Abstract
In the present study, the residual antibacterial activity, or substantivity, of three concentrations of Tetraclean (Ogna Laboratori Farmaceutici, Muggiò, Italy) was assessed in bovine root dentin in vitro. One hundred ten dentin tubes prepared from bovine incisor teeth were infected in vitro for 14 days with Enterococcus faecalis. Thereafter, the specimens were divided into five groups as follows: 100% Tetraclean, 10% Tetraclean, 1% Tetraclean, sterile dentin tubes (negative control), and infected dentin tubes (positive control). Dentin chips were collected with round burs into tryptic soy broth and, after culturing, the number of colony-forming units (CFU) was counted. The number of CFU was minimal in the first cultures in all experimental groups, and the results obtained were significantly different in the different groups at all time periods (p<0.05). At all five experimental periods, the 100% Tetraclean group showed the most effective antibacterial action (p<0.05). In each group, the number of CFU increased significantly with time (p<0.05). There was a direct relationship between the concentration of Tetraclean and its substantivity. In conclusion, under the conditions of the study presented here, the substantivity of 100% Tetraclean was significantly higher than that of lower concentrations.
Keywords: Tetraclean, Enterococcus faecalis, Dentin
INTRODUCTION
Microorganisms have long been recognized as the primary cause in the development of pulpal and periradicular diseases.1-3 Numerous measures including the use of various instrumentation techniques, irrigation regimens, and intracanal medicaments have been described to reduce the number of root canal microorganisms. However, despite optimal endodontic therapy, some cases fail because there are areas in the root canal that cannot be adequately debrided and disinfected with existing instrumentation techniques.4-6 In necrotic teeth, as well as in retreatment cases, treatment should be completed in two visits.7 Furthermore, some studies have suggested that calcium hydroxide is ineffective against Enterococcus faecalis.8 An alternative protocol to overcome the above-mentioned problems is to use antimicrobial agents that exhibit substantivity, that is, agents that have a therapeutic effect for a prolonged period.9 Chlorhexidine as well as tetracyclines possess this property of antibacterial substantivity.10 Recently, two doxycycline-based root canal irrigants have been introduced.11 MTAD (Dentsply Tulsa Dental, Tulsa, OK, USA), a mixture of doxycycline, citric acid, and a detergent (Tween 80), was introduced as a final irrigant for disinfection of the root canal system.12 The substantivity of MTAD has been demonstrated for up to 4 weeks.13 Furthermore, it has been demonstrated that the substantivity of MTAD has a direct relationship to its concentration.14 Tetraclean (Ogna Laboratori Farmaceutici, Muggiò, Italy), like MTAD, is a doxycycline-based endodontic irrigant composed of an antibiotic, an acid, and a detergent.15 However, the concentration of the antibiotic, doxycycline (50 mg ml-1) and the type of detergent (polypropylene glycol) differ from those of MTAD.11 Mohammadi et al.16 showed that the substantivity of Tetraclean was significantly higher than that of MTAD. The relationship between the concentration of Tetraclean and its substantivity has not yet been assessed. Therefore, the purpose of this study was to compare the antibacterial substantivity of three concentrations of Tetraclean (100%, 10%, and 1%) against E. faecalis in bovine root dentin in vitro.
