Abstract
The OSCAR study was a multicenter, prospective randomized open-label blinded end-point study of 1164 Japanese elderly hypertensive patients comparing the efficacy of angiotensin II receptor blocker (ARB) uptitration to an ARB plus calcium channel blocker (CCB) combination. In this prospective study, we performed prespecified subgroup analysis according to baseline estimated glomerular filtration rate (eGFR) with chronic kidney disease (CKD) defined as an eGFR <60 ml/min per 1.73 m2. Blood pressure was lower in the combined therapy than in the high-dose ARB cohort in both groups with and without CKD. In patients with CKD, significantly more primary events (a composite of cardiovascular events and noncardiovascular death) occurred in the high-dose ARB group than in the combination group (30 vs. 16, respectively, hazard ratio 2.25). Significantly more cerebrovascular and more heart failure events occurred in the high-dose ARB group than in the combination group. In patients without CKD, however, the incidence of primary events was similar between the two treatments. The treatment-by-subgroup interaction was significant. Allocation to the high-dose ARB was a significant independent prognostic factor for primary events in patients with CKD. Thus, the ARB plus CCB combination conferred greater benefit in prevention of cardiovascular events in patients with CKD compared with high-dose ARB alone. Our findings provide new insight into the antihypertensive strategy for elderly hypertensive patients with CKD.
Keywords: cardiovascular disease, CKD, combination therapy, high-dose ARB, hypertension
Chronic kidney disease (CKD), manifested by decreased glomerular filtration rate (GFR), is a worldwide public health problem, and an older population has a higher prevalence of CKD.1, 2, 3, 4, 5 A large amount of evidence shows that decreased GFR is an independent risk factor for cardiovascular events and all-cause mortality in the general population1, 2, 4, 5, 6 and high-risk populations such as the elderly,3 as well as in patients with hypertension,7 diabetes,8 heart failure,9 or myocardial infarction.10 The National Kidney Foundation Task Force11 and a statement from the American Heart Association Councils12 have recommended that patients with CKD should be considered as a high-risk group for subsequent cardiovascular events and that treatment recommendation based on cardiovascular risk stratification should take into account the highest-risk status of patients with CKD.
It has been established that the renin–angiotensin system (RAS) has a pivotal role in the pathophysiology of CKD and cardiovascular disease. Inhibition of RAS by angiotensin II receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors confers cardiovascular protection in both diabetic13, 14 and nondiabetic hypertensive patients with CKD,7, 15 and is regarded as the first-line therapeutic strategy for CKD in Western countries16, 17 and Japan.18 Furthermore, previous evidence indicates that uptitration of an ARB provides greater suppression of renal events in hypertensive patients with diabetic nephropathy19 and greater reduction of cardiovascular events in heart failure patients20 compared with lower dose of ARB, thereby showing the benefit of high-dose ARB therapy in prevention of cardiovascular and renal events. On the other hand, the combination of ARB and calcium channel blocker (CCB) is recommended as one of the preferred combination therapies for the general hypertensive population.16, 17, 18 However, it remains to be elucidated which antihypertensive strategy is more effective for the prevention of cardiovascular morbidity and mortality in high-risk subjects with CKD compared with those without CKD at baseline; that is, is ARB plus CCB combination therapy or high-dose ARB therapy more effective?
We conducted the OlmeSartan and Calcium Antagonists Randomized (OSCAR) study comparing the preventive effect of ARB plus CCB combination therapy versus high-dose ARB therapy on cardiovascular morbidity and mortality in Japanese elderly hypertensive patients with baseline cardiovascular disease and/or type 2 diabetes, and have recently reported the principal results of the OSCAR study.21 In the present study, to determine whether CKD might influence the relative effectiveness of ARB plus CCB combination versus high-dose ARB in the prevention of cardiovascular morbidity and mortality, we performed a subgroup analysis of the OSCAR study according to baseline estimated GFR (eGFR), which we had prespecified as described in our protocol paper.22
RESULTS
Categorization of patients according to baseline eGFR
Eighty-six patients were excluded from the present analysis because no serum creatinine value at baseline was available. As a result, 1078 patients of a total of 1164 patients originally enrolled in the OSCAR study were included in the present analysis (Table 1). The number of patients with an eGFR of <60 ml/min per 1.73 mm2 (defined as patients with CKD) was 353; of these, 334 (94.6%) patients had an eGFR of 30–59 ml/min per 1.73 mm2 and 19 patients (5.4%) had an eGFR <30 ml/min per 1.73 mm2. The number of patients having an eGFR ⩾60 ml/min per 1.73 mm2 (patients without CKD) was 725; of these, 629 (86.8%) patients had an eGFR of 60–89 ml/min per 1.73 mm2 and 96 patients (13.2%) had an eGFR ⩾90 ml/min per 1.73 mm2.
Table 1. Baseline characteristics.
