Abstract
This manuscript reports on findings of three open label, pilot studies and it reviews studies using rTMS as a maintenance treatment for any disorder. The first pilot study examined whether a patient’s original treatment response to 1 Hz rTMS over temporal cortex could be replicated by stimulating a homologous region of the opposite hemisphere. The second study examined whether a patient’s response to 1Hz rTMS could be replicated by applying 10 Hz rTMS over the same treatment site. The third study applied a 3-day course of maintenance rTMS, either at 1 or 10 Hz, when subjects indicated that the benefit of their last course of treatment was waning. Patients with bilateral subjective tinnitus of at least 6 months duration were recruited from a prior, sham controlled study with treatment crossover that applied 1Hz rTMS over temporal cortex. Both treatment responders and non-responders were recruited. Results indicated, first, that the original treatment response, both positive and negative, is replicated after stimulating a homologous region of the opposite hemisphere; second, patients respond similarly to 1 and 10 Hz stimulation of the same treatment site (an exception was one patient who initially failed 1 Hz stimulation but responded positively to 10 Hz stimulation); and, third, maintenance rTMS had a sustained and additive benefit for tinnitus among treatment responders. Conclusions are that rTMS-induced effects on tinnitus are neither hemisphere specific nor frequency dependent; although, different frequencies of rTMS may have greater potency for a given subject. Maintenance treatment is a well tolerated approach with demonstrated feasibility for managing chronic tinnitus in persons who respond positively to an initial course of treatment.
Keywords: Tinnitus, rTMS, maintenance treatment, within subject, 1 Hz, 10 Hz
1 Introduction
Repetitive transcranial magnetic stimulation (rTMS) can temporarily decrease subjective tinnitus perception in about 50% of patients (Langguth, et al., 2008; Mennemeier, et al., 2011); although, it is unclear how rTMS decreases tinntus. Subjective tinnitus (hereafter, tinnitus) is defined as the perception of sound in the absence of an internal or external sound source. Fifty million people in the US are affected by tinnitus. Approximately 12 million seek treatment and 3 million are disabled (Shargorodsky, et al., 2010). There is no universal cure that benefits all tinnitus patients. Repetitive TMS is a non-invasive method of regional brain stimulation in which magnetic pulses are delivered over the scalp to induce electric currents in the brain that activate neurons (Bohning, 2000). Most studies of tinnitus apply rTMS over auditory processing areas in either the left or right temporal cortex once per day for one or two weeks. Repetitive TMS treatments for tinnitus have a very good safety margin and their effect is generally greater than placebo (Langguth, et al., 2008) with some exceptions noted (Piccirillo, et al., 2011). It is probably most accurate to state that a subset of tinnitus patients respond positively to rTMS and a subset does not (Mennemeier, et al., 2011).
A positive response to rTMS is operationally defined as a significant decrease from baseline on a tinnitus questionnaire or visual analogue rating of tinnitus loudness or annoyance following treatment (VARL and VARA, respectively). Individual responses to rTMS can range from no response (about 50%) to reports of no remaining tinnitus three months after rTMS treatment (Khedr, et al., 2010). Most patients who respond positively to rTMS treatment report a temporary reduction in tinnitus that lasts for days to weeks, in one or both ears, before tinnitus returns [see for example (Plewnia, et al., 2007)]. Whereas the response rate to rTMS varies from study to study, undoubtedly being influenced by sample size and composition and how tinnitus is assessed, the 50% average is reasonably consistent across studies even when different hemispheres are stimulated and when different frequencies of rTMS are used to treat tinnitus (Khedr, et al., 2008; Khedr, et al., 2010; Langguth, et al, 2010). Additionally, stimulating the hemisphere contralateral to the ear with loudest tinnitus may be more efficacious that targeting either the left or right hemisphere for treatment (De Ridder, et al., 2005; Khedr, et al., 2010).
It is presently unclear who responds positively to rTMS treatment or how rTMS induces a treatment effect. Patients with less chronic or severe tinnitus and with better preserved hearing may be more likely to respond positively to rTMS (De Ridder, et al., 2005; Kleinjung, et al., 2007); however, negative outcomes can be obtained in patients who fit these criteria and positive outcomes can be obtained in patients who do not (Dornhoffer & Mennemeier, 2010). Patients also respond to rTMS in ways that defy our understanding of how rTMS should work to improve tinnitus. For example, low frequency rTMS, which is thought to inhibit cortical activity immediately following stimulation, has often been used to “treat” regions of temporal cortex that are hyperactive and thought to be responsible for tinnitus perception [see for example (Mennemeier, et al., 2011)]. In contrast to this hypothesis, it has been shown that high frequency rTMS, which should have an opposite effect, can not only improve tinnitus perception but may also be more effective than low frequency rTMS (Khedr, et al., 2009; Khedr, et al., 2010). Current decisions over which frequency of rTMS to apply for tinnitus have no firm theoretical basis. In fact, contemporary models of rTMS, which explain the immediate effects of rTMS (i.e., low frequency inhibits and high frequency excites neuronal activity)(George, et al., 2002), appear inadequate to explain how both low and high frequencies rTMS can decrease tinnitus perception for days, weeks and months after treatment.
To advance rTMS as a clinical treatment for tinnitus, it is important to investigate factors that can potentially increase the response rate and/or prolong the duration of its treatment effect. It is also theoretically important to address observations that challenge our understanding of how rTMS induces a treatment effect. For example, while it is known that delivering stimulation to either the right or left hemisphere can decrease tinnitus, it is not known whether a patient who fails to respond to stimulation of one hemisphere would respond positively to stimulation of the other, as nearly all rTMS treatment studies treat only one hemisphere. Additionally, while it is known that both low (1 Hz) and high (10 & 25 Hz) frequencies of rTMS applied over temporal cortex can decrease tinnitus, it is not known whether a patient who fails to respond to one frequency of stimulation would respond positively to another, as stimulation frequencies are rarely compared within subjects. Finally, only a few studies have attempted to reapply rTMS after the initial benefit wanes (Langguth, et al., 2003; Mennemeier, et al., 2008; Mennemeier & Dornhoffer, 2008); so it is not known whether relapse or “maintenance treatment” can improve or prolong the benefit of a single course of rTMS. A review of studies using rTMS as a maintenance treatment suggests that it can.
