated in the hind leg demonstrated the greatest survival benefit (Fig 1B).
CD8 T cell priming is suppressed through an anatomical gradient
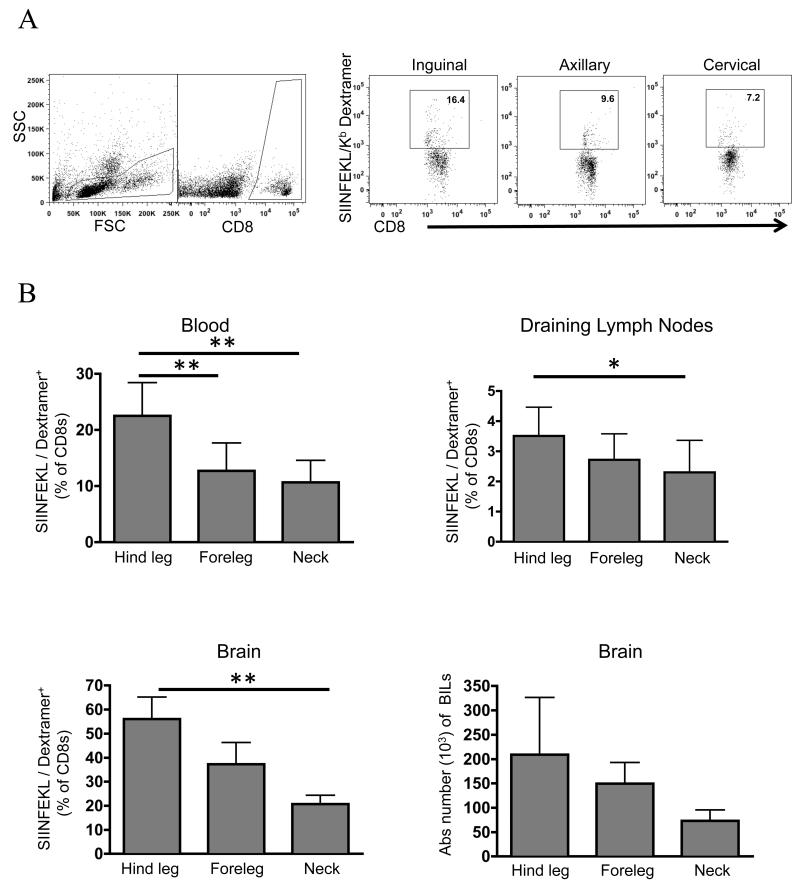
We measured priming of an endogenous CD8 T cell response against the OVA antigen by flow cytometry using a SIINFEKL/H2-Kb dextramer. Mice were inoculated with GL261-OVA and vaccinated in the back of the neck, foreleg, or the hind leg for drainage into the cervical, axillary, or inguinal lymph nodes, respectively. In all tissues analyzed, there was a stepwise decline in the frequency of dextramer-binding CD8 T cells as the vaccination site approached the tumor in the following order: hind leg>foreleg>neck (Fig 2B). There were several notable differences in CD8 T frequency in each tissue. In the blood, dextramer-binding CD8 T cells averaged from ~11-28% of the CD8 T cell compartment depending on the site of priming. Mice primed in the rear leg had roughly twice as many dextramer-staining cells compared to other sites. In the lymph nodes draining the respective vaccination site, CD8 T cell frequency was much lower than blood, averaging ~1.5-2.8% depending on the site of priming. The brain infiltrating lymphocytes (BILs) followed a similar trend compared to the vaccine draining lymph nodes, but at much higher frequency, ranging from 18-60% of the CD8 T cells being dextramer-binding. There was a three-fold increase in the dextramer-binding CD8 T cell frequency in mice vaccinated in the hind leg compared to back of the neck. There was also a change in the absolute number of BILs that paralleled the trend of CD8 T cell frequency, but this failed to reach statistical significance due to high animal-to-animal variability (Fig 2B).
FIGURE 2. T-cell priming is suppressed as vaccination site approaches the tumor.