|
Overall |
CKD (+) |
CKD (−) |
||||||
|---|---|---|---|---|---|---|---|---|
| Total patients (n=1078) | CKD (+) (n=353) | CKD (−) (n=725) | P-value | High-dose ARB (n=181) | ARB+CCB (n=172) | High-dose ARB (n=354) | ARB+CCB (n=371) | |
| eGFR (ml/min per 1.73 m2) | 67.2±18.7 | 47.8±9.1 | 76.7±14.4 | <0.0001 | 47.3±8.9 | 48.3±9.3 | 76.4±13.9 | 76.9±14.8 |
| Age (years) | 73.7±5.4 | 75.5±5.2 | 72.8±5.3 | <0.0001 | 75.2±5.1 | 75.8±5.4 | 73.0±5.4 | 72.7±5.3 |
| Male, n (%) | 477 (44.3) | 167 (47.3) | 310 (42.8) | 0.16 | 80 (44.2) | 87 (50.6) | 157 (44.4) | 153 (41.2) |
| BMI (kg/m2) | 24.1±3.6 | 23.9±3.2 | 24.2±3.7 | 0.17 | 24.2±3.4 | 23.5±3.0a | 24.3±3.8 | 24.1±3.7 |
| BMI ⩾25 kg/m2, n (%) | 403 (37.5) | 118 (33.4) | 285 (39.4) | 0.06 | 67 (37.0) | 51 (29.7) | 144 (40.8) | 141 (38.1) |
| Systolic BP (mm Hg) | 158.0±11.9 | 158.9±12.0 | 157.5±11.8 | 0.07 | 158.9±12.8 | 158.9±11.2 | 158.3±12.3 | 156.7±11.3 |
| Diastolic BP (mm Hg) | 84.6±9.8 | 84.8±9.7 | 84.6±9.8 | 0.79 | 85.0±9.8 | 84.5±9.7 | 84.8±9.8 | 84.4±9.8 |
| Heart rate (b.p.m) | 73±10 | 73±10 | 73±10 | 0.76 | 74±10 | 73±10 | 74±10 | 73±9 |
| Hyperlipidemia, n (%) | 528 (49.0) | 177 (50.1) | 351 (48.4) | 0.59 | 96 (53.0) | 81 (47.1) | 173 (48.9) | 178 (48.0) |
| Current smoker, n (%) | 107 (9.9) | 26 (7.4) | 81 (11.2) | 0.05 | 16 (8.9) | 10 (5.8) | 43 (12.2) | 38 (10.2) |
| Current alcohol intake, n (%) | 350 (32.6) | 125 (35.7) | 225 (31.0) | 0.12 | 55 (30.7) | 70 (40.9) | 114 (32.2) | 111 (29.9) |
| Serum values | ||||||||
| Creatinine (mg/dl) | 0.8±0.3 | 1.1±0.3 | 0.7±0.1 | <0.0001 | 1.1±0.3 | 1.1±0.3 | 0.7±0.1 | 0.7±0.1 |
| Potassium (mmol/l) | 4.3±0.5 | 4.4±0.5 | 4.3±0.4 | <0.0001 | 4.4±0.4 | 4.5±0.5 | 4.3±0.4 | 4.3±0.4 |
| Total cholesterol (mg/dl) | 199±35 | 195±35 | 200±35 | 0.03 | 196±36 | 194±35 | 198±34 | 203±36 |
| HDL cholesterol (mg/dl) | 56.6±15.2 | 53.9±15.2 | 57.9±15.0 | <0.0001 | 54.9±17.0 | 52.9±13.0 | 56.8±14.6 | 58.9±15.3 |
| Fasting plasma glucose (mg/dl) | 117±44 | 116±46 | 117±43 | 0.72 | 119±48 | 113±45 | 120±48 | 115±38 |
| Casual plasma glucose (mg/dl) | 145±59 | 140±56 | 147±60 | 0.25 | 138±54 | 144±59 | 144±55 | 150±64 |
| Previous antihypertensive treatment, n (%) | ||||||||
| No. of agents | ||||||||
| 0 | 300 (27.8) | 66 (18.7) | 234 (32.3) | <0.0001 | 33 (18.2) | 33 (19.2) | 115 (32.5) | 119 (32.1) |
| 1 | 473 (43.9) | 163 (46.2) | 310 (42.8) | 83 (45.9) | 80 (46.5) | 150 (42.4) | 160 (43.1) | |
| 2 | 253 (23.5) | 104 (29.5) | 149 (20.6) | 52 (28.7) | 52 (30.2) | 72 (20.3) | 77 (20.8) | |
| ⩾3 | 52 (4.8) | 20 (5.7) | 32 (4.4) | 13 (7.2) | 7 (4.1) | 17 (4.8) | 15 (4.0) | |
| Oral glucose-lowering agents, n (%) | 417 (38.7) | 134 (38.0) | 283 (39.0) | 0.73 | 71 (39.2) | 63 (36.6) | 144 (40.7) | 139 (37.5) |
| Insulin treatment, n (%) | 68 (6.3) | 24 (6.8) | 44 (6.1) | 0.64 | 12 (6.6) | 12 (7.0) | 19 (5.4) | 25 (6.7) |
| Lipid-lowering agents, n (%) | 399 (37.0) | 133 (37.7) | 266 (36.7) | 0.75 | 66 (36.5) | 67 (39.0) | 134 (37.9) | 132 (35.6) |
| Antiplatelet agents, n (%) | 343 (31.8) | 142 (40.2) | 201 (27.7) | <0.0001 | 65 (35.9) | 77 (44.8) | 103 (29.1) | 98 (26.4) |
Abbreviations: ARB, angiotensin II receptor blocker; BMI, body mass index; BP, blood pressure; CCB, calcium channel blocker; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein.
Data are mean±s.d. for continuous variables and number (%) for categorical variables. P-value was calculated using t-tests for continuous variables and χ2 tests for categorical variables. CKD (+), patients with CKD (with eGFR of <60 ml/min per 1.73 m2) at baseline; CKD (−), patients without CKD (with eGFR of ⩾60 ml/min per 1.73 m2) at baseline; High-dose ARB, patients allocated to high-dose ARB therapy; ARB+CCB, patients allocated to ARB+CCB combination therapy.
P=0.03 versus high-dose ARB.
Demographic and baseline characteristics of patients with and without CKD
Importantly, there was no significant difference regarding any of the baseline characteristics between the patients (n=1078) included in the present analysis and total patients (n=1164)21, 22 originally enrolled in the OSCAR study.
As shown in Table 1, eGFR (mean±s.d.) in patients with and without CKD was 47.8±9.1 and 76.7±14.4 ml/min per 1.73 mm2, respectively (P<0.0001 between the two groups). Compared with patients without CKD, patients with CKD were older, had higher serum creatinine, and lower serum high-density lipoprotein cholesterol; a lower proportion of them had a history of previous antihypertensive agents and a higher proportion had a history of antiplatelet agents.
In patients with CKD (Table 1), baseline characteristics were well balanced between high-dose ARB and ARB plus CCB treatment groups, except for a slight difference in body mass index between the two groups (24.2±3.4 vs. 23.5±3.0 kg/m2, P=0.03). In patients without CKD (Table 1), there was no significant difference between the two treatment groups in any of the baseline characteristics.
Table 2 shows that a higher proportion of patients with CKD had cardiovascular disease at baseline compared with those without CKD (80.5 vs. 66.9%, P<0.0001), whereas a slightly lower proportion of them had type 2 diabetes at baseline (49.0 vs. 55.9%, P=0.03).
