Abstract
BACKGROUND
The Chronic Care Model is an effective framework for improving chronic disease management. There is scarce literature describing this model for people living with HIV. Decision Support (DS) and Clinical Information Systems (CIS) are two components of this model that aim to improve care by changing health care provider behavior.
OBJECTIVE
Our aim was to assess the effectiveness of DS and CIS interventions for individuals with HIV, through a systematic literature review.
DESIGN
We performed systematic electronic searches from 1996 to February 2011 of the medical (E.g. Medline, EMBASE, CINAHL) and grey literature. Effectiveness was measured by the frequency of statistically significant outcome improvement. Data and key equity indicator extraction and synthesis was completed.
PARTICIPANTS AND INTERVENTIONS
We included comparative studies of people living with HIV that examined the impact of DS or CIS interventions on outcomes.
MAIN MEASURES
The following measures were assessed: outcome (immunological/virological, medical, psychosocial, economic measures) and health care process/performance measures.
KEY RESULTS
Records were screened for relevance (n = 10,169), full-text copies of relevant studies were obtained (n = 123), and 16 studies were included in the review. Overall, 5/9 (55.6%) and 17/41 (41.5%) process measures and 5/12 (41.7%) and 3/9 (33.3%) outcome measures for DS and CIS interventions, respectively, were statistically significantly improved. DS–explicit mention of implementation of guidelines and CIS-reminders showed the most frequent improvement in outcomes. DS-only interventions were more effective than CIS-only interventions in improving both process and outcome measures. Clinical, statistical and methodological heterogeneity among studies precluded meta-analysis. Primary studies were methodologically weak and often included multifaceted interventions that made assessment of effectiveness challenging.
CONCLUSIONS
Overall, DS and CIS interventions may modestly improve care for people living with HIV, having a greater impact on process measures compared to outcome measures. These interventions should be considered as part of strategies to improve HIV care through changing provider performance.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2145-y) contains supplementary material, which is available to authorized users.
KEY Words: HIV/AIDS, chronic disease management, Chronic Care Model, decision support, clinical information systems, practice guidelines, systematic review
INTRODUCTION
While the global incidence of HIV infection has stabilized, the overall number of people living with HIV has steadily increased, as HIV treatments extend life.1 In the developed world, mortality from non-AIDS events now exceeds that of AIDS-defining opportunistic diseases in individuals receiving effective antiretroviral therapy (ART).2,3 There is an increasing role, particularly in high-resource settings, for shifting our approach to HIV care from one of tertiary/specialist care to one that includes the prevention and treatment of common diseases.4–6 As HIV infection moves into the realm of a chronic disease managed primarily in the ambulatory setting, it is important to understand how the principles of chronic disease management can be applied to this population.
The Chronic Care Model (CCM; Wagner Model)7 is a well-established framework for effective, evidence-based clinical and quality improvement in chronic disease management. CCM initiatives have become the foundation of patient care for ischemic heart disease,8,9 congestive heart failure,10–12 diabetes,10,11,13 asthma,10,11,13 chronic obstructive pulmonary disease (COPD),14 and depression,10 and have been shown to improve patient-reported health status and quality of life in primary care settings.15 However, literature on the application of the CCM framework, and its elements for HIV management in the primary care setting, is limited.
Decision Support (DS) and Clinical Information Systems (CIS) are two elements of the CCM that specifically target changing provider behavior to improve patient care. DS interventions, such as the distribution of educational materials, use of clinical practice guidelines, and case discussions, emphasize the integration of evidence-based guidelines into clinical practice. CIS interventions are based on establishing information systems to organize patient data in order to improve the delivery of care, such as by developing rosters of patients with certain conditions and providing reminders.7 Several systematic reviews have attempted to determine the effectiveness of these elements on patient care, although clinical, methodological and statistical heterogeneity of the included studies made data synthesis and generalizability difficult.10,16–18 As technology advances, DS and CIS interventions have become intertwined.16,18,19 In fact, computerization of decision support tools is likely an important feature contributing to their effectiveness.13,19 Some systematic reviews have examined the DS and CIS elements explicitly in the context of the CCM framework,10,13 in addition to many that have not directly identified these components, but have studied interventions that clearly fall under these categories.17–21 These reviews demonstrate that DS and CIS interventions are often successful,10,13,17–22 although the magnitude of effect may be modest17 and also more clearly improve provider performance than patient health measures.10,13,18
The purpose of this systematic review is to describe the application and effectiveness of DS and CIS interventions for persons living with HIV, and to identify the successful characteristics of these interventions.
METHODS
Protocol
A protocol for record eligibility was developed a priori. However, record screening began prior to the launch of PROSPERO, a prospective register of systematic review protocols.
