Abstract
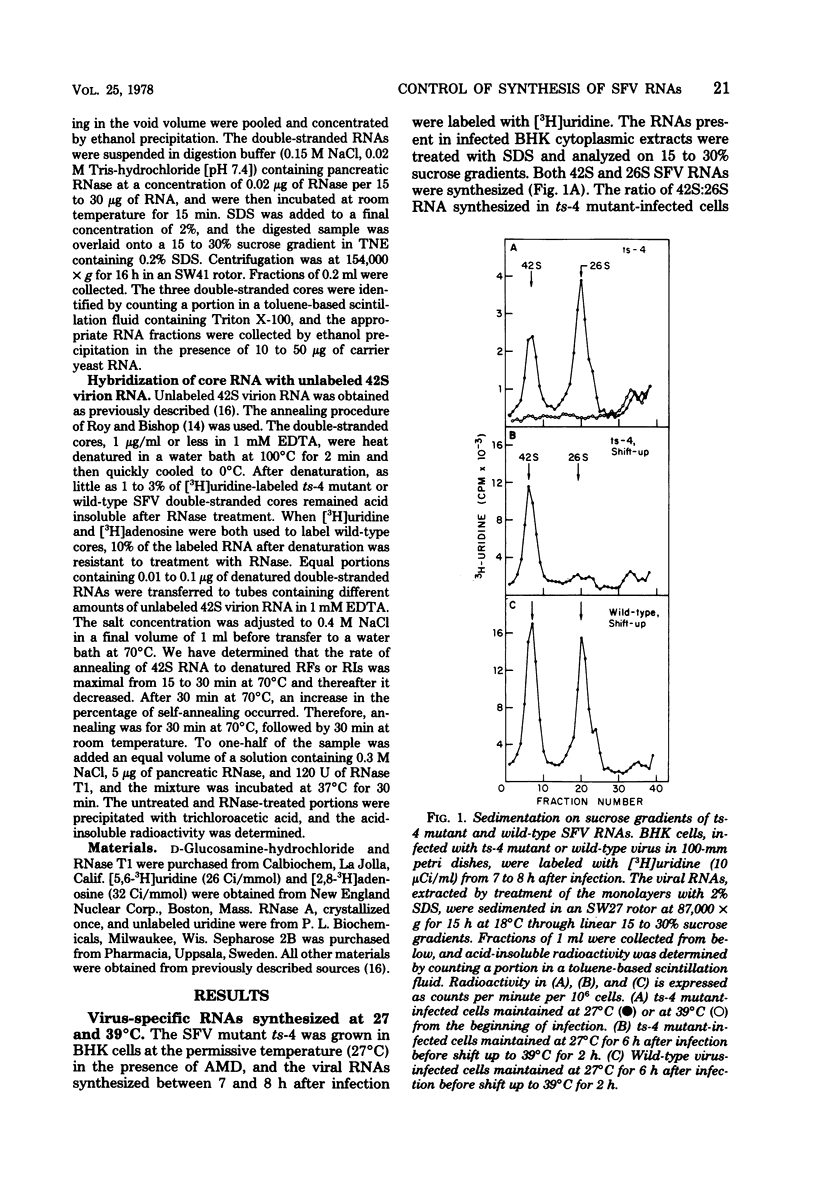
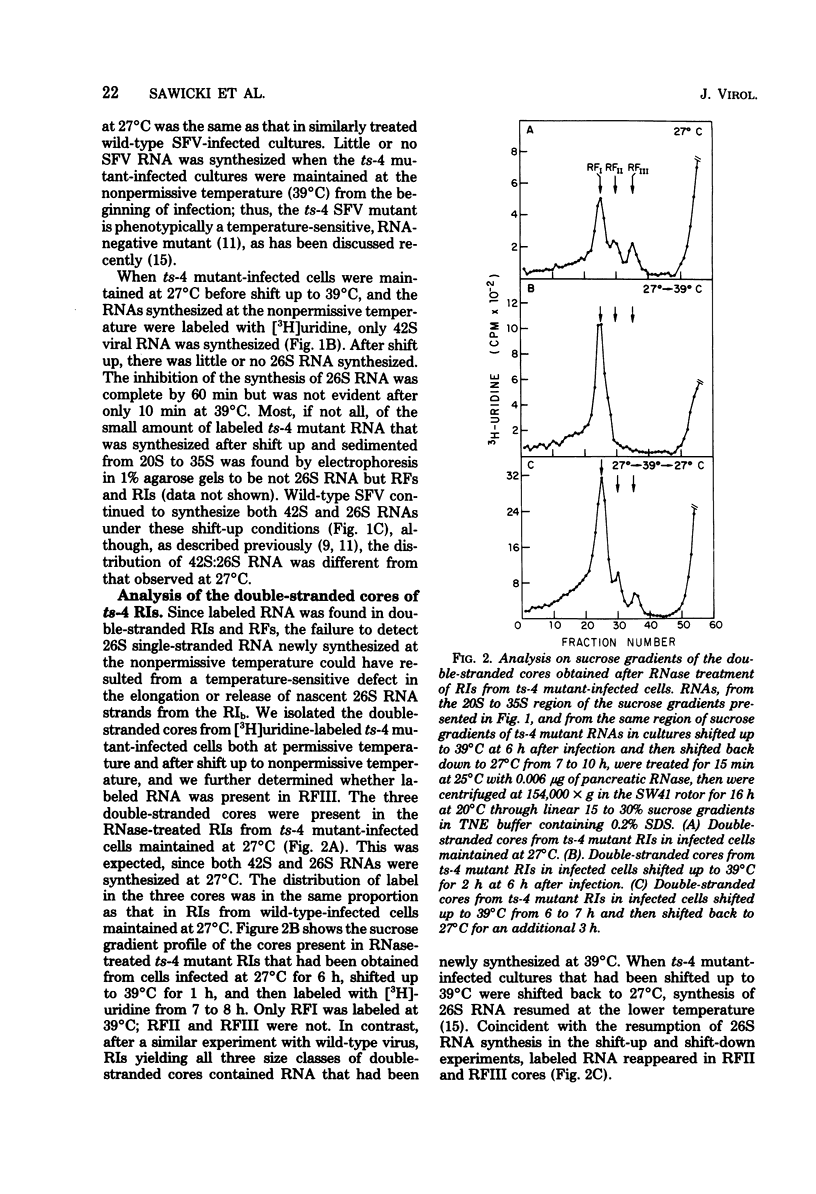
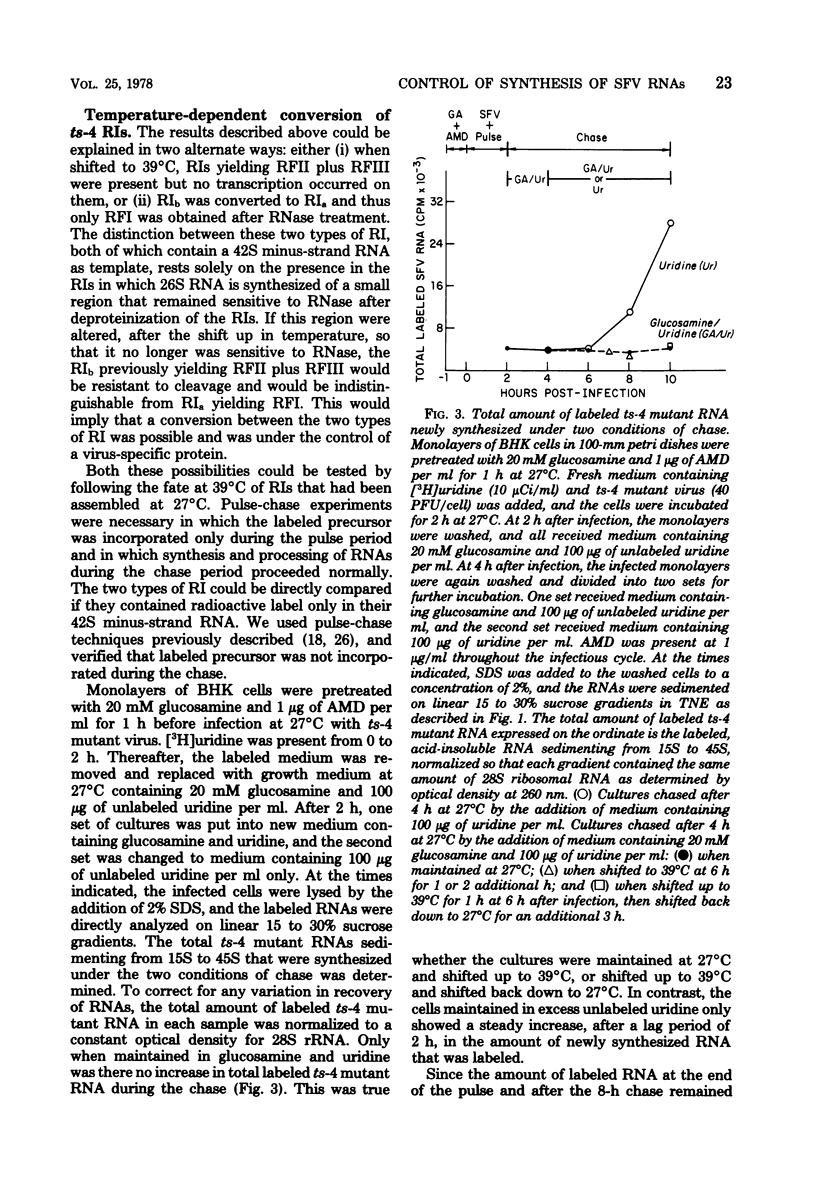
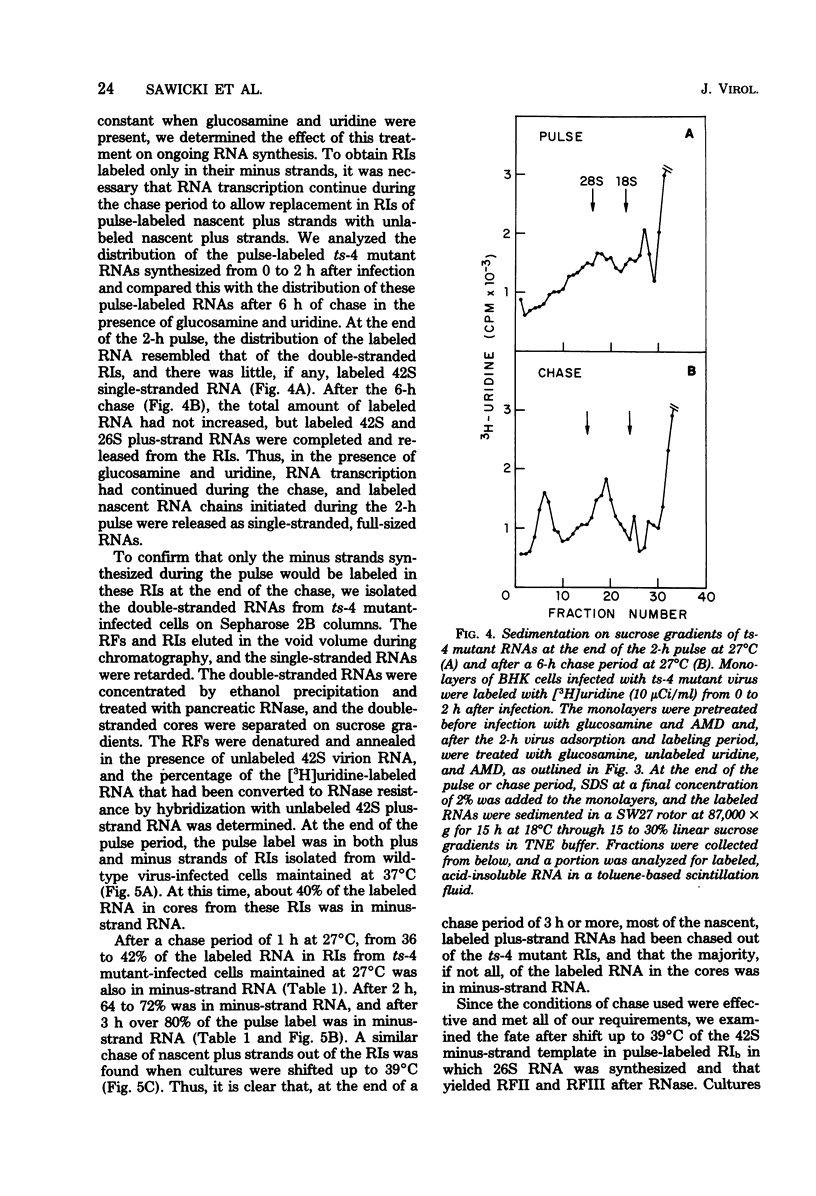
When cells infected with the Semliki Forest virus (SFV) mutant ts-4 were shifted to the nonpermissive temperature, synthesis of 26S RNA ceased, whereas synthesis of 42S RNA continued normally. These two single-stranded SFV RNAs are synthesized in two types of replicative intermediate (RI), 26S RNA in RIb and 42S RNA in RIa. Cessation of 26S RNA synthesis after shift up in temperature was accompanied by loss of RIb. When infected cells were shifted back down to 27°C, 26S RNA synthesis resumed, coincident with the reappearance of RIb. In both types of RI, the 42S minus-strand RNA is template for synthesis of plus-strand RNA. In pulse-chase experiments, we obtained RIs labeled only in their minus-strand RNA, and thus could follow the fate of RIs assembled at 27°C when they were shifted to 39°C. Our results show that, after shift up to 39°C, there was a quantitative conversion of RIs in which 26S RNA had been synthesized to RIs in which 42S RNA was synthesized. This conversion of RIb to RIa was reversible, since RIs in which 26S RNA was synthesized reappeared when the infected cultures were shifted back down to 27°C. We propose that, associated with RIb, in which 26S RNA is synthesized, there is a