Table 2. Baseline cardiovascular disease and type 2 diabetes of overall patients and patients with or without CKD.
|
Overall |
CKD (+) |
CKD (−) |
||||||
|---|---|---|---|---|---|---|---|---|
| Total patients (n=1078) | CKD (+) (n=353) | CKD (−) (n=725) | P-value | High-dose ARB (n=181) | ARB+CCB (n=172) | High-dose ARB (n=354) | ARB+CCB (n=371) | |
| Previous cardiovascular disease, n (%) | 769 (71.3) | 284 (80.5) | 485 (66.9) | <0.0001 | 149 (82.3) | 135 (78.5) | 238 (67.2) | 247 (66.6) |
| Stroke | 193 (17.9) | 74 (21.0) | 119 (16.4) | 0.07 | 37 (20.4) | 37 (21.5) | 69 (19.5) | 50 (13.5)a |
| Transient ischemic attack | 52 (4.8) | 27 (7.7) | 25 (3.5) | 0.003 | 16 (8.8) | 11 (6.4) | 9 (2.5) | 16 (4.3) |
| Asymptomatic cerebrovascular disease | 196 (18.2) | 58 (16.4) | 138 (19.0) | 0.3 | 33 (18.2) | 25 (14.5) | 68 (19.2) | 70 (18.9) |
| Myocardial infarction | 35 (3.3) | 22 (6.2) | 13 (1.8) | 0.0001 | 11 (6.1) | 11 (6.4) | 5 (1.4) | 8 (2.2) |
| Angina pectoris | 121 (11.2) | 44 (12.5) | 77 (10.6) | 0.37 | 23 (12.7) | 21 (12.2) | 39 (11.0) | 38 (10.2) |
| Heart failure | 83 (7.7) | 34 (9.6) | 49 (6.8) | 0.1 | 17 (9.4) | 17 (9.9) | 21 (5.9) | 28 (7.6) |
| Left ventricular hypertrophy | 189 (17.5) | 56 (15.9) | 133 (18.3) | 0.31 | 32 (17.7) | 24 (14.0) | 64 (18.1) | 69 (18.6) |
| Aortic aneurysm | 4 (0.4) | 3 (0.9) | 1 (0.1) | 0.07 | 1 (0.6) | 2 (1.2) | 0 (0) | 1 (0.3) |
| Arteriosclerotic peripheral arterial occlusive disease | 24 (2.2) | 10 (2.8) | 14 (1.9) | 0.35 | 5 (2.8) | 5 (2.9) | 4 (1.1) | 10 (2.7) |
| Serum creatinine outside normal range | 73 (6.8) | 68 (19.3) | 5 (0.7) | <0.0001 | 34 (18.8) | 34 (19.8) | 3 (0.9) | 2 (0.5) |
| Proteinuria | 126 (11.7) | 69 (19.6) | 57 (7.9) | <0.0001 | 39 (21.6) | 30 (17.4) | 26 (7.3) | 31 (8.4) |
| Previous type 2 diabetes, n (%) | 578 (53.6) | 173 (49.0) | 405 (55.9) | 0.03 | 85 (47.0) | 88 (51.2) | 197 (55.7) | 208 (56.1) |
Abbreviations: ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; CKD, chronic kidney disease.
Data are number (%). P-value was calculated using χ2 tests. CKD (+), patients with CKD at baseline; CKD (−), patients without CKD at baseline; high-dose ARB, patients allocated to high-dose ARB therapy; ARB+CCB, patients allocated to ARB+CCB combination therapy.
P=0.03 versus high-dose ARB.
As shown in Table 2, in patients with CKD and without CKD, no significant difference was found between the two treatment groups in proportion of patients with previously existing cardiovascular disease or type 2 diabetes, except for a slight difference between the treatment groups in the percentage of previous stroke in patients without CKD.
Blood pressure (BP) during follow-up period
Table 3 shows the number and percentage of patients who received additional antihypertensive drug(s) in the two treatment groups in the subgroups with CKD or without CKD, throughout the follow-up period. In all groups, diuretics, β-blockers, and α-blockers were mainly prescribed as additional antihypertensive medications, and diuretics were the most prescribed among all additional antihypertensive drugs. The percentage of patients who received these additional antihypertensive drugs was greater in the high-dose ARB group than in the ARB plus CCB combination group, in both the subgroups with CKD and that without CKD.
Table 3. Number of patients who received additional antihypertensive drug(s) throughout the follow-up period.
|
CKD (+) |
CKD (−) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
High-dose ARB |
ARB+CCB |
High-dose ARB |
ARB+CCB |
|||||||||||||||||
| After allocation | N | None | D | β | α | N | None | D | β | α | N | None | D | β | α | N | None | D | β | α |
| 6 mos | 180 | 112 (62) | 34 (19) | 21 (12) | 18 (10) | 170 | 138 (81) | 13 (7) | 6 (4) | 6 (4) | 350 | 246 (70) | 55 (16) | 30 (9) | 32 (9) | 371 | 292 (79) | 34 (9) | 10 (3) | 6 (2) |
| 12 mos | 171 | 91 (53) | 44 (26) | 28 (16) | 15 (9) | 165 | 130 (79) | 16 (10) | 6 (4) | 9 (5) | 336 | 211 (63) | 74 (22) | 39 (12) | 38 (11) | 361 | 280 (78) | 38 (11) | 17 (5) | 11 (3) |
| 18 mos | 163 | 86 (53) | 49 (30) | 28 (17) | 17 (10) | 158 | 119 (75) | 17 (11) | 8 (5) | 6 (4) | 325 | 204 (63) | 77 (24) | 38 (12) | 36 (11) | 341 | 262 (77) | 42 (12) | 22 (6) | 11 (3) |
| 24 mos | 156 | 77 (49) | 55 (35) | 30 (19) | 19 (12) | 156 | 114 (73) | 20 (13) | 11 (7) | 9 (6) | 311 | 184 (59) | 80 (26) | 39 (13) | 42 (14) | 327 | 242 (74) | 43 (13) | 24 (7) | 11 (3) |
| 30 mos | 152 | 65 (43) | 57 (38) | 30 (20) | 16 (11) | 154 | 113 (73) | 20 (13) | 10 (6) | 6 (4) | 302 | 163 (54) | 83 (27) | 41 (14) | 39 (13) | 321 | 229 (71) | 44 (14) | 24 (7) | 10 (3) |
| 36 mos | 148 | 65 (44) | 52 (35) | 30 (20) | 15 (10) | 147 | 103 (70) | 22 (15) | 9 (6) | 9 (6) | 297 | 163 (55) | 92 (31) | 42 (14) | 38 (13) | 315 | 218 (69) | 50 (16) | 27 (9) | 9 (3) |
Abbreviations: ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; CKD, chronic kidney disease; D, diuretics; mos, months; none, no additional drug; β, β-blockers; α, α-blockers.
Data represent number (%) of patients. CKD (+), patients with CKD at baseline; CKD (−), patients without CKD at baseline; high-dose ARB, patients allocated to high-dose ARB therapy; ARB+CCB, patients allocated to ARB+CCB combination therapy.
