Abstract
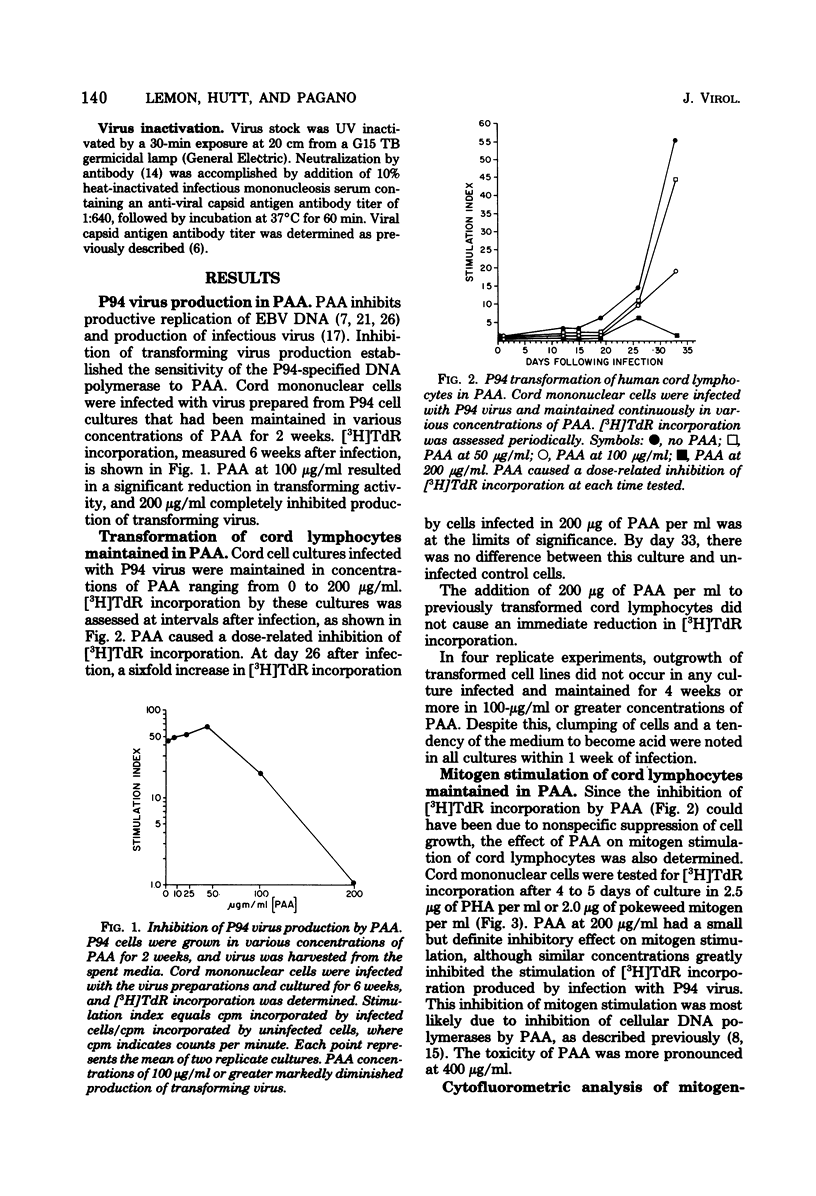
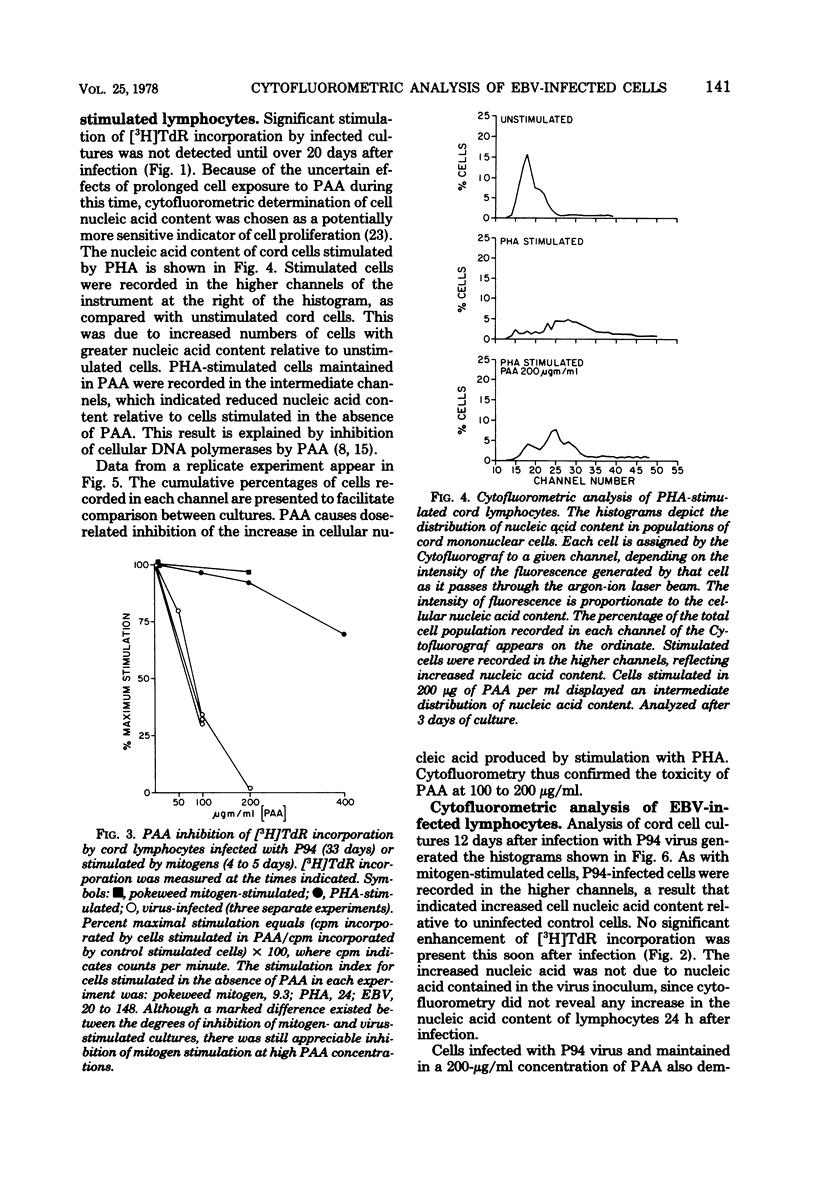
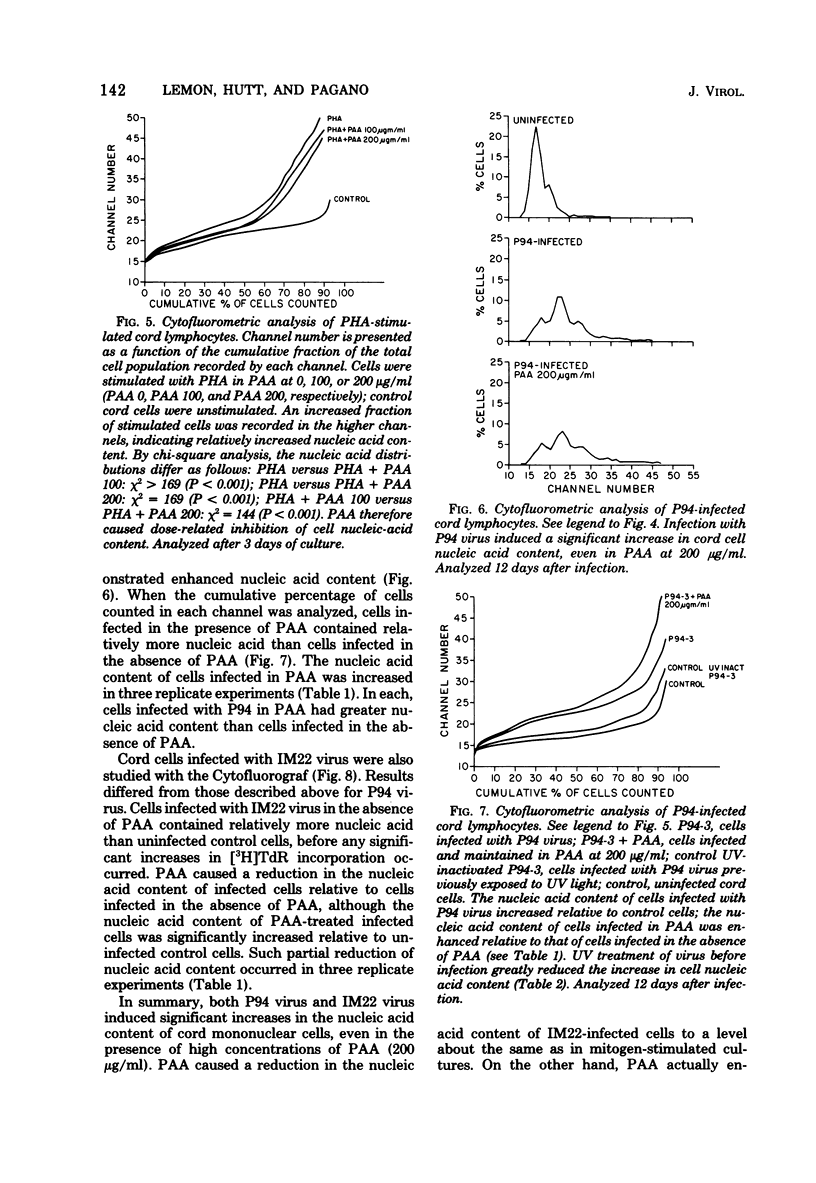
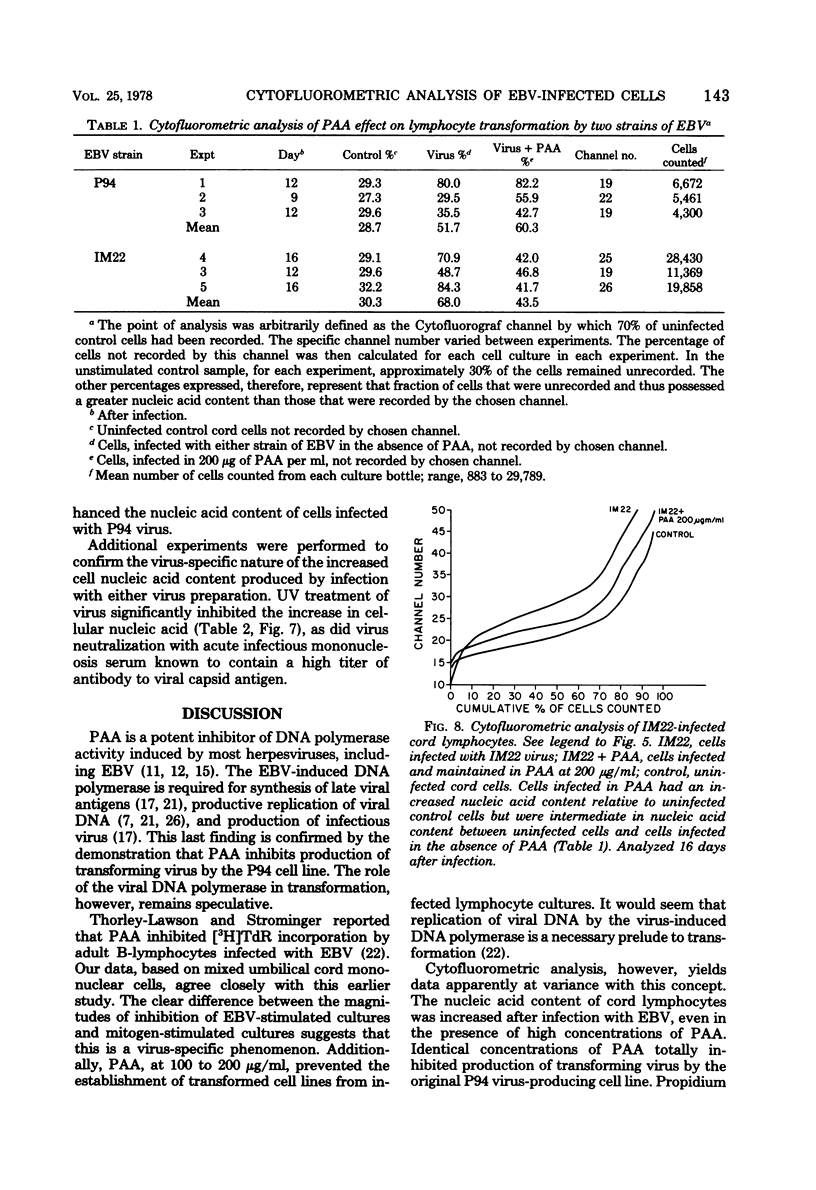
DNA synthesis in Epstein-Barr virus (EBV)-infected lymphocytes was inhibited by phosphonoacetic acid (PAA) as measured by [3H]thymidine incorporation. PAA, at a concentration of 200 microgram/ml, inhibited [3H]thymidine incorporation by human umbilical cord lymphocytes infected with EBV strain P94 but had little effect on DNA synthesis in mitogen-stimulated cells. Transformed cell lines did not develop from infected cord cell cultures treated with 100 microgram of PAA per ml. Cytofluorometric analysis showed marked increases in cellular nucleic acid content (RNA plus DNA) as early as 9 days after infection of cord cells in the absence of PAA and before significant enhancement of [3H]thymidine incorporation became apparent. Moreover, EBV led to increases in cellular nucleic acid even when 200 microgram of PAA per ml was added to cell cultures before infection. The apparent discrepancy between results obtained by [3H]thymidine incorporation and cytofluorometry is explained either by significant inhibition of cellular DNA polymerases by PAA or by a block at the G2 + M phase of the cell cycle. The data suggest that EBV