Abstract
The purpose of endodontic therapy is to preserve the patient's natural teeth without compromising the patient's local or systemic health. Calcium hydroxide has been included in several materials and antimicrobial formulations that are used in several treatment modalities in endodontics, such as inter-appointment intracanal medicaments. The purpose of this article was to review the antimicrobial properties of calcium hydroxide in endodontics. Calcium hydroxide has a high pH (approximately 12.5-12.8) and is classified chemically as a strong base. The lethal effects of calcium hydroxide on bacterial cells are probably due to protein denaturation and damage to DNA and cytoplasmic membranes. Calcium hydroxide has a wide range of antimicrobial activity against common endodontic pathogens but is less effective against Enterococcus faecalis and Candida albicans. Calcium hydroxide is also a valuable anti-endotoxin agent. However, its effect on microbial biofilms is controversial.
Keywords: Biofilm, Calcium hydroxide, Candida albicans, Endotoxin
INTRODUCTION
Calcium hydroxide was originally introduced to the field of endodontics by Herman in 1920 as a pulp-capping agent.1 It is a white odorless powder with the formula Ca(OH)2 (Fig. 1) and has a molecular weight of 74.08. Ca(OH)2 has low solubility in water (about 1.2 g·L-1 at 25 ℃), which decreases as the temperature rises.2 The dissociation coefficient of Ca(OH)2 (0.17) permits a slow, controlled release of both calcium and hydroxyl ions. The low solubility is a good clinical characteristic because a long period is necessary for Ca(OH)2 to become soluble in tissue fluids when in direct contact with vital tissues.3 Ca(OH)2 has a high pH (about 12.5-12.8), is insoluble in alcohol, and is chemically classified as a strong base. Its main actions result from the ionic dissociation of the Ca2+ and OH-ions and their effect on vital tissues, such as inducing hard tissue deposition and being antibacterial.2 Ca(OH)2 dissociates into calcium and hydroxyl ions on contact with aqueous fluids.4 Ca(OH)2 in water has a thixotropic behavior, which means that it will be very fluid when agitated.3 When Ca(OH)2 is exposed to carbon dioxide (CO2) or carbonate ions (CO3-) in biological tissue, the dissociation of the chemical leads to the formation of calcium carbonate (CaCO3) and an overall consumption of Ca2+ ions. However, one study showed that after 30 days of exposure to carbon dioxide, six preparations of Ca(OH)2 still maintained a purportedly bactericidal pH within the root canal.2
FIG. 1.
Structure of the calcium hydroxide molecule.
MECHANISM OF ANTIMICROBIAL ACTIVITY
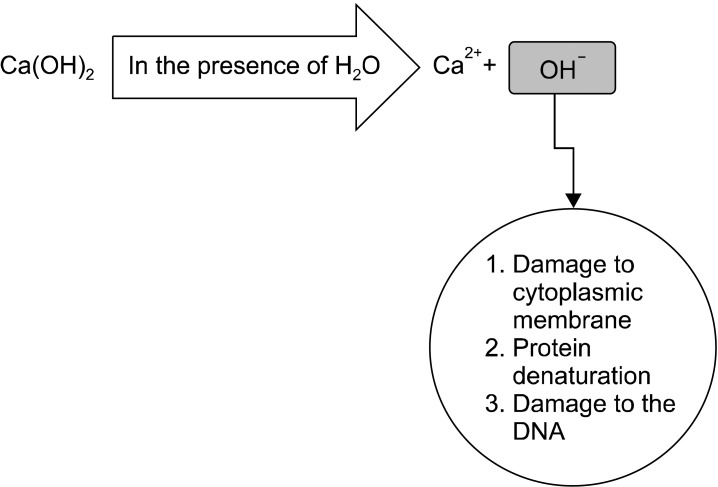
According to Siqueira,5 the antimicrobial activity of Ca(OH)2 is dependent on the release of hydroxyl ions in an aqueous environment. Furthermore, Siqueira and Lopes6 stated that hydroxyl ions are highly oxidant free radicals that show extreme reactivity with several biomolecules. This reactivity is indiscriminate; thus, this free radical rarely diffuses away from sites of generation.6 The lethal effects of hydroxyl ions on bacterial cells are probably due to damage to the bacterial cytoplasmic membrane, denaturation of proteins, or damage to the DNA (Fig. 2).
FIG. 2.
A schematic view of the mechanisms of the antibacterial activity of calcium hydroxide.
Although these three mechanisms may occur, it is difficult to establish, in a chronological sense, which is the main mechanism involved in the death of bacterial cells after exposure to a strong base.6 Hydroxyl ions from Ca(OH)2 exert their mechanism of action in the cytoplasmic membrane because that is where enzymatic sites are located.7 Extracellular enzymes act on nutrients, carbohydrates, proteins, and lipids that, through hydrolysis, favor digestion. Intracellular enzymes located in the cell favor the respiratory activity of the cellular wall structure. The pH gradient of the cytoplasmic membrane is altered by the high concentration of hydroxyl ions from calcium hydroxide acting on the proteins of the membrane (protein denaturation). The high pH of Ca(OH)2 alters the integrity of the cytoplasmic membrane by means of chemical injury to the organic components and transport of nutrients or by means of the destruction of phospholipids or unsaturated fatty acids of the cytoplasmic membrane during the peroxidation process, which is a saponification reaction.8
Adjustment of intracellular pH is influenced by different cellular processes, such as cellular metabolism, alterations in shape, mobility, adjustment of transporters and polymerization of cytoskeleton components, activation of cellular proliferation and growth, conductivity and transport through the membrane, and isosmotic cellular volume. Thus, many cellular functions can be affected by pH, including the enzymes that are essential for cellular metabolism.9 Estrela et al.10 found that bacterial enzymatic inactivation under extreme conditions of pH for a long period of time is irreversible.