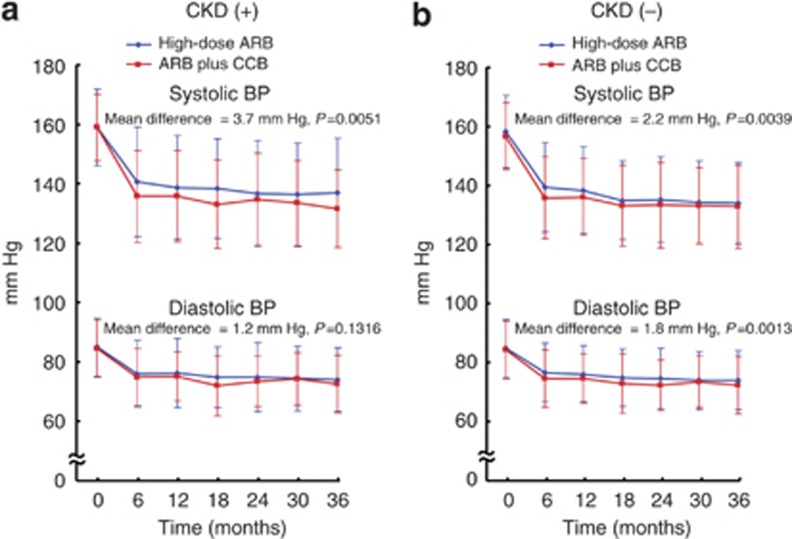
In patients with CKD (Figure 1a), systolic BP of the ARB plus CCB combination group was lower than that of the high-dose ARB group throughout the follow-up period, and the mean difference in systolic BP between the groups during follow-up period was 3.7 mm Hg (P=0.0051). In patients without CKD (Figure 1b), both systolic BP and diastolic BP were lower in the ARB plus CCB combination group than in the high-dose ARB group, and the mean difference in systolic BP and diastolic BP between the groups was 2.2 mm Hg (P=0.0039) and 1.8 mm Hg (P=0.0013), respectively.
Figure 1.
Time course of systolic and diastolic blood pressure (BP) in high-dose angiotensin II receptor blocker (ARB) and ARB plus calcium channel blocker (CCB) combination groups in patients with chronic kidney disease (CKD) and without CKD. (a) Indicates patients with CKD and (b) indicates patients without CKD.
The proportion of patients who achieved target BP of <140/90 mm Hg was significantly higher in the ARB plus CCB combination group than in the high-dose ARB group, both in patients with CKD (77.3 vs. 60.2%, P=0.0005) and those without CKD (75.7 vs. 67.8%, P=0.0174).
Incidence of primary outcome events in patients with or without CKD
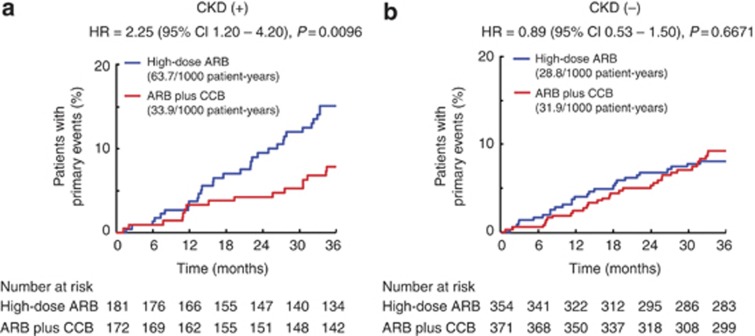
Figure 2 indicates the incidence of primary outcome events in the high-dose ARB and ARB plus CCB combination groups in patients with CKD and without CKD. There was a significant interaction between the subgroup of patients with CKD and those without CKD for the incidence of primary outcome events, and the treatment-by-subgroup interaction was statistically significant (P=0.0293 for interaction).
Figure 2.
Kaplan–Meier curves for primary composite end points during the follow-up period in patients with chronic kidney disease (CKD) and without CKD. In (a), the number of patients in high-dose angiotensin II receptor blocker (ARB) and ARB plus calcium channel blocker (CCB) combination groups were 181 and 172, respectively, and there were 30 and 16 primary end points in high-dose ARB and ARB plus CCB combination groups, respectively. In (b), the number of primary end points was 27 in 354 patients assigned high-dose ARB and 32 in 371 patients assigned ARB plus CCB combination. CI, confidence interval; HR, hazard ratio.
In patients with CKD (Figure 2a), 30 patients (16.6% 63.7 per 1000 patient-years) of 181 patients assigned to high-dose ARB and 16 patients (9.3% 33.9 per 1000 patient-years) of 172 patients assigned to ARB plus CCB combination experienced primary events: the difference (56.1%) in relative risk between the two groups was significant (hazard ratio (HR)=2.25; 95% confidence interval (CI)=1.20–4.20; P=0.0096). On the other hand, in patients without CKD (Figure 2b), there was no significant difference between high-dose ARB and ARB plus CCB combination groups regarding the incidence of primary events (27 (7.6%) vs. 32 (8.6%) events: 28.8 per 1000 patient-years vs. 31.9 per 1000 patient-years): HR=0.89: 95% CI=0.53–1.50; P=0.6671.
Incidence of secondary end point in patients with or without CKD
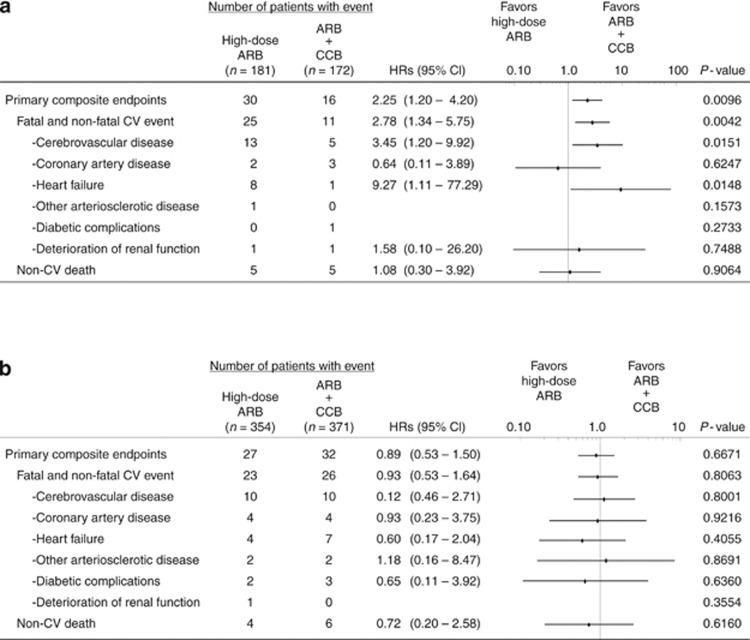
In patients with CKD (Figure 3a), the ARB plus CCB combination group had a lower incidence of cerebrovascular disease (HR=3.45; 95% CI=1.20–9.92; P=0.0151) and heart failure (HR=9.27; 95% CI=1.11–77.29; P=0.0148) than the high-dose ARB group.
