Abstract
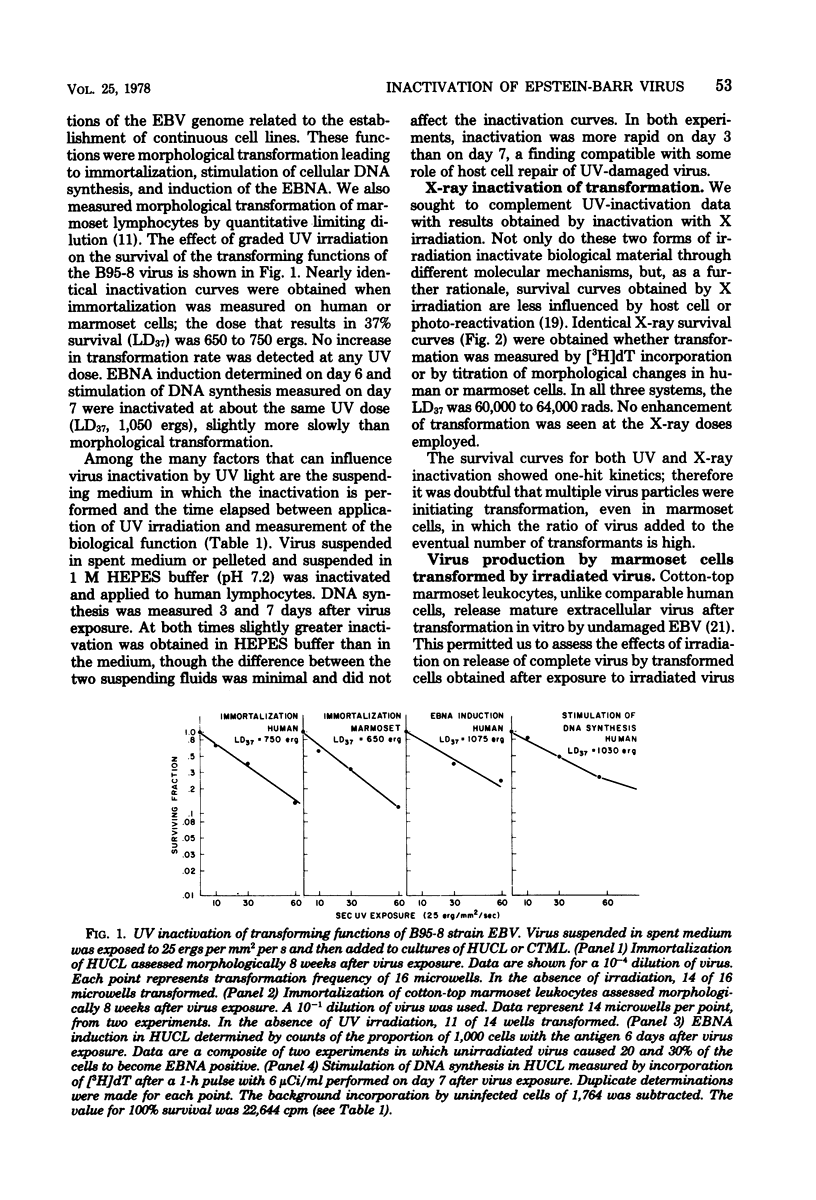
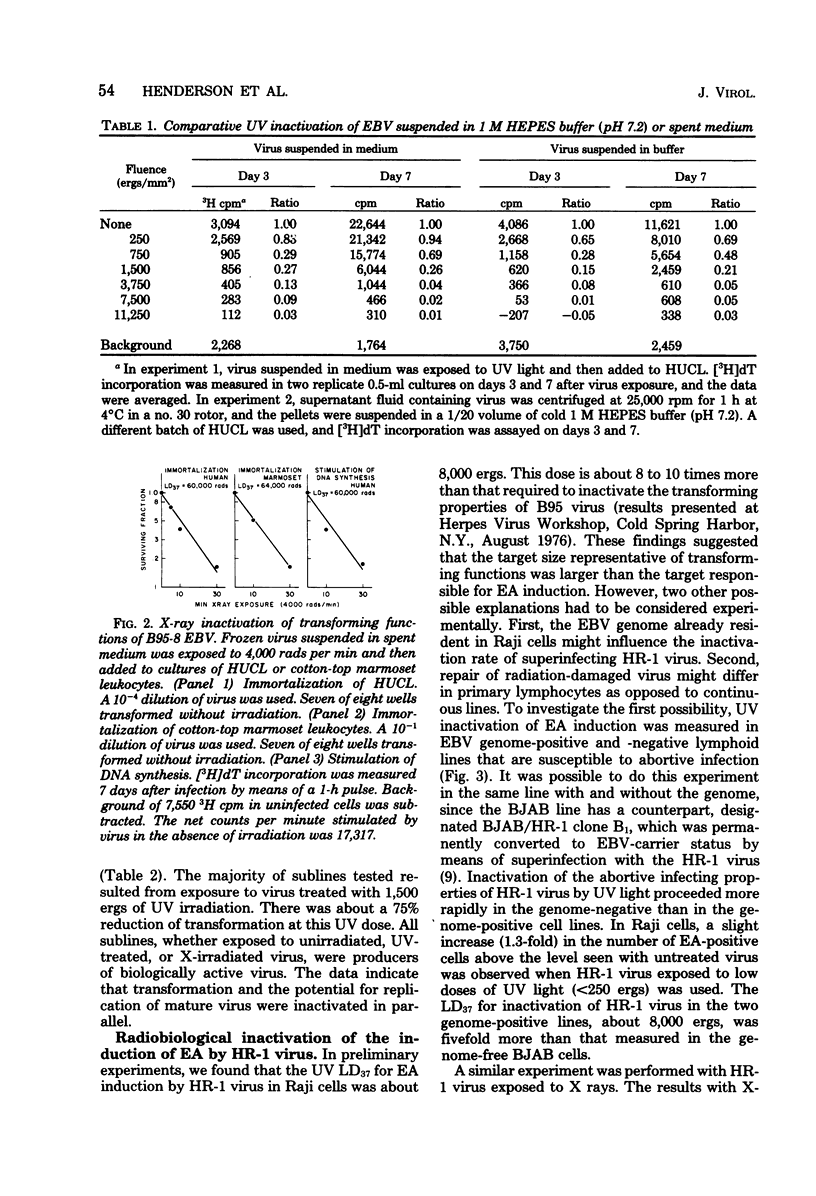
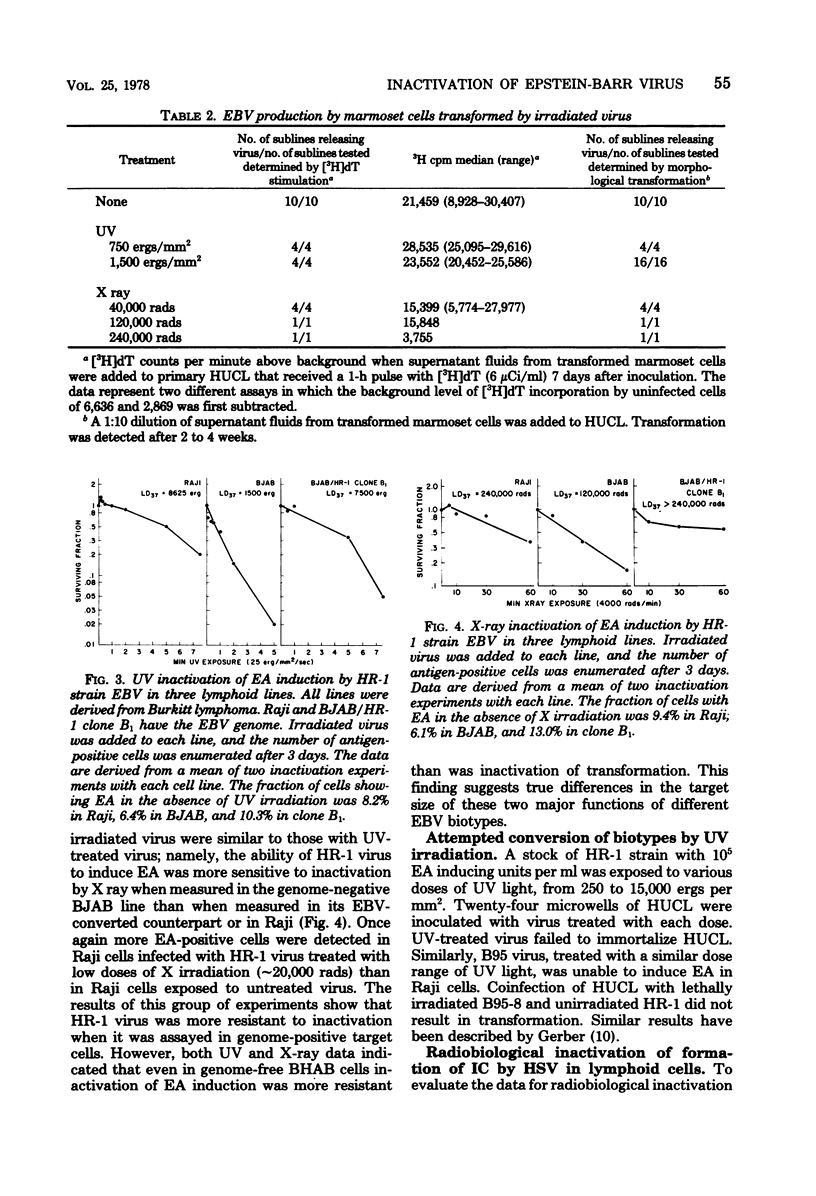
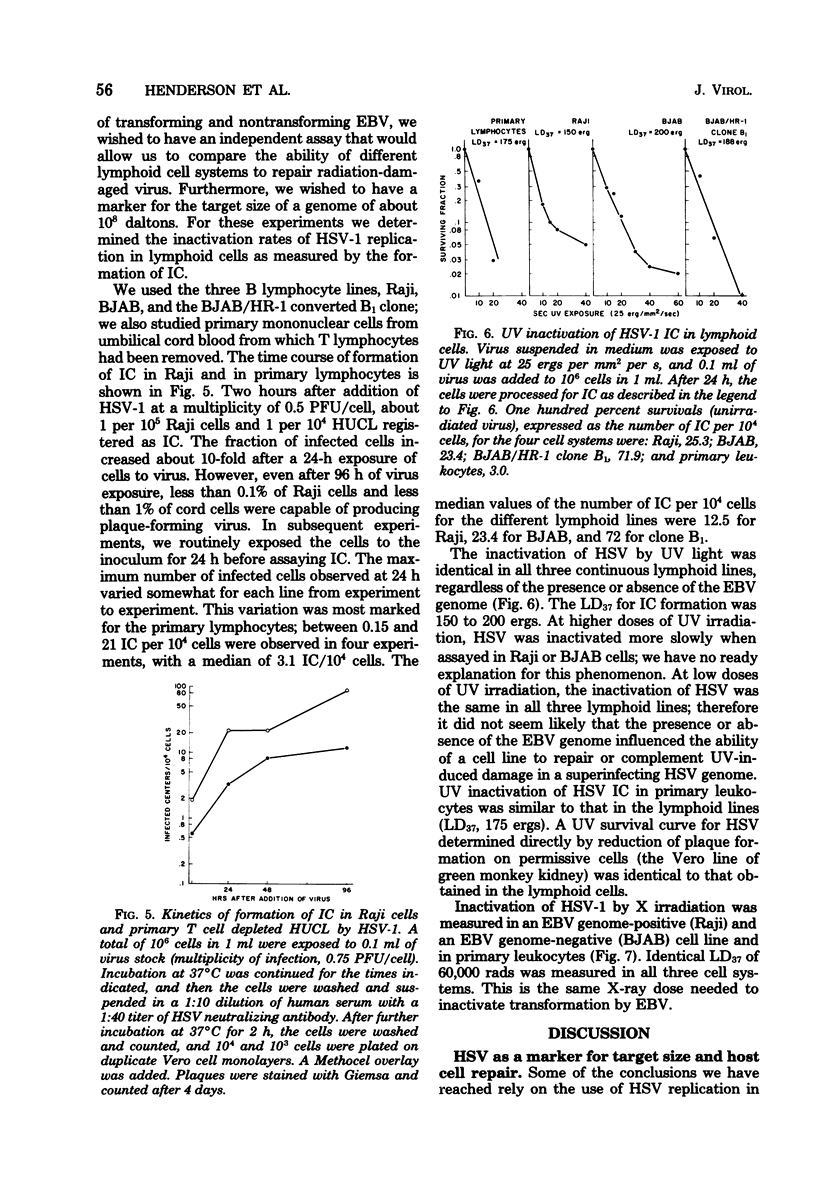
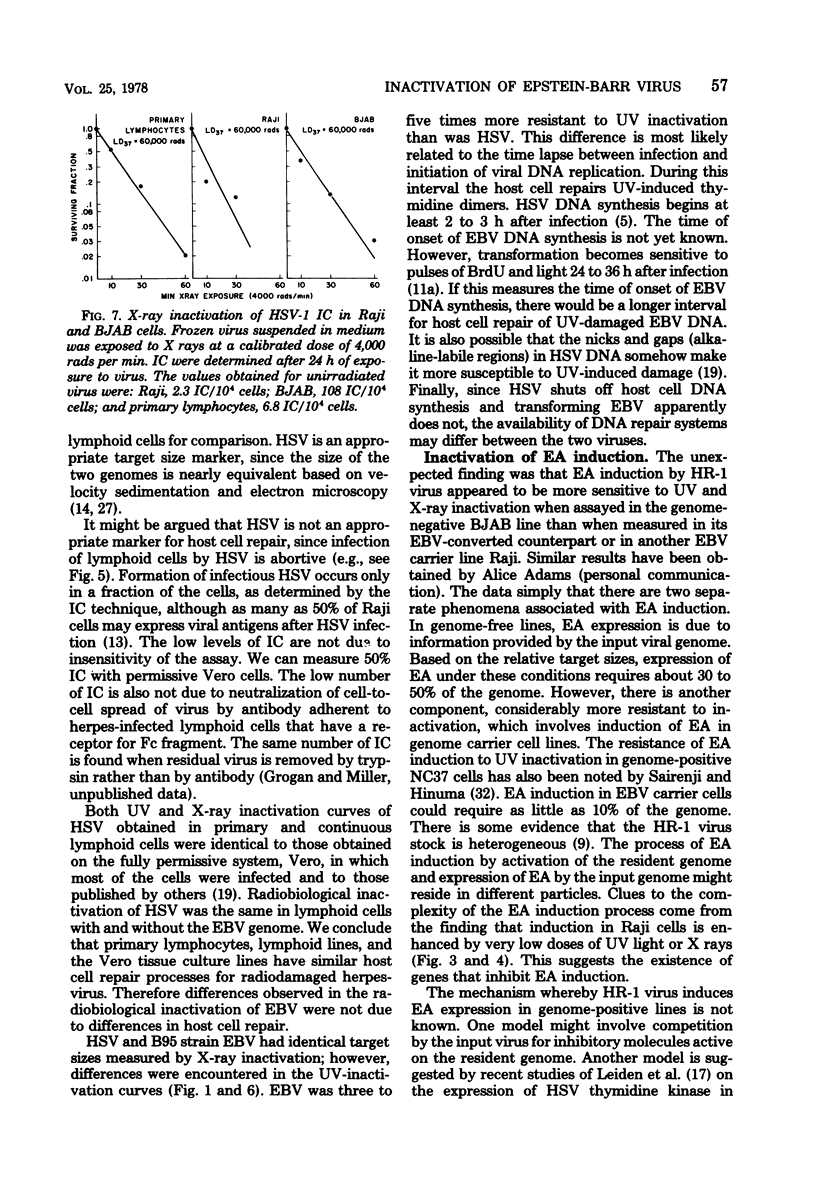
Lymphocyte transforming properties of B95-8 strain Epstein-Barr virus (EBV) are very sensitive to inactivation by either UV or X irradiation. No dose of irradiation increases the transforming capacity of EBV. The X-ray dose needed for inactivation of EBV transformation (dose that results in 37% survival, 60,000 rads) is similar to the dose required for inactivation of plaque formation by herpes simplex virus type 1 (Fischer strain). Although herpes simplex virus is more sensitive than EBV to UV irradiation, this difference is most likely due to differences in the kinetics or mechanisms of repair of UV damage to the two viruses. The results lead to the hypothesis that a large part, or perhaps all, of the EBV genome is in some way needed to initiate transformation. The abilities of EBV to stimulate host cell DNA synthesis, to induce nuclear antigen, and to immortalize are inactivated in parallel. All clones of marmoset cells transformed by irradiated virus produce extracellular transforming virus. These findings suggest that the abilities of the virus to transform and to replicate complete progeny are inactivated together. The amounts of UV and X irradiation that inactivate transformation by B95-8 virus are less than the dose needed to inactivate early antigen