Glioma-bearing mice were vaccinated for 4 consecutive days and boosted on day 7. On day 8, mice were sacrificed and analyzed using flow cytometry A, gating scheme used, B whole blood, lymph nodes, and brain infiltrating lymphocytes were analyzed for an endogenous CD8 T cell response. Error bars are representative of standard deviation (n=8 mice/group for blood and lymph nodes, n=4 for BILs; *P < 0.05; ** P < 0.01 by t-test. Data are representative of two independent experiments).
Presence of the tumor locally alters the affinity of the T cell receptor
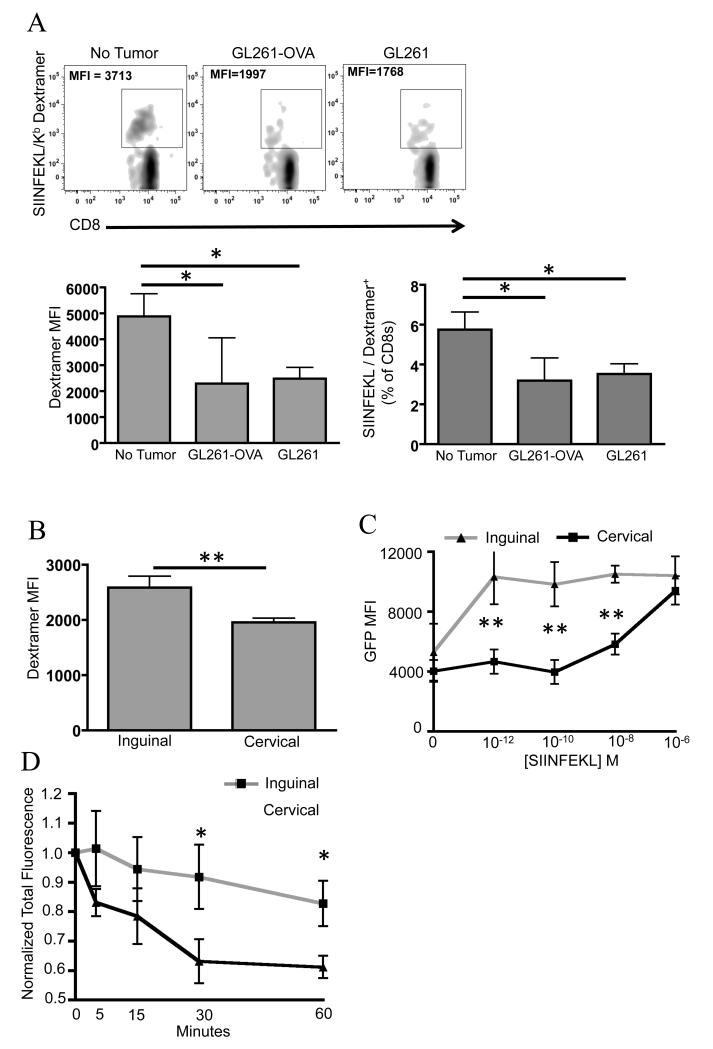
Subsequent experiments were conducted to determine if the lower T cell priming was due to an intrinsic bias of the cervical lymph nodes themselves or from the presence of the tumor. These experiments also addressed how the presence of the cognate antigen (OVA) being expressed in the tumor impacted priming. To answer these questions, mice were inoculated with the GL261-OVA or the parental GL261cells; saline injection was performed in the “no tumor” group of mice to control for brain injury. Mice were vaccinated with OVA plus Poly:ICLC in the back of the neck for drainage into the cervical lymph nodes. Whole blood was collected and analyzed as before. As illustrated in Figure 3A, the presence of the tumor reduced the dextramer-binding CD8 T cell frequency by half regardless of whether the tumor expressed OVA or not. GL261 and GL261-OVA groups were not significantly different. Thus, the presence of the tumor in the brain is responsible for the suppression in priming in the cervical lymph nodes. Furthermore, the change in priming is not dependent on expression of the antigen used for vaccination in the tumor.