Figure 3.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for the primary composite end points and secondary end points in patients with chronic kidney disease (CKD) and patients without CKD at baseline. (a) Indicates patients with CKD and (b) indicates patients without CKD. The HRs and 95% CIs were derived from a stratified Cox proportional hazards model, taking into account sex, age, and baseline cardiovascular disease and type 2 diabetes. The P-values were derived from a log-rank test, stratified by sex, age, and baseline cardiovascular disease and type 2 diabetes. ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; CV, cardiovascular.
On the other hand, in patients without CKD (Figure 3b), there was no significant difference between the two treatment groups with regard to any secondary end point.
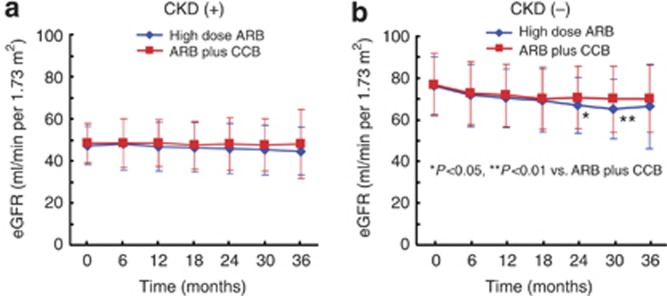
Time course of changes in eGFR throughout the follow-up period
Figure 4 depicts the time course of eGFR changes in the subgroups with CKD or without CKD. In the subgroup with CKD, compared with baseline eGFR, eGFR slightly declined in both the high-dose ARB group and ARB plus CCB combination group during the follow-up period. eGFR (mean±s.d.) at the end of the study tended to be lower in the high-dose ARB group than in the ARB plus CCB combination group (44.6±11.4 vs. 48.1±16.5 ml/min per 1.73 m2; P=0.291), although the difference did not reach statistical significance. In the subgroup without CKD, eGFR at the end of the study did not significantly differ between high-dose ARB and ARB plus CCB combination groups (66.5±19.9 vs. 70.0±16.1 ml/min per 1.73 m2; P=0.12), although eGFR in the high-dose ARB group was significantly lower than that in the ARB plus CCB combination group at 24 months (66.8±13.4 vs. 70.6±15.0 ml/min per 1.73 m2; P=0.01) and 30 months (65.2±14.2 vs. 69.9±15.9 ml/min per 1.73 m2; P=0.002).
Figure 4.
Time course of changes in estimated glomerular filtration rate (eGFR) in patients with chronic kidney disease (CKD) and without CKD during the follow-up period. (a) Indicates patients with CKD and (b) indicates patients without CKD. ARB, angiotensin II receptor blocker; CCB, calcium channel blocker. Data represent the mean±s.d.
Association of prognostic factors with primary outcome events
Table 4 shows the results of multivariable Cox regression analysis for overall patients, the subgroup with CKD, and the subgroup without CKD. For overall patients, high-dose ARB treatment was not significantly associated with primary outcome events (P=0.239), whereas sex (P=0.004), age (P=0.001), and baseline cardiovascular disease (P=0.042) were significantly associated with primary outcome events.
Table 4. Adjusted hazard ratios of prognostic factor for primary outcome events.
|
Overall patients |
CKD (+) |
CKD (−) |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| High-dose ARB | 1.26 (0.86–1.86) | 0.239 | 2.27 (1.22–4.23) | 0.010 | 0.86 (0.51–1.44) | 0.561 |
| Male gender | 1.79 (1.21–2.64) | 0.004 | 1.66 (0.92–3.01) | 0.092 | 1.96 (1.15–3.34) | 0.013 |
| Age⩾75 years | 2.00 (1.35–2.97) | 0.001 | 2.76 (1.40–5.43) | 0.003 | 1.61 (0.96–2.71) | 0.072 |
| Previous CV disease | 1.76 (1.02–3.04) | 0.042 | 1.60 (0.65–3.95) | 0.304 | 1.93 (0.96–3.85) | 0.064 |
| Previous diabetes | 1.26 (0.81–1.94) | 0.310 | 1.31 (0.68–2.52) | 0.422 | 1.33 (0.73–2.43) | 0.351 |
Abbreviations: ARB, angiotensin II receptor blocker; CKD, chronic kidney disease; CV, cardiovascular; HR, hazard ratio; 95% CI, 95% confidence interval.
CKD (+), patients with CKD at baseline; CKD (−), patients without CKD at baseline; high-dose ARB, allocation to high-dose ARB therapy.
In patients with CKD, high-dose ARB treatment (P=0.010), as well as age (P=0.003), but not baseline cardiovascular disease (P=0.304), was significantly associated with primary outcome events. In patients without CKD, only sex (P=0.013) was associated with primary outcome events.
BP was not significantly associated with primary outcome events for overall patients or patients with CKD or without CKD.
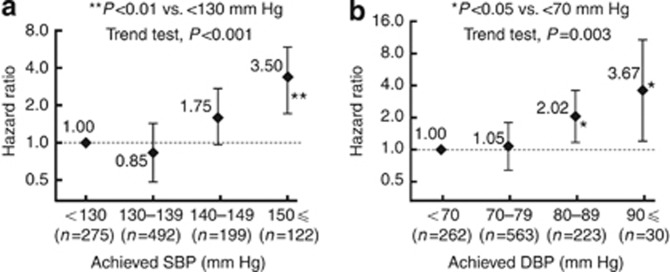
Relationship between achieved BP and incidence of primary events
Figure 5 indicates the adjusted relative risk at different levels of achieved BP in overall patients with available eGFR data (n=1078). The incidence of primary events was significantly higher in the subgroup with systolic BP⩾150 mm Hg (HR=3.50, 95% CI=1.93–6.34, P<0.01) than that in the subgroup with systolic BP<130 mm Hg. The incidence of primary events was significantly higher in the subgroups that achieved diastolic BP of 80–89 mm Hg (HR=2.02, 95% CI=1.16–3.52, P<0.05) or diastolic BP⩾90 mm Hg (HR=3.67, 95% CI=1.36–9.90, P<0.05) compared with the subgroup with diastolic BP<70 mm Hg.
Figure 5.
Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of achieved systolic blood pressure (BP) and diastolic BP levels for incidence of primary composite end points in total patients with available estimated glomerular filtration rate data (n=1078). The achieved BP on a per-patient basis was defined as the mean value of all BP values during the follow-up period. The reference category was (a) <130 mm Hg for systolic BP and (b) <70 mm Hg for diastolic BP. DBP, diastolic BP; SBP, systolic BP.