Eligibility Criteria
Population
Individuals known to be living with HIV with no restrictions based on age, gender, geography, setting, or transmission group.
Types of Interventions
Similar to the method of Zwar et al.,13 intervention strategies for people living with HIV were categorized according to the Effective Practice and Organization of Care (EPOC) taxonomy of interventions (http://epoc.cochrane.org/information-specific-epoc-reviews, accessed June 1, 2012), pertaining to the DS or CIS elements of the CCM. The EPOC taxonomy was used because of its focus on interventions designed to improve professional practice and delivery of health care, which fits with the scope of this review. This taxonomy allows mapping of the interventions to the elements of the CCM, which facilitated a descriptive categorization of the interventions.
Types of Comparison Groups
Studies without a comparison group were excluded. Comparators included usual care, another (non-CCM) intervention, or both.
Types of Assessment Measures
The effectiveness of interventions in this review was evaluated by assessing improvement in “outcome” measures and “healthcare process/provider performance” measures, as defined by Adair et al.23
Primary Measures of Assessment
We identified a priori the following “outcome” measures for included studies:
1) Immunological or virological outcomes: CD4 count or viral load
2) Medical outcomes: mortality of patients, progression to AIDS, opportunistic infections and cancers, hospitalizations, functional status/disability, adherence to medication, and change in at-risk behaviors
3) Psychosocial outcomes: an outcome measure for quality of life or psychological health and well-being
5) Economic outcomes: information about healthcare utilization (length of stay, emergency department visits, visits to providers), costs of treating patients, and costs to patients of healthcare received
“Healthcare process/provider performance” measures were also assessed. While these were not selected a priori, they included any measures of processes that are assumed to improve patient care, such as:
Health care professional adherence to guidelines
Proportion of patients on antiretrovirals
Proportion of patients on indicated prophylaxis
Rates of screening for HIV-related illness
Provision of counseling
Rates of appropriate vaccination
Identification of at-risk behaviors
Patient or provider satisfaction with care
Types of Studies
Randomized clinical trials (RCTs), controlled clinical trials (CCTs), cohort studies, case-control studies, and controlled before and after designs were included.
Information Sources
The comprehensive literature search strategy was informed by previous systematic reviews based on the CCM13 and the HIV/AIDS5 literature (See Appendix 1, available online), and was developed by an experienced medical librarian.
We searched the literature from 1996 (the advent of modern ART) to February 2011. First, we conducted a search of electronic databases that cover international literature in medical/health sciences, psychology, social sciences and social work (including, but not restricted to, MEDLINE, EMBASE, CINAHL, and Cochrane Library). Second, hand searching was performed, as needed, within reference lists of included studies. Third, we used an Internet search strategy of grey literature to identify other published and unpublished literature. Since some studies that were published in 1996 or later included data collected prior to 1996, a post-hoc decision was made to exclude these studies; as they were pre-ART, interventions and outcomes were not deemed relevant to current practice.
Study selection
Records were combined into Distiller SR (http://systematic-review.net/), a web-based systematic review reference management software program. Next, the database was filtered for duplications to derive a unique set of records. We used five stages to review the articles:
Stage 1: Screening
The titles and abstracts of records identified by the search were screened using a checklist within Distiller SR to eliminate titles/topics that were not pertinent to the research question. Due to the large number of records to be screened, 40 records were first screened independently by two reviewers (CK, RD). The weighted kappa score between reviewers was 0.99, thus subsequent records were screened by only one reviewer. If there was any uncertainty regarding inclusion/exclusion, the article proceeded to Stage 2.
Stage 2: Verification of Eligibility Criteria
Using a priori study eligibility criteria, full-text copies of the potentially eligible studies were assessed by two reviewers (CK, AP, RD) to determine whether they fulfilled the inclusion criteria. The third reviewer resolved any disagreements.
Stage 3: Data Extraction
Data were extracted from each included study using a standardized data extraction form. The form was developed based on relevant literature in the area and derived by one reviewer (RD). While the data extraction form was not formally piloted and compared between reviewers, a small sample of studies was used by one reviewer (AP) to ensure that the form was comprehensive and easy to understand.24 Data extracted included participant demographics, study design, description of the DS or CIS interventions, and measures of assessment.
Stage 4: Data Synthesis
Summary tables containing all information abstracted from eligible studies were created. Comparability of studies was assessed by careful review of the population, interventions, comparators, and outcomes by one reviewer (AP). If two or more comparable studies were identified, a pooled estimate of effect was calculated in a meta-analysis to explore the effectiveness of the intervention. For studies looking at dichotomous data, relative risk (RR), odds ratio (OR), and/or risk difference are provided where reported. For continuous data, means and standard deviations or standard errors were extracted where available. For studies that were not comparable in terms of population, intervention, comparator, or assessment measures, a qualitative summary is provided. Outcomes were deemed significant if the 95% confidence interval (CI) of their effect estimate did not include unity. Where CIs were not reported, we used a p-value of ≤ 0.05 (comparing intervention and control groups) as indicating statistical significance. We also described the improvement across studies by the category of outcome and by taxonomy of DS or CIS interventions.
