Abstract
Purpose
We evaluated the predictive value of preoperative lung spirometry test for postoperative morbidity and the nature of complications related to an abnormal pulmonary function after gastric cancer surgery.
Methods
Between February 2009 and March 2010, 538 gastric cancer patients who underwent laparoscopic (n = 247) and open gastrectomy (n = 291) were divided into the normal (forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] ≥ 0.7, n = 441) and abnormal pulmonary function group (FEV1/FVC < 0.7, n = 97), according to the preoperative lung spirometry test. The predictive value of lung spirometry for postoperative morbidity was evaluated using the univariate and multivariate analysis.
Results
After surgery, the abnormal pulmonary function group showed a significantly increased incidence of local (29.9% vs. 18.1%, P = 0.009) and systemic complications (8.2% vs. 2.0%, P = 0.005) than the normal group. Of local complications, anastomosis leakage and wound complication were found to be more common in the abnormal pulmonary function group. In the univariate and multivariate analysis, an abnormal pulmonary function was an independent predictor for postoperative local complication (odds ratio, 1.75; 95% confidence interval, 1.03 to 2.97) after adjusted by old age, total gastrectomy, open surgery, and tumor-node-metastasis stage. Meanwhile, an old age and a history of pulmonary disease were independent predictors for systemic complication.
Conclusion
Preoperative lung spirometry is a simple and useful means to predict postoperative morbidity after gastric cancer surgery. In view of its simplicity and low cost, we recommend adding preoperative lung spirometry test to assess the operative risk and aid in proper perioperative treatment planning.
Keywords: Stomach neoplasms, Morbidity, Spirometry, Chronic obstructive pulmonary disease, Respiratory function test
INTRODUCTION
Gastric carcinoma is largely a disease of old age, and thus surgical patients often have various underlying comorbidities at the time of operation [1]. Accordingly, a thorough preoperative assessment of underlying disease is crucial for making proper treatment decision and predicting possible postoperative complications [2]. Pulmonary comorbidity is one of the most common underlying problems, which is widely believed to increase the risk of postoperative respiratory complications [3]. Although it is assumed that pulmonary disease may also increase the risk of surgical complications due to the impaired wound healing process associated with tissue hypoxia, only a few studies have demonstrated the association between pulmonary comorbidity and surgical complications in patients undergoing major abdominal surgery [4].
Preoperative screening of pulmonary disease usually depends on the history taking of patients' previous medical history. However, pulmonary disease is seldom diagnosed and treated until patients visit physicians with overt respiratory symptoms. Meanwhile, lung spirometry allows patients with pulmonary abnormality to be objectively screened, and could be useful for predicting operative risk related to an abnormal pulmonary function [5]. In the field of thoracic surgery, preoperative lung spirometry is widely used to select surgical candidates and predict the occurrence of postoperative respiratory complications [6]. However, lung spirometry is not commonly recommended for patients with major abdominal surgery because of its uncertainty about predictive value for postoperative complications [7,8]. In the present study, we have investigated the value of preoperative lung spirometry test for predicting postoperative complications after gastric cancer surgery. We have also sought to investigate which complications may arise after gastric cancer surgery related to an abnormal pulmonary function.
METHODS
Patients and data collection
From February 2009 through March 2010, 582 consecutive gastric cancer patients underwent pulmonary function test using lung spirometry prior to surgery. Preoperative examinations included endoscopy with biopsy and an abdominal computed tomography (CT) scan, along with basic blood testing, chest radiography, and electrocardiography. Chest CT, liver magnetic resonance imaging, or endoscopic ultrasonography was performed in selected patients for tumor staging. Of the patients, 46 were excluded as following reasons; 1) underwent an operation under an emergency condition, such as, for bleeding or perforation (n = 14), 2) underwent exploratory or bypass surgery for unresectable disease (n = 16), or 3) received preoperative systemic chemotherapy (n = 16), were excluded from the investigation. Finally, 538 patients who underwent elective gastric cancer surgery were included in the present study.