Incidence of adverse events
The number of adverse events was 1, 3, 1, and 2 for hyperkalemia; 7, 5, 5, and 3 for increase in serum creatinine; 1, 0, 1, and 2 for hypotension; and 2, 7, 8, and 13 for cancer in the high-dose ARB/CKD, ARB plus CCB/CKD, high-dose ARB/without CKD, and ARB plus CCB/without CKD groups, respectively. There was no significant difference in these adverse events between high-dose ARB and ARB plus CCB groups, regardless of whether the patients had CKD.
DISCUSSION
The major findings of the current work were as follows: ARB plus CCB combination therapy was more effective in the prevention of cardiovascular morbidity and mortality for Japanese elderly high-risk hypertensive patients with baseline CKD compared with high-dose ARB therapy, whereas there was no significant difference in the incidence of primary events between these treatment groups for patients without baseline CKD. Furthermore, we found that the treatment-by-subgroup interaction between patients with CKD and without CKD was significant. Our present findings provided novel information indicating the superiority of ARB plus CCB combination over high-dose ARB in elderly hypertensive high-risk patients with CKD at baseline.
Compelling evidence shows that reduced eGFR is an independent risk factor for cardiovascular disease and all-cause mortality in a wide range of populations,1, 2, 4, 5, 6 including the elderly3 and hypertensive patients.7 Generally, an eGFR <60 ml/min per 1.73 mm2 is used as the cutoff value for definition of CKD, because this level of eGFR is associated with an increased prevalence of cardiovascular disease.11, 23 In the current study, almost all patients with CKD manifested stage 3 CKD (eGFR of 30–59 ml/min per 1.73 mm2), indicating that these patients had moderate renal dysfunction. In the OSCAR study, most of the primary events in patients with CKD were cardiovascular events, although the occurrence of deterioration of renal function was low, being consistent with previous evidence that patients with CKD have a much higher prevalence of cardiovascular disease than of renal events.6 It is noteworthy that ARB plus CCB combination therapy reduced the incidence of primary events in patients with CKD more than did high-dose ARB, and reduced the incidence of cerebrovascular disease and of heart failure in patients with CKD more than high-dose ARB. These observations demonstrate that ARB plus CCB combination is superior to high-dose ARB in the prevention of cardiovascular events in elderly high-risk hypertensive patients with CKD. On the other hand, no difference in the incidence of primary events was noted between the two treatment groups in patients without CKD. Importantly, there was a significant interaction between treatment groups and CKD category. These findings provided evidence that renal function significantly influences the effectiveness of these two therapies on cardiovascular events in elderly hypertensive patients. However, it cannot be completely ruled out that the lack of difference in the incidence of primary events between the two treatment groups in patients without CKD might be attributed to an insufficient sample size in the OSCAR study, because the number of patients in the subgroup was relatively small.
Similar to the results with overall patients of the OSCAR study,21 ARB plus CCB combination reduced BP more than high-dose ARB both in patients with CKD and without CKD. Furthermore, the proportion of patients who achieved target BP was higher in the ARB plus CCB combination group than in the high-dose ARB group, even though a higher percentage of patients received additional antihypertensive drugs in the high-dose ARB group. Our current findings are consistent with the findings of a meta-analysis showing that the combination of drugs from two different classes is more effective in BP lowering than doubling the dose of one drug.24
The present study did not allow us to define the precise contribution of better BP control with ARB plus CCB combination to the lower incidence of primary events in the subgroup with CKD, because of the small number of patients in this subgroup. Accumulating evidence indicates that strict BP control is associated with the reduction of cardiovascular events in a variety of populations with CKD.7, 15 Large-scale clinical studies25, 26 demonstrate that lower BP is closely associated with a lower incidence of stroke and heart disease. Furthermore, the present analysis on the association of achieved BP with the incidence of primary events shows that lower BP is associated with lower incidence of primary events, being consistent with previous evidence. In the subgroup with CKD, eGFR during the follow-up period did not significantly differ between the two treatment groups, suggesting that the effect on renal function might not be significantly different between the two treatments. Collectively, it is likely that better BP control by ARB plus CCB combination compared with high-dose ARB is responsible for greater benefit of ARB plus CCB combination in prevention of cardiovascular events in patients with CKD. However, the Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial provided the evidence that in patients with hypertension at high risk for cardiovascular events a RAS blocker plus CCB combination reduced the progression of CKD,27 as well as cardiovascular morbidity and mortality,28 more effectively than a RAS blocker plus hydrochlorothiazide. Therefore, it cannot be completely excluded that the BP-independent beneficial effect or renal protective effect of ARB plus CCB combination might partially contribute to the prevention of cardiovascular events in patients with CKD.
Recently, we have reported the results of the OSCAR study21 indicating that there was no significant difference between ARB plus CCB combination and high-dose ARB in the prevention of primary events in overall patients, although ARB plus CCB combination reduced the incidence of primary events in patients with baseline cardiovascular disease more than high-dose ARB. Thus, our previous report has revealed the superiority of ARB plus CCB combination over high-dose ARB in patients with baseline cardiovascular disease.21 In the current subanalysis, a greater proportion of patients with CKD manifested coexisting baseline cardiovascular disease compared with those without CKD. Therefore, we further performed multivariable Cox regression analysis for overall patients, and for patients with CKD and without CKD. We found that allocation to high-dose ARB, but not the presence of baseline cardiovascular disease, was an independent prognostic factor for primary outcome events in patients with CKD. Thus, CKD itself contributes significantly to the superiority of ARB plus CCB combination in prevention of cardiovascular events.
Study limitation
There are several study limitations for the present analysis. First, it is well established that albuminuria, as well as reduced eGFR, is an independent risk factor for cardiovascular disease and death.1, 2, 3, 4, 5, 6, 8, 9, 10, 11 In the current study, we did not examine urinary albumin excretion, because the primary purpose of the OSCAR study was to examine the effect of the two treatments on the incidence of cardiovascular events. Therefore, the precise effect of these two treatments on renal function or the detailed role of renal function in the incidence of primary events was not elucidated in the current study. Second, the degree of sodium intake significantly influences the effects of RAS blockers or CCB in patients with CKD.29, 30 However, in the OSCAR study, we did not mandate salt restriction and did not monitor urinary sodium levels. Therefore, we had no information on the amount of salt intake in the OSCAR study. Third, in patients with CKD, dosing time of antihypertensive drugs or the pattern of night-time BP significantly affects the risk of cardiovascular events.31 However, in this study, we did not have available accurate information on when (in the morning or at night) the patients took the medications, and did not take 24-h BP measurements. Finally, it is unclear whether the present findings on elderly hypertensive patients can be applied to nonelderly hypertensive patients.