Consideration of Equity in Data Extraction and Synthesis25
It is likely that the success of DS and CIS interventions aiming to improve health and health outcomes is affected by the social determinants of health, and the context in which the interventions are implemented. During data collection, information was collected on the study patient populations across those PROGRESS + dimensions most likely to contribute to inequities (Appendix 2, available online), as well as the transmission risk group which is relevant to HIV care.18,23,35,42
Stage 5: Risk of Bias
Each included study was assessed by one reviewer (AP) for methodological quality, using standardized risk of bias checklists. The Cochrane Collaboration’s tool for assessing risk of bias was used for RCTs, clinical controlled trials, and comparison before and after studies. The Newcastle-Ottawa scales were used for cohort studies and case control studies. As there are no instruments validated for non-comparative studies, no tool was used to assess risk of bias. Summary figures were used to depict risk of bias in included studies.24
RESULTS
We identified a total of 10,169 records (Figure 1). Our review process eliminated 10,046 records. Two articles were irretrievable, following extensive searches conducted by two medical librarians. Sixteen articles remained and were included.
Figure 1.
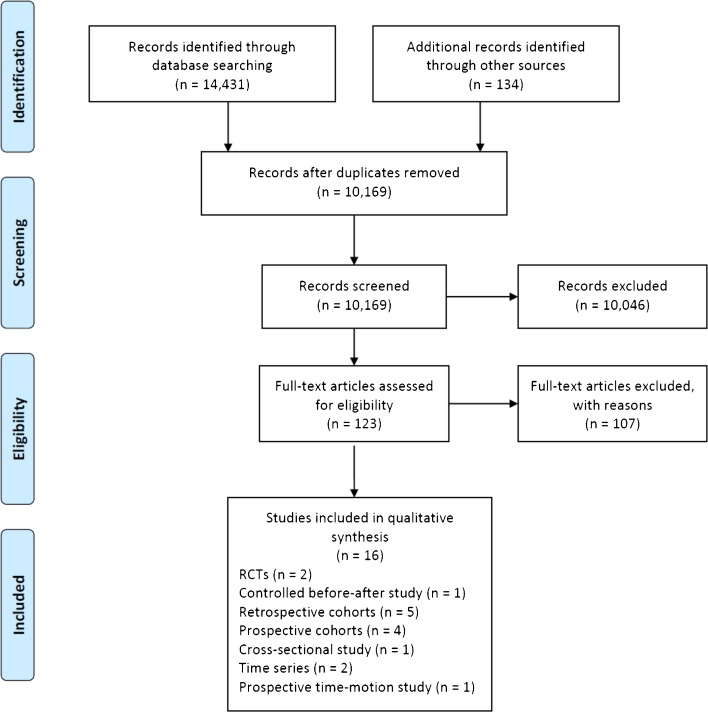
Flow Diagram of Included Studies.
Participants of Included Studies
The total number of HIV positive patients included in this review is 29,897 across 13 studies. Two studies26,27 compared groups based on clinic/providers without reporting numbers of patients, and one28 did not report the total number of patients in the study. Of the studies reporting patients by gender, all but one study that examined cervical screening29 comprised a sample of mostly males. The age range of patients in all but one pediatric study was under 50 years.
Settings of Included Studies
Ten studies were conducted in the USA,2,26,29–36 three in the UK/Europe,37–39 and three in sub-Saharan Africa.27,28,40 All studies examined care in the ambulatory setting.
Design of Included Studies
Thirteen of the 16 studies (81.3%) were observational in design. Further details on the study designs can be found in Figure 1 and Table 1.
Table 1.