virus-specific protein that functions to promote initiation of 26S RNA transcription at an internal site on the 42S minus-strand RNA and to block transcription on the minus strand in this region by the SFV RNA polymerase that had bound and was copying the minus-strand RNA from its 3′ end. A ribonuclease-sensitive region would thus result in the sequence adjacent to the one that was complementary to 26S RNA. This virus-specific protein is not a component of the SFV RNA polymerase that continues to transcribe 42S RNA, and it is temperature sensitive in ts-4 mutant-infected cells. When this virus-specific protein is not present on RIs, the SFV polymerase transcribes the whole 42S minus-strand RNA and yields 42S plus-strand RNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bracha M., Leone A., Schlesinger M. J. Formation of a Sindbis virus nonstructural protein and its relation of 42S mRNA function. J Virol. 1976 Dec;20(3):612–620. doi: 10.1128/jvi.20.3.612-620.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton C. J., Kennedy S. I. Semliki Forest virus intracellular RNA: properties of the multi-stranded RNA species and kinetics of positive and negative strand synthesis. J Gen Virol. 1975 Jul;28(1):111–127. doi: 10.1099/0022-1317-28-1-111. [DOI] [PubMed] [Google Scholar]
- Clegg J. C., Kennedy S. I. Polyadenylic acid sequences in the virus RNA species of cells infected with Semliki Forest Virus. J Gen Virol. 1974 Mar;22(3):331–345. doi: 10.1099/0022-1317-22-3-331. [DOI] [PubMed] [Google Scholar]
- Deborde D. C., Leibowitz R. D. Polyadenylic acid size and position found in Sindbis virus genome and mRNA species. Virology. 1976 Jul 1;72(1):80–88. doi: 10.1016/0042-6822(76)90313-5. [DOI] [PubMed] [Google Scholar]
- Dobos P., Faulkner P. Molecular weight of Sindbis virus ribonucleic acid as measured by polyacrylamide gel electrophoresis. J Virol. 1970 Jul;6(1):145–147. doi: 10.1128/jvi.6.1.145-147.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton B. T., Donaghue T. P., Faulkner P. Presence of poly (A) in the polyribosome-associated RNA of Sindbis-infected BHK cells. Nat New Biol. 1972 Jul 26;238(82):109–111. doi: 10.1038/newbio238109a0. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Levy H. B., Carter W. B. Replication of semliki forest virus: three forms of viral RNA produced during infection. Proc Natl Acad Sci U S A. 1966 Aug;56(2):440–446. doi: 10.1073/pnas.56.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaariainen L., Gomatos P. J. A kinetic analysis of the synthesis in BHK 21 cells of RNAs specific for Semliki Forest virus. J Gen Virol. 1969 Sep;5(2):251–265. doi: 10.1099/0022-1317-5-2-251. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Isolation and basic characterization of temperature-sensitive mutants from Semliki Forest virus;. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):810–820. doi: 10.1111/j.1699-0463.1974.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Keränen S., Lachmi B., Söderlund H., Tuomi K., Ulmanen I. Replication of Semliki Forest virus. Med Biol. 1975 Oct;53(5):342–351. [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. A., Burke D. C. The replication of Semliki Forest virus. J Gen Virol. 1974 Jul;24(1):45–66. doi: 10.1099/0022-1317-24-1-45. [DOI] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Isolation and properties of poliovirus minus strand ribonucleic acid. J Virol. 1970 Nov;6(5):604–609. doi: 10.1128/jvi.6.5.604-609.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D. L., Gomatos P. J. Replication of semliki forest virus: polyadenylate in plus-strand RNA and polyuridylate in minus-strand RNA. J Virol. 1976 Nov;20(2):446–464. doi: 10.1128/jvi.20.2.446-464.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Inhibition of interjacent ribonucleic acid (26S) synthesis in cells infected by Sindbis virus. J Virol. 1969 Aug;4(2):117–122. doi: 10.1128/jvi.4.2.117-122.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C. Detection of an unstable RNA in chick fibroblasts after reduction of the UTP pool by glucosamine. Eur J Biochem. 1971 Dec;24(2):358–365. doi: 10.1111/j.1432-1033.1971.tb19694.x. [DOI] [PubMed] [Google Scholar]