Given that the therapeutic effects of rTMS appear to outlast the immediate inhibitory and excitatory physiological effects of stimulation, and given that symptoms eventually return after initial treatment, there is reason to believe that repeated treatment, as per a maintenance protocol, might have efficacy for managing chronic tinnitus. Towards that end, several small-scale, open label studies have investigated the therapeutic benefits of rTMS maintenance therapy for a variety of psychiatric and neurological disorders. A review of these studies (table 1) indicates that maintenance rTMS can be a well tolerated and effective method of treating the symptoms of medication-resistant depression (Benadhira, et al., 2005; Demirtas-Tatlidede, et al., 2008; Loo, et al., 2007; O’Reardon, et al., 2007; O’Reardon, et al., 2005), bipolar disorder (Dell’Osso, et al., 2009; Li, et al., 2004), schizophrenia (Poulet, et al., 2008; Thirthalli, et al., 2008), and aphasia (Kakuda, et al., 2010). Additionally, two case studies demonstrate the feasibility of using maintenance rTMS for tinnitus (Langguth, et al., 2003; Mennemeier, et al., 2008; Mennemeier & Dornhoffer, 2008); although, a larger series of tinnitus cases has not been published to our knowledge. Even though substantial variability exists among studies in the schedule protocols and rTMS parameters used for maintenance treatment, taken together, these studies advise a new conceptual approach that emphasizes a prophylactic, maintenance model of administration – an approach similar in its logic to scheduled immunotherapy for an allergy. The following summary statements can be tentatively drawn based on a review of these open label studies:
Table 1.
Review of maintenance rTMS treatment studies
| Authors | Disorder (sample size) | Stimulation frequency & intensity | Number of stimuli per session | Initial treatment schedule | Maintenance treatment schedule | Stated result of maintenance treatment | Target area |
|---|---|---|---|---|---|---|---|
| O’Reardon et al. (2007) | MDD and HA (n=2) | 10 Hz at 120% MT | 3,000 | q.d. for 12 weeks | PRN @ 2 sessions QWK for 15–18 months | HA amelioration | Left DPFC |
| Loo et al. (2007) | MDD (n=22) | 10 Hz at 110% MT | 1,500 30 trains, 5 sec on/25 off |
b.i.d for 2 weeks | q.d. for 6 weeks | Added improvement | Left PFC |
| O’Reardon et al. (2005) | MDD (n=10) | 10 Hz at 100% MT | 3,000 40–60 trains, 5 sec on/25 off |
Not defined | 1–3 sessions QWK over 6 years | Marked or moderate improvement | Left PFC |
| Demirtas-Tatlidede et al. (2008) | MDD (n=16) | 10 Hz at 90% MT | 1,600 20 trains, 8 sec on/52 off |
q.d. for 9 days | Variable with mean of 4 courses over 20 months | 50% of patients with improvement | Left DPFC |
| Benadhira et al. (2005) | MDD (n=11) | 10 Hz at 80% MT | 1,600 26 trains |
q.d. for 2 weeks then 3 QWK for 2 weeks | 1 QWK for 1 month then 1 session biweekly for 1 month | Initial improvement sustained | Left DPFC |
| Li et al. (2004) | BD (n=7) | 5 Hz at 110% MT | 1,600 40 trains, 8 sec on |
q.d. for 2 weeks | Approximately. 1 session QWK over 12 months | Relapse prevented in some patients | Left PFC |
| Dell’osso (2009) | BD (n=1) | 1 Hz at 110% MT | 300 | q.d. for 3 weeks | Periodic sessions over 6 months | Initial therapeutic gains maintained | Right DPFC |
| Poulet et al. (2008) | SCHZ (n=1) | 1 Hz at 100% MT | 1,000 | b.i.d. for 5 days | Initial Tx plus b.i.d. once per month | Remission and stabilization of AH | Left TP |
| Thirthalli et al. (2008) | SCHZ (n=1) | 1 Hz at 100% MT | 900 | q.d. for 1 week | 1 session QWK for 6 weeks then 1 session biweekly for 12 weeks then 1 session per month for 3 months | Remission of AH | Left T3-P3 |
| Kakuda et al. (2009) | Aphasia (n=2) | 1 Hz at 90% MT | 1,200 | 10 sessions over 6 days | 1 session QWK for 3 months | Safe and feasible for aphasia treatment | Left PSTG |
| Mennemeier et al. (2008) | Tinnitus (n=1) | 1 Hz at 110% MT | 1,800 | 5 sessions per week | PRN @ 3 sessions biweekly over 1 month | Sustained reduction of tinnitus loudness and obtrusiveness. | Right SLTG |
| Langguth et al. (2008) | Tinnitus (n=1) | 1 Hz at 110% MT | 2,000 | q.d. for 2 weeks | q.d. for 1 week then every 3 months over 36 months | Suppression of tinnitus | Left TC |
MDD – major depressive disorder, HA – head ache, BD – bipolar disorder, SCHZ – schizophrenia, AH – auditory hallucinations, PRN – as needed, q.d. – once per day, b.i.d. – two times per day, QWK – every week, PFC – prefrontal cortex, DPFC – dorsolateral PFC, TP – temporoparietal, T3 & T4 – coordinates of 10–20 system for EEG electrode placement, PSTG – posterior superior temporal gyrus, SLTG – superior lateral temporal gyrus, TC – temporal cortex.
Maintenance treatment appears well tolerated; there is no current evidence to indicate that maintenance rTMS carries a greater risk for an adverse event than would a single course or session of rTMS.
Maintenance treatment appears to work best for those who have a strong positive response to an initial course of treatment.
There is no consensus concerning a “best” or even a “typical” schedule for maintenance treatment; however, the effect of maintenance treatment can be additive and last months before retreatment is necessary.
Larger, controlled trials of maintenance treatment are warranted.
In this manuscript, we report on three open-label, pilot studies using within subject designs to investigate, first, whether stimulation of the left and right hemispheres and, second, whether stimulation at high and low frequencies of rTMS alter treatment outcome in the same subjects. Finally, we report on whether maintenance rTMS can have added or sustained benefit for the treatment of tinnitus. The main purpose of these pilot studies was to guide the design of a larger, controlled clinical trial by establishing concept feasibility and estimating effect size. All of the patients included in these studies were recruited after completing a placebo controlled, crossover study that examined the efficacy of 5 days of 1 Hz rTMS (1800 pulses per day @ 110% MT) delivered over left or right temporal cortex for chronic tinnitus (Mennemeier, et al., 2011). These patients had already been defined as either treatment responders, who achieved a 33% reduction in the VARL from baseline in at least one ear following active but not sham treatment, or as non-responders who failed this criterion. We attempted to recruit equal numbers of responders and non-responders, except for the study of maintenance treatment which enrolled only treatment responders. The first pilot study examined whether a patient’s original response to 1 Hz treatment could be replicated by applying the same stimulation to a homologous region of the opposite hemisphere. The second study examined whether the original treatment response to 1 Hz rTMS could be replicated by applying 10 Hz rTMS over the same treatment site. The third study applied a 3-day course of maintenance rTMS, either at 1 or 10 Hz depending on which worked best for a given subject, whenever the benefit of the last course of treatment was waning. The timing of maintenance treatment was purposely left open-ended to learn when retreatment would be necessary.