In conclusion, in Japanese elderly high-risk hypertensive patients, ARB plus CCB combination therapy was more effective in prevention of cardiovascular events in patients with CKD compared with high-dose ARB therapy. It is likely that better BP control with ARB plus CCB combination is responsible for greater benefit of ARB plus CCB combination in prevention of primary events. CKD defined by eGFR contributes significantly to the relative effectiveness of ARB plus CCB combination versus high-dose ARB in elderly high-risk hypertensive patients. Our present work provided novel insight into the antihypertensive strategy for patients with CKD.
MATERIALS AND METHODS
The rationale, study design, treatment protocol, organization, and management of the OSCAR study (see Appendix) have been previously reported.21, 22 In brief, the OSCAR study was a multicenter prospective randomized open-label blinded end-point study of 1164 hypertensive patients aged 65–84 years who had cardiovascular disease (cerebrovascular disease, cardiac disease, vascular disease, or renal dysfunction) and/or type 2 diabetes at baseline. In the run-in period, all eligible patients received olmesartan (an ARB) monotherapy at a dose of 20 mg/day (a standard dose). If the target BP control (<140/90 mm Hg) was not achieved by olmesartan (20 mg/day) monotherapy and the treatment was well tolerated, patients were randomized to one of the two treatment arms, and received either (1) a doubled dose (40 mg/day) of olmesartan (high-dose ARB monotherapy) or (2) a CCB (amlodipine or azelnidipine) in addition to 20 mg/day olmesartan (ARB plus CCB combination therapy). If further additional antihypertensive treatment was required to achieve the target BP, other antihypertensive drugs such as diuretics or beta blockers could be added, but ARBs, angiotensin-converting enzyme inhibitors, and CCBs were prohibited. The follow-up period was 3 years. This study was conducted under the Declaration of Helsinki and was approved by the Institutional Review Board at each trial site. All patients gave written informed consent.
The primary end point was the time to the first event. The primary end-point events were a composite of fatal and nonfatal cardiovascular events, including cerebrovascular disease (cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage, unspecified stroke, and transient ischemic attack), coronary artery disease (sudden death, myocardial infarction, angina pectoris, and asymptomatic myocardial ischemia), heart failure, other arteriosclerotic diseases (aortic aneurysm, aortic dissection, and atherosclerotic diseases), diabetic microvascular complications (neuropathy, retinopathy, and nephropathy), deterioration of renal function (doubling of serum creatinine, end-stage renal disease as defined by initiation of hemodialysis, or renal transplantation), and noncardiovascular death. The secondary end points were incidence of each cardiovascular event, BP change during the follow-up period, and serious adverse events other than the primary end points. The Endpoint Committee adjudicated, without knowledge of the treatment assignment, all cases of cardiovascular events and death.
Subgroup analysis according to eGFR
The subgroup analysis according to baseline eGFR was prespecified, as described in our protocol paper.22 Baseline eGFR was calculated for patients whose serum creatinine value at baseline was available. The patients without serum creatinine data at baseline were excluded from this analysis. In this study, eGFR was estimated using the new three-variable Japanese equation,32 as follows: eGFR (ml/min per 1.73 m2 of body surface area)=194 × serum creatinine−1.094 × age−0.287 × 0.739 (if female) instead of the abbreviated Modification of Diet in Renal Disease study equation.23 The referenced equation used in this study is recommended for estimation of GFR in Japanese patients, because eGFR calculated by this equation has been demonstrated to be more accurate for Japanese patients than that obtained by other equations.32 In this study, CKD was defined as an eGFR of <60 ml/min per 1.73 m2.11, 23 The study population was divided into two groups based on baseline eGFR: (1) patients with CKD (eGFR of <60 ml/min per 1.73 m2) and (2) those without CKD (eGFR of ⩾60 ml/min per 1.73 m2).
Statistical analyses
Sample size and power of the study were estimated as previously described.22
Primary analyses were performed in compliance with the intention-to-treat principle. For the primary end points, we compared between groups using the log-rank test stratified by sex, age, and risk factors. By using a stratified proportional hazards model, the HR and 95% CI were calculated for each treatment group. Time-to-first event curves were estimated by the Kaplan–Meier method. Repeated measures analysis of variance was used to compare between the groups for BP during the follow-up period, and the least square method was used to estimate the mean difference in BP between the groups. Time course of eGFR change between groups was compared using the unpaired t-test adjusted by Holm's method to avoid multiplicity at multiple time points.
To estimate the heterogeneity of the HR for the prespecified CKD category, the interaction between treatment group and CKD subgroup was assessed using the interaction terms in a stratified Cox proportional hazards model.
Multivariable Cox proportional hazards analyses were performed to determine the association of each prognostic factor with the incidence of primary outcome events, adjusted for the following covariates: sex, age, treatment group, baseline cardiovascular disease, baseline diabetes, and adjusted for systolic BP for a time-dependent covariate.
The achieved systolic BP and diastolic BP were classified into four levels: systolic BP: <130, 130–139, 140–149, and ⩾150 mm Hg; diastolic BP:<70, 70–79, 80–89, and ⩾90 mm Hg. Adjusted HRs and 95% CIs were calculated using the multiple Cox proportional hazard model adjusted by sex, age, baseline cardiovascular disease, and diabetes.
For all analyses, the overall significance level was determined as 5%, and two-sided tests were used.
Acknowledgments
This work was supported by a grant from the Japan Heart Foundation. This trial was registered with ClinicalTrails.gov, number NCT00134160.
