Abstract
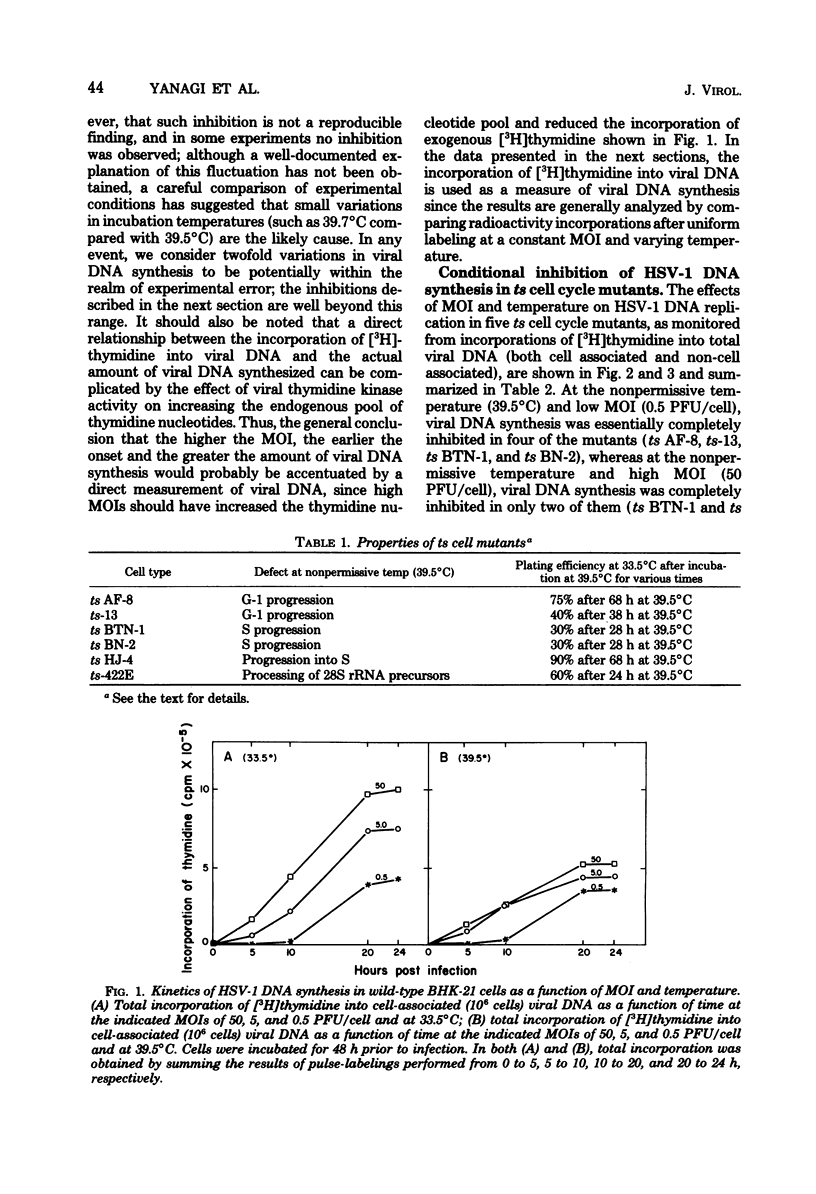
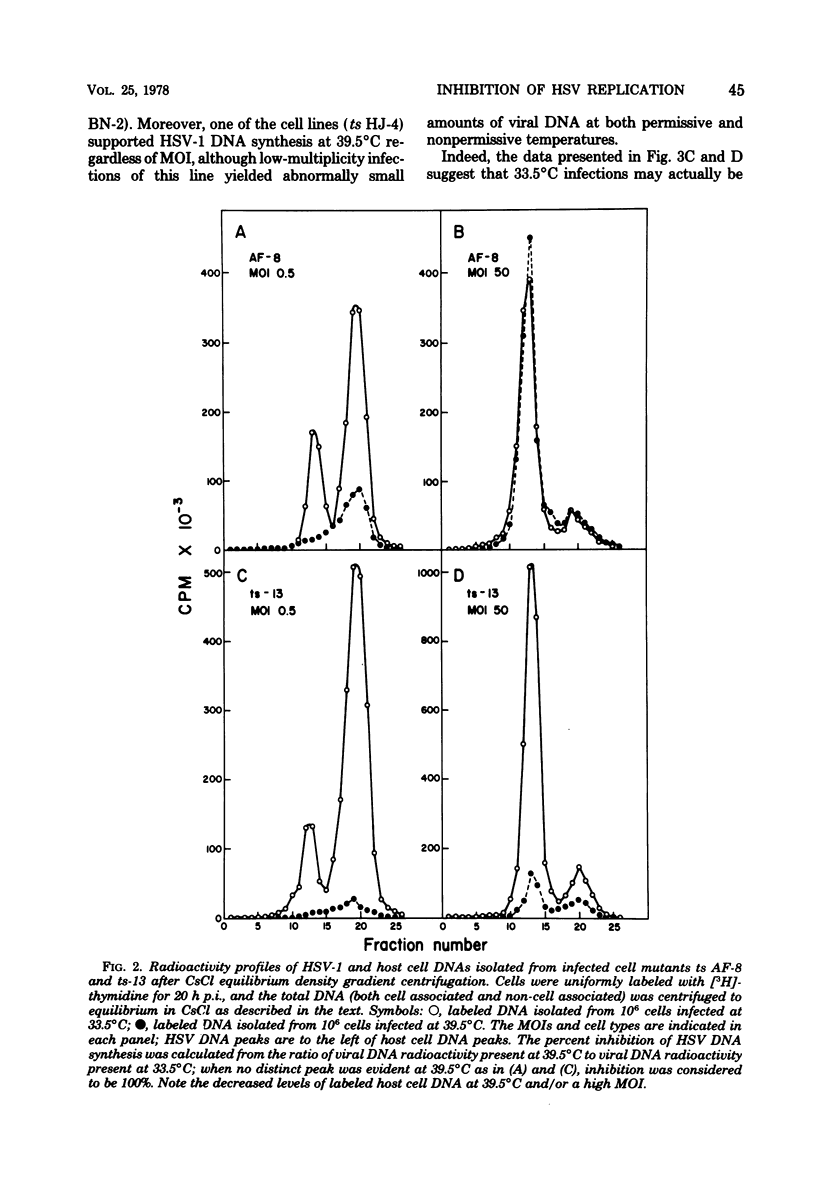
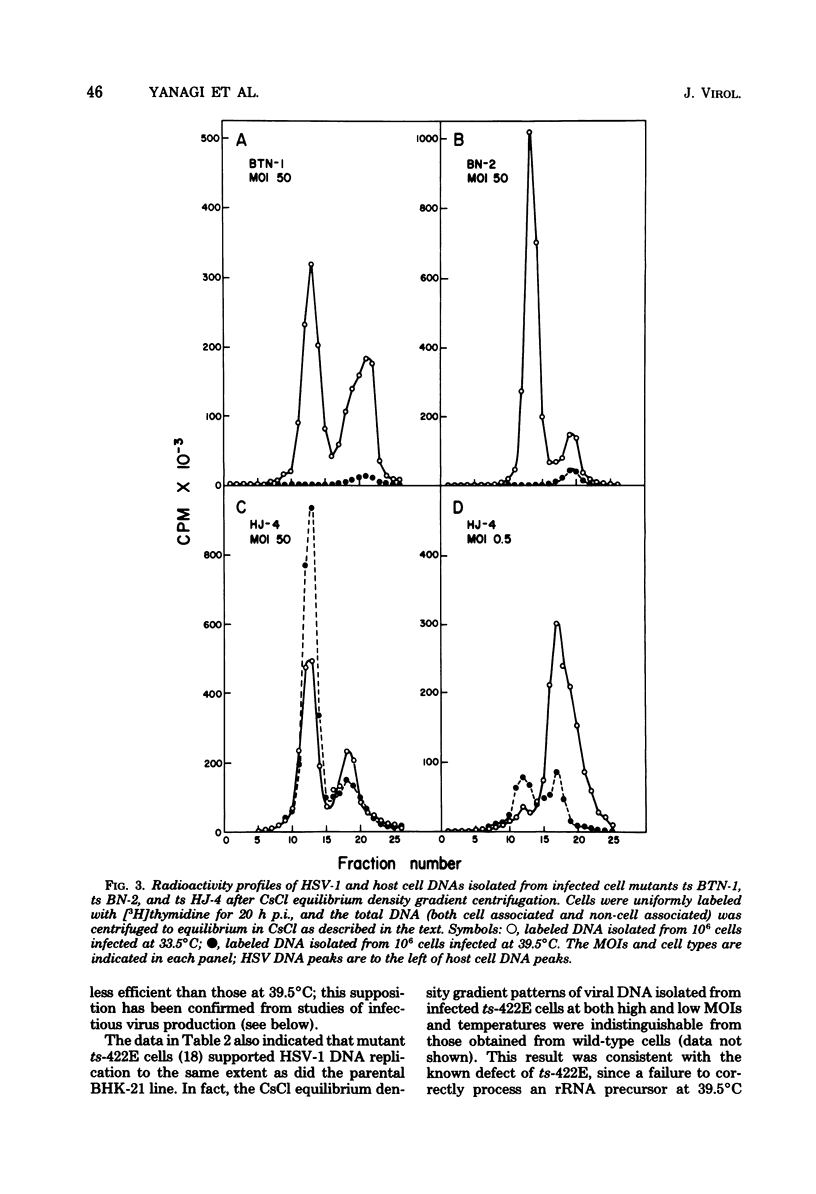
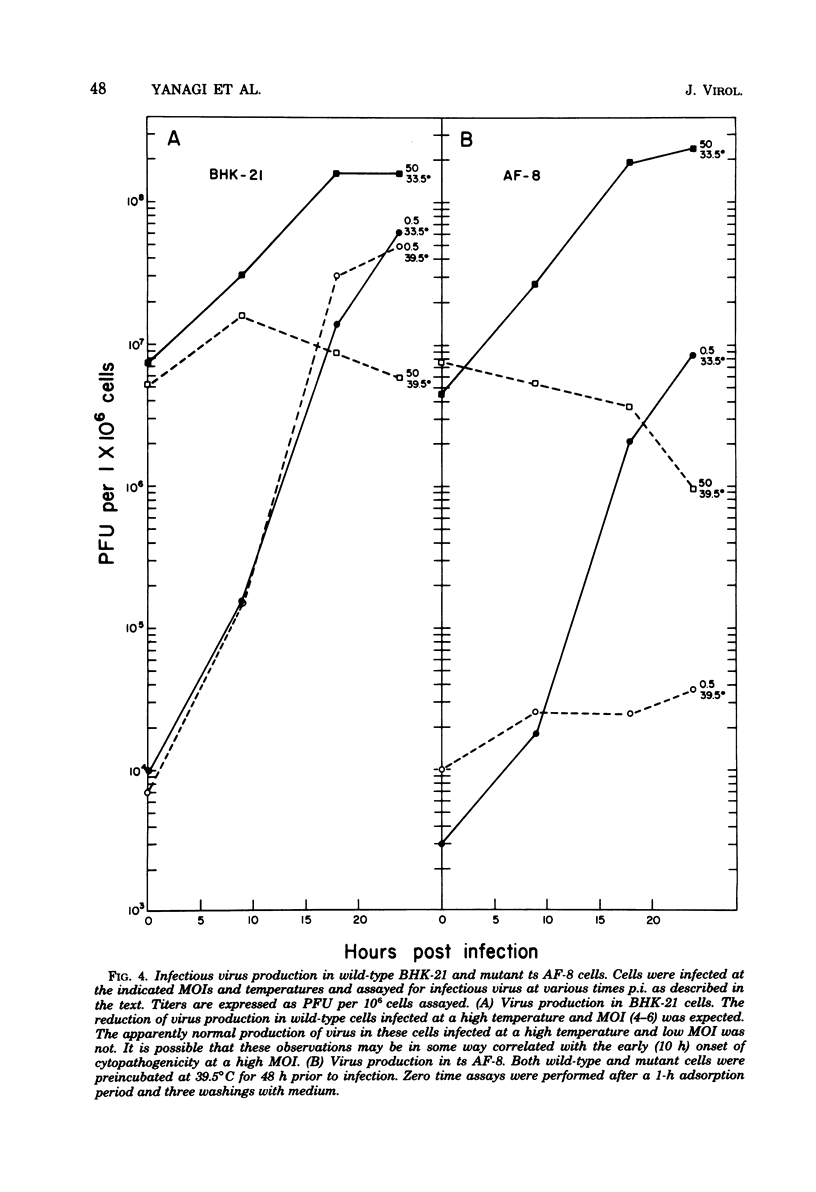
Herpes simplex virus type 1 DNA synthesis and infections progeny production were studied in five different conditional hamster (BHK-21) cell cycle mutants. At the nonpermissive temperature (39.5 degrees C), both events were strongly inhibited in four of these cell lines. The degree of inhibition was a reproducible characteristic of each cell mutant and in two cases was dependent upon the multiplicity of infection. Experiments involving shifts to the nonpermissive temperature at least 3 h postinfection at 33.5 degrees C suggested that the defects in viral replication were not due to faulty adsorption, penetration, or uncoating, whereas experiments involving shifts of infected cells from the nonpermissive temperature to 33.5 degrees C revealed the reversible nature of the inhibition.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstin S. J., Meiss H. K., Basilico C. A temperature-sensitive cell cycle mutant of the BHK cell line. J Cell Physiol. 1974 Dec;84(3):397–408. doi: 10.1002/jcp.1040840308. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Vaughan R. K., Lawrence W. C. Deoxyribonucleic acid synthesis in synchronized mammalian KB cells infected with herpes simplex virus. J Virol. 1971 Jun;7(6):783–791. doi: 10.1128/jvi.7.6.783-791.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARNHAM A. E., NEWTON A. A. The effect of some environmental factors on herpes virus grown in HeLa cells. Virology. 1959 Apr;7(4):449–461. doi: 10.1016/0042-6822(59)90073-x. [DOI] [PubMed] [Google Scholar]
- Falke D., Heicke B., Bässler R. The effect of arabinofuranosyl-cytosine upon the synthesis of Herpesvirus hominis. Arch Gesamte Virusforsch. 1972;39(1):48–62. doi: 10.1007/BF01241528. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The effect of the temperature of incubation on the formation and release of herpes simplex virus in infected FL cells. Virology. 1959 Aug;8:508–524. doi: 10.1016/0042-6822(59)90052-2. [DOI] [PubMed] [Google Scholar]
- Lancz G. J. Effect of pH on the kinetics of herpes simplex virus inactivation at 36 degrees. Virology. 1976 Dec;75(2):488–491. doi: 10.1016/0042-6822(76)90048-9. [DOI] [PubMed] [Google Scholar]
- Levitt J., Becker Y. The effect of cytosine arabinoside on the replication of herpes simplex virus. Virology. 1967 Jan;31(1):129–134. doi: 10.1016/0042-6822(67)90016-5. [DOI] [PubMed] [Google Scholar]