Summary of Included Studies
| Study (year) | Study design | Country | Number of centers | Sample size (CCM Intervention/Control) | Intervention | Measures of performance reported (Effective*/Total (%)) | |
|---|---|---|---|---|---|---|---|
| Process Measures | Outcome Measures | ||||||
| Bucher (2010) | RCT | Switzerland | 7 | Physicians randomized (57/60) | CIS– Audit/feedback | 0/4 | – |
| Patients randomized (1634/1632) | |||||||
| Pyne (2011) | RCT | U.S.A | 3 | 138/138 | DS– Communication and case discussion | – | 2/8 (25%) |
| Medical: 0/4 | |||||||
| Psych: 2/4 (50.0%) | |||||||
| Landon (2004) | CBA | U.S.A | 44 intervention/25 control clinics | 6406/3580 | CIS– Presence of quality monitoring | 0/6 | 0/2 |
| Imm/Vir: 0/1 | |||||||
| Economic: 0/1 | |||||||
| Fonquernie (2010) | PCS | France | 1 | 1717/1717 | DS– Explicit mention of implementation of guidelines CIS– Reminders, Change in medical records systems | 4/8 (50%) | 2/2 (100%) |
| Medical: 1/1 (100%) | |||||||
| Psych: 1/1 (100%) | |||||||
| Gardner (2008) | PCS | U.S.A | 17 | 1091/1091 | DS– Educational meetings | 1/1 (100%) | 1/1 (100%) |
| CIS– Audit/feedback | Medical: 1/1 (100%) | ||||||
| Horswell (2008) | PCS | U.S.A | 8 | 3708/3708 | CIS– Reminders, Change in medical records systems | 2/6 (33.3%) | 1/4 (25%) |
| Imm/vir: 1/2 (50%) | |||||||
| Medical: 0/1 | |||||||
| Economic: 0/1 | |||||||
| Kitahata (2003) | PCS | U.S.A | 1 | 1204/1204 | CIS– Reminders | 4/12 (33.3%) | – |
| Belperio (2009) | RCS | U.S.A | Not reported | 7220/7220 | DS– Distribution of educational materials | 1/4 (25%) | – |
| Brown (2002) | RCS | U.S.A | 1 | 73/73 | CIS– Reminders | 5/5 (100%) | – |
| Ma (2010) | RCS | U.S.A | 1 | 75/75 | DS– Communication and case discussion | – | 3/4 (75%) |
| Imm/Vir: 2/3 (66.7%) | |||||||
| Medical: 1/1 (100%) | |||||||
| Natha (2008) | RCS | U.K. | 1 | 100/100 | DS– Explicit mention of implementation of guidelines | 4/5 (80%) | – |
| Shuter (2003) | RCS | U.S.A | 1 | 1026/1026 | CIS– Audit/feedback | 1/1 (100%) | – |
| Magnus (2009) | SCS | U.S.A | 8 | Not reported | CIS– Reminders, Change in medical records systems | 1/3 (33.3%) | – |
| Morris (2009) | TS | Zambia | 19 | Not reported | DS– Educational meetings | 0/3 | – |
| CIS– Audit/feedback, Presence of quality monitoring | |||||||
| Youngleson (2010) | TS | South Africa | 17 | Not reported | CIS– Presence of quality monitoring | 4/4 (100%) | – |
| Were (2010) | TM | Uganda | 1 | 94/88 | CIS– Reminders, Change in medical records systems | – | 2/3 (66.7%) |
| Economic: 2/3 | |||||||
| Total | 27/62 (43.5%) | 11/24 (45.8%) | |||||
| Imm/Vir: 3/6 (50.0%) | |||||||
| Medical: 3/8 (37/5%) | |||||||
| Psych: 3/5 (60.0%) | |||||||
| Economic: 2/5 (40.0%) | |||||||
*Effective: Process or outcome measures showing statistically significant improvement
RCT: Randomized Clinical Trial, CCT: Controlled Clinical Trial, PCS or RCS: Prospective or Retrospective Cohort Study, CBA: Controlled before and after design, SCS: Serial Cross-sectional Study, TS: Time series (Interrupted), TM: Time-motion Study (Prospective)
Psych: Psychosocial measures, Imm/Vir: Immunological/Virological measures
Risk of Bias and Methodological Quality of Included Studies
Risk of bias for included RCTs and controlled before and after studies is presented in Appendix 3, Table 1 (available online). Both RCTs used an appropriate method for randomization, although it was unclear whether allocation concealment was adequate in either. The risk of bias for cohort, time-motion, and prospective time-series studies was assessed using the Newcastle-Ottawa scale, and results are presented in Appendix 3, available online, Table 2. Six of these studies did not provide adequate information regarding follow-up of patients,26,27,31,38–40 and another seven did not provide any details regarding the control of important confounding variables between the intervention and control groups.26,27,31,34,36,38,39
Effects of DS and CIS Interventions
Nine out of 16 (56%) studies assessed only health care process/performance measures, while three studies (19%) examined only outcome measures. The remaining four out of 16 studies assessed both types of measures. There was significant heterogeneity of study design, patient populations, types of interventions and outcomes examined among the studies (Table 1). Thus, a narrative summary of the evidence is presented in the summary of included studies in Appendix 4 (available online). While heterogeneity makes comparisons across studies impossible, we have included any effect estimates reported in the included studies for maximum transparency.