induction by the nontransforming P3HR-1 strain of EBV. Based on radiobiological inactivation, 10 to 50% of the genome is needed for early antigen induction. Inactivation of early antigen induction is influenced by the cells in which the assay is performed. Inactivation proceeds more rapidly in EBV genome-free cells than in genome carrier Raji or in P3HR-1 converted EBV genome-free cells clone B1. These results indicate that the resident EBV genome participates in the early antigen induction process. Variation in radio-biological killing of B95-8 and P3HR-1 EBV is not attributable to variations in the repair capacities of the cells in which the viruses were assayed, since inactivation of HSV was the same in primary lymphocytes and in all lymphoid cell lines tested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Effect of ultraviolet irradiation on the survival of simian virus 40 functions in human and mouse cells. J Virol. 1970 Oct;6(4):393–399. doi: 10.1128/jvi.6.4.393-399.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A., Lindahl T. Epstein-Barr virus genomes with properties of circular DNA molecules in carrier cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1477–1481. doi: 10.1073/pnas.72.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C., Di Mayorca G. Radiation target size of the lytic and the transforming ability of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):125–127. doi: 10.1073/pnas.54.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Relative target sizes for the inactivation of the transforming and reproductive abilities of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):121–124. doi: 10.1073/pnas.54.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Vaughan R. K., Lawrence W. C. Deoxyribonucleic acid synthesis in synchronized mammalian KB cells infected with herpes simplex virus. J Virol. 1971 Jun;7(6):783–791. doi: 10.1128/jvi.7.6.783-791.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defendi V., Jensen F. Oncogenicity by DNA tumor viruses: enhancement after ultraviolet and cobalt-60 radiations. Science. 1967 Aug 11;157(3789):703–705. doi: 10.1126/science.157.3789.703. [DOI] [PubMed] [Google Scholar]
- Duff R., Knight P., Rapp F. Variation in oncogenic and transforming potential of PARA (defective SV40)-adenovirus 7. Virology. 1972 Mar;47(3):849–853. doi: 10.1016/0042-6822(72)90579-x. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster embryo cells after exposure to inactivated herpes simplex virus type 1. J Virol. 1973 Aug;12(2):209–217. doi: 10.1128/jvi.12.2.209-217.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresen K. O., Hausen H. Establishment of EBNA-expressing cell lines by infection of Epstein-Barr virus (EBV)-genome-negative human lymphoma cells with different EBV strains. Int J Cancer. 1976 Feb 15;17(2):161–166. doi: 10.1002/ijc.2910170203. [DOI] [PubMed] [Google Scholar]
