Abstract
Background
The interpretation of uterine cancer rates is hindered by the inclusion of women whose uterus has been surgically removed in the population at risk. Hysterectomy prevalence varies widely by state and race/ethnicity, exacerbating this issue.
Methods
We estimated hysterectomy-corrected, age-adjusted uterine corpus cancer incidence rates by race/ethnicity for 49 states and the District of Columbia during 2004-2008 using case counts obtained from population-based cancer registries; population data from the U.S. Census Bureau; and hysterectomy prevalence data from the Behavioral Risk Factor Surveillance System. Corrected and uncorrected incidence rates were compared with regard to geographic and racial/ethnic disparity patterns and the association with obesity.
Results
Among non-Hispanic whites, uterine cancer incidence rates (per 100,000 woman-years) uncorrected for hysterectomy prevalence ranged from 17.1 in Louisiana to 32.1 in New Jersey, mirrored regional hysterectomy patterns, and were not correlated with obesity prevalence (Pearson’s correlation coefficient, r = 0.06, two-sided p = 0.68). In comparison, hysterectomy-corrected rates were higher by 30% (District of Columbia) to more than 100% (Mississippi, Louisiana, Alabama, and Oklahoma), displayed no discernible geographic pattern, and were moderately associated with obesity (r = 0.37, two-sided p = 0.009). For most states, hysterectomy correction diminished or reversed the black/white deficit and accentuated the Hispanic/white deficit.
Conclusion
Failure to adjust uterine cancer incidence rates for hysterectomy prevalence distorts true geographic and racial patterns and substantially underestimates the disease burden, particularly for Southern states.
Impact
Correction for hysterectomy is necessary for the accurate evaluation of uterine cancer rates.
Keywords: endometrium, uterus, hysterectomy, geographic pattern, disparity
Introduction
Women whose uterus has been surgically removed are not at risk for cancer of the uterus. Routine reporting of incidence rates for uterine cancer does not take hysterectomy prevalence into account [1, 2], thus underestimating the true burden of disease among those at risk [3-7]. Previous studies in the U.S. using data from Surveillance, Epidemiology, and End Results (SEER) cancer registries have shown that uterine corpus cancer incidence rates corrected for hysterectomy prevalence are 53% to 67% higher than uncorrected rates [5, 7].
Hysterectomy is one of the most frequently performed surgeries in the United States. Approximately 600,000 women undergo the procedure each year, with utilization highest among women ages 35 to 49 years [8-10]. Rates vary widely by region and are almost 50% higher in the South than in the Northeast [9, 10]. Hysterectomy prevalence also differs by race and ethnicity [11, 12]. A recent analysis of the population-based CARDIA cohort found that black women were three times more likely to have had a hysterectomy than white women [13].
It is likely that differences in hysterectomy prevalence confound observed variations in uterine cancer incidence rates. As generally reported, uterine corpus cancer incidence rates appear higher among white women than among black or Hispanic women [2]. Sherman et al. have shown that correcting for hysterectomy prevalence attenuates racial disparities in uterine endometrial cancer [7]. Similarly, it is likely that state variation in uncorrected incidence rates to some extent reflects differences not in cancer occurrence but in hysterectomy utilization.
Geographic patterns of cancer occurrence provide clues for etiologic study vis á vis the relationship between acquired (versus hereditary) factors and the neoplastic pathway. The increased coverage of cancer surveillance in the U.S. in recent years expands this potential. However, the interpretation of reported uterine cancer incidence rates is hindered by the inclusion of large numbers of women without an intact uterus in the population at risk. Therefore, we estimated state-level uterine corpus (i.e., excluding cervix, hereafter uterine) cancer incidence rates corrected for hysterectomy prevalence to reveal the true geographic and racial/ethnic patterns of disease and provide a more accurate representation of the current cancer burden.
Materials and Methods
We identified invasive uterine cancer cases among non-Hispanic white (henceforth abbreviated as white), black, and Hispanic women diagnosed during 2004 to 2008 using incidence data obtained from the North American Association of Central Cancer Registries (NAACCR) for 49 states and the District of Columbia. NAACCR certifies population-based central cancer registries participating in the National Cancer Institute’s Surveillance, Epidemiology, and End Results program and/or the Centers for Disease Control and Prevention’s National Program of Cancer Registries, and aggregates and distributes surveillance data submitted by the registries for epidemiologic research [14, 15]. Consistent with NAACCR and SEER convention, incidence rates are presented as an aggregate over the most recent five years of available data at the time of the study, in this case 2004 through 2008, for increased stability. Age-specific case counts for site codes C54.0-C54.9 (corpus uterus) and C55.9 (uterus, not otherwise specified) (according to the International Classification of Diseases for Oncology, 3rd edition) [16] were retrieved using SEER*Stat software version 7.0.5 [17]. SEER*Stat contains a modified version of the annual time series July 1 county population estimates by sex, age, race, and Hispanic origin as provided by the U.S. Census Bureau [18].