ANTIBACTERIAL ACTIVITY
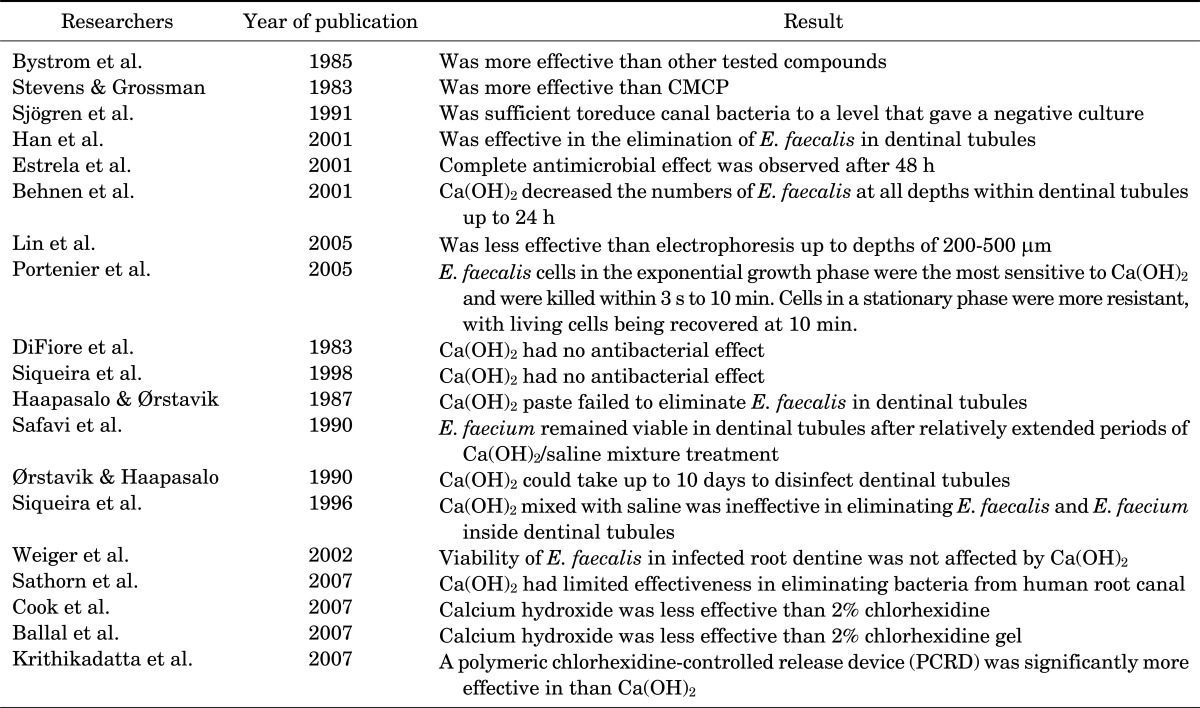
Calcium hydroxide exerts antibacterial effects in the root canal system as long as a high pH is maintained.6 An in vivo study showed that root canals treated with Ca(OH)2 had fewer bacteria than did those dressed with camphorated phenol or camphorated monochlorophenol.11 Another study reported Ca(OH)2 to be effective in preventing the growth of microorganisms but to a limited extent when compared to camphorated chlorophenol, stressing the necessity of direct contact to achieve the optimum antibacterial effect.12 It was shown that a 7-day application of a Ca(OH)2 medicament was sufficient to reduce canal bacteria to a level that gave a negative culture.13 It has also been shown that an aqueous Ca(OH)2 paste and a silicone oil-based Ca(OH)2 paste are effective in the elimination of Enterococcus faecalis in dentinal tubules.14
Estrela et al.15 demonstrated that both the direct exposure test and the agar diffusion test are useful in establishing the antimicrobial spectrum of Ca(OH)2 and in developing improved infection control protocols. A complete antimicrobial effect was observed after 48 h with both tests, irrespective of the Ca(OH)2 paste vehicle.15 Another study showed that Ca(OH)2 decreased the numbers of E. faecalis at all depths within dentinal tubules up to 24 h and that less viscous preparations of Ca(OH)2 were more effective in the elimination of E. faecalis from dentinal tubules than were viscous preparations.16
In a study to evaluate the effect of electrophoretically activated Ca(OH)2 on bacterial viability in dentinal tubules, Lin et al.17 reported that treatment with electrophoresis was significantly more effective than pure Ca(OH)2 up to depths of 200 to 500 µm. Specimens treated with electrophoretically activated Ca(OH)2 revealed no viable bacteria in dentinal tubules to a depth of 500 µm from the root canal space within 7 days.17
E. faecalis cells in the exponential growth phase have been shown to be the most sensitive to Ca(OH)2 and are killed within 3 s to 10 min. Cells in a stationary phase were more resistant, with living cells being recovered at 10 min. However, cells in a starvation phase were the most resistant and were not totally eliminated during a 10-minute test period.18
By contrast, several studies have attested to the ineffectiveness of Ca(OH)2 in eliminating bacterial cells. Two studies revealed that Ca(OH)2 had no antibacterial effect as a paste or as the commercial preparation Pulpdent when used against Streptococcus sanguis.19,20 It was also shown that a Ca(OH)2 paste (Calasept; Speiko, Darmstadt, Germany) failed to eliminate, even superficially, E. faecalis in dentinal tubules.21 Safavi et al.22 indicated that E. faecium remained viable in dentinal tubules after relatively extended periods of Ca(OH)2/saline mixture treatment. Another study demonstrated that Ca(OH)2 could take up to 10 days to disinfect dentinal tubules infected by facultative bacteria.23 Siqueira and Uzeda24 demonstrated that, after 1 week of contact, Ca(OH)2 mixed with saline was ineffective in eliminating E. faecalis and E. faecium inside dentinal tubules. Estrela et al.8 found that Ca(OH)2 in infected dentinal tubules had no antimicrobial effect on S. faecalis, S. aureus, Bacillus subtilis, Pseudomonas aeruginosa, or on the bacterial mixture used throughout the experiment. It has been revealed that the viability of E. faecalis in infected root dentine was not affected by Ca(OH)2.25 In a systematic review to assess the antibacterial efficacy of Ca(OH)2, Sathorn et al.26 showed that Ca(OH)2 had limited effectiveness in eliminating bacteria from human root canal when assessed by culture techniques.