Patient data regarding demographic characteristics, operative results, pathologic findings, hospital courses, and postoperative complications were prospectively collected. Regarding postoperative complications, when a complication occurred near the operation field, it was considered as a local complication, and when a complication was not associated with the operation field, it was considered as a systemic complication. The following postoperative surgical complications were included in the data-recording protocol: abdominal bleeding, luminal bleeding, abdominal infection, pancreatic fistula, pancreatitis, anastomosis leakage, paralytic ileus, duodenal stump leakage, ascites, and wound infection. Other complications were recorded as considered appropriate. Anastomosis leaks were diagnosed based on the CT scan or upper gastrointestinal findings of clinically suspected patients. Abdominal infection was diagnosed radiologically when a clinical infection sign was accompanied by abdominal fluid collection. Pancreatic fistula was diagnosed based on a continued high amylase concentration in drainage fluid from the peripancreatic area for more than 7 days. Bleeding was diagnosed based on abdominal CT findings or clinical suspicion, due to, for example, bloody abdominal drainage or melana. Types of complications were judiciously defined and recorded according to the diagnostic criteria, and the severities were classified based on the Accordion Severity Grading System of surgical complications [9]. In-hospital mortality was defined as operation-related death within 30 days of surgery or during the period of hospitalization.
To evaluate the predictive value of lung spirometry, a comparative analysis was carried out between the normal (n = 441) and the abnormal pulmonary function group (n = 97) with respect to postoperative morbidity and mortality. The ability of pulmonary function test results to predict postoperative morbidity was determined using the univariate and multivariate analysis. This study was approved by the Institutional Review Board at the Chonnam National University Hospital, South Korea.
Preoperative pulmonary function testing
Preoperative pulmonary function testing was performed using the SensorMedics Vmax22 diagnostic spirometer (SensorMedics, Loma Linda, CA, USA). Based on the postbronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] ratios and FEV1 values, pulmonary function airway limitations were classified as normal (FEV1/FVC ≥ 0.7), mild (FEV1/FVC < 0.7, FEV1 ≥ 80% predicted), moderate (FEV1/FVC < 0.7, 50% ≤ FEV1 < 80% predicted), severe (FEV1/FVC < 0.7, 30% ≤ FEV1 < 50%), or very severe (FEV1/FVC < 0.7, FEV1 < 30% predicted), according to the spirometric classification of chronic obstructive pulmonary disease (COPD) [10].
Operative procedure and perioperative care
Patients underwent distal or total gastrectomy with regional lymph node dissection, as described by the gastric cancer treatment guideline issued by the Japanese Gastric Cancer Association [11]. Laparoscopic surgery was indicated based on the preoperative tumor staging (cT1-2N0). Billroth I reconstruction was the primary procedure after distal gastrectomy, and Billroth II or Roux-en Y gastrojejunostomy was considered in selected cases, such as wide resection of the stomach. Roux-en Y esophagojejunostomy was performed routinely after total gastrectomy.
All the patients were managed using a standardized clinical pathway protocol. For example, no preoperative mechanical bowel preparation or nasogastric tube was employed. Postoperatively, patients were administered an oral diet from postoperative day 1, and intravenous fluid (20 to 25 mL/kg/day) was administered during the first three postoperative days. Prophylactic antibiotics were administered before skin incision and were continued until 12 hours after surgery. Thromboprophylaxis was performed using a pneumatic intermittent compression device. Hospital discharge was decided based on the fulfillment of the following objective discharge criteria; no evidence of a postoperative complication, the ability to ambulate without assistance, tolerable pain on administration of oral analgesia, the ability to take more than 70% of any given meal, and a willingness to go home.
Statistical analysis
The Student's t-test was used to analyze continuous data, and the chi-square test or Fisher's exact test was used to analyze categorical data. During the analysis of predictors of postoperative morbidity, all continuous data were dichotomized, and thus, the chi-square test or Fisher's exact test was used for univariate comparisons. Statistically significant factors (P < 0.05) by univariate analysis were used as covariates for multivariate analysis to identify predictors of postoperative morbidity. All the statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA), and P values of < 0.05 were regarded significant.