Incidence data for Maryland, Nevada, and the District of Columbia are limited by availability and/or quality based on NAACCR criteria for high quality during one or more years during 2004 to 2008. Specifically, cancer incidence rates presented for the District of Columbia are for diagnosis years 2004 to 2007 and rates for Nevada are for diagnosis years 2004 to 2006; rates for Maryland were not available for any year during 2004 to 2008. Incidence rates for Alabama, Louisiana, Mississippi, and Texas exclude data from July through December 2005 because of substantial migrations of populations as a result of Hurricane Katrina in September 2005. Incidence data for white women in Wisconsin were not available exclusive of Hispanic origin. According to U.S. Census Bureau population estimates, approximately 4.4% of white females residing in Wisconsin during 2004-2008 were Hispanic.
State hysterectomy prevalence (available for women 18 years of age and older) by age and race/ethnicity for survey years 2004, 2006, and 2008 was obtained from the Behavioral Risk Factor Surveillance System (BRFSS) [19]; the three years of data were aggregated to increase the stability of the estimates, particularly for black and Hispanic women. The BRFSS is an ongoing, state-based, random digit-dialed telephone survey of the non-institutionalized population of adults (aged > 18 year) in the US. BRFSS collects self-reported information on health risk behaviors and use of health services. Hysterectomy status was determined by a woman’s positive answer to the question ‘Have you had a hysterectomy’? Statistical software was used to account for the complex sampling design (SUDAAN, release 9.2; Research Triangle Institute, Research Triangle Park, North Carolina) and to derive weighted prevalence estimates and standard errors.
Uterine cancer incidence rates were corrected by reducing the population at risk (the denominator) by the hysterectomy prevalence for each of eight age groups (<20, 20-29, 30-39, 40-49, 50-59, 60-69, 70-79, and > 80 years) for whites and five age groups (<20, 20-39, 40-59, 60-79, >80 years) for blacks and Hispanics. Corrected age-specific rates were then used to calculate overall rates, age-adjusted to the 2000 U.S. standard population. Age-adjusted rates uncorrected for hysterectomy prevalence were calculated using the same method for comparison purposes. States with a BRFSS sample size < 500 or fewer than 20 incident cancer cases, which occurred only for blacks and Hispanics, were not presented. After exclusions, we analyzed data for 157,890 incident cases of uterine cancer diagnosed among white women in 49 states and the District of Columbia during 2004 to 2008; 17,588 cases among black women in 27 states and the District of Columbia; and 12,361 cases among Hispanic women in 18 states. Rates for the total U.S. included all states with the following exceptions: Maryland because of unavailable data (see above); Nevada because data for 2008 were not available and data for 2007 were not high-quality based on NAACCR criteria; and the District of Columbia because data for 2008 were not high-quality based on NAACCR criteria. Wisconsin was excluded from the total U.S. rate for whites and Hispanics due to the lack of Hispanic origin data.
United States maps were generated to compare uncorrected and corrected rates for whites using ArcGIS software, version 10.0 [20]. States were categorized using the Jenks optimization method, which identifies natural breaks in the data set such that the variance is minimized within groups and maximized between groups. Because obese women have a uterine cancer risk two to three times that of normal weight women [21], incidence rates for whites were tested for an association with state obesity prevalence (defined as body mass index (BMI) of >30 kg/m2) using data for non-Hispanic white women 18 years and older obtained from the BRFSS for survey years 1994-1995 and Pearson’s correlation coefficient weighted by the state non-Hispanic white female population. Changes in the risk of uterine cancer among black and Hispanic women relative to white women as a result of hysterectomy correction were quantified using rate ratios with 95% confidence intervals as estimated by the delta method [22].
Results
As expected, hysterectomy prevalence varied considerably across states and was lowest in the Northeast and highest in the South for all three racial/ethnic groups considered in our analysis. This variation was stronger for whites than for blacks or Hispanics (Table 1). For example, there was a three-fold difference between the lowest (10.1% in the District of Columbia) and highest (31.4% in Alabama) hysterectomy prevalence among whites, compared to a two-fold difference among blacks (15.1% in New York versus 28.4% in Alabama). Although in general blacks were more likely than whites to have had a hysterectomy, in many Southern states prevalence was higher among whites. Hispanic women were generally least likely to have had a hysterectomy.
Table 1.