In a polymerase chain reaction (PCR) study to evaluate the effect of root canal filling with or without prior Ca(OH)2 or 2% chlorhexidine (CHX) on the persistence of bacterial DNA in infected dentinal tubules, Cook et al.27 showed that 2% CHX treatment followed by canal filling was more effective in removing the DNA of E. faecalis than placement of Ca(OH)2 or immediate canal filling. Furthermore, in failed root canal treatments, a 2% CHX gel was a more effective intracanal medicament than Ca(OH)2 paste against E. faecalis.28 Krithikadatta et al.29 indicated that 2% CHX gel alone was more effective against E. faecalis than was Ca(OH)2. Another study found that a polymeric chlorhexidine-controlled release device was significantly more effective in reducing intradentinal bacteria than was Ca(OH)2 (Table 1).30
TABLE 1.
Studies on the antibacterial activity of calcium hydroxide
EFFECTS ON ENDOTOXIN
Endotoxin, which is present on all Gram-negative bacteria, is composed of polysaccharides, lipids, and proteins and is referred to as lipopolysaccharide (LPS), emphasizing its chemical structure.31,32 Lipid A is the region of the endotoxin molecule responsible for its toxic effects. When free to act, endotoxins do not cause cell or tissue pathosis directly but instead stimulate competent cells to release chemical mediators.33 Macrophages are the main target of endotoxins.33 Therefore, endotoxins are not intrinsically toxic.
During root canal treatment, LPS is released during multiplication or bacterial death, thus causing a series of biological effects34 that lead to an inflammatory reaction32 and periapical bone resorption.35,36
Currently, one of the concerns in endodontics is the treatment of teeth with necrotic pulps and periapical pathosis, because treatment failure is higher than in cases without periapical disease.37,38 In teeth with chronic periapical lesions, there is a greater prevalence of Gram-negative anaerobic bacteria disseminated throughout the root canal system (dentinal tubules, apical resorptive defects, and cementum lacunae), including apical bacterial biofilm.37-40 Because these areas are not reached by instrumentation, the use of a root canal medicament is recommended to aid in the elimination of these bacteria and to increase the possibility of clinical success.37-39 Teeth with and without radiographically visible periapical disease could be considered as different pathological entities requiring different treatment regimens. Where bone loss has occurred, the use of a root canal medicament between treatment sessions is recommended by some,41 because the success of treatment in cases with periapical pathosis is directly related to the elimination of bacteria from the root canal system. The procedures and medicaments used in root canal treatment should lead not only to bacterial death but also to the inactivation of bacterial endotoxin.33
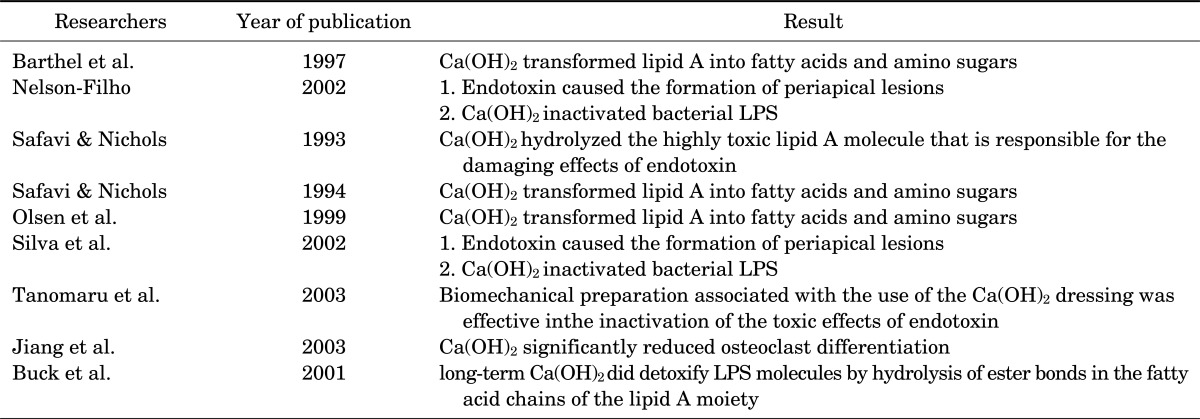
An in vitro study demonstrated that Ca(OH)2 hydrolyzed the highly toxic lipid A molecule that is responsible for the damaging effects of endotoxin.42 Another study showed that Ca(OH)2 transformed lipid A into fatty acids and amino sugars, which are nontoxic components.43 These findings were confirmed by some other in vitro studies.34,44
In two in vivo studies, Nelson-Filho et al.39 as well as Silva et al.45 revealed that endotoxin caused the formation of periapical lesions and that Ca(OH)2 inactivated bacterial LPS. In a study on dogs' teeth, Tanomaru et al.46 showed that a biomechanical preparation with only irrigating solutions did not inactivate the endotoxin; however, the same treatment associated with the use of the Ca(OH)2 dressing was effective in inactivation of the toxic effects of this endotoxin. Another study indicated that Ca(OH)2 significantly reduced osteoclast differentiation.47 It has been demonstrated that long-term Ca(OH)2 treatment did detoxify LPS molecules by hydrolysis of ester bonds in the fatty acid chains of the lipid A moiety (Table 2).48
TABLE 2.