2 Material and Method
2.1 Subjects
Twelve patients elected to receive further, open-label treatment in one or more of the pilot studies. The only restriction to recruitment was that a patient had to live close enough to the treatment center to return as needed. No other selection criteria were applied for studies 1 and 2 other than to obtain equal numbers of responders and non-responders. For study 3, only patients who indicated on a Likert-type rating scale that their tinnitus improved after receiving either 1 or 10 Hz rTMS were eligible for maintenance treatment. Table 2 provides characteristics of the treatment sample and indicates which patients participated in which studies. The mean age for the sample was 50 years (SD=15). All patients were diagnosed with subjective, bilateral tinnitus of at least six months duration. No subject had normal hearing. The average total percent score on the Tinnitus Handicap Questionnaire was equal to the 70th percentile for a large sample of patients with tinnitus (Kuk, et al., 1990). On average, few symptoms of depression were reported (Beck, et al., 1961). All subjects completed an informed consent process and signed a written informed consent to participate. The studies were approved by the University Institutional Review Board governing the use of human subjects in biomedical research. All subjects met the inclusion criteria and did not meet the exclusion criteria for the parent study (Mennemeier, et al., 2011).
Table 2.
Sample Characteristics
| Subject | Classification | Tinnitus | Questionnaires | Studies | |||
|---|---|---|---|---|---|---|---|
| Severity | Years | THQ | TSI | BDI | |||
| 1 | R | B, Max = 60 dB/6 kHz | 10.0 | 29 | 4 | 3 | |
| 2 | R | B, Max = 45 dB/8 kHz | 0.5 | 26 | 5 | 3 | |
| 3 | NR | B, Max = 60 dB/4 kHz | 6.0 | 100 | 27 | 1 | |
| 4f | R | B, Max = 65 dB/6 kHz | 13.0 | 38 | 1 | 1 & 3 | |
| 5f | NR | B, Max = 35 dB/8 kHz | 2.0 | 32 | 3 | 1 | |
| 6 | R | L, Max = 70 dB/8 kHz | 12.0 | 35 | 35 | 5 | 2 & 3 |
| 7 | R | B, Max = 45 dB/8 kHz | 3.5 | 66 | 38 | 17 | 3 |
| 8 | R | B, Max = 75 dB/4 kHz | 23.0 | 34 | 28 | 1 | 3 |
| 9 | R | B, Max = 50 dB/8 kHz | 2.0 | 66 | 35 | 12 | 1 |
| 10 | NR | R, Max = 70 dB/4 kHz | 5.0 | 55 | 15 | 0 | 1 & 2 |
| 11 | NR | B, Max = 65 dB/4 kHz | 20.0 | 91 | 39 | 17 | 1 & 2 |
| 12 | R | B, Max = 50 dB/4k Hz | 5.0 | 32 | 27 | 6 | 1, 2 & 3 |
| Mean | 8.50 | 54.90 | 30.2 | 8.17 | |||
| SD | 7.27 | 25.28 | 7.50 | 8.33 | |||
R = responder and NR = non-responder.
denotes female. Hearing thresholds reported in dB 1969 ANSI. All patients had down sloping sensory neural hearing loss from a mild level to the maximum loss indicated. B=bilateral, R=right, L=left, M=maximum hearing loss, & K= frequency in Hz. Tinnitus duration is reported in years. THQ = Tinnitus Handicap Questionnaire total score; TSI=Tinnitus Severity Index; BDI=Beck Depression Inventory. Studies column indicates the pilot studies, 1–3, in which each subject participated.
2.2 Apparati
Active stimulation was delivered using a Magstim Super Rapid stimulator and a Magstim air-film, figure-of-eight, 70-mm stimulating coil. Coil placement was guided using the Brainsight Frameless Stereotaxy (Rogue Research) and a CT scan of the brain. Motor evoked potentials (MEP) of the thenar muscle were recorded in response to single pulses of TMS delivered to the contralateral motor cortex from surface electrodes fixed on the skin in a belly-tendon montage. The EMG signal was amplified and filtered (10 Hz–1 kHz bandpass) (Precision World Instruments) and analyzed in LabView.
2.3 Measures
The primary outcome measure was the VARL (0=tinnitus absent; 100=extremely or painfully loud tinnitus) obtained on each day of treatment before rTMS was administered. A secondary outcome measure, the VARA (0= no annoyance; 100=extremely annoying), was obtained at the same time; however, baseline data for the VARA measure was missing for three subjects because it was added to the protocol after the VARL. Tests of neuropsychological function, i.e. the Digit Symbol Test, the three-words-at-five-minutes test, and the finger tapping test (Spreen, O. & Strauss, E., 1991) were assessed as a safety measure to determine if rTMS adversely affected cognition.
3 Procedures and findings common to all experiments
A detailed description of our experimental procedures has been published (Mennemeier, et al., 2011). To summarize these procedures, patients wore electrodes for recording the MEP and they wore earplugs to protect hearing during TMS. Patients could elect to wear a bite guard to prevent jaw movement. The TMS coil was guided to the same motor threshold and treatment sites as in the parent study (Mennemeier, et al., 2011), i.e., in or adjacent to Brodmann area 22. Motor threshold (MT) was determined prior to each treatment by recording MEPs in response to single pulses of increasing intensity until a MEP of at least 50 μvolts was recorded in 3 of 6 trials. One hertz stimulation was delivered at 110% of the MT for 30 minutes (1800 total pulses per session) for five consecutive days (9000 total pulses per week). Ten hertz rTMS was delivered at 110% of the MT in 90, two-second trains with 20 seconds separating each train over 33 minutes (1800 total pulses per session) for five consecutive days (9000 total pulses per week). In study 3 on maintenance treatment, treatment was delivered for three rather than five days. Patients were asked whether they experienced facial or dental pain, headache, or changes in hearing after every rTMS session. VARL and VARA ratings and the neuropsychological tests were performed before and after every rTMS session to assess whether rTMS had any immediate adverse effect on tinnitus or cognition. The primary outcome measure, however, focused on ratings made only before treatment to avoid any temporary changes in tinnitus perception that might be associated the immediate effects and sensory stimulation of rTMS. Analyses of the primary and secondary outcome measures focused on the percent difference score from the baseline, averaged between ears, in the parent study (i.e., [(baseline − treatment/baseline) ×100] where a negative value indicates a decrease in the VARL and VARA). Difference scores allow for meaningful comparisons across subjects. The baseline score from the parent study establishes a stable reference point across experiments.
Treatment was well tolerated, consistent with most rTMS studies of tinnitus(Langguth, B. et al., 2008), as there were no serious adverse effects and no detrimental changes in tinnitus or neuropsychological function immediately following rTMS. There were no reported changes in hearing. Patients occasionally reported discomfort of the scalp beneath the stimulation coil (approximately 2% of sessions) or felt dental pain (< 1% of sessions). In each case, the coil position was adjusted, either to reposition the center of the coil or to move the wings of the coil away from an irritated area of the scalp, and stimulation continued. No one elected to stop stimulation during a test session. No one withdrew from the study and only two sessions were missed by a subject across all experiments.