RESULTS
Patient characteristics
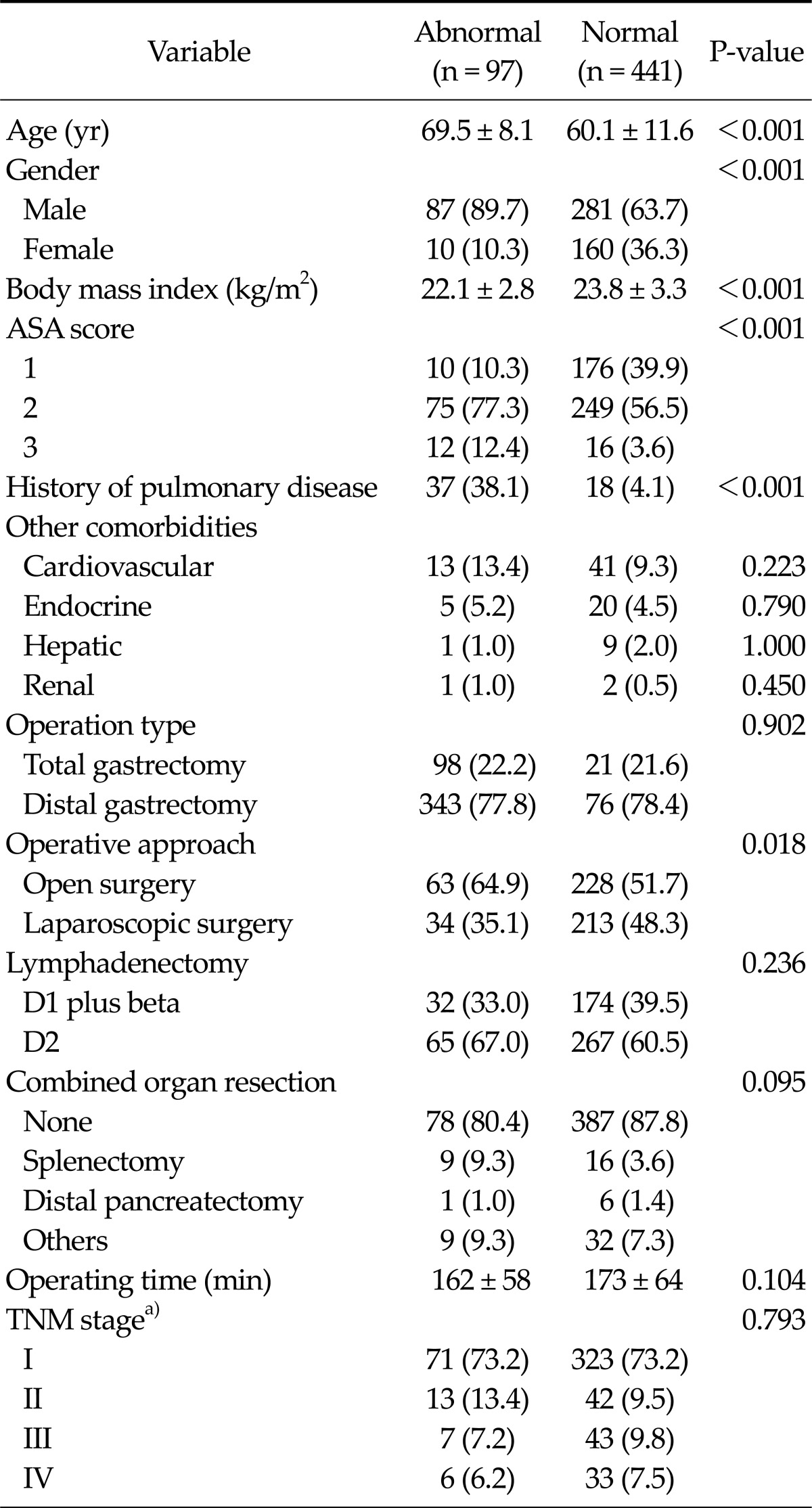
Table 1 presents the clinicopathological characteristics of patients included in the present study. The study subjects consisted of 368 males and 170 females of mean age (standard deviation) of 61.8 (11.6) years, and 419 patients (77.9%) underwent distal gastrectomy and 119 patients (22.1%) underwent total gastrectomy. Laparoscopic surgery was performed in 247 patients (45.9%). Pathologic examinations revealed that 394 patients (73.2%) were of stage I, 55 (10.2%) were of stage II, 50 (9.3%) were of stage III, and 39 (7.2%) were of stage IV, according to the sixth edition of the Union for International Cancer Control tumor-node-metastasis (TNM) classification.