Studies on the anti-endotoxin activity of calcium hydroxide
ANTI-FUNGAL ACTIVITY
Fungi have occasionally been found in primary root canal infections,49,50 but they are more common in filled root canals in teeth that have become infected some time after treatment or in those that have not responded to treatment.51 Overall, the occurrence of fungi in infected root canals varies between 1% and 17%.52 A large number of other yeasts have also been isolated from the oral cavity, including Candida glabrata, C. guilliermondii, C. parapsilosis, C. krusei, C. inconspicua, C. dubliniensis, C. tropicalis, and Saccharomyces species.51
It has been demonstrated that C. albicans cells are highly resistant to Ca(OH)2 and that all Candida species (C. albicans, C. glabrata, C. guilliermondii, C. krusei, and C. tropicalis) are either equally high or had higher resistance to aqueous calcium hydroxide than did E. faecalis.53,54 Because C. albicans survives at a wide range of pH values, the alkalinity of saturated Ca(OH)2 solution may not have any effect on C. albicans. In addition, Ca(OH)2 pastes may provide the Ca2+ ions necessary for the growth and morphogenesis of Candida. These mechanisms may explain why Ca(OH)2 has been found to be ineffective against C. albicans.51
Siqueira et al.55 investigated the antifungal ability of several medicaments against C. albicans, C. glabrata, C. guilliermondii, C. parapsilosis, and Saccharomyces cerevisiae. They reported that whereas the paste of Ca(OH)2 in camphorated paramonochlorophenol (CPMC)/glycerin had the most pronounced antifungal effects, Ca(OH)2 in glycerin or chlorhexidine and chlorhexidine in detergent also had antifungal activity, but at a lower level than the paste of Ca(OH)2 in CPMC/glycerin.55 In another study, Ferguson et al.56 evaluated the in vitro susceptibility of C. albicans to various irrigants and medicaments. The minimum inhibitory concentrations of NaOCl, hydrogen peroxide, chlorhexidine digluconate, and aqueous Ca(OH)2 were determined. The results showed that NaOCl, hydrogen peroxide, and chlorhexidine digluconate were effective against C. albicans, even when diluted significantly.56 Furthermore, aqueous Ca(OH)2 had no antifungal activity when maintained in direct contact with C. albicans cells, whereas Ca(OH)2 paste and CPMC were effective antifungal agents.56
Valera et al.57 showed that, as an intra-canal medicament, CPMC was more effective against C. albicans than Ca(OH)2/CPMC paste. Siqueira et al.58 evaluated the effectiveness of four intracanal medicaments in disinfecting the root dentine of bovine teeth experimentally infected with C. albicans. Infected dentine cylinders were exposed to 4 different medicaments: Ca(OH)2/glycerin, Ca(OH)2/0.12% chlorhexidine digluconate, Ca(OH)2/CPMC/glycerin, and 0.12% chlorhexidine digluconate/zinc oxide. The specimens were left in contact with the medicaments for 1 hour, 2 days, and 7 days. The specimens treated with Ca(OH)2/CPMC/glycerin paste or with chlorhexidine/zinc oxide paste were completely disinfected after 1 hour of exposure. Only Ca(OH)2/glycerin paste consistently eliminated C. albicans infection after 7 days of exposure. Ca(OH)2 mixed with chlorhexidine was ineffective in disinfecting dentine even after 1 week of exposure. Of the medicaments tested, the Ca(OH)2/CPMC/glycerin paste and chlorhexidine digluconate mixed with zinc oxide were the most effective in eliminating C. albicans cells from dentine specimens.58
EFFECTS ON BIOFILMS
The term biofilm was introduced to designate the thin-layered (sessile) condensations of microbes that may occur on various surface structures in nature.59 In endodontics, the biofilm concept was initially discussed mainly within the framework of bacteria on the root tips of teeth with necrotic and infected pulps or pulpless and infected root canal systems.60,61 Such bacterial aggregations have been thought to be the cause of therapy-resistant apical periodontitis.61,62 Using transmission electron microscopy (TEM), Nair60 examined the root canal contents of 31 teeth that had gross coronal caries and to which the periapical inflammatory lesion was attached upon extraction. In addition to his observations of the microstructure of the inflammatory tissue, he noted that the major bulk of the organisms existed as loose collections of cocci, rods, filaments, and spirochetes.60 Whereas most of these organisms appeared to be suspended in what was described as a moist canal space, dense aggregates were also observed sticking to the canal walls and forming layers of bacterial condensations. Amorphous material filled the interbacterial spaces and was interpreted as an extracellular matrix of bacterial origin. When they occurred, the bacterial condensations had a palisade structure similar to the one for dental plaque on external tooth surfaces, suggesting similar mechanisms for bacterial attachment as those for dental plaque.60 Sen et al.63 examined untreated extracted teeth with apical periodontitis by scanning electron microscopy (SEM) and found that the root canals were heavily infected, with microorganisms being observed in all areas of the canal. Cocci and rods predominated and formed colonies on the root canal walls and also, to a varying degree, penetrated the dentinal tubules.63 Nair et al.61 found that even after instrumentation, irrigation, and canal filling in a one-visit treatment, microbes existed as biofilms in untouched locations in the main canal, isthmuses, and accessory canals in most of the specimens.
Antimicrobial agents have often been developed and optimized for their activity against fast-growing, dispersed populations containing a single microorganism.59,64 However, microbial communities grown in biofilms are remarkably difficult to eradicate with antimicrobial agents, and microorganisms in mature biofilms can be notoriously resistant for reasons that have yet to be adequately explained.60,65 Some reports have shown that microorganisms grown in biofilms could be 2-fold to 1000-fold more resistant than the corresponding planktonic form of the same organisms.59 Using scanning electron microscopy and scanning confocal laser microscopy, Distel et al.66 reported that despite intracanal dressing with Ca(OH)2, E. faecalis formed biofilms in root canals. Another study showed that Ca(OH)2 was 100% effective in eliminating E. faecalis biofilm.67 Brändle et al.68 evaluated the effects of growth condition (planktonic, mono- and multi-species biofilms) on the susceptibility of E. faecalis, Streptococcus sobrinus, C. albicans, Actinomyces naeslundii, and Fusobacterium nucleatum to alkaline stress. The findings showed that planktonic microorganisms were most susceptible; only E. faecalis and C. albicans survived in saturated solution for 10 minutes, and the latter also survived for 100 minutes.68 Dentine adhesion was the major factor in improving the resistance of E. faecalis and A. naeslundii to calcium hydroxide, whereas the multispecies context in a biofilm was the major factor in promoting resistance of S. sobrinus to the disinfectant. In contrast, the C. albicans response to calcium hydroxide was not influenced by growth conditions.68
COMBINATION OF Ca(OH)2 AND CHLORHEXIDINE
Chlorhexidine (CHX) is a cationic biguanide whose optimal antimicrobial activity is achieved within a pH range of 5.5 to 7.0.69 Therefore, it is likely that alkalinizing the pH by adding Ca(OH)2 to CHX will lead to precipitation of CHX molecules, thereby decreasing its effectiveness.70 It has been demonstrated that the alkalinity of Ca(OH)2 when mixed with CHX remains unchanged.71 Therefore, the usefulness of mixing Ca(OH)2 with CHX remains unclear and controversial.69
When used as an intracanal medicament, CHX was more effective than Ca(OH)2 in eliminating E. faecalis from inside dentinal tubules.69 One report revealed that all of the chlorhexidine formulations studied, including a CHX/Ca(OH)2 50:50 mix, were effective in eliminating E. faecalis from dentinal tubules, with a 1% CHX gel working better than the other preparations.72 These findings were corroborated by two other studies in bovine dentine and73 and human dentine.74
Haenni et al.71 found no additive antibacterial effect by mixing Ca(OH)2 powder with 0.5% CHX. They indicated that CHX had a reduced antibacterial action. However, Ca(OH)2 did not lose its antibacterial properties in such a mixture.71 An in vitro study showed that 2% CHX gel was the most effective agent against E. faecalis inside dentinal tubules, followed by a Ca(OH)2/2% CHX mixture, whereas Ca(OH)2 alone was totally ineffective, even after 30 days.75 The 2% CHX gel was also significantly more effective than the Ca(OH)2/2% CHX mixture against C. albicans at 7 days, although there was no significant difference at 15 and 30 days. Ca(OH)2 alone was completely ineffective against C. albicans.75 In an in vivo study using primary teeth, Onçag et al.76 showed that a 1% CHX-gluconate gel, both with and without Ca(OH)2, was more effective against E. faecalis than Ca(OH)2 alone over a 48-hour period.