4 Experiments
4.1 Targeting the opposite temporal lobe for treatment
Seven patients, three treatment responders and four non-responders, chose to have 1 Hz stimulation of a homologous region in the opposite hemisphere (defined anatomically on their CT scan and targeted using Brainsight). Two patients originally received stimulation of the right and four patients received left hemisphere stimulation before crossing over to stimulation of the opposite hemisphere. The amount of time separating this experiment from completion of the parent study ranged from 11 to 37 days.
4.1.1 Results
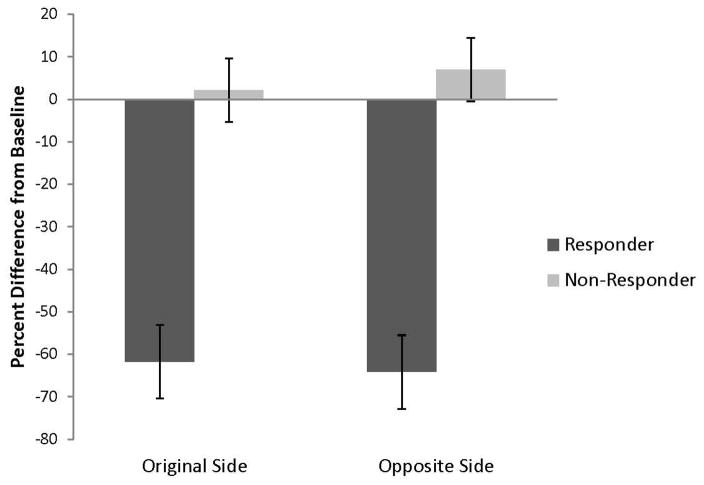
Means of the percent difference scores from baseline for the VARL and VARA were examined using a two factor, mixed model ANOVA. Patient classification (responder/non-responder) was the between subjects factor and site of stimulation (original versus opposite hemisphere) was the within subjects factor. For the VARL, the main effect of classification was significant F (1,5) = 50.92, p<.01. Percent difference scores for the responders were significantly decreased relative to the non-responders (i.e., responder mean (M) = −62.91, standard error (SE) = 7.15, lower confidence limit (LCL) = −81.30, upper confidence limit (UCL) = −44.53; non-responders M = 4.59, SE = 6.19, LCL = −11.33, UCL = 20.51 (Figure 1). The achieved power for this effect was > .99 and the effect size was very large (Cohen’s f = 3.19). In contrast, the effect of original versus opposite hemisphere stimulation was not significant F (1, 5) = .036, p=.86. Achieved power was low = .065 and the effect size was very small (Cohen’s f = .084). Respective means were as follows: original hemisphere M = −29.77, SE = 5.47, LCL = −43.82, UCL = −15.72; opposite hemisphere M = −28.55, SE = 5.98, LCL = −43.91, UCL = −13.19. The interaction of classification and site of stimulation was not significant.
Figure 1.
Bar graph of the mean percent change in the VARL from baseline (with pooled SE bars) for subjects who either responded or did not respond to an original course of 1Hz rTMS treatment. Original side refers to the hemisphere receiving the first course of 1Hz rTMS treatment and opposite side refers to treating the opposite hemisphere. Stimulation of each hemisphere had a similar effect on tinnitus in both responders and non-responders.
Means values for the VARA could not be analyzed using ANOVA because only one responder and two non-responders completed the VARA. Therefore, we report only the mean difference scores for the responder (i.e., original hemisphere M= −64.57; opposite hemisphere M= −33.56) and those for the non-responders (original hemisphere M = 14.86, SE=5.4, LCL = − 55.62, UCL = 83.79; opposite M = 9.63, SE = 8.22, LCL = −94.87, UCL = 114.15).
Because extreme scores can unduly influence the results of ANOVA, especially with small sample sizes, we examined the Studentized residuals resulting from the ANOVA to determine if statistical outliers were present. A Studentized residual is a raw residual divided by an estimate of its standard deviation (i.e., a form of a Student’s t-statistic). Studentized residuals with an absolute value greater than two are considered outliers. We did not observe any outliers. The ranges of Studentized residuals were between −1.53 to 1.25 and −1 to 1 for the VARL and VARA scores, respectively.
4.2 Comparing 1 and 10 Hz stimulation
Five patients elected to receive five days of 10 Hz rTMS treatment at the same site as for 1Hz stimulation. Two patients were treatment responders and two were non-responders. One patient could not be unambiguously defined as a treatment responder according to our criteria so his data was removed from the analysis. This patient’s case is interesting, however and is mentioned further in the discussion. The amount of time separating this experiment and the end of the parent study ranged from 18 to 529 days (1.5 years).
4.2.1 Results
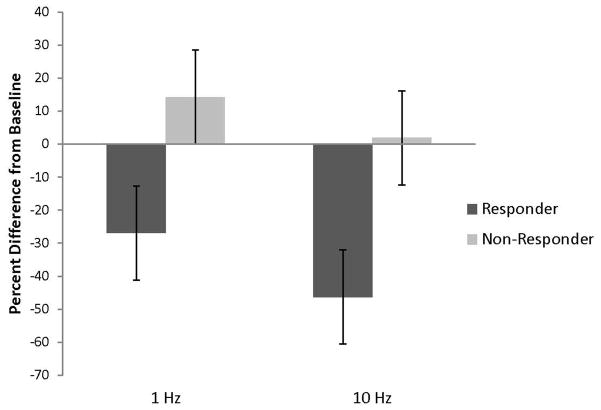
Means of the percent difference scores from baseline for the VARL and VARA were examined using a two factor, mixed model ANOVA. Patient classification (responder/non-responder) was the between subjects factor and stimulation frequency (1 versus 10 Hz) was the within subjects factor. For the VARL, the main effect of classification failed to reach significance F (1,2) = 4.95, p=.16, f=1.57; however, this result was likely due to the small sample size as the achieved power was moderate (.50) and the effect size was very large (Cohen’s f = 1.57) (Figure 2). A power calculation indicated that a sample size of only six subjects would yield power equal to .80 to detect a difference between groups. Numerically, difference scores were decreased for responders relative to non-responders (i.e., responders M = −36.59, SE = 14.22, LCL = −97.78, UCL = 24.60; non-responders M = 8.13, SE = 14.22, LCL = −53.06, UCL = 69.32). Similar outcomes were observed for the within subject effect of stimulation frequency which also failed to reach significance, F (1,2) = 2.10, p=.28, but the achieved power was moderate (.58) and the effect size was large (Cohen’s f = 1.03). A sample size of six would also yield power equal to .80 to detect a within subject difference. Numerically, the difference scores for 10 Hz stimulation were decreased relative to 1 Hz stimulation (10 Hz M = −38.28, SE = 6.97, LCL = −68.26, UCL = −8.23; 1 Hz M = −14.06, SE = 3.66, LCL= −29.82, UCL = 1.69). The interaction of classification and frequency was not significant.