initiates alterations in cellular nucleic acid synthesis or cell division without prior replication of viral DNA by virus-induced DNA polymerases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Bjursell G., Kaschka-Dierich C., Lindahl T. Circular Epstein-Barr virus genomes of reduced size in a human lymphoid cell line of infectious mononucleosis origin. J Virol. 1977 May;22(2):373–380. doi: 10.1128/jvi.22.2.373-380.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Crissman H. A., Steinkamp J. A. Rapid, simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J Cell Biol. 1973 Dec;59(3):766–771. doi: 10.1083/jcb.59.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Tanaka A., Nonoyama M., Derge J. G. Replication of the resident repressed Epstein-Barr virus genome during the early S phase (S-1 period) of nonproducer Raji cells. Proc Natl Acad Sci U S A. 1974 Mar;71(3):631–633. doi: 10.1073/pnas.71.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E., Miller G., Robinson J., Heston L. Efficiency of transformation of lymphocytes by Epstein-Barr virus. Virology. 1977 Jan;76(1):152–163. doi: 10.1016/0042-6822(77)90292-6. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Huang C. H., Huong S. M., Selgrade M. Preferential inhibition of herpes-group viruses by phosphonoacetic acid: effect on virus DNA synthesis and virus-induced DNA polymerase activity. Yale J Biol Med. 1976 Mar;49(1):93–99. [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J Virol. 1975 Dec;16(6):1560–1565. doi: 10.1128/jvi.16.6.1560-1565.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt L. M., Huang Y. T., Dascomb H. E., Pagano J. S. Enhanced destruction of lymphoid cell lines by peripheral blood leukocytes taken from patients with acute infectious mononucleosis. J Immunol. 1975 Jul;115(1):243–248. [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Mode of inhibition of herpes simplex virus DNA polymerase by phosphonoacetate. Biochemistry. 1975 Dec 16;14(25):5475–5479. doi: 10.1021/bi00696a015. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E., Overby L. R. Inhibition of DNA polymerase from herpes simplex virus-infected wi-38 cells by phosphonoacetic Acid. J Virol. 1975 May;15(5):1281–1283. doi: 10.1128/jvi.15.5.1281-1283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Coope D., Niederman J., Pagano J. Biological properties and viral surface antigens of Burkitt lymphoma- and mononucleosis- derived strains of Epstein-Barr virus released from transformed marmoset cells. J Virol. 1976 Jun;18(3):1071–1080. doi: 10.1128/jvi.18.3.1071-1080.