Uterine corpus cancer incidence rates before and after correction for hysterectomy prevalence by state and race/ethnicity, 2004-2008
| Non-Hispanic White |
Black |
Hispanic |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hysterectomy prevalence |
Uncorrected rate |
Corrected rate |
Percent increase |
Hysterectomy prevalence |
Uncorrected rate |
Corrected rate |
Percent increase |
Hysterectomy prevalence |
Uncorrected rate |
Corrected rate |
Percent increase |
||||
| State | SE | SE | SE | ||||||||||||
| Alabama | 31.4 | 0.62 | 18.4 | 38.4 | 108.8% | 28.4 | 1.05 | 20.4 | 40.8 | 100.6% | |||||
| Alaska | 21.5 | 0.97 | 23.6 | 40.4 | 71.4% | ||||||||||
| Arizona | 22.1 | 0.69 | 19.3 | 33.9 | 75.9% | 21.2 | 1.74 | 16.1 | 26.9 | 66.9% | |||||
| Arkansas | 28.8 | 0.53 | 18.7 | 36.0 | 92.6% | 26.0 | 1.33 | 20.0 | 40.0 | 100.0% | |||||
| California | 16.6 | 0.41 | 24.3 | 37.6 | 54.8% | 21.1 | 1.73 | 20.3 | 36.5 | 80.0% | 15.7 | 0.90 | 18.4 | 26.5 | 44.1% |
| Colorado | 21.1 | 0.40 | 20.1 | 34.5 | 72.2% | 23.0 | 1.14 | 16.1 | 27.1 | 68.2% | |||||
| Connecticut | 13.0 | 0.32 | 29.6 | 40.8 | 38.0% | 17.3 | 1.53 | 26.2 | 41.8 | 59.6% | 13.8 | 1.42 | 21.8 | 27.7 | 27.4% |
| Delaware | 16.0 | 0.59 | 29.0 | 42.4 | 46.3% | 18.8 | 1.86 | 28.0 | 41.3 | 47.6% | |||||
| Washington DC* | 10.1 | 0.54 | 17.2 | 22.4 | 29.9% | 17.4 | 0.71 | 26.4 | 40.7 | 54.2% | |||||
| Florida | 21.3 | 0.44 | 21.3 | 35.7 | 67.2% | 23.5 | 1.28 | 24.1 | 44.5 | 84.8% | 15.6 | 1.02 | 21.8 | 30.1 | 38.1% |
| Georgia | 24.8 | 0.53 | 18.6 | 34.3 | 84.2% | 24.5 | 0.92 | 19.3 | 38.3 | 98.4% | |||||
| Hawaii | 13.5 | 0.65 | 25.4 | 35.8 | 41.3% | ||||||||||
| Idaho | 25.8 | 0.46 | 22.7 | 45.3 | 99.2% | ||||||||||
| Illinois | 16.6 | 0.40 | 28.0 | 41.8 | 49.4% | 22.0 | 1.23 | 23.3 | 41.9 | 79.8% | 17.4 | 1.97 | 21.1 | 30.1 | 42.8% |
| Indiana | 22.3 | 0.43 | 26.3 | 43.6 | 65.5% | 22.7 | 1.52 | 20.5 | 38.3 | 87.0% | |||||
| Iowa | 18.0 | 0.38 | 28.6 | 43.3 | 51.3% | ||||||||||
| Kansas | 24.4 | 0.34 | 23.3 | 40.9 | 75.5% | 25.4 | 1.76 | 22.2 | 42.5 | 91.6% | 22.2 | 1.62 | 23.3 | 39.5 | 69.3% |
| Kentucky | 25.6 | 0.46 | 24.4 | 43.8 | 79.8% | 22.7 | 2.22 | 20.3 | 35.5 | 74.5% | |||||
| Louisiana | 30.0 | 0.45 | 17.1 | 36.0 | 110.5% | 25.8 | 0.77 | 18.5 | 33.8 | 82.2% | |||||
| Maine | 18.1 | 0.42 | 31.2 | 46.9 | 50.3% | ||||||||||
| Maryland† | 16.6 | 0.38 | - | - | - | 21.0 | 0.90 | - | - | - | - | - | - | - | |
| Massachusetts | 12.2 | 0.27 | 30.3 | 40.7 | 34.2% | 19.6 | 1.43 | 23.9 | 47.1 | 96.7% | 14.2 | 1.07 | 25.9 | 34.4 | 32.9% |
| Michigan | 18.6 | 0.38 | 28.1 | 42.9 | 52.7% | 21.9 | 1.24 | 24.0 | 41.7 | 73.8% | |||||
| Minnesota | 16.2 | 0.39 | 27.8 | 40.6 | 46.3% | ||||||||||
| Mississippi | 31.1 | 0.51 | 18.7 | 39.7 | 112.4% | 25.3 | 0.70 | 23.4 | 41.7 | 78.4% | |||||
| Missouri | 20.8 | 0.52 | 24.8 | 39.8 | 60.5% | 25.3 | 1.91 | 20.7 | 37.7 | 82.0% | |||||