Figure 2.
Bar graph of the mean percent change in the VARL from baseline (with pooled SE bars) for subjects who either responded or did not respond to an original course of 1Hz rTMS treatment. 1Hz refers to results for the initial course of treatment and 10Hz refers treating the same brain site with at 10 Hz frequency. The main effect of stimulation frequency was not significant in this small sample (n=4) but the effect size was large.
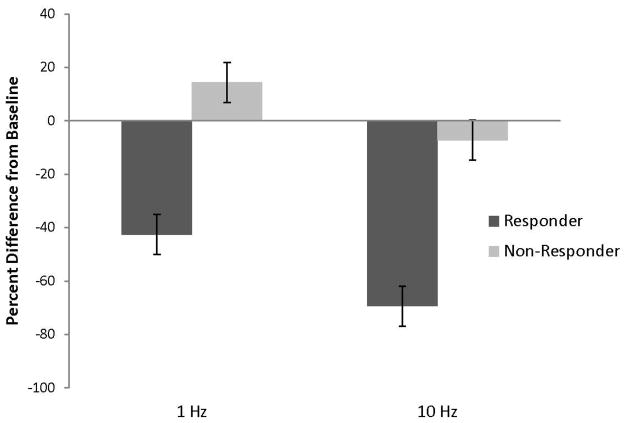
For the VARA, the main effect of classification was significant, F (1,2) = 34.93, p<.05, achieved power was high (>.99), and the effect size was large (Cohen’s f = 1.02) (Figure 3). Difference scores for responders were decreased relative to non-responders (responders M = −55.93, SE=7.12, LCL=−86.56, UCL=−25.29; non-responders M = 3.58, SE = 7.12, LCL = −27.05, UCL = 34.22). The within subject effect of stimulation frequency was also significant, F (1,2) = 26.03, p<.05, observed power was high (>.99), and the effect size was very large (Cohen’s f = 3.62). Difference scores decreased from 1 Hz to 10 Hz stimulation (1 Hz M = −14.06, SE= 3.66, LCL=−29.82, UCL=1.69; 10 Hz M = −38.28, SE=6.97, LCL=−68.26, UCL=−8.23). The interaction of classification and frequency was not significant. Examination of the Studentized residuals, which ranged from −1.16 to 1.16 and from −1.41 to 1.41, respectively, for the VARL or VARA, showed no outliers.
Figure 3.
Bar graph of the mean percent change in the VARA from baseline (with pooled SE bars) for subjects who either responded or did not respond to an original course of 1Hz rTMS treatment. 1Hz refers to results for the initial course of treatment and 10Hz refers treating the same brain site with at 10 Hz frequency. Stimulation at 10 Hz led to significant decrease in perception of tinnitus annoyance.
4.3 Maintenance treatment
Seven patients, all treatment responders, entered maintenance rTMS treatment. These patients were instructed to contact the study coordinator when and if their tinnitus started to worsen after their latest course of treatment. Electronic mail reminders were sent to patients every two weeks. Two patients elected to receive maintenance treatment at 10 Hz and five received maintenance treatment at 1 Hz because they indicated on the Likert ratings that these frequencies worked best. Three daily sessions constituted one course of maintenance treatment.
4.3.1 Results
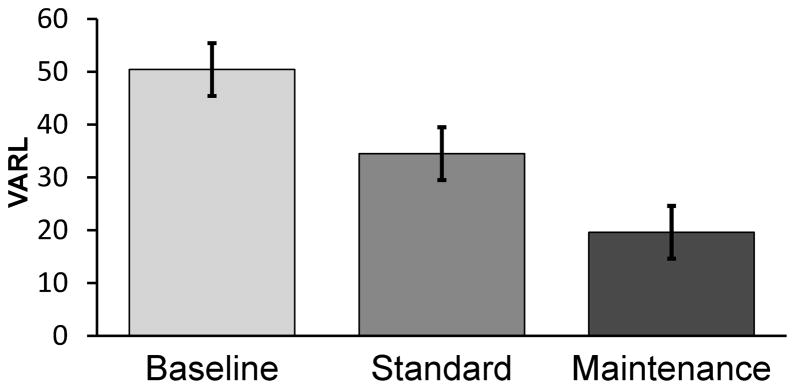
Patients completed between 2 and 6 courses of maintenance treatment over a 9 month observation period. On average, subjects requested retreatment every 2 to 3 months; however, it should be noted that some patients reported delaying retreatment due to schedule constraints. Retreatment was 100% reliable as all subjects reported a drop in tinnitus loudness and annoyance from the beginning to the end of each course of maintenance treatment. On average, the VARL on the last day of a course of treatment fell 32% from baseline (mean of the raw VARL=50, SE=7.3) by the end of the standard treatment week (mean=34, 8.7) and 61% by the end of their last maintenance treatment (mean=19, 4.7) (F=8.7, df=2,12 p<.004) (Cohen’s f=1.26) (Figure 4).
Figure 4.
Bar graph of the mean VARL ratings (with pooled SE bars) for subjects in the maintenance treatment study showing ratings obtained at baseline, on the last day of the initial treatment week (standard), and on the last day of maintenance treatment. Continued maintenance treatment led to a significant decrease in ratings of tinnitus loudness.