MATERIALS AND METHODS
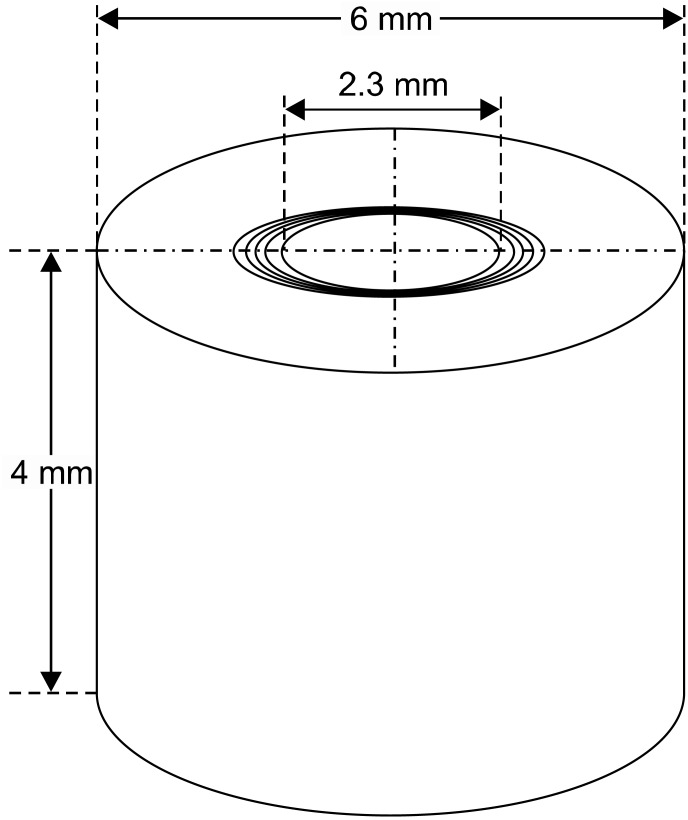
Forty intact bovine central incisors were selected for this study. The specimens were kept in 0.5% NaOCl solution for no longer than 7 days. The apical 5 mm and two-thirds of the crown were removed from each tooth with a rotary diamond saw at 1000 rpm (Isomet Plus precision saw; Buehler, Lake Bluff, IL, USA) under water cooling. The cementum was removed by using polish paper (Ecomet 3 variable-speed grinder-polisher; Buehler), which resulted in a center-holed piece of root dentin with a 6-mm outer diameter (Fig. 1). The roots were then cut into 4-mm thick slices with a diamond saw as above. The canals of the 4-mm blocks were enlarged with an ISO 023 round bur by using slow speed. All teeth and dentin slices were preserved during the procedures in vials containing tap water to avoid dehydration. The dentin tubes (n=110) were individually treated with 5.25% NaOCl and 17% EDTA (with pH 7.2) to remove the smear layer. The specimens were placed in BHI broth (Oxoid, Basingstoke, UK) and autoclaved. They were then kept in an incubator at 37℃ for 24 hours to check the efficacy of the sterilization. A total of 110 specimens were randomly divided into five groups as follows: Group 1 (30 specimens), 100% Tetraclean; Group 2 (30 specimens), 10% Tetraclean; Group 3 (30 specimens), 1% Tetraclean; Group 4 (10 specimens), positive control (infected dentin tubes); and Group 5 (10 specimens), negative control (sterile dentin tubes).
FIG. 1.
Schematic view of used dentin tubes (Adapted from Mohammadi14).
Isolated 24-hour colonies of pure cultures of E. faecalis (ATCC 29212) were suspended in 5 ml of BHI. The turbidity of the inoculum, prepared in BHI, was adjusted to the turbidity of a 0.5 McFarland Standard (1.5×108 cells/ml).
The bottles containing each specimen in groups 1, 2, 3, and 4 were opened under laminar flow. Sterile pipettes were used to remove 2 ml of sterile BHI and to replace it with 2 ml of bacterial inoculum. The bottles were closed and kept at 37℃ for 14 days, with the replacement of 1 ml of contaminated BHI for 1 ml of freshly prepared BHI every 2 days to avoid medium saturation. After the contamination period, each specimen was removed from its bottle under aseptic conditions and the canal was irrigated with 5 ml of sterile saline and dried with sterile paper points. The outer surface of the specimens was covered with two layers of nail varnish to prevent contact of the medicament with the external surface. Then, specimens were fixed at the bottom of wells of 24-well cell culture plates with decontaminated sticky wax, which also obliterated the apical surface of the root canal. Finally, the irrigation solutions were inserted into the canal lumen with sterile 3-ml plastic syringes and 27-gauge needles until the dentin tubes were totally filled. Five minutes after placement of the irrigants, solutions were removed by using sterile paper points. The specimens were then incubated at 37℃ for 28 days to maintain humidity. Dentin chips were removed from the canals with sequential, sterile, low-speed round burs with increasing diameters of ISO sizes (025, 027, 029, 031, and 033) at experimental times of 0, 7, 14, 21, and 28 days. Each bur removed approximately 0.1 mm of dentin around the canal. The powder dentin samples obtained with each bur were immediately collected in separate test tubes containing 3 ml of freshly prepared TSB. Thereafter, 100 µl from each test tube was cultured on blood agar. Growing colonies were counted and recorded as CFU. SPSS 16.0 statistical software was used to conduct the statistical analysis (SPSS Inc, Chicago, IL, USA). Data were analyzed by using analysis of variance and covariance with repeated measures (ANOVA) to indicate differences between the experimental groups and the positive control. One-way ANOVA (Tukey's method) was used to indicate differences within each layer.