| Montana | 21.2 | 0.42 | 23.9 | 40.0 | 67.7% | ||||||||||
| Nebraska | 20.7 | 0.34 | 26.7 | 44.2 | 65.6% | 20.7 | 1.90 | 20.9 | 37.5 | 79.9% | |||||
| Nevada‡ | 21.8 | 0.70 | 19.0 | 33.2 | 74.4% | 18.6 | 2.08 | 16.3 | 25.2 | 54.4% | |||||
| New Hampshire | 15.4 | 0.34 | 29.9 | 43.0 | 43.8% | ||||||||||
| New Jersey | 11.4 | 0.24 | 32.1 | 42.4 | 32.2% | 17.2 | 0.79 | 23.0 | 37.0 | 61.1% | 13.8 | 0.85 | 21.5 | 28.9 | 34.2% |
| New Mexico | 20.9 | 0.49 | 22.1 | 36.5 | 65.0% | 19.6 | 0.65 | 17.7 | 27.2 | 54.0% | |||||
| New York | 13.2 | 0.33 | 31.0 | 42.3 | 36.5% | 15.1 | 1.05 | 27.0 | 38.2 | 41.5% | 15.5 | 1.30 | 24.0 | 34.6 | 44.1% |
| North Carolina | 22.5 | 0.30 | 22.1 | 37.8 | 70.8% | 24.9 | 0.63 | 22.0 | 41.2 | 87.1% | 15.1 | 1.50 | 12.4 | 16.8 | 35.1% |
| North Dakota | 19.7 | 0.48 | 26.8 | 41.7 | 55.5% | ||||||||||
| Ohio | 19.8 | 0.59 | 28.2 | 44.1 | 56.1% | 25.5 | 1.70 | 20.5 | 39.5 | 92.4% | |||||
| Oklahoma | 27.5 | 0.41 | 19.9 | 40.5 | 103.0% | 26.0 | 1.47 | 18.1 | 36.1 | 99.4% | 23.5 | 2.02 | 21.8 | 41.7 | 91.0% |
| Oregon | 22.0 | 0.43 | 24.4 | 42.8 | 75.4% | ||||||||||
| Pennsylvania | 17.3 | 0.34 | 31.7 | 47.4 | 49.3% | 19.8 | 1.34 | 22.3 | 37.1 | 66.6% | |||||
| Rhode Island | 15.4 | 0.41 | 30.6 | 43.2 | 41.0% | ||||||||||
| South Carolina | 25.3 | 0.43 | 19.3 | 35.8 | 85.2% | 27.3 | 0.84 | 23.3 | 46.4 | 99.5% | |||||
| South Dakota | 19.1 | 0.38 | 24.3 | 38.0 | 56.2% | ||||||||||
| Tennessee | 26.3 | 0.53 | 19.8 | 36.6 | 85.0% | 27.5 | 1.52 | 17.3 | 33.6 | 93.7% | |||||
| Texas | 25.6 | 0.61 | 18.8 | 36.0 | 91.1% | 27.9 | 1.50 | 17.8 | 36.6 | 105.4% | 20.2 | 0.82 | 18.3 | 27.7 | 51.0% |
| Utah | 25.0 | 0.50 | 22.4 | 43.1 | 92.2% | 19.1 | 2.03 | 15.9 | 22.7 | 43.1% | |||||
| Vermont | 14.0 | 0.30 | 32.0 | 43.5 | 36.0% | ||||||||||
| Virginia | 17.8 | 0.49 | 23.7 | 36.1 | 52.6% | 24.1 | 1.22 | 19.6 | 37.1 | 89.3% | |||||
| Washington | 20.0 | 0.22 | 24.7 | 41.0 | 65.6% | 17.4 | 1.25 | 15.3 | 22.5 | 47.2% | |||||
| West Virginia | 23.1 | 0.49 | 29.1 | 48.0 | 64.8% | ||||||||||
| Wisconsin§ | 17.3 | 0.43 | 27.7 | 41.2 | 48.5% | 21.7 | 1.75 | 24.2 | 46.4 | 91.4% | |||||
| Wyoming | 25.3 | 0.44 | 20.9 | 38.2 | 83.0% | ||||||||||
| US¶ | 19.9 | 0.08 | 25.1 | 40.2 | 60.5% | 23.0 | 0.28 | 21.8 | 38.9 | 78.4% | 17.3 | 0.39 | 19.5 | 28.7 | 46.9% |
Incidence rates (all ages) and hysterectomy prevalence (ages 20+) were age-adjusted to the 2000 US standard population.
States were excluded if case counts were < 20 or BRFSS sample size was < 500.
Incidence rate based on cases diagnosed during 2004 to 2007.
Incidence data unavailable.
Incidence rate based on cases diagnosed during 2004 to 2006.
Incidence rate for non-Hispanic whites not exclusive of Hispanic origin.
Incidence rates exclude DC, MD, and NV; rates for non-Hispanic whites and Hispanics also exclude WI.