Table 1.
Clinicopathological characteristics of the normal and abnormal pulmonary function groups
Values are presented as mean ± standard deviation or number (%)
a)Sixth edition of Union for International Cancer Control tumornode-metastasis (TNM) classification.
ASA, American Society of Anesthesiologists.
Based on preoperative lung spirometry, 97 patients (18.0%) had an abnormal pulmonary function, which means, 44 (8.2%) had mild, 40 (7.4%) moderate, and 13 (2.4%) severe airway limitation, but none of them had very severe airway limitation according to the spirometric classification of airway limitation. Of the 97 patients with an abnormal pulmonary function, 37 (38.1%) have had a previous medical history of pulmonary disease, such as, COPD or asthma, and 18 (4.1%) of 441 patients with a normal pulmonary function had a history of pulmonary disease.
The abnormal pulmonary function group had a greater mean age (69 years vs. 60 years, P < 0.001), more number of male patients (89.7% vs. 63.7%, P < 0.001), a higher American Society of Anesthesiologists (ASA) score (ASA score of ≥2, 89.7% vs. 60.1%, P < 0.001), and a lower body mass index (22.1 kg/m2 vs. 23.8 kg/m2, P < 0.001) than the normal pulmonary function group. However, no significant intergroup differences were found for other comorbidities, such as, cardiovascular, endocrine, hepatic, or renal diseases. Despite no consideration of pulmonary function during decision making regarding operative approaches, a greater proportion of the abnormal pulmonary function group underwent open surgery (64.9% vs. 51.7%, P = 0.018).
Postoperative morbidity and mortality
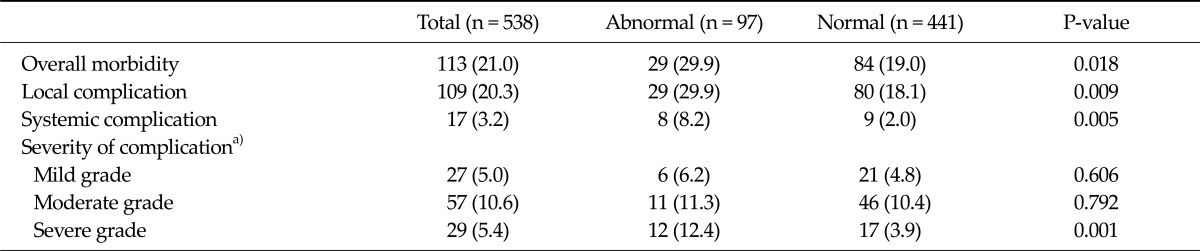
Table 2 presents the postoperative morbidities and mortalities of the two study groups. After surgery, 113 (21.0%) of the study subjects developed postoperative complications, which included 109 surgical and 17 systemic complications. The severities of these postoperative complications were as follows; 27 (5.0%) mild, 57 (10.6%) moderate, and 29 (5.4%) severe. Not even a single case of in-hospital mortality occurred after surgery.
Table 2.
Postoperative morbidities in the normal and abnormal pulmonary function groups
Values are presented as number (%).
a)Based on the Accordion Severity Classification System of surgical complications [9].
Patients with an abnormal pulmonary function had a significantly greater incidence of postoperative complication when compared to normal patients, in terms of both surgical (29.9% vs. 18.1%, P = 0.009) and systemic complications (8.2% vs. 2.0%, P = 0.005). When the severities of postoperative complications were compared, the incidences of mild and moderate complications were similar in the two groups, but the rate of severe grade complications was significantly higher in patients with an abnormal pulmonary function (12.4% vs. 3.9%, P = 0.001) (Table 2).