RESULTS
The number of CFU obtained from five consecutive dentinal layers is presented in Table 1. The number of CFU in all three experimental groups was minimal in the first cultures. The positive control group showed viable bacteria at all experimental times, which indicated the efficiency of the method. In contrast, the negative control group showed no viable bacteria at any experimental time. At all experimental times, days 0, 7, 14, 21, and 28, the 100% Tetraclean group showed the most effective antibacterial action (p<0.05).
TABLE 1.
Mean of the CFU and the standard deviations of E. faecalisin the experimental groups
Values are mean±SD. CFU: colony-forming units.
DISCUSSION
With current instrumentation techniques, many areas of the root canal walls are left completely untouched by instruments. Therefore, an irrigation solution is required to improve the debridement of the root canal system.4,5 The reasons for choosing E. faecalis in the present study were as follows: it is a well-recognized pathogen associated with persistent apical periodontitis in endodontically treated teeth. Furthermore, it readily colonizes dentinal tubules in bovine roots and can penetrate the entire width of the circumpulpal dentin within 2 days of inoculation. In addition, it is nonfastidious, easy to culture, and grows rapidly.17
Tetraclean is a mixture of doxycycline, citric acid, and propylene glycol.15 Doxycycline is a bacteriostatic antibiotic that inhibits the growth of bacteria but does not kill them.11 Therefore, endotoxin is not produced. Citric acid is another component of Tetraclean. Its efficacy on endodontic bacteria has been demonstrated. Propylene glycol is the third component of Tetraclean that was added to reduce surface tension and increase the penetration depth. The antibacterial activity of Tetraclean appears to be due to the synergistic effect of doxycycline and citric acid. Furthermore, by reducing the surface tension, propylene glycol increases the penetration depth of Tetraclean into the irregularities of the root canal system.11,15
Khademi et al.9 compared the antibacterial substantivity of 2% chlorhexidine (CHX), 100 mg/ml doxycycline, and 2.6% NaOCl in bovine root dentin in vitro. They found that the substantivity of CHX was significantly greater than that of doxycycline and NaOCl, which is in contrast with the findings of the study presented here. In the study presented here, the antibacterial substantivity of three concentrations of Tetraclean (100%, 10%, and 1%) was monitored for 28 days. At all experimental periods, the 100% Tetraclean (undiluted) solution had the most effective antibacterial action, which stresses the ability of Tetraclean to adsorb to hydroxyapatite with prolonged gradual release at therapeutic levels. Mohammadi et al.16 found that the residual antibacterial activity of Tetraclean was significantly greater than that of MTAD. In another study, Mohammadi assessed the residual antibacterial activity of three concentrations of MTAD.14 The findings showed that there was a direct relationship between the concentration of MTAD and its substantivity. It seems that the presence of a detergent (Tween 80) in MTAD increases the depth of penetration of this material into dentinal tubules by decreasing surface tension.
Another variable concerning the substantivity of root canal irrigants is the time of dentin treatment to induce substantivity.10 There is considerable debate in the literature regarding the time of dentin treatment to induce substantivity. Some studies have shown that dentin should be treated for 1 week to induce substantivity. On the other hand, some works have demonstrated that treatment for only 5 to 10 min with CHX induces substantivity for even 12 weeks.10 Rosenthal et al.18 found that treatment with a 2% solution of CHX induced substantivity for up to 12 weeks, which is in contrast with the findings of the present study. However, White et al.19 concluded that the antimicrobial activity of 2% CHX as a canal irrigant lasted 72 hours. In an in vivo study to evaluate the substantivity of 2% CHX as a root canal irrigating solution, Leonardo et al.20 found that CHX prevents microbial activity with residual effects in the root canal system up to 48 h, whereas the present study showed that the substantivity of 2% CHX was remained for 28 days. Komorowski et al.21 reported that for induction of substantivity, dentin should be treated with CHX for 7 days and that 5-min treatment with CHX did not induce substantivity, which is in contrast with our findings. Lin et al.22 attributed the limited antibacterial effect of CHX irrigation to the ability of dentin to absorb the medication during the first hour and stated that only after the saturation point after the first hour did the antibacterial capability of CHX increase with time.