FIGURE 3. Presence of the tumor alters TCR affinity following vaccination.
Wildtype mice were vaccinated on days 3-6 and boosted on day 10 post-tumor inoculations. Saline injection was used to control for brain injury in tumor-free mice. Mice were sacrificed on day 11. A, whole blood was analyzed using flow cytometry for an endogenous CD8 T cell response. B, Mean fluorescent intensity (MFI) of the CD8+SIINFEKL/Kb+ Dextramer population was measured on splenocytes from Nur77GFP mice and C, analyzed for GFP expression as a readout of TCR signaling. D Tetramer fall off assay conducted on splenocytes used in C to further document TCR affinity. Error bars are representative of standard deviation (n=12/group for figure A and n=7/group for figures B-D; *P < 0.05; ** P < 0.01 by t-test. Data are representative of two independent experiments for figure A).
We then focused on qualitative differences in CD8 T cells primed in the rear leg or neck, exclusively in tumor-bearing mice, because this is most relevant to a clinical scenario. The MFI of dextramer-binding CD8 T cells was significantly lower when vaccination was administered in the neck compared to the rear leg (Fig 3B), suggesting that TCR affinity might be altered. To test TCR affinity we first utilized a novel Nur77GFP reporter mouse that was recently described (37). In this mouse model, GFP expression is a surrogate for TCR signaling strength and is not affected by co-stimulation delivered by the antigen presenting cell (37). Glioma-bearing Nur77GFP mice were repeatedly vaccinated in the neck or rear leg. One day after the last vaccine mice were sacrificed and their splenocytes were stimulated with various concentrations of SIINFEKL peptide. Dextramer-binding CD8 T cells were gated on and analyzed for GFP expression. Importantly, there was no appreciable difference in GFP expression in the absence of exogenous SIINFEKL peptide, demonstrating that the baseline TCR signaling was comparable in the splenocytes harvested from each group (Fig 3C). There was a striking decrease in TCR signaling in CD8 T cells from the cervical group relative to the inguinal group when antigen concentration was limiting; over two-fold differences at 10−12 to 10−8 molar SIINFEKL. To confirm that GFP expression truly reflected TCR affinity, we also conducted a standard tetramer fall-off assay on a portion of splenocytes harvested from the same Nur77GFP mice. Figure 3D shows that dextramer-binding CD8 T cells primed by vaccination draining to the cervical lymph nodes had a significantly faster rate of tetramer fall off compared to the inguinal group. Collectively, these data reveal a qualitative change in TCR affinity as affected by the location of vaccination in relation to the tumor draining lymph nodes.
Effector function of CD8 T cells is changed as a consequence of vaccination site