- Henderson E., Robinson J., Frank A., Miller G. Epstein-Barr virus: transformation of lymphocytes separated by size or exposed to bromodeoxyuridine and light. Virology. 1977 Oct 1;82(1):196–205. doi: 10.1016/0042-6822(77)90042-3. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G., zur Hausen H. Effect of herpes simplex virus on cultured Burkitt tumor cells and its failure to influence the Epstein-Barr virus carrier state. Cancer Res. 1969 Feb;29(2):489–494. [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATARJET R., MORENNE P., BERGER R. Un appareil simple pour le dosage des rayonnements ultraviolets émis par les lampes germicides. Ann Inst Pasteur (Paris) 1953 Aug;85(2):175–184. [PubMed] [Google Scholar]
- Leiden J. M., Buttyan R., Spear P. G. Herpes simplex virus gene expression in transformed cells. I. Regulation of the viral thymidine kinase gene in transformed L cells by products of superinfecting virus. J Virol. 1976 Nov;20(2):413–424. doi: 10.1128/jvi.20.2.413-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Rubin H. Radiation studies of avian tumor viruses and of Newcastle disease virus. Virology. 1966 Apr;28(4):533–542. doi: 10.1016/0042-6822(66)90238-8. [DOI] [PubMed] [Google Scholar]
- Lytle C. D., Aaronson S. A., Harvey E. Host-cell reactivation in mammalian cells. II. Survival of herpes simplex virus and vaccinia virus in normal human and xeroderma pigmentosum cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Aug;22(2):159–165. [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975 May;92(2):478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Replication of viral deoxyribonucleic acid and breakdown of cellular deoxyribonucleic acid in Epstein-Barr virus infection. J Virol. 