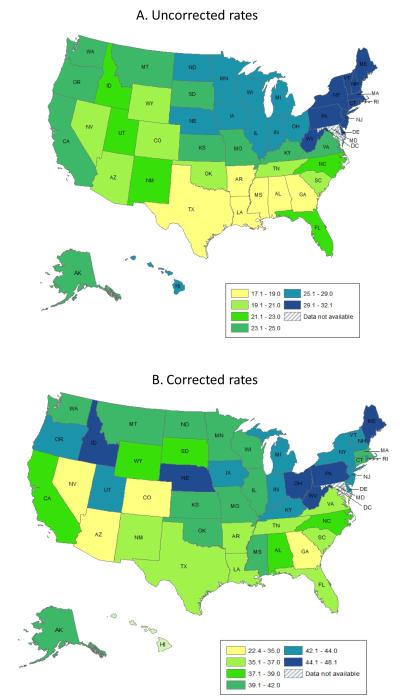
Among white women, age-adjusted uterine cancer incidence rates (per 100,000 woman-years) uncorrected for hysterectomy prevalence ranged from 17.1 in Louisiana to 32.1 in New Jersey (Table 1), with the highest rates concentrated in the Northeast and the lowest rates in the South (Figure 1A); this incidence pattern was not associated with obesity prevalence (r = 0.06; p = 0.68). The increase in incidence rates after accounting for hysterectomy prevalence ranged from 30% in DC to greater than100% in Oklahoma, Alabama, Louisiana, and Mississippi (Table 1). In contrast to uncorrected rates, corrected rates exhibited no discernible geographic pattern (Figure 1B) and were moderately correlated with obesity (r = 0.37, two-sided p = 0.009).
Figure 1.
Geographic patterns in uterine corpus cancer incidence rates A) uncorrected and B) corrected for hysterectomy prevalence, 2004-2008. Note: The range for the lowest category is wide because the rate for the District of Columbia was an extreme outlier, 10.8 cases per 100,000 less than the second lowest state (Nevada).
Among black women, uncorrected uterine cancer rates ranged from 17.3 in Tennessee to 28.0 in DC among the 28 states with sufficient data for analysis (Table 1). Hysterectomy correction increased rates among blacks from 42% (New York) to 105% (Texas) and reduced the number of states with significantly higher rates among white than black women from 13 to three (Table 2). In some states, the excess cancer burden appeared to shift from whites to blacks. In Massachusetts, for example, the rate ratio increased from 0.79 (95% CI, 0.69-0.90) to 1.16 (95% CI, 0.93-1.19).
Table 2.
Uterine corpus cancer risk among black and Hispanic women compared to non-Hispanic white women before and after hysterectomy correction (for states with sufficient data)
| Black | Hispanic | |||||||
|---|---|---|---|---|---|---|---|---|
| Uncorrected |
Corrected |
Uncorrected |
Corrected |
|||||
| State | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI |
| Alabama | 1.11 | (1.01-1.22) | 1.06 | (0.93-1.21) | ||||
| Arizona | 0.84 | (0.75-0.93) | 0.79 | (0.67-0.95) | ||||
| Arkansas | 1.07 | (0.92-1.24) | 1.11 | (0.91-1.36) | ||||
| California | 0.83 | (0.79-0.89) | 0.97 | (0.81-1.16) | 0.76 | (0.73-0.79) | 0.71 | (0.65-0.76) |
| Colorado | 0.80 | (0.70-0.92) | 0.79 | (0.66-0.93) | ||||
| Connecticut | 0.89 | (0.77-1.02) | 1.02 | (0.84-1.24) | 0.74 | (0.62-0.88) | 0.68 | (0.56-0.82) |
| Delaware | 0.96 | (0.78-1.18) | 0.97 | (0.76-1.24) | ||||
| Washington DC | 1.53 | (1.16-2.02) | 1.82 | (1.41-2.35) | ||||
| Florida | 1.13 | (1.07-1.19) | 1.25 | (1.10-1.42) | 1.02 | (0.97-1.07) | 0.84 | (0.78-0.91) |
| Georgia | 1.04 | (0.97-1.11) | 1.12 | (0.99-1.26) | ||||
| Illinois | 0.83 | (0.78-0.89) | 1.00 | (0.88-1.14) | 0.75 | (0.69-0.83) | 0.72 | (0.61-0.85) |
| Indiana | 0.78 | (0.68-0.88) | 0.88 | (0.70-1.11) | ||||
| Kansas | 0.95 | (0.76-1.20) | 1.04 | (0.78-1.38) | 1.00 | (0.78-1.29) | 0.96 | (0.71-1.31) |
| Kentucky | 0.83 | (0.71-0.99) | 0.81 | (0.63-1.04) | ||||
| Louisiana | 1.08 | (0.98-1.20) | 0.94 | (0.83-1.06) | ||||
| Massachusetts | 0.79 | (0.69-0.90) | 1.16 | (0.93-1.44) | 0.85 | (0.74-0.99) | 0.84 | (0.72-0.99) |
| Michigan | 0.85 | (0.79-0.92) | 0.97 | (0.86-1.10) | ||||
| Mississippi | 1.25 | (1.12-1.40) | 1.05 | (0.93-1.19) | ||||