Another study showed that that 2% CHX-gluconate was significantly more effective against E. faecalis than Ca(OH)2 used alone or a mixture of the two.74 Although this was also confirmed by Lin et al.,77 a study by Evans et al.78 using bovine dentine concluded that 2% CHX with Ca(OH)2 was more effective than Ca(OH)2 in water. An animal study demonstrated that teeth dressed with CHX for 4 weeks had reduced inflammatory reactions in the periodontium (both apically and marginally) and less root resorption.79 Waltimo et al.53 reported that 0.5% CHX-acetate was more effective at killing C. albicans than was saturated Ca(OH)2, whereas Ca(OH)2 combined with CHX was more effective than Ca(OH)2 used alone.
BUFFERING EFFECT OF DENTINE ON THE ANTIBACTERIAL ACTIVITY OF Ca(OH)2
The root canal milieu is a complex mixture of a variety of organic and inorganic components. Hydroxyapatite is the major representative of the inorganic components, whereas pulp tissue, microorganisms, and inflammatory exudate rich in proteins such as albumin are the major organic components.80 The relative importance of the various organic and inorganic compounds in the inactivation of root canal disinfectants has been studied to a limited extent only.81 Difficulties in designing experiments that will give reliable and comparable data have been some of the greatest challenges. Haapasalo et al.81 introduced a new dentine powder model for studying the inhibitory effect of dentine on various root canal irrigants and medicaments. They concluded that dentine powder effectively abolished the killing of E. faecalis by Ca(OH)2.81 Hydroxyapatite had an effect similar to dentine on Ca(OH)2, preventing the killing of E. faecalis.82
The substantial effect of dentine on the antibacterial activity of Ca(OH)2 can be attributed to the buffering action of dentine against alkali.83 Ca(OH)2 is used as a thick paste in vivo; however, its solubility is low and saturation is achieved at a relatively low concentration of hydroxyl ions. Both laboratory and in vivo studies have shown that buffering by dentine, particularly in the subsurface layers of the root canal walls, might be the main factor behind the reduced antibacterial effect of Ca(OH)2. It is possible that deeper in dentine (outside the main root canal), Ca(OH)2 is present as a saturated solution or at concentrations even below that level.81 Besides dentine, remnants of necrotic pulp tissue as well as inflammatory exudate might affect the antibacterial potential of endodontic disinfectants.80
In conclusion, (1) The antimicrobial activity of Ca(OH)2 is related to the release of highly reactive hydroxyl ions in an aqueous environment, which mainly affects cytoplasmic membranes, proteins, and DNA.
(2) Although some clinical studies have supported the efficacy of Ca(OH)2 as an intracanal medicament, others studies have questioned its efficacy and have indicated CHX instead of Ca(OH)2.
(3) Endotoxin, a component of the cell wall of Gram-negative bacteria, plays a fundamental role in the genesis and maintenance of periapical lesions due to the induction of inflammation and bone resorption. Ca(OH)2 inactivates endotoxin, in vitro and in vivo, and appears currently to be the only clinically effective medicament for inactivation of endotoxin.
(4) Fungi have occasionally been found in primary root canal infections, but they appear to occur more often in filled root canals of teeth in which treatment has failed. C. albicans is by far the fungal species most commonly isolated from infected root canals. It seems that the combinations of Ca(OH)2 with camphorated paramonochlorophenol or chlorhexidine have the potential to be used as effective intracanal medicaments for cases in which fungal infection is suspected.
(5) The few studies conducted on the antimicrobial potential of Ca(OH)2 on biofilms have demonstrated inconsistent results. Further studies are required to elucidate the anti-biofilm efficacy of Ca(OH)2.
(6) Although the usefulness of mixing Ca(OH)2 with CHX remains unclear and controversial, it seems that by mixing Ca(OH)2 with CHX, the antimicrobial activity of Ca(OH)2 is increased. In other words, the descending order of the antimicrobial activity of Ca(OH)2, CHX, and their combination is as follows: CHX, Ca(OH)2/CHX, and Ca(OH)2.
(7) It seems that dentine, hydroxyapatite, and remnants of necrotic pulp tissue as well as inflammatory exudate decrease the antibacterial potential of Ca(OH)2. In other words, Ca(OH)2 is likely to be effective under laboratory conditions but relatively ineffective as a medicament in vivo.