Studies Evaluating only Decision Support Interventions
There were four studies that looked at DS-only interventions: one assessed the distribution of educational materials,34 one included explicit mention of implementation of guidelines into practice,37 and two involved communication and case discussion35,36 interventions (Table 1). Two studies reported process measures,34,37 and found 5/9 (55.6%) of measures were significantly improved. Two studies reported outcome measures,35,36 and found 5/12 (41.7%) of measures were significantly improved (2/3 immunological/virological, 1/5 medical, 2/4 psychosocial). The DS intervention reporting the highest proportion of significantly improved outcomes was the explicit mention of implementation of guidelines, with improvement in four out of five (80%) healthcare process/performance measures.
Studies Evaluating only Clinical Information Systems Interventions
There were nine included studies that looked at CIS-only interventions: two examined reminders,30,41 two examined audit and feedback,29,33,38 two examined presence of quality monitoring28,31 and three studies examined both reminders and changes in medical records systems.26,32,40 Of the eight studies reporting process measures,26,28–32,38,41 17/41 (41.5%) were statistically significantly improved. For the three studies reporting outcome measures,31,32,40 3/9 (33.3%) of outcomes were improved, and there was no discernable pattern regarding the type of outcome measures that improved or not. With a total of 9/17 (52.9%) improved outcomes, the use of reminders was the most effective CIS intervention.
Studies Evaluating Interventions Combining Decision Support and Clinical Information Systems Interventions
Three studies27,33,39 implemented both DS and CIS interventions. One study (Gardner et al.33) used primarily provider training (DS-educational meetings, along with CIS-audit and feedback at one site only). Another (Morris et al.27) performed a complex, task-shifting traineeship that was primarily DS-educational meetings, but included CIS-quality monitoring and CIS-audit and feedback. The third study (Fonquernie et al.39) combined primarily CIS interventions (CIS-reminders and CIS-changes to medical records systems) with DS-explicit implementation of guidelines. Two of these studies assessed both process and outcome measures, while one looked at process measures only. For process measures, the findings varied with a total of 5/12 (41.7%) of measures improved; one study reported 4/8 (50%) measures as having improved, another reporting 1/1 measure as improved, and the third reporting no improvement in process measures (0/3). All outcome measures assessed in these studies showed statistically significant improvement (3/3; two medical and one psychosocial).
Study Features Relating to Outcome Improvement
As heterogeneity precluded meta-analysis, we examined whether study design, number of participants or study setting was associated with effectiveness. In the two RCTs, 0/4 of process measures and 2/8 (25%) of outcome measures were improved, compared to 27/58 (46.6%) and 9/16 (56.3%) of process and outcome measures, respectively, in the observational studies. In studies with >1000 patients, 13/42 (30.1%) of process measures and 4/9 (44.4%) of outcome measures were improved, compared to 16/23 (69.6%) and 5/12% (41.7%) of process and outcome measures in smaller (<1000 patients) studies. In addition, for the three largest studies with several thousand participants each,31,32,34 only 3/16 (18.8%) of process measures and 1/6 (16.7%) of outcome measures were significantly improved. Finally, the three studies conducted in Africa27,28,40 versus Europe or North America were all too different to assess the impact of setting on the effectiveness of interventions.
Other Considerations
Equity indicators were poorly reported overall, and details are outlined in Appendix 5 (available online). When reported, these indicators did not provide any significant insights into the populations for which the interventions were more effective compared to others.
DISCUSSION
Our review aimed to determine the effectiveness of Decision Support and Clinical Information Systems interventions in improving the care of persons with HIV, through the influence of provider behavior. There was considerable heterogeneity between study populations, settings, types of interventions, types of measures, and methodological quality of the included studies. This heterogeneity is consistent with existing literature examining the effectiveness of the Chronic Care Model10,16–18 for other conditions. While it is difficult to discern an overall pattern from the results, several observations can be made.
DS interventions were more likely than CIS interventions to improve process and outcome measures. The DS intervention most likely to be effective was the explicit implementation of guidelines, although this intervention on its own was implemented in only one study.37 This is similar to findings by Zwar et al.,13 although these authors also found DS-distribution of educational materials to be more effective in other conditions than we did for HIV, and found DS-educational meetings to be effective, while our review did not identify any studies examining this intervention. The most frequently effective CIS intervention in our review was the implementation of provider reminders, which was reported to improve health care process/provider performance outcomes in two studies.30,41 The least effective CIS intervention was audit and feedback. Identifying reminders as highly effective is consistent with Davis et al.20 and with Garg et al.,18 who found reminders to be the most effective form of computerized decision support. However, this finding is in contrast to Zwar et al.13 who found CIS-audit and feedback to be more effective for other conditions than we did for HIV. The magnitude of effect, which varies among studies, is consistent with previous work defining improvements as significant but of modest effect size.10,17,22
Studies assessing a combination of DS/CIS interventions were less likely than DS-only and equally likely to CIS-only interventions to improve process measures, and more likely to report improved outcome measures, although this number of studies is too small for definitive comparison. These findings add to the debate in the literature regarding whether the CCM must be implemented into practice as a whole, or whether individual elements can improve chronic disease management. The reality is that most interventions are multifaceted,10,11,13,16,17,21 and while some reviews have found that more than one intervention within or across CCM elements may be more effective than a single intervention,11,16,20 others have not.10,13,17
The number of RCTs was small and their risk of bias high, and these studies showed less effectiveness than those in observational study designs. In addition, larger studies were less likely to result in improved measures than smaller studies. Publication bias may have resulted in smaller, negative trials not being published.