1972 Apr;9(4):714–716. doi: 10.1128/jvi.9.4.714-716.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana T., Kieff E. Epstein-barr virus-specific RNA. II. Analysis of polyadenylated viral RNA in restringent, abortive, and prooductive infections. J Virol. 1977 May;22(2):321–330. doi: 10.1128/jvi.22.2.321-330.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owada M., Ihara S., Toyoshima K., Sugino Y., Kozai Y. Ultraviolet inactivation of avian sarcoma viruses: biological and biochemical analysis. Virology. 1976 Feb;69(2):710–718. doi: 10.1016/0042-6822(76)90499-2. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., Huang C. H., Huang Y. T. Epstein-Barr virus genome in infectious mononucleosis. Nature. 1976 Oct 28;263(5580):787–789. doi: 10.1038/263787a0. [DOI] [PubMed] [Google Scholar]
- Pritchett R. F., Hayward S. D., Kieff E. D. DNA of Epstein-Barr virus. I. Comparative studies of the DNA of Epstein-Barr virus from HR-1 and B95-8 cells: size, structure, and relatedness. J Virol. 1975 Mar;15(3):556–559. doi: 10.1128/jvi.15.3.556-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Robinson J. E., Andiman W. A., Henderson E., Miller G. Host-determined differences in expression of surface marker characteristics on human and simian lymphoblastoid cell lines transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1977 Feb;74(2):749–753. doi: 10.1073/pnas.74.