References
- 1.Hermann BW. Calcium hydroxid als Mittelzurn, Behandeln und Fullen von Wurzelkanalen [Thesis] Wurzburg: 1920. [Google Scholar]
- 2.Farhad A, Mohammadi Z. Calcium hydroxide: a review. Int Dent J. 2005;55:293–301. doi: 10.1111/j.1875-595x.2005.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 3.Spångberg L, Haapasalo M. Rationale and efficacy of root canal medicaments and root filling materials with emphasis on treatment outcome. Endod Top. 2002;2:35–58. [Google Scholar]
- 4.Rehman K, Saunders WP, Foye RH, Sharkey SW. Calcium ion diffusion from calcium hydroxide-containing materials in endodontically-treated teeth: an in vitro study. Int Endod J. 1996;29:271–279. doi: 10.1111/j.1365-2591.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 5.Siqueira JF., Jr Strategies to treat infected root canals. J Calif Dent Assoc. 2001;29:825–837. [PubMed] [Google Scholar]
- 6.Siqueira JF, Jr, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999;32:361–369. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 7.Estrela C, Sydney GB, Bammann LL, Felippe O., Jr Estudo do efeito biológico do pH na atividade enzimática de bactérias anaeróbias. Rev Fac Odontol Bauru. 1994;2:29–36. [Google Scholar]
- 8.Estrela C, Pimenta FC, Ito IY, Bammann LL. Antimicrobial evaluation of calcium hydroxide in infected dentinal tubules. J Endod. 1999;25:416–418. doi: 10.1016/S0099-2399(99)80269-6. [DOI] [PubMed] [Google Scholar]
- 9.Putnam RW. Intracellular pH regulation. Cell physiology. San Diego: Academic Press; 1995. pp. 212–229. [Google Scholar]
- 10.Estrela C, Pécora JD, Silva RS. pH analysis of vehicles and calcium hydroxide pastes. Braz Endod J. 1998;3:41–47. [Google Scholar]
- 11.Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1:170–175. doi: 10.1111/j.1600-9657.1985.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 12.Stevens RH, Grossman LI. Evaluation of the antimicrobial potential of calcium hydroxide as an intracanal medicament. J Endod. 1983;9:372–374. doi: 10.1016/S0099-2399(83)80187-3. [DOI] [PubMed] [Google Scholar]
- 13.Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991;24:119–125. doi: 10.1111/j.1365-2591.1991.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 14.Han GY, Park SH, Yoon TC. Antimicrobial activity of Ca(OH)2 containing pastes with Enterococcus faecalis in vitro. J Endod. 2001;27:328–332. doi: 10.1097/00004770-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Estrela C, Rodrigues de Araújo Estrela C, Bammann LL, Pecora JD. Two methods to evaluate the antimicrobial action of calcium hydroxide paste. J Endod. 2001;27:720–723. doi: 10.1097/00004770-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Behnen MJ, West LA, Liewehr FR, Buxton TB, McPherson JC., 3rd Antimicrobial activity of several calcium hydroxide preparations in root canal dentin. J Endod. 2001;27:765–767. doi: 10.1097/00004770-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Lin S, Tsesis I, Zukerman O, Weiss EI, Fuss Z. Effect of electrophoretically activated calcium hydroxide on bacterial viability in dentinal tubules--in vitro. Dent Traumatol. 2005;21:42–45. doi: 10.1111/j.1600-9657.2004.00293.x. [DOI] [PubMed] [Google Scholar]
- 18.Portenier I, Waltimo T, Ørstavik D, Haapasalo M. The susceptibility of starved, stationary phase, and growing cells of Enterococcus faecalis to endodontic medicaments. J Endod. 2005;31:380–386. doi: 10.1097/01.don.0000145421.84121.c8. [DOI] [PubMed] [Google Scholar]
- 19.DiFiore PM, Peters DD, Setterstrom JA, Lorton L. The antibacterial effects of calcium hydroxide apexification pastes on Streptococcus sanguis. Oral Surg Oral Med Oral Pathol. 1983;55:91–94. doi: 10.1016/0030-4220(83)90313-4. [DOI] [PubMed] [Google Scholar]
- 20.Siqueira JF, Jr, Lopes HP, de Uzeda M. Recontamination of coronally unsealed root canals medicated with camphorated paramonochlorophenol or calcium hydroxide pastes after saliva challenge. J Endod. 1998;24:11–14. doi: 10.1016/S0099-2399(98)80204-5. [DOI] [PubMed] [Google Scholar]
- 21.Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66:1375–1379. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 22.Safavi KE, Spangberg LS, Langeland K. Root canal dentinal tubule disinfection. J Endod. 1990;16:207–210. doi: 10.1016/s0099-2399(06)81670-5. [DOI] [PubMed] [Google Scholar]
- 23.Orstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol. 1990;6:142–149. doi: 10.1111/j.1600-9657.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 24.Siqueira JF, Jr, de Uzeda M. Disinfection by calcium hydroxide pastes of dentinal tubules infected with two obligate and one facultative anaerobic bacteria. J Endod. 1996;22:674–676. doi: 10.1016/S0099-2399(96)80062-8. [DOI] [PubMed] [Google Scholar]
- 25.Weiger R, de Lucena J, Decker HE, Löst C. Vitality status of microorganisms in infected human root dentine. Int Endod J. 2002;35:166–171. doi: 10.1046/j.1365-2591.2002.00465.x. [DOI] [PubMed] [Google Scholar]
- 26.Sathorn C, Parashos P, Messer H. Antibacterial efficacy of calcium hydroxide intracanal dressing: a systematic review and meta-analysis. Int Endod J. 2007;40:2–10. doi: 10.1111/j.1365-2591.2006.01197.x. [DOI] [PubMed] [Google Scholar]
- 27.Cook J, Nandakumar R, Fouad AF. Molecular- and culture-based comparison of the effects of antimicrobial agents on bacterial survival in infected dentinal tubules. J Endod. 2007;33:690–692. doi: 10.1016/j.joen.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Ballal V, Kundabala M, Acharya S, Ballal M. Antimicrobial action of calcium hydroxide, chlorhexidine and their combination on endodontic pathogens. Aust Dent J. 2007;52:118–121. doi: 10.1111/j.1834-7819.2007.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 29.Krithikadatta J, Indira R, Dorothykalyani AL. Disinfection of dentinal tubules with 2% chlorhexidine, 2% metronidazole, bioactive glass when compared with calcium hydroxide as intracanal medicaments. J Endod. 2007;33:1473–1476. doi: 10.1016/j.joen.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y, Han SH, Hong SH, Lee JK, Ji H, Kum KY. Antimicrobial efficacy of a polymeric chlorhexidine release device using in vitro model of Enterococcus faecalis dentinal tubule infection. J Endod. 2008;34:855–858. doi: 10.1016/j.joen.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Westphal O. Bacterial endotoxins. The second carl prausnitz memorial lecture. Int Arch Allergy Appl Immunol. 1975;49:1–43. [PubMed] [Google Scholar]