Our work is consistent with previous reviews describing that DS and CIS interventions improve care, although we found a smaller proportion of improved outcomes than other reviews.17,18 Certain features of DS and CIS interventions improve their effectiveness, including the intensity of the intervention;16,22 the presence of a preceding practice-specific needs assessment;20 the involvement of the providers in the development of guidelines,13 process integration,13,19,20 the use of computer-based systems;16,19 and the provision of the reminder to patients themselves.13 The studies included in this review do not provide sufficient detail to extrapolate these potentially successful features to the setting of HIV/AIDS care. As with previous literature, we found that, overall, DS and CIS interventions improve processes of care, but that these improvements do not clearly translate into improved patient outcomes.10,13,18,22,42
This systematic review has several important limitations. First, our summary and synthesis is limited by the methods used in the primary studies, most of which were of observational design and lacked detailed descriptions of the interventions, a common pitfall of health care research.43 Second, studies included in the review did not monitor the long-term impact of the interventions that would make them more applicable for use in routine clinical practice.44 Third, there were a limited number of studies examining each intervention and outcome. The clinical heterogeneity of these interventions, outcomes and populations precluded our ability to perform a meta-analysis. Finally, many of the studies included multiple interventions, often across both DS and CIS categories, or were multifaceted to the extent that attributing effectiveness to one component of the intervention was difficult.45
Overall, DS and CIS interventions may modestly improve care for people living with HIV, with process measures more likely to be improved than definitive outcome measures. However, the limitations of the included studies precluded us from delineating the ingredients that influence effectiveness. Future studies aiming to change provider behavior through the implementation of DS and CIS interventions for people with HIV should use experimental, rather than observational, methodologies in larger samples of patients. Interventions should be described in detail43 and attention paid to known facilitating characteristics of effective DS and CIS interventions, especially when implemented in a broader quality improvement or CCM initiative. In addition, the importance of equity indicators in the design, implementation, and evaluation of interventions should be considered and reported.25 Finally, in addition to intermediate measures of process of care, studies should be extended to determine whether sustained and clinically significant differences can be found in patient-level outcomes.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
(DOCX 1478 kb)
Contributors
I would like to thank Dr. William Hogg for his mentorship during this project. Thank you also to Angela Eady for her medical searching expertise, Dr. Nicholas A. Zwar for his assistance with intervention categorization, Dr. Richard H. Glazier for his insight into systematic reviews of the HIV/AIDS care literature, and Dr. Mark Petticrew for his guidance regarding equity indicators.
Funders
The first author (AP) received funding from the Élisabeth Bruyère Research Institute for a summer studentship. The corresponding author (CK) received an Ontario HIV Treatment Network Health Care Provider Scholarship and Canadian Institutes of Health Research Fellowship, which supported the conduct of this systematic review.
Prior presentations
An abstract on this project will be presented in poster format at the North American Primary Care Research Group Annual Meeting and the Ontario HIV Treatment Network Research Conference in November 2011.
Conflict of Interest
The authors declare that they have no conflicts.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Abbreviations
- ART
Antiretroviral therapy
- CCM
Chronic Care Model
- CIS
Clinical Information Systems
- DS
Decision Support
- EPOC
Effective Practice and Organization of Care
- HIV/AIDS
Human Immunodeficiency virus/Acquired Immune Deficiency Syndrome
Contributor Information
Anjori Pasricha, Email: apasr005@uottawa.ca.
Roo T. M. Deinstadt, Email: roo.deinstadt@gmail.com.
David Moher, Email: dmoher@ohri.ca.
Amanda Killoran, Email: Amanda.Killoran@nice.org.uk.
Sean B. Rourke, Email: srourke@ohtn.on.ca.
Claire E. Kendall, Phone: +1-613-5626262, FAX: +1-613-5626099, Email: ckendall@uottawa.ca.
References
- 1.Unaids. Report on the Global AIDS Epidemic2008 Contract No.: Report.