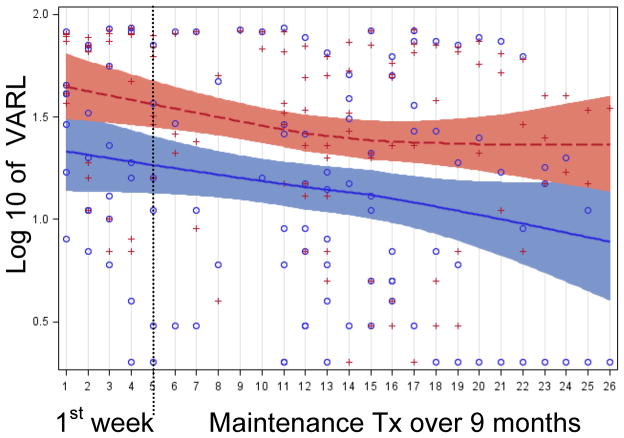
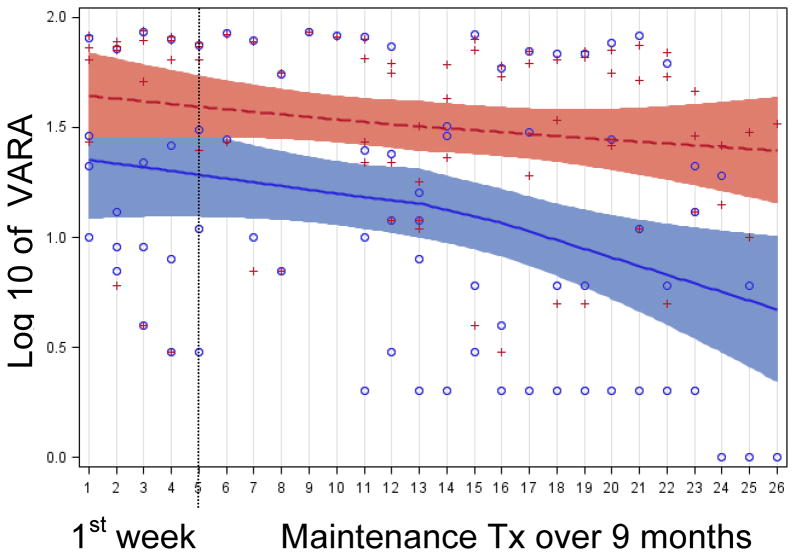
Figure 5 shows a Loess curve fitted to the log-VARL ratings accumulated for every maintenance treatment session and every subject over a total of 21 sessions (Figure 6 shows a Loess curve fitted to the log-VARA ratings). Loess is a hybrid regression technique that fits simple models to localized data subsets to build up a smooth curve through a set of data (i.e., a regression line with 95% cis). Improvement was observed for both ears - ipsilateral and contralateral to the stimulation coil. Further, the effect of maintenance treatment was additive, decreasing by twice as much as the initial week-long course of active treatment, and it was sustained in that tinnitus did not return to the baseline level during maintenance treatment.
Figure 5.
Loess curves fit to the VARL ratings made at each standard and maintenance treatment session. Circles denote ratings for the ear contralateral to stimulation and crosses denote ratings for the ipsilateral ear. The dashed line is the Loess curve for the contralateral ear and the solid line is the Loess curve for the ipsilateral ear. Shaded bands around each Loess curve indicate 95% confidence limits for the Loess curve.
Figure 6.
Loess curves fit to the VARA ratings during standard and maintenance treatment (calculated identical to figure 5).
5 Discussion
We examined different approaches to rTMS treatment for tinnitus to determine whether they could increase either the response rate or duration of treatment effects. Study 1 compared the effects of stimulating homologous regions of both hemispheres using 1 Hz rTMS. Whereas previous studies compared right and left hemisphere stimulation in parallel groups of subjects (Khedr, et al., 2010); study one is unique in using a within subjects design to study treatment responders and non-responders. Results indicated that treating the opposite hemisphere tends to replicate the initial treatment effect, both positive and negative, without converting a non-responder to a responder (or vice versa). If anything, stimulating the opposite hemisphere might have aggravated tinnitus loudness and annoyance temporarily among the non-responders, but tinnitus eventually returned to baseline in all cases. Treating both hemispheres sequentially, however, could be a useful approach for treatment responders as stimulation to both are likely to improve tinnitus.
Study two is also unique for comparing different frequencies of stimulation at the same site within responders and non-responders. In general, patients who responded positively to 1 Hz rTMS over temporal cortex also responded positively to 10 Hz stimulation, and patients who failed to respond to 1 Hz rTMS also failed to respond to 10 Hz stimulation. One exception is the patient who was not included in the analysis because he could not be unambiguously classified as a treatment responder. This patient generally failed to report any changes in tinnitus perception from baseline during the week of 1 or 10 Hz rTMS treatment but he did report a strong positive response the week following 10 Hz stimulation. Another exception was that 10 Hz stimulation may have been more beneficial than 1 Hz for ratings of tinnitus annoyance and possibly loudness. Several limitations of our study might account for this finding. One and ten Hz treatment always occurred in the same order, so the added benefit of 10 Hz treatment could represent a cumulative rather than frequency dependent effect. The period separating treatments was as short as 11 days in one subject, so carry forward effects of 1 Hz treatment could be present. Finally, the sample size was small, so it is uncertain whether the large effect size would be replicated in a larger sample. Alternatively, 10 Hz stimulation might have greater potency than 1 Hz stimulation for some patients. Several findings are consistent with this interpretation. First, we observed during maintenance treatment that 1 Hz stimulation appeared to work better for some subjects and that 10 Hz stimulation worked better for others even though all of the patients responded positively to both low and high frequency stimulation. Second, a similar observation has been reported in a maintenance treatment study of depression that delivered high frequency stimulation over prefrontal cortex (O’Reardon, et al., 2005). One patient who had a suboptimal response to 10 Hz rTMS subsequently responded to 20 Hz stimulation. Frequency dependent responses to rTMS treatment for depression have also been reported, within subjects, in group studies (Kimbrell, et al., 1999; Speer, et al., 2000). Finally, a group study that compared tinnitus patients who received different frequencies of rTMS found that 1, 10 and 25 Hz stimulation could all decrease tinnitus but that 10 Hz stimulation may have had greater potency (Khedr, et al., 2008). These findings are important because they suggest that a patient who fails to respond to one frequency of stimulation may respond to another. As outlined below, these findings are also important for theoretical reasons.
Finding that 1 and 10Hz rTMS can have similar, long term treatment effects on tinnitus challenges our understanding of how rTMS induces a treatment effect. Whereas immediate effects of rTMS, which last seconds to minutes after stimulation, appear to be frequency dependent (George, et al., 2002), treatment effects which lasts for weeks and months may not. A frequency dependent model, however, is not adequate to explain how low and high frequencies of rTMS induce similar rather than opposite behavioral effects on tinnitus perception. A more appropriate model will need to account for how different frequencies of rTMS can have similar rather than opposite behavioral effects.
Study three is the only study, to our knowledge, that applied maintenance rTMS in a series of patients with tinnitus; however, the findings replicate those of studies using maintenance rTMS for other psychiatric and neurological disorders. Maintenance treatment, at both 1 and 10 Hz, was well tolerated and effective for persons who responded positively to an initial course of treatment. The effect of maintenance treatment was additive over a standard course of treatment and the benefits were sustained over time. On average, subjects requested additional maintenance treatment every two to three months; however, we recommend that future clinical trials incorporate fixed treatment schedules, perhaps once per month, with fixed observation periods between treatment that are sufficiently long (e.g., 3–4 weeks) to determine if maintenance treatment has additive and sustained benefits over time. We found, as well, that unilateral maintenance treatment was beneficial for tinnitus perception bilaterally even though ratings were consistently lower for the ear ipsilateral rather than contralateral to stimulation (tinnitus was typically worse for the contralateral ear at the start of the study).