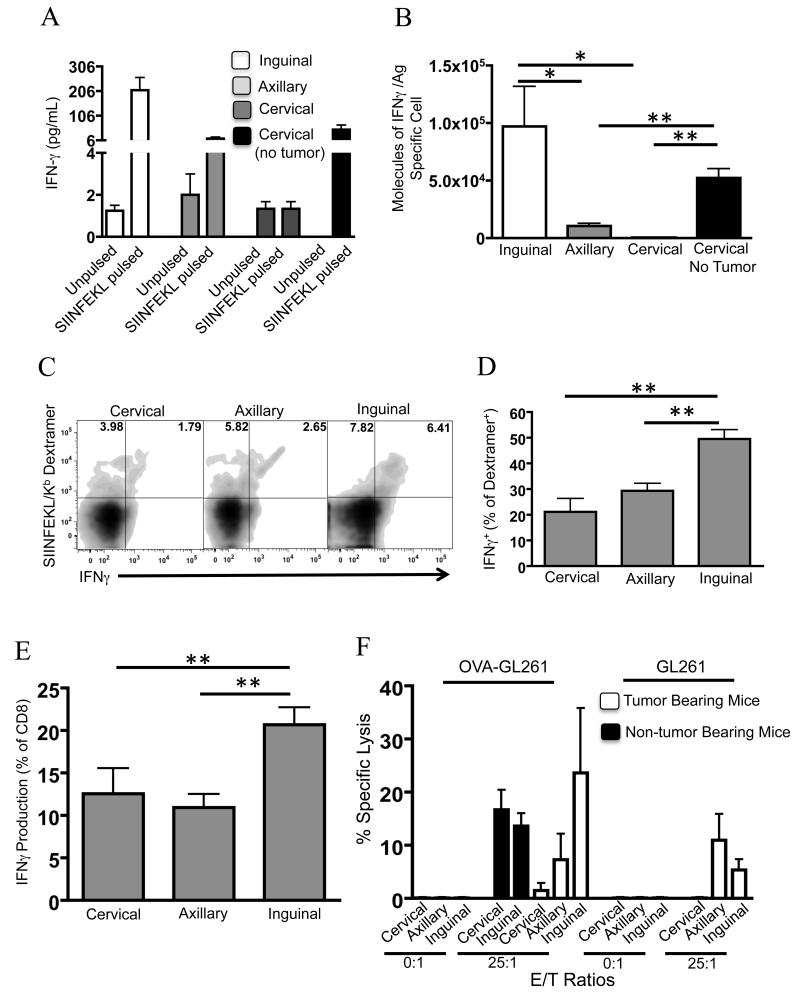
We investigated how vaccination site alters effector cytokine elaboration and cytotoxic function of CD8 T cells. Mice were primed at multiple sites as before, and the lymph nodes draining the vaccination sites were harvested and used as a source of effector cells. Lymphocytes were stimulated for 12 hours in vitro with the OVA-derived SIINFEKL peptide or unpulsed as a control. There was a greater than ten-fold increase in soluble IFNγ measured in responder cells from the inguinal lymph node compared to axillary (Fig 4A). Cells from the cervical lymph node completely failed to elaborate IFNγ in response to stimulation with SIINFEKL.
FIGURE 4. Impaired effector function of CD8 T cells primed near the tumor.
Tumor-bearing and tumor-free mice were vaccinated on days 3-6 and boosted on day 10 post-tumor inoculation. Lymphocytes were isolated from draining lymph nodes on day 11, stimulated with a SIINFEKL peptide and A, analyzed for IFNγ secretion. B, The molecules of IFNγ elaborated per antigen specific cell was determined by calculating the number of dextramer-binding cells in the tissue culture in A. C, Intracellular flow cytometry was performed on the cultures in A. D, aggregate data from C. E, total intracellular IFNγ production, and F CTL assays using lymphocytes isolated from the indicated draining lymph nodes as effectors. Error bars are representative of standard deviation (n=4/group for figures A & D, n=5 for Figure E, and n=8 for figure F; *P < 0.05; ** P < 0.01 by t-test. Data are representative of two independent experiments).
To better characterize the responding cells, we used flow cytometry to determine the frequency of dextramer-binding cells in the CD8 gate secreting IFNγ, and the amount of IFNγ produced per cell, following peptide stimulation in vitro. The number of soluble IFNγ molecules elaborated per dextramer-binding CD8 T cell was calculated (Fig 4B), revealing large differences in functional capacity on a per cell basis as affected by vaccination site. Consistent with previous experiments, mice primed in the neck in the absence of a tumor had a significant restoration of effector function as measured by IFNγ response to SIINFEKL stimulation. Intracellular flow cytometry confirmed that dextramer-binding CD8 T cells were the source of IFNγ (Fig 4C). To assess the in vivo effector function of tumoricidal T cells, unstimulated lymphocytes were isolated from the various lymph nodes and analyzed intracellularly for IFNγ production. Mice primed in the hind leg had a significant increase of IFNγ compared to the other treatment groups (Fig 4D). This response correlated with previous experiments demonstrating that priming near the tumor environment had suppressed effector function.