- 2.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145(6):397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 4.Aberg JA, Kaplan JE, Libman H, Emmanuel P, Anderson JR, Stone VE, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2009;49(5):651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 5.Handford CD, Tynan AM, Rackal JM, Glazier RH. Setting and organization of care for persons living with HIV/AIDS. Cochrane database of systematic reviews. 2006;3. [DOI] [PMC free article] [PubMed]
- 6.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7(2):69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 7.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff. 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 8.Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta-analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med. 2005;143(9):659–672. doi: 10.7326/0003-4819-143-9-200511010-00010. [DOI] [PubMed] [Google Scholar]
- 9.Clark AM, Haykowsky M, Kryworuchko J, MacClure T, Scott J, DesMeules M, et al. A meta-analysis of randomized control trials of home-based secondary prevention programs for coronary artery disease. European Journal of Cardiovascular Prevention and Rehabilitation: Official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2010;17(3):261–270. doi: 10.1097/HJR.0b013e32833090ef. [DOI] [PubMed] [Google Scholar]
- 10.Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag C. 2005;11(8):478–488. [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the Chronic Care Model in the new millennium. Health Aff. 2009;28(1):75–85. doi: 10.1377/hlthaff.28.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44(4):810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 13.Zwar N, Harris M, Griffiths R, Roland M, Dennis S, Powell DaviesG, et al. A systematic review of chronic disease management. Research Centre for Primary Health Care and Equity, School of Public Health and Community Medicine. University of New South Wales. 2006.
- 14.Adams SG, Smith PK, Allan PF, Anzueto A, Pugh JA, Cornell JE. Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med. 2007;167(6):551–561. doi: 10.1001/archinte.167.6.551. [DOI] [PubMed] [Google Scholar]
- 15.Hung DY, Glasgow RE, Dickinson LM, Froshaug DB, Fernald DH, Balasubramanian BA, et al. The chronic care model and relationships to patient health status and health-related quality of life. Am J Prev Med. 2008;35(5 Suppl):S398–S406. doi: 10.1016/j.amepre.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998;317(7156):465–468. doi: 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimshaw J, Eccles M, Thomas R, MacLennan G, Ramsay C, Fraser C, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966-1998. J Gen Intern Med. 2006;21(Suppl 2):S14–S20. doi: 10.1111/j.1525-1497.2006.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis DA, Taylor-Vaisey A. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. CMAJ. 1997;157(4):408–416. [PMC free article] [PubMed] [Google Scholar]
- 21.Weingarten SR, Henning JM, Badamgarav E, Knight K, Hasselblad V, Gano A, Jr, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ. 2002;325(7370):925. doi: 10.1136/bmj.325.7370.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342(8883):1317–1322. doi: 10.1016/0140-6736(93)92244-N. [DOI] [PubMed] [Google Scholar]
- 23.Adair CE, Simpson E, Casebeer AL, Birdsell JM, Hayden KA, Lewis S. Performance measurement in healthcare: part I–concepts and trends from a State of the Science Review. Healthc Pol. 2006;1(4):85–104. [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://www.cochrane-handbook.org accessed June 1, 2012.
- 25.Tugwell P, Petticrew M, Kristjansson E, Welch V, Ueffing E, Waters E, et al. Assessing equity in systematic reviews: realising the recommendations of the Commission on Social Determinants of Health. BMJ. 2010;341:c4739. doi: 10.1136/bmj.c4739. [DOI] [PubMed] [Google Scholar]
- 26.Magnus M, Herwehe J, Andrews L, Gibson L, Daigrepont N, De Leon JM, et al. Evaluating health information technology: provider satisfaction with an HIV-specific, electronic clinical management and reporting system. AIDS Patient Care STDS. 2009;23(2):85–91. doi: 10.1089/apc.2008.0053. [DOI] [PubMed] [Google Scholar]