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Niederman J. C., Stitt D. A. Infectious mononucleosis: appearance of neutralizing antibody to Epstein-Barr virus measured by inhibition of formation of lymphoblastoid cell lines. J Infect Dis. 1972 Apr;125(4):403–406. doi: 10.1093/infdis/125.4.403. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Glaser R., Rapp F. Studies of an Epstein-Barr virus-induced DNA polymerase. Virology. 1977 Feb;76(2):494–502. doi: 10.1016/0042-6822(77)90232-x. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Detection of Epstein-Barr viral genome in nonproductive cells. Nat New Biol. 1971 Sep 22;233(38):103–106. doi: 10.1038/newbio233103a0. [DOI] [PubMed] [Google Scholar]
- Nyormoi O., Thorley-Lawson D. A., Elkington J., Strominger J. L. Differential effect of phosphonoacetic acid on the expression of Epstein-Barr viral antigens and virus production. Proc Natl Acad Sci U S A. 1976 May;73(5):1745–1748. doi: 10.1073/pnas.73.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Gerber P. Selective transformation of B lymphocytes by E.B. virus. Lancet. 1973 Jul 14;2(7820):93–94. doi: 10.1016/s0140-6736(73)93286-8. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Robinson J. Assay for Epstein-Barr virus based on stimulation of DNA synthesis in mixed leukocytes from human umbilical cord blood. J Virol. 