We next investigated CD8 T cell tumoricidal function in tumor-bearing mice primed with OVA and Poly:ICLC vaccination. Lymphocytes isolated from lymph nodes draining the vaccination site were used as a source of CTLs. CTLs from mice vaccinated in the hind leg had significantly greater tumoricidal function compared to mice vaccinated in the foreleg (Fig 4E). Cells isolated from the cervical lymph nodes had negligible killing activity, but tumoricidal function was restored in tumor-free mice. Killing was mostly dependent on the expression of OVA because parental GL261 targets had diminished specific lysis. However, cells primed by vaccination in the hind leg or foreleg had tumoricidal function above background against parental GL261, implying spontaneous epitope spreading to endogenous GL261 antigens in these lymph nodes. Also consistent with this explanation is the finding that CTLs from tumor-free mice that were vaccinated with OVA had no tumoricidal function against parental GL261 targets (Fig 4E). Additionally, no significant killing activity was measured within saline-vaccinated mice at any site, ruling out differences in baseline killing function in cervical, axillary, and inguinal lymph nodes (data not shown).
Discussion
Tumor-induced immune suppression consists of several functionally distinct mechanisms, most of which have been reported to corrupt not only the tumor microenvironment but also local secondary lymphoid tissues in direct drainage of the tumor site (41, 42). In this study we examined the relationship between vaccination site and treatment efficacy in a glioma model for the first time. From an efficacy standpoint measured by survival, the results were generalizable to different vaccine adjuvants (CpG or Poly:ICLC) and different antigen sources (lysate or single protein). Our data demonstrate a profound suppression of CD8 T cell priming within the cervical lymph nodes. This suppression is tumor-dependent, but is not dependent on expression of the vaccine antigen in the tumor. This suppressive response is location related, decreasing in severity as the distance from the tumor increases. Such suppression is likely to occur from secreted tumor-derived factors that reach secondary lymphoid organs rather than direct contact with tumor cells because GL261 does not metastasize to extracranial sites. A clear limitation to our study is the use of mice with recently established tumors to explore these questions. At best, this is a model of minimal residual disease in a clinical setting. How this animal model relates to humans who have co-evolution of the immune system and tumor for months is an open question. Nonetheless, the results are informative and raise intriguing questions that can be explored in human subjects.
Tumor vaccines break tolerance by reversing anergy and/or cross priming naïve CD8 T cells. Crucial to both processes is presentation of antigen on MHC I and induction of costimulatory signals by dendritic cells (DCs) (43). We sought to characterize the suppressive mechanisms present in our tumor model from site-based differences in response to vaccination. We found no difference in expression of CD80, CD86, or OX40L in DCs in the cervical lymph nodes of glioma-bearing and tumor-free mice (data not shown). Differential expression of other costimulatory molecules cannot be ruled out, although a myriad of other suppressive mechanisms exists. Hyperactivation of immune checkpoint molecules such as CTLA-4 or PD-1 may occur from the local presence of tumor-derived factors. Such a mechanism has been associated with anergic or tolerant CD8 T cells whose tumoricidal function can be restored by antibody-mediated blockade (44). In addition, studies report increases in DC expression of PD-L1 in tumor draining lymph nodes (45). Additionally, local downregulation of MHC II expression has been shown to limit CD4 help to tumor-specific CD8 T cells (46), but we did not detect differences in expression of MHC II by DCs in our studies (data not shown). Regulatory T cells are often increased in tumor draining lymph nodes (47, 48). We have not tested regulatory T cells levels in our model. Clearly many additional studies are needed to more fully characterize the mechanisms of immune suppression in the cervical lymph nodes of glioma-bearing mice.