Our studies share the limitations of other open label trials. Lacking subject and experimenter blinds, these studies may be biased toward finding positive results. Lacking controls, these studies cannot be considered conclusive but can only show feasibility. Although we attempted to recruit equal numbers of subjects who either responded or did not respond positively to rTMS, selection biases could still be present as subjects elected to participate in further experiments after receiving rTMS. Finally, a combination of selection bias and a small sample size could exaggerate findings if subjects are not truly representative of the target population. These concerns can only be resolved by conducting larger, controlled trials - a view expressed in nearly every manuscript on maintenance rTMS. Limitations notwithstanding, our pilot studies indicate that future controlled, clinical trials would benefit from defining treatment responders and non-responders, empirically, in order to learn if subtypes of patients exist who may be more or less likely to respond to rTMS. The efficacy of rTMS could then be examined in light of these subtypes. Future trials would also benefit from incorporating within-subject designs that comparing the efficacy of different frequencies of rTMS for tinnitus. Our data suggests that patients may respond differently to different frequencies of stimulation. For example, tinnitus duration might interact with stimulation frequency as indicated in one study (De Ridder, D. et al., 2005). Finally, future clinical trials can improve the treatment potential of rTMS by focusing on ways to increase treatment duration. Maintenace rTMS may be particularly useful in this regard for patients who respond to an initial course of active rTMS. Our maintenance treatment study parallels other maintenance treatment studies with regard to the ambiguity over how maintenance treatment should be applied. There is no uniformity. Most studies applied several sessions per week either as a response to symptom relapse [see for example (Fitzgerald, Paul B. et al., 2006)] or as a regularly scheduled treatment that might maintain the benefit of previous treatments. We chose 3 sessions per week, as needed, because this schedule appeared sufficient to replicate the treatment effect and because we wanted to learn how often retreatment would be necessary. We think the advantages of a relapse treatment approach are that fewer applications of rTMS are required and that the maintenance schedule can be individually tailored. Disadvantages of this approach, however, are that subjects tend to “put off” retreatment, which may make maintenance less effective, and it is difficult to evaluate treatment efficacy when schedules are not consistent across subjects. Alternatively, regularly scheduled maintenance treatments may have the advantages of greater efficacy by not allowing symptoms to return to a previous level, by better monitoring due to regular patient contact, and by ease of analyzing outcome. Our experience suggests that retreating patients several times a week, e.g., every 3 to 6 weeks, may be a reasonable starting point for maintenance treatment in tinnitus.
6 Conclusions
The rTMS induced effect on tinnitus does not appear to be hemisphere specific or frequency dependent though different frequencies of stimulation may have greater potency for some subjects than for others. Subjects who respond positively to an initial course of rTMS treatment may get additive and sustained benefit from maintenance treatment. Administering maintenance treatment every 3 to 6 weeks would appear to be a reasonable treatment schedule; however, additional studies are needed to validate this conclusion.
Highlights.
Maintenance rTMS has added benefit for tinnitus among treatment responders.
The rTMS-effect on tinnitus was not hemisphere specific or frequency dependent.
Maintenance treatment every 3 to 6 weeks can be a reasonable treatment schedule.
Future controlled trials of maintenance rTMS treatment for tinnitus are warranted.
Acknowledgments
This study was supported by the NIH: National Center for Research Resources Centers of Biomedical Research Excellence (COBRE) Grant Number RR20146 and the NCRR grant UL1RR029884; National Institute of Deafness and other Communication Disorders DC011824; National Institute of Child Health and Human Development HD055677 and HD055269; and by a Tinnitus Research Consortium Grant-in-Aid. The funding source(s) had no involvement in designing the study, in data collection, analysis and interpretation, or in writing or deciding to submit the article for publication.
Abbreviations
- VARL
visual analogue rating of loudness
- VARA
visual analogue rating of annoyance
- rTMS
repetitive transcranial magnetic stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benadhira R, Saba G, Samaan A, Dumortier G, Heloise L, Gastal D, Kalalou K, Verdon C, Januel D. Transcranial magnetic stimulation for refractory depression. American Journal of Psychiatry. 2005;162:193. doi: 10.1176/appi.ajp.162.1.193. [DOI] [PubMed] [Google Scholar]
- Bohning DE. Introduction and overview of TMS physics. In: George MS, Belmaker RH, editors. Transcranial magnetic stimulatioin in neuropsychiatry. Washington, D.C: American Psychiatric Press; 2000. pp. 13–44. [Google Scholar]
- De Ridder D, Verstraeten E, Kelen K, De Mulder G, Sunaert S, Verlooy J, Heyning P, Moller A. Transcranial magnetic stimulation for tinnitus: influence of tinnitus duration on stimulation parameter choice and maximal tinnitus suppression. Otol Neurotol. 2005;26:616–619. doi: 10.1097/01.mao.0000178146.91139.3c. [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Mundo E, D’Urso N, Possoli S, Buoli M, Ciabatti M, Rosanova M, Massimini M, Bellina V, Mariotti M, Carlo Altamura A. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug resistant bipolar depression. Bipolar Disorders. 2009;11:76–81. doi: 10.1111/j.1399-5618.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Mechanic-Hamiltion D, Press DZ, Pearlman C, Stern WM, Thall M, Pascual-Leone A. An open label, prospective study of repetitive transcranial magnetic stimulation (rTMS) in the long term treatment of refractory deperssion: reproducibility and duration of the antidepressant effect in medication free-patients. Journal of Clincal Psychiatry. 2008;69:930–934. doi: 10.4088/jcp.v69n0607. [DOI] [PubMed] [Google Scholar]
- Dornhoffer J, Mennemeier M. Repetative transcranial magnetic stimulation as a treatment of tinnitus. The Hearing Journal. 2010;63:16–18. doi: 10.1097/01.HJ.0000390816.71876.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Rothwell JC, ElA One-year follow up of patients with chronic tinnitus treated with left temporoparietal rTMS. European Journal of Neurology. 2009;16:404–408. doi: 10.1111/j.1468-1331.2008.02522.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Benitez J, De Castella AR, Brown TL, Daskalakis ZJ, Kulkarni J. Naturalistic study of the use of transcranial magnetic stimulation in the treatment of depressive relapse. Australian and New Zealand Journal of Psychiatry. 2006;40:764–768. doi: 10.1080/j.1440-1614.2006.01881.x. [DOI] [PubMed] [Google Scholar]