- Segal S., Sreevalsan T. Sindbis virus replicative intermediates: purification and characterization. Virology. 1974 Jun;59(2):428–442. [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. I. Relative size and genetic content of 26 s and 49 s RNA. J Mol Biol. 1972 Nov 28;71(3):599–613. [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. II. Multiple forms of double-stranded RNA isolated from infected cells. J Mol Biol. 1972 Nov 28;71(3):615–631. doi: 10.1016/s0022-2836(72)80027-5. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Sonnabend J. A., Martin E. M., Mécs E. Viral specific RNAs in infected cells. Nature. 1967 Jan 28;213(5074):365–367. doi: 10.1038/213365a0. [DOI] [PubMed] [Google Scholar]
- Waite M. R. Protein synthesis directed by an RNA temperature-sensitive mutant of Sindbis virus. J Virol. 1973 Feb;11(2):198–206. doi: 10.1128/jvi.11.2.198-206.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Localization of the 26-S RNA sequence on the viral genome type 42-S RNA isolated from SFV-infected cells. Virology. 1976 Aug;73(1):190–199. doi: 10.1016/0042-6822(76)90073-8. [DOI] [PubMed] [Google Scholar]
- Wertz G. W. Method of examining viral RNA metabolism in cells in culture: metabolism of vesicular stomatitis virus RNA. J Virol. 1975 Nov;16(5):1340–1344. doi: 10.1128/jvi.16.5.1340-1344.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