TCR diversity exists in the CD8 T cell precursor clones capable of responding to vaccination. Differential expansion of clones occurs in a hierarchy according to TCR affinity, enabling high affinity clones to preferentially expand and dominate the response (39, 49, 50). We found relatively weaker TCR affinity of CD8 T cells specific for the OVA antigen when vaccination drained to the cervical lymph nodes of mice with gliomas. It is unlikely that thymic emigrant populations could contribute to this effect, as one week elapsed from time of tumor implantation to vaccination. Rather, this difference may be due to other factors including relatively greater expansion of cells with weaker binding to cognate antigen, destabilized TCR co-receptor complex (51-53), or perhaps more likely is that cells with stronger TCR signaling are selectively deleted. Additional work is needed to address these possibilities. This study is the first to demonstrate a meaningful difference in TCR affinity as it relates to vaccination site in tumor-bearing hosts. We postulate that differences in TCR affinity may underlie the inferior effector functions of CD8 T cells from mice vaccinated near the tumor draining lymph nodes, as only high levels of antigen triggered TCR signaling ex vivo in these mice (Fig 3C). Accordingly, due to impaired TCR signaling, it is unlikely that levels of peptide-MHC I are sufficiently high in situ to trigger CD8 T cell tumoricidal function in animals vaccinated near the tumor. Lower TCR affinity and poor effector function would account for the inferior survival of animals vaccinated nearer to the tumor draining lymph nodes.
This study has potential implications for the design of translational research and clinical trials, where injection site is variable and often arbitrarily chosen. These data provide guidelines for a strategic choice of vaccination site in humans and raise several questions. For example, in metastatic melanoma, would better immune responses be achieved if vaccination sites were consistently altered to be farthest away from active tumors? What is the immune competence of such distal lymph nodes related to those that have been studied to date, which are only located around the tumor and resected as part of standard of care? Our results call for testing if anatomically graded suppression occurs in humans, whose anatomic scale may follow different rules than that of mice.
Acknowledgements
We are indebted to Dan Sloper, Jovany Cortes, Joe Lalli, Nick Erickson, and Rob Shaver for assisting in the harvest of tissues and with survival studies.
This work was supported in part by NIH grants T32 GM008244 (MSTP), T32 CA009138 (Cancer Biology Training Grant), and Torske Klubben Fellowship for Minnesota Residents to BMA; NIH grants R01 CA154345, R01 CA160782, the American Cancer Society grant RSG-09-189-01-LIB, and generous support from the Minnesota Medical Foundation, the Hedberg Family Foundation, and the Children’s Cancer Research Fund to JRO, and the Randy Shaver Foundation to MRO.
References
- 1.Chang CN, Huang YC, Yang DM, Kikuta K, Wei KJ, Kubota T, Yang WK. A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J Clin Neurosci. 2011;18:1048–1054. doi: 10.1016/j.jocn.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 2.Okada H, Lieberman FS, Edington HD, Witham TF, Wargo MJ, Cai Q, Elder EH, Whiteside TL, Schold SC, Jr., Pollack IF. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of recurrent glioblastoma: preliminary observations in a patient with a favorable response to therapy. J Neurooncol. 2003;64:13–20. doi: 10.1007/BF02700016. [DOI] [PubMed] [Google Scholar]
- 3.Ardon H, Van Gool SW, Verschuere T, Maes W, Fieuws S, Sciot R, Wilms G, Demaerel P, Goffin J, Van Calenbergh F, Menten J, Clement P, Debiec-Rychter M, De Vleeschouwer S. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-012-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T, Narita M, Takahashi M, Tanaka R. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89:1172–1179. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 2001;50:337–344. doi: 10.1007/s002620100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 7.Cochran AJ, Balda BR, Starz H, Bachter D, Krag DN, Cruse CW, Pijpers R, Morton DL. The Augsburg Consensus. Techniques of lymphatic mapping, sentinel lymphadenectomy, and completion lymphadenectomy in cutaneous malignancies. Cancer. 2000;89:236–241. [PubMed] [Google Scholar]