| Missouri | 0.83 | (0.75-0.93) | 0.95 | (0.80-1.13) | ||||
| Nebraska | 0.78 | (0.52-1.18) | 0.85 | (0.49-1.46) | ||||
| Nevada | 0.86 | (0.66-1.11) | 0.76 | (0.55-1.04) | ||||
| New Jersey | 0.72 | (0.66-0.77) | 0.87 | (0.79-0.97) | 0.67 | (0.61-0.73) | 0.68 | (0.61-0.76) |
| New Mexico | 0.80 | (0.70-0.91) | 0.75 | (0.65-0.86) | ||||
| New York | 0.87 | (0.83-0.91) | 0.90 | (0.83-0.98) | 0.77 | (0.73-0.82) | 0.82 | (0.73-0.91) |
| North Carolina | 0.99 | (0.93-1.06) | 1.09 | (1.00-1.19) | 0.56 | (0.43-0.72) | 0.44 | (0.33-0.60) |
| Ohio | 0.73 | (0.67-0.79) | 0.90 | (0.77-1.05) | ||||
| Oklahoma | 0.91 | (0.74-1.11) | 0.89 | (0.70-1.14) | 1.09 | (0.84-1.43) | 1.03 | (0.72-1.48) |
| Pennsylvania | 0.70 | (0.65-0.76) | 0.78 | (0.69-0.90) | ||||
| South Carolina | 1.20 | (1.10-1.31) | 1.30 | (1.16-1.46) | ||||
| Tennessee | 0.88 | (0.79-0.97) | 0.92 | (0.77-1.09) | ||||
| Texas | 0.94 | (0.88-1.01) | 1.02 | (0.87-1.18) | 0.97 | (0.92-1.02) | 0.77 | (0.71-0.83) |
| Utah | 0.71 | (0.53-0.95) | 0.53 | (0.38-0.73) | ||||
| Virginia | 0.83 | (0.76-0.90) | 1.03 | (0.90-1.17) | ||||
| Washington | 0.62 | (0.50-0.76) | 0.55 | (0.43-0.70) | ||||
| US | 0.87 | (0.86-0.88) | 0.97 | (0.94-1.00) | 0.78 | (0.77-0.79) | 0.71 | (0.69-0.74) |
RR=rate ratio, non-Hispanic white women as referent group; CI=confidence interval.
Among Hispanic women, uncorrected uterine cancer incidence rates ranged from 12.4 in North Carolina to 25.9 in Massachusetts in the 18 states with sufficient data (Table 1); correcting rates for hysterectomy prevalence resulted in increases from 27% (Connecticut) to 91% (Oklahoma). In contrast to blacks, adjusting rates for hysterectomy increased the number of states with a lower risk among Hispanics compared to whites from 12 to 14 (Table 2). In Texas, for example, the uncorrected incidence rate was similar among whites (18.8) and Hispanics (18.3) whereas the corrected rate was 30% higher among whites, 36.0 versus 27.7 in Hispanics (Table 1).
Discussion
We found that state-level uterine cancer incidence rates that are not corrected for hysterectomy prevalence substantially underestimate the risk of disease and distort geographic and racial/ethnic patterns. As expected, hysterectomy correction had the largest effect on rates in the South, where hysterectomy prevalence was highest irrespective of race. For example, the incidence rate doubled among white women in Alabama, Louisiana, Mississippi, and Oklahoma and among black women in Alabama, Arkansas, and Texas.
Consistent with previous findings, correction for hysterectomy prevalence modified racial disparity patterns in uterine cancer incidence [7]. The excess risk among whites compared to blacks for the U.S. overall before correction (25.1 versus 21.8 cases per 100,000 woman-years) was reduced to statistically insignificant after correction (40.2 versus 38.9 cases per 100,000). Likewise, for 12 of 28 states, the excess risk among whites compared to blacks before hysterectomy correction was eliminated after rates were corrected. Hysterectomy correction had a lesser, but opposite effect on the risk difference between Hispanics and whites. In Florida and Texas, two of the most populous Hispanic states, uterine cancer risk that was identical for Hispanic and white women before correction became significantly higher among whites after accounting for hysterectomy prevalence.