1975 May;15(5):1065–1072. doi: 10.1128/jvi.15.5.1065-1072.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976 Apr;18(1):151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D., Strominger J. L. Transformation of human lymphocytes by Epstein-Barr virus is inhibited by phosphonoacetic acid. Nature. 1976 Sep 23;263(5575):332–334. doi: 10.1038/263332a0. [DOI] [PubMed] [Google Scholar]
- Utsinger P. D., Yount W. J., Fallon J. G., Logue M. J., Fuller C. R., Elliott D. Cytofluorometric analysis of the kinetics of lymphocyte transformation after phytohemagglutinin stimulation: comparison with the kinetics of thymidine incorporation. Blood. 1977 Jan;49(1):33–46. [PubMed] [Google Scholar]
- Van Dilla M. A., Trujillo T. T., Mullaney P. F., Coulter J. R. Cell microfluorometry: a method for rapid fluorescence measurement. Science. 1969 Mar 14;163(3872):1213–1214. doi: 10.1126/science.163.3872.1213. [DOI] [PubMed] [Google Scholar]
- Weissbach A. Vertebrate DNA polymerases. Cell. 1975 Jun;5(2):101–108. doi: 10.1016/0092-8674(75)90017-3. [DOI] [PubMed] [Google Scholar]
- Yajima Y., Tanaka A., Nonoyama M. Inhibition of productive replication of Epstein-Barr virus DNA by phosphonoacetic acid. Virology. 1976 May;71(1):352–354. doi: 10.1016/0042-6822(76)90119-7. [DOI] [PubMed] [Google Scholar]