- George M, Nahas Z, Kozel A, Xingbao L, Denslow S, Yamanaka K, Mishory A, Foust MJ, Bohning DE. Mechanisms and state of the art of transcranial magnetic stimulation. The Journal of ECT. 2002;18:170–181. doi: 10.1097/00124509-200212000-00002. [DOI] [PubMed] [Google Scholar]
- Kakuda W, Abo M, Uruma G, Kaito N, Watanabe M. Low-frequency rTMS with language therapy over a 3-month period for sensory-dominant aphasia: Case series of two post-stroke Japanese patients. Brain Injury. 2010;24:1113–1117. doi: 10.3109/02699052.2010.494587. [DOI] [PubMed] [Google Scholar]
- Khedr E, Rothwell J, Ahmed M, El-Atar A. Effect of daily repetitive transcranial magnetic stimulation for treatment of tinnitus: comparison of different stimulus frequencies. J Neurol Neurosurg Psychiatry. 2008;79:212–215. doi: 10.1136/jnnp.2007.127712. [DOI] [PubMed] [Google Scholar]
- Khedr EM, bo-Elfetoh N, Rothwell JC, El-Atar A, Sayed E, Khalifa H. Contralateral versus ipsilateral rTMS of temporoparietal cortex for the treatment of chronic unilateral tinnitus: comparative study. European Journal of Neurology. 2010;17:976–983. doi: 10.1111/j.1468-1331.2010.02965.x. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EW, Repella JD, Danielson AL, Willis MW, Benson BE, Speer AM, Osuch E, George MS, Post RM. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS): a function of baseline cerebral glucose metabolism. Biological Psychiatry. 1999;46:603–613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- Kleinjung T, Steffens T, Londero A, Langguth B. Which tinnitus patients benefit from Transcranial magnetic stimulation? Otolaryngol Head Neck Surg. 2007;137:589–595. doi: 10.1016/j.otohns.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Kuk FK, Tyler RS, Russell D, Jordan H. The psychometric properties of a tinnitus handicap questionnaire. Ear & Hearing. 1990;11:434–445. doi: 10.1097/00003446-199012000-00005. [DOI] [PubMed] [Google Scholar]
- Langguth B, De Ridder D, Dornhoffer J, Eichhammer P, Folmer RL, Frank E, Fregni F, Gerloff C, Khedr E, Kleinjung T, Landgrebe M, Lee S, Lefaucheur JP, Londero A, Marcondes R, Moller AR, Pascual A, Plewnia C, Rossi S, Sanchez T, Sand P, Schlee W, Steffens T, van dHP, Hajak G. Controversy: Does repetitive transcranial magnetic stimulation/transcranial direct current stimulation show efficacy in treating tinnitus patients? Brain Stimulation. 2008;1:192–205. doi: 10.1016/j.brs.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Langguth B, Eichhammer P, Wiegand R, Marienhegen J, Maenner P, Jacob P, Hajak G. Neuronavigated rTMS in a patient with chronic tinnitus. Effects of 4 weeks treatment. Neuro Report. 2003;14:977–980. doi: 10.1097/00001756-200305230-00014. [DOI] [PubMed] [Google Scholar]
- Langguth B, Kleinjung T, Landgrebe M, De Ridder D, Hajak G. rTMS for the treatment of tinnitus: The role of neuronavigation for coil positioning. Neurophysiologie Clinique/Clinical Neurophysiology. 2010;40:45–58. doi: 10.1016/j.neucli.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Li X, Nahas Z, Anderson B, Kozel FA, George MS. Can left prefrontal rTMS be used as a maintenance treatment for bipolar depression? Depression and Anxiety. 2004;20:98–100. doi: 10.1002/da.20027. [DOI] [PubMed] [Google Scholar]
- Loo CK, Mitchell PB, McFarquahar TF, Malhi GS, Sachdev PS. A sham-controlled trial of the efficacy and safety of twice-daily rTMS in major depression. Psychol Med. 2007;37:341–349. doi: 10.1017/S0033291706009597. [DOI] [PubMed] [Google Scholar]
- Mennemeier M, Chelette KC, Allen S, Bartel TB, Triggs W, Kimbrell T, Crew J, Munn T, Brown GJ, Dornhoffer J. Variable changes in PET activity before and after rTMS treatment for tinnitus. The Laryngoscope. 2011;121:815–822. doi: 10.1002/lary.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennemeier M, Chelette KC, Myhill J, Taylor-Cooke P, Bartel T, Triggs W, Kimbrell TA, Dornhoffer J. Maintenance Repetitive Transcranial Magnetic Stimulation Can Inhibit the Return of Tinnitus. Laryngoscope. 2008;118:1228–1232. doi: 10.1097/MLG.0b013e318170f8ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennemeier MS, Dornhoffer J. In Reference to Maintenance Repetitive Transcranial Magnetic Stimulation can Inhibit the Return of Tinnitus. The Laryngoscope. 2008;118:2264–2265. doi: 10.1097/MLG.0b013e318170f8ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reardon JP, Fontecha JF, Cristancho MA, Newman S. Unexpected reduction in migraine and psychogenic headache following rTMS treatment for major depression: a report of two cases. CNS Spectr. 2007;12:921–925. doi: 10.1017/s1092852900015716. [DOI] [PubMed] [Google Scholar]
- O’Reardon JPBKH, Peshek AD, Pradilla RR, Pimiento PC. Long-term maintenance therapy for major depressive disorder with rTMS. Journal of Clincal Psychiatry. 2005;66:1524–1528. doi: 10.4088/jcp.v66n1205. [DOI] [PubMed] [Google Scholar]
- Piccirillo JF, Garcia KS, Nicklaus J, Pierce K, Burton H, Vlassenko AG, Mintun M, Duddy D, Kallogjeri D, Spitznagel EL., Jr Low-Frequency Repetitive Transcranial Magnetic Stimulation to the Temporoparietal Junction for Tinnitus. Archives of Otolaryngology--Head & Neck Surgery. 2011;137:221–228. doi: 10.1001/archoto.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewnia C, Reimold M, Najib A, Reischl G, Plontke SK, Gerloff C. Moderate therapeutic efficacy of PET-navigated transcranial magnetic stimulation against chronic tinnitus: a randomised, controlled pilot study. J Neurol Neurosurg Psychiatry. 2007;78:152–156. doi: 10.1136/jnnp.2006.095612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet EMM, Brunelin JER, Kallel LAS, D’Amato THI, Saouid MOHA. Maintenance Treatment With Transcranial Magnetic Stimulation in a Patient With Late-Onset Schizophrenia. American Journal of Psychiatry. 2008;165:537–538. doi: 10.1176/appi.ajp.2007.07060868. [DOI] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, Farwell WR. Prevalence and Characteristics of Tinnitus among US Adults. The American Journal of Medicine. 2010;123:711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Speer AM, Kimbrell TA, Wasserman EM, Repella JD, Willis MW, Hajak G, Herscovitch P, Post RM. Opposite effects of high and low frequency rTMS on regional brain activity in mood disorder patients. Biological Psychiatry. 2000;48:1133–1144. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. New York: Oxford University Press; 1991. [Google Scholar]
- Thirthalli J, Bharadwaj B, Kulkarni S, Kharawala S, Andrade C, Gangadhar BN. Successful use of maintenance rTMS for 8-ámonths in a patient with antipsychotic-refractory auditory hallucinations. Schizophrenia Research. 2008;100:351–352. doi: 10.1016/j.schres.2008.01.003. [DOI] [PubMed] [Google Scholar]