- 8.Cochran AJ, Morton DL, Stern S, Lana AM, Essner R, Wen DR. Sentinel lymph nodes show profound downregulation of antigen-presenting cells of the paracortex: implications for tumor biology and treatment. Mod Pathol. 2001;14:604–608. doi: 10.1038/modpathol.3880358. [DOI] [PubMed] [Google Scholar]
- 9.Lana AM, Wen DR, Cochran AJ. The morphology, immunophenotype and distribution of paracortical dendritic leucocytes in lymph nodes regional to cutaneous melanoma. Melanoma Res. 2001;11:401–410. doi: 10.1097/00008390-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Matsuura K, Yamaguchi Y, Ueno H, Osaki A, Arihiro K, Toge T. Maturation of dendritic cells and T-cell responses in sentinel lymph nodes from patients with breast carcinoma. Cancer. 2006;106:1227–1236. doi: 10.1002/cncr.21729. [DOI] [PubMed] [Google Scholar]
- 11.Hoon DS, Bowker RJ, Cochran AJ. Suppressor cell activity in melanoma-draining lymph nodes. Cancer Res. 1987;47:1529–1533. [PubMed] [Google Scholar]
- 12.Hoon DS, Korn EL, Cochran AJ. Variations in functional immunocompetence of individual tumor-draining lymph nodes in humans. Cancer Res. 1987;47:1740–1744. [PubMed] [Google Scholar]
- 13.Wen DR, Hoon DS, Chang C, Cochran AJ. Variations in lymphokine generation by individual lymph nodes draining human malignant tumors. Cancer Immunol Immunother. 1989;30:277–282. doi: 10.1007/BF01744894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farzad Z, Cochran AJ, McBride WH, Gray JD, Wong V, Morton DL. Lymphocyte subset alterations in nodes regional to human melanoma. Cancer Res. 1990;50:3585–3588. [PubMed] [Google Scholar]
- 15.Ostroukhova M, Qi Z, Oriss TB, Dixon-McCarthy B, Ray P, Ray A. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin Invest. 2006;116:996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc’h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 19.Tran Thang NN, Derouazi M, Philippin G, Arcidiaco S, Di Berardino-Besson W, Masson F, Hoepner S, Riccadonna C, Burkhardt K, Guha A, Dietrich PY, Walker PR. Immune infiltration of spontaneous mouse astrocytomas is dominated by immunosuppressive cells from early stages of tumor development. Cancer research. 2010;70:4829–4839. doi: 10.1158/0008-5472.CAN-09-3074. [DOI] [PubMed] [Google Scholar]
- 20.Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, Valet F, Kissenpfennig A, Sparwasser T, Malissen B, Fetler L, Amigorena S. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Gordon LB, Knopf PM, Cserr HF. Ovalbumin is more immunogenic when introduced into brain or cerebrospinal fluid than into extracerebral sites. J Neuroimmunol. 1992;40:81–87. doi: 10.1016/0165-5728(92)90215-7. [DOI] [PubMed] [Google Scholar]
- 22.Harling-Berg CJ, Park TJ, Knopf PM. Role of the cervical lymphatics in the Th2-type hierarchy of CNS immune regulation. J Neuroimmunol. 1999;101:111–127. doi: 10.1016/s0165-5728(99)00130-7. [DOI] [PubMed] [Google Scholar]
- 23.Gordon LB, Nolan SC, Cserr HF, Knopf PM, Harling-Berg CJ. Growth of P511 mastocytoma cells in BALB/c mouse brain elicits CTL response without tumor elimination: a new tumor model for regional central nervous system immunity. J Immunol. 1997;159:2399–2408. [PubMed] [Google Scholar]
- 24.Gordon LB, Nolan SC, Ksander BR, Knopf PM, Harling-Berg CJ. Normal cerebrospinal fluid suppresses the in vitro development of cytotoxic T cells: role of the brain microenvironment in CNS immune regulation. J Neuroimmunol. 1998;88:77–84. doi: 10.1016/s0165-5728(98)00077-0. [DOI] [PubMed] [Google Scholar]