- Macnab J. C. Transformation of rat embryo cells by temperature-sensitive mutants of herpes simplex virus. J Gen Virol. 1974 Jul;24(1):143–153. doi: 10.1099/0022-1317-24-1-143. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Garfinkle B., Adler R., Devinney D., Florkiewicz R., Shaw J. E. Transformation of cells by herpes simplex virus--fact or fantasy? Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):765–772. doi: 10.1101/sqb.1974.039.01.088. [DOI] [PubMed] [Google Scholar]
- Meiss H. K., Basilico C. Temperature sensitive mutants of BHK 21 cells. Nat New Biol. 1972 Sep 20;239(90):66–68. doi: 10.1038/newbio239066a0. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Falke D., Zahn R. K. DNA-dependent DNA polymerase pattern in noninfected and herpesvirus infected rabbit kidney cells. Arch Gesamte Virusforsch. 1973;42(3):278–284. doi: 10.1007/BF01265653. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Okubo C. K., Raskas H. J. Abortive replication of adenovirus 2 in the temperature-sensitive hamster cell mutant tsAF8. Virology. 1977 May 1;78(1):1–10. doi: 10.1016/0042-6822(77)90074-5. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Raskas H. J., Basilico C. Temperature-sensitive cell mutations that inhibit adenovirus 2 replication. Proc Natl Acad Sci U S A. 1975 Jan;72(1):328–332. doi: 10.1073/pnas.72.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Li J. L. Demonstration of the oncogenic potential of herpes simplex viruses and human cytomegalovirus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):747–763. doi: 10.1101/sqb.1974.039.01.087. [DOI] [PubMed] [Google Scholar]
- Roubal J. Cytosine-arabinoside does not inhibit incorporation of 3H-thymidine in herpes simplex virus transformed cells. Arch Virol. 1977;53(3):255–259. doi: 10.1007/BF01314669. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Yamanishi K. Transformation of hamster embryo and human embryo cells by temperature sensitive mutants of herpes simplex virus type 2. Virology. 1974 Sep;61(1):306–311. doi: 10.1016/0042-6822(74)90267-0. [DOI] [PubMed] [Google Scholar]
- Talavera A., Basilico C. Temperature sensitive mutants of BHK cells affected in cell cycle progression. J Cell Physiol. 1977 Sep;92(3):425–436. doi: 10.1002/jcp.1040920310. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Meiss H. K., Basilico C. A temperature-sensitive mutation affecting 28S ribosomal RNA production in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1273–1277. doi: 10.1073/pnas.70.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]