- 27.Morris MB, Chapula BT, Chi BH, Mwango A, Chi HF, Mwanza J, et al. Use of task-shifting to rapidly scale-up HIV treatment services: experiences from Lusaka, Zambia. BMC Health Serv Res. 2009;9:5. doi: 10.1186/1472-6963-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youngleson MS, Nkurunziza P, Jennings K, Arendse J, Mate KS, Barker P. Improving a mother to child HIV transmission programme through health system redesign: quality improvement, protocol adjustment and resource addition. PLoS One. 2010;5(11):e13891. doi: 10.1371/journal.pone.0013891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuter J, Kalkut GE, Pinon MW, Bellin EY, Zingman BS. A computerized reminder system improves compliance with Papanicolaou smear recommendations in an HIV care clinic. Int J STD AIDS. 2003;14(10):675–680. doi: 10.1258/095646203322387938. [DOI] [PubMed] [Google Scholar]
- 30.Brown CL, Tangsinmankong N, Emmanuel PJ. Improving care of HIV-infected patients in the outpatient setting with patient data flow sheets. J Assoc Nurses AIDS Care. 2002;13(4):58–63. doi: 10.1016/S1055-3290(06)60371-3. [DOI] [PubMed] [Google Scholar]
- 31.Landon BE, Wilson IB, McInnes K, Landrum MB, Hirschhorn L, Marsden PV, et al. Effects of a quality improvement collaborative on the outcome of care of patients with HIV infection: the EQHIV study. Ann Intern Med. 2004;140(11):887–896. doi: 10.7326/0003-4819-140-11-200406010-00010. [DOI] [PubMed] [Google Scholar]
- 32.Horswell R, Butler MK, Kaiser M, Moody-Thomas S, McNabb S, Besse J, et al. Disease management programs for the underserved. Dis Man. 2008;11(3):145–152. doi: 10.1089/dis.2007.0011. [DOI] [PubMed] [Google Scholar]
- 33.Gardner LI, Marks G, O’Daniels CM, Wilson TE, Golin C, Wright J, et al. Implementation and evaluation of a clinic-based behavioral intervention: positive steps for patients with HIV. AIDS Patient Care STDS. 2008;22(8):627–635. doi: 10.1089/apc.2007.0210. [DOI] [PubMed] [Google Scholar]
- 34.Belperio PS, Mole LX, Boothroyd DB, Backus LI. Provider prescribing of 4 antiretroviral agents after implementation of drug use guidelines in the Department of Veterans Affairs. J Manag Care Pharm. 2009;15(4):323–334. doi: 10.18553/jmcp.2009.15.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma A, Chen DM, Chau FM, Saberi P. Improving adherence and clinical outcomes through an HIV pharmacist’s interventions. AIDS Care. 2010;22(10):1189–1194. doi: 10.1080/09540121003668102. [DOI] [PubMed] [Google Scholar]
- 36.Pyne JM, Fortney JC, Curran GM, Tripathi S, Atkinson JH, Kilbourne AM, et al. Effectiveness of collaborative care for depression in human immunodeficiency virus clinics. Arch Intern Med. 2011;171(1):23–31. doi: 10.1001/archinternmed.2010.395. [DOI] [PubMed] [Google Scholar]
- 37.Natha M, Sheehy C, Pollard M, Pakianathan M, Prime K. An audit of completeness of HIV clinical histories: before and after introduction of an HIV proforma. Int J STD AIDS. 2008;19(2):127–128. doi: 10.1258/ijsa.2007.007174. [DOI] [PubMed] [Google Scholar]
- 38.Bucher HC, Rickenbach M, Young J, Glass TR, Vallet Y, Bernasconi E, et al. Randomized trial of a computerized coronary heart disease risk assessment tool in HIV-infected patients receiving combination antiretroviral therapy. Antivir Ther. 2010;15(1):31–40. doi: 10.3851/IMP1475. [DOI] [PubMed] [Google Scholar]
- 39.Fonquernie F, Lacombe K, Vincensini JP, Boccara F, Clozel S, Ayouch Boda A, et al. How to improve the quality of a disease management program for HIV-infected patients using a computerized data system. The Saint-Antoine Orchestra program. AIDS Care. 2010;22(5):588–596. doi: 10.1080/09540120903280893. [DOI] [PubMed] [Google Scholar]
- 40.Were MC, Shen C, Bwana M, Emenyonu N, Musinguzi N, Nkuyahaga F, et al. Creation and evaluation of EMR-based paper clinical summaries to support HIV-care in Uganda, Africa. Int J Med Inf. 2010;79(2):90–96. doi: 10.1016/j.ijmedinf.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitahata MM, Dillingham PW, Chaiyakunapruk N, Buskin SE, Jones JL, Harrington RD, et al. Electronic human immunodeficiency virus (HIV) clinical reminder system improves adherence to practice guidelines among the University of Washington HIV Study Cohort. Clin Infect Dis. 2003;36(6):803–811. doi: 10.1086/368085. [DOI] [PubMed] [Google Scholar]
- 42.Solberg LI, Crain AL, Sperl-Hillen JM, Hroscikoski MC, Engebretson KI, O’Connor PJ. Care quality and implementation of the chronic care model: a quantitative study. Ann Fam Med. 2006;4(4):310–316. doi: 10.1370/afm.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glasziou P, Chalmers I, Altman DG, Bastian H, Boutron I, Brice A, et al. Taking healthcare interventions from trial to practice. BMJ. 2010;341:c3852. doi: 10.1136/bmj.c3852. [DOI] [PubMed] [Google Scholar]
- 44.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000;321(7262):694–696. doi: 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1478 kb)