Appendix
OSCAR Study Group
Aichi; Kenji Yamada. Akita; Goro Namekawa, Yasushi Suzuki. Aomori; Kenichi Kimura, Morio Aihara. Chiba; Akiko Soyama, Michiko Yonemitsu, Tomotane Shishikura, Toshiyuki Imasawa. Ehime; Masahiro Hasui. Fukuoka; Hidenori Urata, Hiroshi Ikezono, Masahiko Seki, Masaki Munekiyo, Takatoshi Otonari, Tetsuya Ohtsubo, Yasunori Sawayama, Yoichi Hanaoka, Yoshinori Takajo, Yuji Taira. Fukushima; Kuniyoshi Shima. Gifu; Hiroyuki Ohbayashi. Hiroshima; Kazuya Shigenobu. Hokkaido; Chieko Imamoto, Hiromitsu Yokota, Kazuo Yamagata, Kouichi Kanda, Tateo Ogura, Toshio Tsubokura. Hyogo; Akira Kosaka, Akira Tabuchi, Masaharu Shigenobu, Takatoshi Takamiya, Yasuki Makino, Yoshikazu Irie. Kagawa; Hideyasu Kiyomoto, Hirofumi Hitomi. Kagoshima; Yasuhiro Hashiguchi, Yoshihiro Fukuoka, Yoshitaka Shintomi. Kanagawa; Fusahiro Nonaka, Hiroshi Takeda, Masato Nishimura, Nariaki Kanemoto, Takayuki Furuki. Kumamoto; Akira Maki, Akira Sato, Eiichiro Tanaka, Etsuro Tsutsumi, Hajime Shono, Haruo Takeda, Hideaki Jinnouchi, Hirofumi Kann, Hiromi Fujii, Hiroyuki Shono, Hisao Fujimoto, Hisayasu Terazaki, Junichi Matsubara, Kazuhiko Yamada, Kazuhiro Nishigami, Keiichiro Tsuruta, Kenichi Koyama, Kenji Azuma, Koichiro Kataoka, Koji Sasaki, Kouji Honjio, Kunihiro Ohmori, Kunio Idegami, Masakazu Matsukawa, Masamitsu Toihata, Mikiko Suematsu, Motoko Tanaka, Osamu Hashiguchi, Ryo Fukami, Seiko Fujimoto, Shinichi Uemura, Shiro Mimori, Shojiro Naomi, Shouji Maruta, Shuichi Matsuo, Sunao Kojima, Taiji Sekigami, Takashi Fukunaga, Takashi Kudoh, Takashi Ono, Takeshi Koga, Tomio Wakita, Tomohiro Sawada, Toshihiko Sakanashi, Toshihiro Higashi, Yasuhiro Nagayoshi, Yasuhiro Sakamoto, Yoshihiro Kimura, Yuji Miyao, Yutaka Horio, Kyoto; Ken Takenaka. Miyazaki; Hiroshi Senokuchi, Hirotsugu Ohta, Juniti Miyata, Naoto Yokota, Takeshi Yamamoto. Nagasaki; Hiroyuki Oka, Yoshito Tanioka, Niigata; Toshihide Shu. Okayama; Hirohiko Asonuma, Naoki Kashihara, Naruya Tomita, Takehiko Tokura, Tamaki Sasaki. Osaka; Hidenori Koyama, Katsuo Suyama, Kenei Shimada, Masahito Imanishi, Masanori Emoto, Masayuki Hosoi, Masayuki Nagata, Nobuo Wakaki, Shiro Yanagi, Takao Yoshioka, Takeshi Horio, Tetsuya Hayashi. Saga; Kazuo Moroe, Shiro Hata. Saitama; Hideto Muranaka, Masaru Arai, Shouji Mashiba, Souichirou Ishimoto, Tadahiko Ogasawara, Tomoya Fujino, Tomoyuki Okudaira. Shimane; Yuko Yamane. Shizuoka; Masako Waki. Tokushima; Akira Ota. Kazuto Okagawa, Kenzo Motoki, Takashi Iwase. Tokyo; Akihiko Hachiya, Hiromi Takekawa, Kenzo Matsumura, Masato Yamamoto, Minoru Hojo, Shiho Kaku, Tetsuya Taniguchi, Yasunaga Hiyoshi, Yutaka Shimizu. Yamaguchi; Hideaki Hanamiya.
SK-M has received consultancy fees/honoraria from AstraZeneca, Astellas, Bayer, Boerhinger Ingelheim, Daiichi-Sankyo, Kyowa Hakko Kirin, Novartis, Pfizer, Takeda, Shionogi, and Servier. HO has received grants from the Japan Heart Foundation, and has received consultancy fees/honoraria from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Eisai, Kowa, Kyowa Hakko Kirin, MSD, Novartis, Pfizer, Sanofi-Aventis, Schering-Plough, and Takeda. All other authors declare that they have no conflict of interest.
References
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Irie F, Iso H, Sairenchi T, et al. The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int. 2006;69:1264–1271. doi: 10.1038/sj.ki.5000284. [DOI] [PubMed] [Google Scholar]
- Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63:1121–1129. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- Ninomiya T, Kiyohara Y, Kubo M, et al. Chronic kidney disease and cardiovascular disease in a general Japanese population: the Hisayama Study. Kidney Int. 2005;68:228–236. doi: 10.1111/j.1523-1755.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- Ruilope LM, Salvetti A, Jamerson K, et al. Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. J Am Soc Nephrol. 2001;12:218–225. doi: 10.1681/ASN.V122218. [DOI] [PubMed] [Google Scholar]
- Mann JF, Gerstein HC, Pogue J, et al. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- Hillege HL, Girbes AR, de Kam PJ, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Rice MM, AJ K, et al. Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the Prevention of Events with ACE inhibition (PEACE) trial. Circulation. 2006;114:26–31. doi: 10.1161/CIRCULATIONAHA.105.592733. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Mancia G, De Backer G, Dominiczak A, et al. Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of hypertension (ESH) and of the European Society of cardiology (ESC) Eur Heart J. 2007;2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- Ogihara T, Kikuchi K, Matsuoka H, et al. The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2009) Hypertens Res. 2009;32:3–107. [PubMed] [Google Scholar]
- Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1840–1848. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Kim-Mitsuyama S, Matsui K, et al. Angiotensin II receptor blocker-based therapy in Japanese elderly high-risk hypertensive patients Am J Med 2012. e-pub ahead of print 16 April 2012. [DOI] [PubMed]
- Ogawa H, Kim-Mitsuyama S, Jinnouchi T, et al. Rationale, design and patient baseline characteristics of OlmeSartan and calcium antagonists randomized (OSCAR) study: a study comparing the incidence of cardiovascular events between high-dose angiotensin II receptor blocker (ARB) monotherapy and combination therapy of ARB with calcium channel blocker in Japanese elderly high-risk hypertensive patients (ClinicalTrials. gov no. NCT00134160) Hypertens Res. 2009;32:575–580. doi: 10.1038/hr.2009.60. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Reboldi G, Angeli F, et al. Angiotensin-converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention. Hypertension. 2005;46:386–392. doi: 10.1161/01.HYP.0000174591.42889.a2. [DOI] [PubMed] [Google Scholar]
- Bakris GL, Sarafidis PA, Weir MR, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010;375:1173–1181. doi: 10.1016/S0140-6736(09)62100-0. [DOI] [PubMed] [Google Scholar]
- Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- Esnault VL, Ekhlas A, Delcroix C, et al. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol. 2005;16:474–481. doi: 10.1681/ASN.2004060505. [DOI] [PubMed] [Google Scholar]
- Vogt L, Waanders F, Boomsma F, et al. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol. 2008;19:999–1007. doi: 10.1681/ASN.2007060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Mojon A, et al. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22:2313–2321. doi: 10.1681/ASN.2011040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]