This paper is the first to estimate state-level uterine cancer incidence rates among those women at risk. The substantial shift in the geographic pattern of uterine cancer after adjusting for hysterectomy prevalence highlights the importance of using corrected rates to compare and interpret state-specific disease burdens. The geographic pattern of uncorrected rates reflected differences in hysterectomy prevalence more than uterine cancer occurrence. Cancer surveillance data are the basis for much epidemiologic research for which reliable cancer rates are essential. For example, landmark studies confirming the association between occupational exposures of shipyard workers and increased lung cancer risk circa World War II were instigated by the high lung cancer death rates along the Atlantic coast revealed in the first Atlas of Cancer Mortality published in 1975 [23, 24, 25]. Similarly, geographic variations in breast and colorectal cancer have led to greater understanding of the contribution of lifestyle factors in the developmental pathway of these malignancies [26, 27]. Our findings confirm that inferences about uterine cancer based on rates unadjusted for hysterectomy prevalence may be flawed.
Obese women have a risk of uterine cancer that is two to three times that of normal weight women [21]. Adipose tissue mediates cancer development by increasing levels of endogenous estrogen, which promotes uterine tumor growth [28]. Although obesity is estimated to account for almost 40% of uterine cancer cases [21], a modest association between state-level incidence and obesity prevalence became apparent only after rates were corrected for hysterectomy prevalence.
Although the BRFSS is the only available source for state-level hysterectomy prevalence, these data have several limitations. First, response rates have been moderate relative to other health surveys, which may affect representativeness of the population at large. For example, in 2008 the median response was 53.3%. Second, only those households containing a residential telephone line are available for sampling. While almost 98% of the U.S. population has home telephone service, coverage varies by state [29]. Third, BRFSS data are self-reported, and therefore subject to recall bias. However, because accuracy of recall increases with the significance of the exposure of interest [30] and hysterectomy is a major medical procedure resulting in fertility loss, misclassification due to recall bias was probably minimal. Moreover, the self-reported patterns of hysterectomy prevalence we observed were consistent with those of previous reports [9-12]. Fourth, the indication for hysterectomy cannot be determined from the survey data; therefore, it is likely that some of the women who reported a history of hysterectomy underwent the procedure as the result of a uterine cancer diagnosis, which would overestimate corrected incidence estimates. However, only about 10% of hysterectomies in the United States are performed to treat a malignancy (i.e., ovarian, cervical, or uterine cancer) [9, 10], so this issue is unlikely to have affected the interpretation of our findings.
Although cancer surveillance coverage has improved dramatically over the past decade, three state registries did not have high quality data for all five years included in our analysis, and thus were excluded from the total U.S. incidence rate estimates. However, these states represented only 2.9% of the female population during 2004 to 2008. In addition, in a sensitivity analysis excluding those states for which we had data but that did not meet NAACCR criteria for high quality (DC and Nevada), the correlations between uterine cancer incidence (uncorrected and corrected for hysterectomy prevalence) and obesity did not change.
Accurate knowledge of the cancer burden at the state level is crucial for cancer control planning and etiologic research. Our study found that conventionally reported uterine cancer incidence rates that do not account for hysterectomy prevalence in the population at risk substantially underestimate disease burden and distort true geographic and racial disparity patterns. This issue also highlights the importance of enhancing reliable, multi-level, population-wide surveillance systems like the BRFSS for monitoring health behaviors and conditions and providing necessary information for public health advocacy efforts.
Acknowledgment
The authors would like to thank Deepa Naishadham, M.A., M.S. for her technical assistance.
Funding: This work was supported by the Intramural Research Department of the American Cancer Society and the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
The authors have no financial disclosures.
References
- 1.Copeland G, Lake A, Firth R, Wohler B, Wu X, Stroup A, et al., editors. Cancer in North America: 2004-2008. Volume One: Combined Cancer Incidence for the United States and Canada. North American Association of Central Cancer Registries, Inc; Springfield, IL: 2011. [Google Scholar]
- 2.Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, et al. Annual Report to the Nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–66. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luoto R, Raitanen J, Pukkala E, Anttila A. Effect of hysterectomy on incidence trends of endometrial and cervical cancer in Finland 1953-2010. British journal of cancer. 2004;90:1756–9. doi: 10.1038/sj.bjc.6601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyon JL, Gardner JW. The rising frequency of hysterectomy: its effect on uterine cancer rates. American journal of epidemiology. 1977;105:439–43. doi: 10.1093/oxfordjournals.aje.a112402. [DOI] [PubMed] [Google Scholar]