- 32.Rietschel ET, Brade H. Bacterial endotoxins. Sci Am. 1992;267:54–61. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- 33.Leonardo MR, Silva RA, Assed S, Nelson-Filho P. Importance of bacterial endotoxin (LPS) in endodontics. J Appl Oral Sci. 2004;12:93–98. doi: 10.1590/s1678-77572004000200002. [DOI] [PubMed] [Google Scholar]
- 34.Barthel CR, Levin LG, Reisner HM, Trope M. TNF-alpha release in monocytes after exposure to calcium hydroxide treated Escherichia coli LPS. Int Endod J. 1997;30:155–159. doi: 10.1046/j.1365-2591.1997.00066.x. [DOI] [PubMed] [Google Scholar]
- 35.Stashenko P. Role of immune cytokines in the pathogenesis of periapical lesions. Endod Dent Traumatol. 1990;6:89–96. doi: 10.1111/j.1600-9657.1990.tb00400.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki M, Nakane A, Kumazawa M, Hashioka K, Horiba N, Nakamura H. Endotoxin and gram-negative bacteria in the rat periapical lesions. J Endod. 1992;18:501–504. doi: 10.1016/S0099-2399(06)81351-8. [DOI] [PubMed] [Google Scholar]
- 37.Leonardo MR, da Silva LA, Leonardo Rde T, Utrilla LS, Assed S. Histological evaluation of therapy using a calcium hydroxide dressing for teeth with incompletely formed apices and periapical lesions. J Endod. 1993;19:348–352. doi: 10.1016/s0099-2399(06)81361-0. [DOI] [PubMed] [Google Scholar]
- 38.Katebzadeh N, Hupp J, Trope M. Histological periapical repair after obturation of infected root canals in dogs. J Endod. 1999;25:364–368. doi: 10.1016/S0099-2399(06)81173-8. [DOI] [PubMed] [Google Scholar]
- 39.Nelson-Filho P, Leonardo MR, Silva LA, Assed S. Radiographic evaluation of the effect of endotoxin (LPS) plus calcium hydroxide on apical and periapical tissues of dogs. J Endod. 2002;28:694–696. doi: 10.1097/00004770-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Trope M, Delano EO, Orstavik D. Endodontic treatment of teeth with apical periodontitis: single vs. multivisit treatment. J Endod. 1999;25:345–350. doi: 10.1016/S0099-2399(06)81169-6. [DOI] [PubMed] [Google Scholar]
- 41.Leonardo MR, Silva LAB, Leonardo RT. Tratamento de canal radicular em sessao unica: crenca vs. ciencia. In: Feller C, Gorab R, editors. Atualizacao na Clinica Odontologica. Sao Paulo: Artes Médicas; 2000. pp. 29–57. [Google Scholar]
- 42.Safavi KE, Nichols FC. Effect of calcium hydroxide on bacterial lipopolysaccharide. J Endod. 1993;19:76–78. doi: 10.1016/S0099-2399(06)81199-4. [DOI] [PubMed] [Google Scholar]
- 43.Safavi KE, Nichols FC. Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. J Endod. 1994;20:127–129. doi: 10.1016/S0099-2399(06)80057-9. [DOI] [PubMed] [Google Scholar]
- 44.Olsen MH, DiFiore PM, Dixit SN, Veis A. The effect of calcium hydroxide inhibition on LPS induced release of IL-1b from human monocytes in whole blood. J Endod. 1999;25:289. [Google Scholar]
- 45.Silva L, Nelson-Filho P, Leonardo MR, Rossi MA, Pansani CA. Effect of calcium hydroxide on bacterial endotoxin in vivo. J Endod. 2002;28:94–98. doi: 10.1097/00004770-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Tanomaru JM, Leonardo MR, Tanomaru Filho M, Bonetti Filho I, Silva LA. Effect of different irrigation solutions and calcium hydroxide on bacterial LPS. Int Endod J. 2003;36:733–739. doi: 10.1046/j.1365-2591.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- 47.Jiang J, Zuo J, Chen SH, Holliday LS. Calcium hydroxide reduces lipopolysaccharide-stimulated osteoclast formation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:348–354. doi: 10.1067/moe.2003.18. [DOI] [PubMed] [Google Scholar]
- 48.Buck RA, Cai J, Eleazer PD, Staat RH, Hurst HE. Detoxification of endotoxin by endodontic irrigants and calcium hydroxide. J Endod. 2001;27:325–327. doi: 10.1097/00004770-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Baumgartner JC, Watts CM, Xia T. Occurrence of Candida albicans in infections of endodontic origin. J Endod. 2000;26:695–698. doi: 10.1097/00004770-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Lana MA, Ribeiro-Sobrinho AP, Stehling R, Garcia GD, Silva BK, Hamdan JS, et al. Microorganisms isolated from root canals presenting necrotic pulp and their drug susceptibility in vitro. Oral Microbiol Immunol. 2001;16:100–105. doi: 10.1034/j.1399-302x.2001.016002100.x. [DOI] [PubMed] [Google Scholar]
- 51.Siqueira JF, Jr, Sen BH. Fungi in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:632–641. doi: 10.1016/S1079210404000046. [DOI] [PubMed] [Google Scholar]
- 52.Waltimo TMT, Haapasalo M, Zehnder M, Meyer J. Clinical aspects related to endodontic yeast infections. Endod Top. 2004;9:66–78. [Google Scholar]
- 53.Waltimo TM, Orstavik D, Sirén EK, Haapasalo MP. In vitro susceptibility of Candida albicans to four disinfectants and their combinations. Int Endod J. 1999;32:421–429. doi: 10.1046/j.1365-2591.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 54.Waltimo TM, Sirén EK, Orstavik D, Haapasalo MP. Susceptibility of oral Candida species to calcium hydroxide in vitro. Int Endod J. 1999;32:94–98. doi: 10.1046/j.1365-2591.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 55.Siqueira JF, Jr, Rôças IN, Magalhães FA, de Uzeda M. Antifungal effects of endodontic medicaments. Aust Endod J. 2001;27:112–114. doi: 10.1111/j.1747-4477.2001.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 56.Ferguson JW, Hatton JF, Gillespie MJ. Effectiveness of intracanal irrigants and medications against the yeast Candida albicans. J Endod. 2002;28:68–71. doi: 10.1097/00004770-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Valera MC, de Moraes Rego J, Jorge AO. Effect of sodium hypochlorite and five intracanal medications on Candida albicans in root canals. J Endod. 2001;27:401–403. doi: 10.1097/00004770-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Siqueira JF, Jr, Rôças IN, Lopes HP, Magalhães FA, de Uzeda M. Elimination of Candida albicans infection of the radicular dentin by intracanal medications. J Endod. 2003;29:501–504. doi: 10.1097/00004770-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Svensater G, Bergenholtz G. J Endod Biofilms in endodontic infections. Endod Top. 2004;9:27–36. [Google Scholar]