- 25.Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, Grau S, Hiraoka N, Eckstein V, Ecker RC, Korff T, von Deimling A, Unterberg A, Beckhove P, Herold-Mende C. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res. 2011;17:4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 26.Barcia C, Jr., Gomez A, Gallego-Sanchez JM, Perez-Valles A, Castro MG, Lowenstein PR, Barcia C, Sr., Herrero MT. Infiltrating CTLs in human glioblastoma establish immunological synapses with tumorigenic cells. Am J Pathol. 2009;175:786–798. doi: 10.2353/ajpath.2009.081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustafson MP, Lin Y, New KC, Bulur PA, O’Neill BP, Gastineau DA, Dietz AB. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12:631–644. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling R, Shi W, Vredenburgh JJ, Bigner DD, Heimberger AB. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13:324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer research. 2011;71:2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei J, Wu A, Kong LY, Wang Y, Fuller G, Fokt I, Melillo G, Priebe W, Heimberger AB. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2011;6:e16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wischhusen J, Friese MA, Mittelbronn M, Meyermann R, Weller M. HLA-E protects glioma cells from NKG2D-mediated immune responses in vitro: implications for immune escape in vivo. J Neuropathol Exp Neurol. 2005;64:523–528. doi: 10.1093/jnen/64.6.523. [DOI] [PubMed] [Google Scholar]
- 34.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nature medicine. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 35.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 36.Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol. 2006;6:659–670. doi: 10.1038/nri1919. [DOI] [PubMed] [Google Scholar]
- 37.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. The Journal of experimental medicine. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu A, S Oh, K Ericson, Demorest ZL, Vengco I, Gharagozlou S, Chen W, Low WC, Ohlfest JR. Transposon-based interferon gamma gene transfer overcomes limitations of episomal plasmid for immunogene therapy of glioblastoma. Cancer Gene Ther. 2007;14:550–560. doi: 10.1038/sj.cgt.7701045. [DOI] [PubMed] [Google Scholar]
- 39.Hommel M, Hodgkin PD. TCR affinity promotes CD8+ T cell expansion by regulating survival. J Immunol. 2007;179:2250–2260. doi: 10.4049/jimmunol.179.4.2250. [DOI] [PubMed] [Google Scholar]
- 40.Olin MR, Andersen BM, Zellmer DM, Grogan PT, Popescu FE, Xiong Z, Forster CL, Seiler C, SantaCruz KS, Chen W, Blazar BR, Ohlfest JR. Superior efficacy of tumor cell vaccines grown in physiologic oxygen. Clin Cancer Res. 2010;16:4800–4808. doi: 10.1158/1078-0432.CCR-10-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shu S, Cochran AJ, Huang RR, Morton DL, Maecker HT. Immune responses in the draining lymph nodes against cancer: implications for immunotherapy. Cancer Metastasis Rev. 2006;25:233–242. doi: 10.1007/s10555-006-8503-7. [DOI] [PubMed] [Google Scholar]
- 42.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 43.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 46.Gerner MY, Casey KA, Mescher MF. Defective MHC class II presentation by dendritic cells limits CD4 T cell help for antitumor CD8 T cell responses. J Immunol. 2008;181:155–164. doi: 10.4049/jimmunol.181.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 48.Hiura T, Kagamu H, Miura S, Ishida A, Tanaka H, Tanaka J, Gejyo F, Yoshizawa H. Both regulatory T cells and antitumor effector T cells are primed in the same draining lymph nodes during tumor progression. J Immunol. 2005;175:5058–5066. doi: 10.4049/jimmunol.175.8.5058. [DOI] [PubMed] [Google Scholar]
- 49.Manuel ER, Charini WA, Sen P, Peyerl FW, Kuroda MJ, Schmitz JE, Autissier P, Sheeter DA, Torbett BE, Letvin NL. Contribution of T-cell receptor repertoire breadth to the dominance of epitope-specific CD8+ T-lymphocyte responses. J Virol. 2006;80:12032–12040. doi: 10.1128/JVI.01479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yachi PP, Ampudia J, Zal T, Gascoigne NR. Altered peptide ligands induce delayed CD8-T cell receptor interaction--a role for CD8 in distinguishing antigen quality. Immunity. 2006;25:203–211. doi: 10.1016/j.immuni.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monu N, Frey AB. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. Cancer Res. 2007;67:11447–11454. doi: 10.1158/0008-5472.CAN-07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang L, Yamagata N, Yadav R, Brandon S, Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP, Breyer RM. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–735. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]