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. Assay for Epstein-Barr virus based on stimulation of DNA synthesis in mixed leukocytes from human umbilical cord blood. J Virol. 1975 May;15(5):1065–1072. doi: 10.1128/jvi.15.5.1065-1072.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. J., Wildy P., Cameron K. R. Formation of small plaques by herpes viruses irradiated with ultraviolet light. Virology. 1971 Sep;45(3):808–812. doi: 10.1016/0042-6822(71)90201-7. [DOI] [PubMed] [Google Scholar]
- Sairenji T., Hinuma Y. Ultraviolet inactivation of Epstein-Barr virus: effect on synthesis of virus-associated antigens. Int J Cancer. 1975 Jul 15;16(1):1–6. doi: 10.1002/ijc.2910160102. [DOI] [PubMed] [Google Scholar]
- Shope T. C., Klein-Robbenhaar J., Miller G. Fatal encephalitis due to Herpesvirus hominis: use of intact brain cells for isolation of virus. J Infect Dis. 1972 May;125(5):542–544. doi: 10.1093/infdis/125.5.542. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Miyagi M., Yajima Y., Nonoyama M. Improved production of Epstein-Barr virus DNA for nucleic acid hybridization studies. Virology. 1976 Oct 1;74(1):81–85. doi: 10.1016/0042-6822(76)90130-6. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H., Schulte-Holthausen H. Presence of EB virus nucleic acid homology in a "virus-free" line of Burkitt tumour cells. Nature. 1970 Jul 18;227(5255):245–248. doi: 10.1038/227245a0. [DOI] [PubMed] [Google Scholar]