- 60.Ramachandran Nair PN. Light and electron microscopic studies of root canal flora and periapical lesions. J Endod. 1987;13:29–39. doi: 10.1016/S0099-2399(87)80089-4. [DOI] [PubMed] [Google Scholar]
- 61.Nair PN, Henry S, Cano V, Vera J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after "one-visit" endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:231–252. doi: 10.1016/j.tripleo.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Wu MK, Dummer PM, Wesselink PR. Consequences of and strategies to deal with residual post-treatment root canal infection. Int Endod J. 2006;39:343–356. doi: 10.1111/j.1365-2591.2006.01092.x. [DOI] [PubMed] [Google Scholar]
- 63.Sen BH, Piskin B, Demirci T. Observation of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod Dent Traumatol. 1995;11:6–9. doi: 10.1111/j.1600-9657.1995.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 64.Gilbert P, Das J, Foley I. Biofilm susceptibility to antimicrobials. Adv Dent Res. 1997;11:160–167. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 65.Bowden GH, Hamilton IR. Survival of oral bacteria. Crit Rev Oral Biol Med. 1998;9:54–85. doi: 10.1177/10454411980090010401. [DOI] [PubMed] [Google Scholar]
- 66.Distel JW, Hatton JF, Gillespie MJ. Biofilm formation in medicated root canals. J Endod. 2002;28:689–693. doi: 10.1097/00004770-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Chai WL, Hamimah H, Cheng SC, Sallam AA, Abdullah M. Susceptibility of Enterococcus faecalis biofilm to antibiotics and calcium hydroxide. J Oral Sci. 2007;49:161–166. doi: 10.2334/josnusd.49.161. [DOI] [PubMed] [Google Scholar]
- 68.Brändle N, Zehnder M, Weiger R, Waltimo T. Impact of growth conditions on susceptibility of five microbial species to alkaline stress. J Endod. 2008;34:579–582. doi: 10.1016/j.joen.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 69.Athanassiadis B, Abbott PV, Walsh LJ. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust Dent J. 2007;52(1 Suppl):S64–S82. doi: 10.1111/j.1834-7819.2007.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 70.Mohammadi Z, Abbott PV. The properties and applications of chlorhexidine in endodontics. Int Endod J. 2009;42:288–302. doi: 10.1111/j.1365-2591.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 71.Haenni S, Schmidlin PR, Mueller B, Sener B, Zehnder M. Chemical and antimicrobial properties of calcium hydroxide mixed with irrigating solutions. Int Endod J. 2003;36:100–105. doi: 10.1046/j.1365-2591.2003.00629.x. [DOI] [PubMed] [Google Scholar]
- 72.Almyroudi A, Mackenzie D, McHugh S, Saunders WP. The effectiveness of various disinfectants used as endodontic intracanal medications: an in vitro study. J Endod. 2002;28:163–167. doi: 10.1097/00004770-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Gomes BP, Vianna ME, Sena NT, Zaia AA, Ferraz CC, de Souza Filho FJ. In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:544–550. doi: 10.1016/j.tripleo.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 74.Schäfer E, Bössmann K. Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulations against Enterococcus faecalis. J Endod. 2005;31:53–56. doi: 10.1097/01.don.0000134209.28874.1c. [DOI] [PubMed] [Google Scholar]
- 75.Ercan E, Dalli M, Dülgergil CT. In vitro assessment of the effectiveness of chlorhexidine gel and calcium hydroxide paste with chlorhexidine against Enterococcus faecalis and Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e27–e31. doi: 10.1016/j.tripleo.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 76.Onçag O, Cogulu D, Uzel A. Efficacy of various intracanal medicaments against Enterococcus faecalis in primary teeth: an in vivo study. J Clin Pediatr Dent. 2006;30:233–237. [PubMed] [Google Scholar]
- 77.Lin YH, Mickel AK, Chogle S. Effectiveness of selected materials against Enterococcus faecalis: part 3. The antibacterial effect of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J Endod. 2003;29:565–566. doi: 10.1097/00004770-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Evans MD, Baumgartner JC, Khemaleelakul SU, Xia T. Efficacy of calcium hydroxide: chlorhexidine paste as an intracanal medication in bovine dentin. J Endod. 2003;29:338–339. doi: 10.1097/00004770-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 79.Lindskog S, Pierce AM, Blomlöf L. Chlorhexidine as a root canal medicament for treating inflammatory lesions in the periodontal space. Endod Dent Traumatol. 1998;14:186–190. doi: 10.1111/j.1600-9657.1998.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 80.Haapasalo M, Qian W, Portenier I, Waltimo T. Effects of dentin on the antimicrobial properties of endodontic medicaments. J Endod. 2007;33:917–925. doi: 10.1016/j.joen.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 81.Haapasalo HK, Sirén EK, Waltimo TM, Ørstavik D, Haapasalo MP. Inactivation of local root canal medicaments by dentine: an in vitro study. Int Endod J. 2000;33:126–131. doi: 10.1046/j.1365-2591.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 82.Portenier I, Haapasalo H, Rye A, Waltimo T, Ørstavik D, Haapasalo M. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int Endod J. 2001;34:184–188. doi: 10.1046/j.1365-2591.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 83.Wang JD, Hume WR. Diffusion of hydrogen ion and hydroxyl ion from various sources through dentine. Int Endod J. 1988;21:17–26. doi: 10.1111/j.1365-2591.1988.tb00949.x. [DOI] [PubMed] [Google Scholar]