- 5.Merrill RM, Feuer EJ. Risk-adjusted cancer-incidence rates (United States) Cancer causes & control : CCC. 1996;7:544–52. doi: 10.1007/BF00051888. [DOI] [PubMed] [Google Scholar]
- 6.Merrill RM, Lyon JL, Wiggins C. Comparison of two methods based on cross-sectional data for correcting corpus uterine cancer incidence and probabilities. BMC cancer. 2001;1:13. doi: 10.1186/1471-2407-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman ME, Carreon JD, Lacey JV, Jr., Devesa SS. Impact of hysterectomy on endometrial carcinoma rates in the United States. Journal of the National Cancer Institute. 2005;97:1700–2. doi: 10.1093/jnci/dji378. [DOI] [PubMed] [Google Scholar]
- 8.Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy Surveillance--United States, 1994-1999. MMWR CDC Surveill Summ. 2002;51:1–8. [PubMed] [Google Scholar]
- 9.Whiteman MK, Hillis SD, Jamieson DJ, Morrow B, Podgornik MN, Brett KM, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. American journal of obstetrics and gynecology. 2008;198:34, e1–7. doi: 10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 10.Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstetrics and gynecology. 2007;110:1091–5. doi: 10.1097/01.AOG.0000285997.38553.4b. [DOI] [PubMed] [Google Scholar]
- 11.Powell LH, Meyer P, Weiss G, Matthews KA, Santoro N, Randolph JF, Jr., et al. Ethnic differences in past hysterectomy for benign conditions. Women’s health issues : official publication of the Jacobs Institute of Women’s Health. 2005;15:179–86. doi: 10.1016/j.whi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Howard BV, Kuller L, Langer R, Manson JE, Allen C, Assaf A, et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative Observational Study. Circulation. 2005;111:1462–70. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- 13.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black-White differences in hysterectomy prevalence: the CARDIA study. American journal of public health. 2009;99:300–7. doi: 10.2105/AJPH.2008.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North American Association of Central Cancer Registries (NAACCR) Incidence - CiNA Analytic File, 1995-2008, for Expanded Races, Standard File, Siegel - site-specific endometrial cancer incidence, NAACCR. NAACCR; Springfield, IL: 2012. Surveillance, Epidemiology and End Results (SEER) Program SEER*Stat Database. [Google Scholar]
- 15.North American Association of Central Cancer Registries (NAACCR): Incidence - CiNA Analytic File, 1995-2008, for NHIAv2 Origin, Standard File, Siegel - site-specific endometrial cancer incidence, NAACCR. NAACCR; Springfield, IL: 2012. Surveillance, Epidemiology and End Results (SEER) Program SEER*Stat Database. [Google Scholar]
- 16.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al., editors. International Classification of Diseases for Oncology. Third ed World Health Organization; 2000. [Google Scholar]
- 17.Statistical Research and Applications Branch. Version 7.0.5 National Cancer Institute; Bethesda: 2011. Seer*Stat software. [Google Scholar]
- 18.U.S. Census Bureau 2009 National Population Estimates. 2011 http://www.census.gov/popest/
- 19.Behavioral Risk Factor Surveillance System 2004, 2006, 2008 . National Center for Chronic Disease Prevention and Health Promotion. Centers for Disease Control and Prevention; Hyattsville, Maryland: 2009. [Google Scholar]
- 20.Environmental Science and Research Institute (ESRI) ArcGIS and ArcMap software. Version 10.0 ESRI; Redlands, CA: 2011. [Google Scholar]
- 21.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev. 2002;11(Suppl 2):S94–100. [PubMed] [Google Scholar]
- 22.Oehlert GW. A note on the delta method. The American Statistician. 1992;46:27–9. [Google Scholar]
- 23.Mason TJ, McKay FW, Hoover R. Atlas of Cancer Mortality for U.S. Counties: 1950-69. Government Printing Office; Washington DC: 1975. al. e. [Google Scholar]
- 24.Blot WJ, Harrington JM, Toledo A, Hoover R, Heath CW, Jr., Fraumeni JF., Jr. Lung cancer after employment in shipyards during World War II. The New England journal of medicine. 1978;299:620–4. doi: 10.1056/NEJM197809212991202. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson WJ, Lilis R, Frank AL, Selikoff IJ. Lung cancer prevalence among shipyard workers. American journal of industrial medicine. 1980;1:191–203. doi: 10.1002/ajim.4700010210. [DOI] [PubMed] [Google Scholar]
- 26.Blot WJ, Fraumeni JF, Jr., Stone BJ, McKay FW. Geographic patterns of large bowel cancer in the United States. Journal of the National Cancer Institute. 1976;57:1225–31. doi: 10.1093/jnci/57.6.1225. [DOI] [PubMed] [Google Scholar]
- 27.Sturgeon SR, Schairer C, Gail M, McAdams M, Brinton LA, Hoover RN. Geographic variation in mortality from breast cancer among white women in the United States. Journal of the National Cancer Institute. 1995;87:1846–53. doi: 10.1093/jnci/87.24.1846. [DOI] [PubMed] [Google Scholar]
- 28.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature reviews Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 29.United States Census Bureau [cited 2012 April 25];American FactFinder. 2010 ( factfinder2.census.gov). [Web-based data retrieval tool] Census:[Available from:
- 30.Coughlin SS. Recall bias in epidemiologic studies. Journal of clinical epidemiology. 1990;43:87–91. doi: 10.1016/0895-4356(90)90060-3. [DOI] [PubMed] [Google Scholar]