In conclusion, under the conditions of the study presented here, the substantivity of 100% Tetraclean was significantly higher than that of lower concentrations.
References
- 1.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–349. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 2.Möller AJ, Fabricius L, Dahlén G, Ohman AE, Heyden G. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89:475–484. doi: 10.1111/j.1600-0722.1981.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 3.Sundqvist G. Ecology of the root canal flora. J Endod. 1992;18:427–430. doi: 10.1016/S0099-2399(06)80842-3. [DOI] [PubMed] [Google Scholar]
- 4.Hess W. Anatomy of root canals in the teeth of the permanent dentition. New York: William Wood & Co; 1925. [Google Scholar]
- 5.Peters OA, Laib A, Göhring TN, Barbakow F. Changes in root canal geometry after preparation assessed by high-resolution computed tomography. J Endod. 2001;27:1–6. doi: 10.1097/00004770-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321–328. doi: 10.1111/j.1600-0722.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 7.Bergenholtz G, Trope M. Microbiological basis for endodontic treatment: can a maximal outcome be achieved in one visit? Endod Topics. 2002;1:40–53. [Google Scholar]
- 8.Haapasalo M, Orstavik D. in vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66:1375–1379. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 9.Khademi AA, Mohammadi Z, Havaee A. Evaluation of the antibacterial substantivity of several intra-canal agents. Aust Endod J. 2006;32:112–115. doi: 10.1111/j.1747-4477.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadi Z, Abbott PV. Antimicrobial substantivity of root canal irrigants and medicaments: a review. Aust Endod J. 2009;35:131–139. doi: 10.1111/j.1747-4477.2009.00164.x. [DOI] [PubMed] [Google Scholar]
- 11.Mohammadi Z, Abbott PV. On the local applications of antibiotics and antibiotic-based agents in endodontics and dental traumatology. Int Endod J. 2009;42:555–567. doi: 10.1111/j.1365-2591.2009.01564.x. [DOI] [PubMed] [Google Scholar]
- 12.Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, et al. A new solution for the removal of the smear layer. J Endod. 2003;29:170–175. doi: 10.1097/00004770-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Mohammadi Z, Shahriari S. Residual antibacterial activity of chlorhexidine and MTAD in human root dentin in vitro. J Oral Sci. 2008;50:63–67. doi: 10.2334/josnusd.50.63. [DOI] [PubMed] [Google Scholar]
- 14.Mohammadi Z. Evaluation of residual antibacterial activity of three concentrations of new root canal irrigation solution. N Y State Dent J. 2008;74:31–33. [PubMed] [Google Scholar]
- 15.Giardino L, Pecora G, Ambu E, Savoldi E. A new irrigant in the treatment of apical periodontitis: from research to clinic; 12th Biennial Congress European Society of Endodontology; Dublin. 2005. pp. 15–17. [Google Scholar]
- 16.Mohammadi Z, Giardino L, Mombeinipour A. Antibacterial substantivity of a new antibiotic-based endodontic irrigation solution. Aust Endod J. 2012;38:26–30. doi: 10.1111/j.1747-4477.2010.00263.x. [DOI] [PubMed] [Google Scholar]
- 17.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–98. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal S, Spångberg L, Safavi K. Chlorhexidine substantivity in root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:488–492. doi: 10.1016/j.tripleo.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 19.White RR, Hays GL, Janer LR. Residual antimicrobial activity after canal irrigation with chlorhexidine. J Endod. 1997;23:229–231. doi: 10.1016/S0099-2399(97)80052-0. [DOI] [PubMed] [Google Scholar]
- 20.Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifácio KC, Ito IY. in vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. J Endod. 1999;25:167–171. doi: 10.1016/s0099-2399(99)80135-6. [DOI] [PubMed] [Google Scholar]
- 21.Komorowski R, Grad H, Wu XY, Friedman S. Antimicrobial substantivity of chlorhexidine-treated bovine root dentin. J Endod. 2000;26:315–317. doi: 10.1097/00004770-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Zuckerman O, Weiss EI, Mazor Y, Fuss Z. Antibacterial efficacy of a new chlorhexidine slow release device to disinfect dentinal tubules. J Endod. 2003;29:416–418. doi: 10.1097/00004770-200306000-00009. [DOI] [PubMed] [Google Scholar]