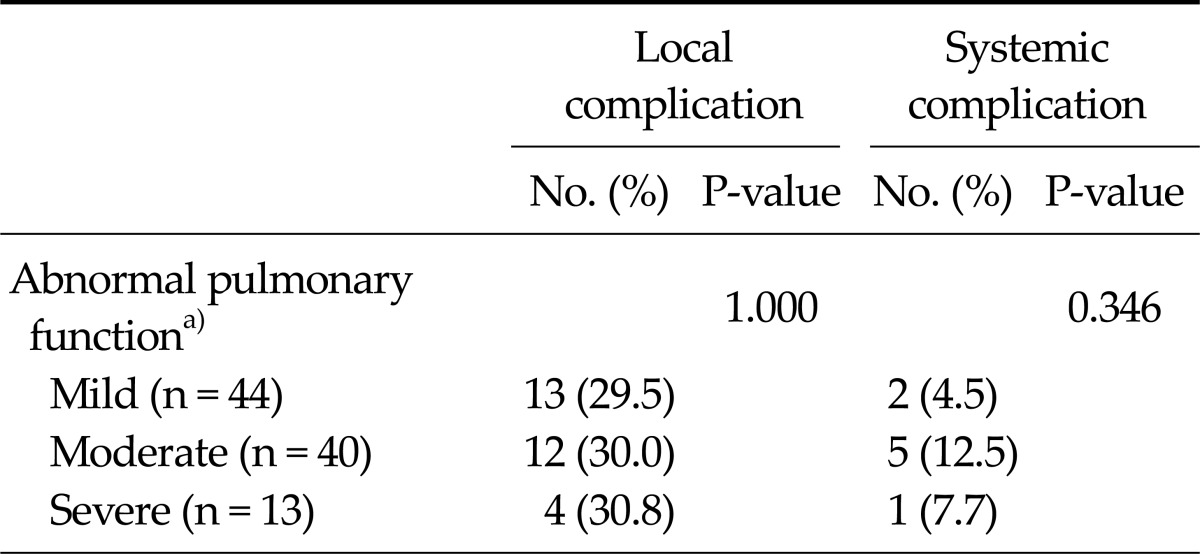
Table 3 provides the details of postoperative morbidities for different airway limitation severities. No significant differences in local (29.5%, 30.0%, and 30.8% for mild, moderate, and severe airway limitation, respectively) or systemic complications (4.5%, 12.5%, and 7.7%, respectively) were observed with respect to the severity of airway limitation.
Table 3.
Postoperative morbidities according to the airway limitation severities
a)Based on the spirometric severity classification of pulmonary function [9].
Predictive value of preoperative lung spirometry for postoperative complications
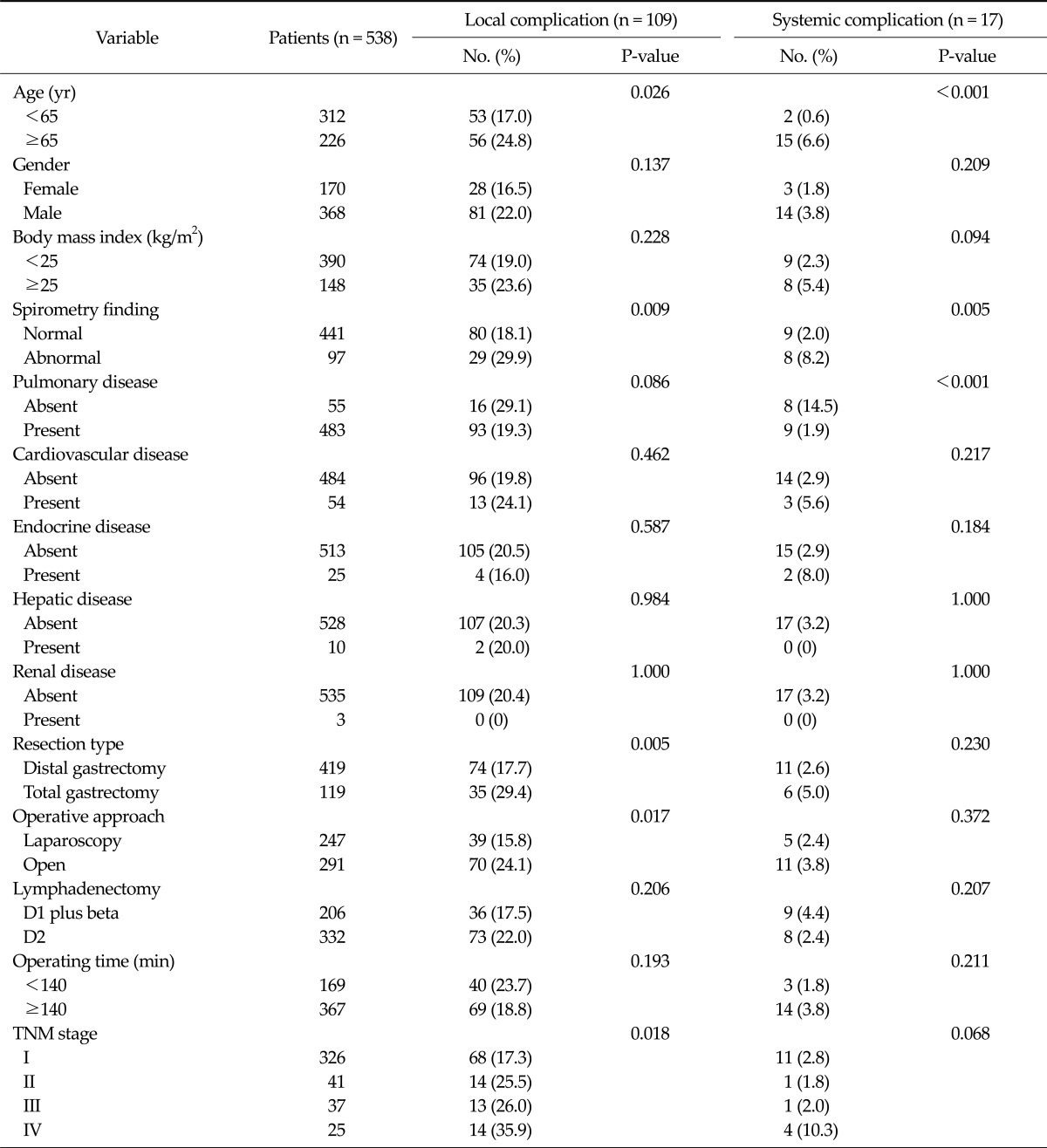
Table 4 presents the results of univariate analysis for predictors of postoperative complications. By univariate analysis, an age of ≥ 65 years (P = 0.026), total gastrectomy (P = 0.005), open surgery (P = 0.017), an abnormal pulmonary function (P = 0.009), and TNM stage (P = 0.018) were found to be significantly associated with the occurrence of postoperative surgical complications, whereas an age of ≥ 65 years (P < 0.001), an abnormal pulmonary function (P = 0.005), and a history of pulmonary disease (P < 0.001) were found to be significantly associated with systemic complications.
Table 4.
Univariate analysis results for predictors of postoperative morbidity
TNM, tumor-node-metastasis.
We performed multivariate analysis adjusting the variables that were statistically significant in the univariate analysis, to further investigate the relationship between abnormal pulmonary function and postoperative complications. Multivariate analysis revealed that an abnormal pulmonary function was an independent predictive factor for postoperative surgical complications (odds ratio [OR], 1.75; 95% confidence interval [CI], 1.03 to 2.97) after adjusted by old age, resection type, operative approach, and TNM stage (Table 5). Meanwhile, an age of ≥ 65 years (OR, 8.12; 95% CI, 1.76 to 37.38) and a history of pulmonary disease (OR, 5.75; 95% CI, 1.67 to 19.82) were independent predictors for systemic complications, whereas an abnormal pulmonary function was not.
Table 5.
Multivariate analysis results for predictors of postoperative morbidity
Multivariate analysis after adjusting the variables with P-value of <0.1 in the univariate analysis.
OR, odds ratio; CI, confidence interval; TNM, tumor-nodemetastasis.
Postoperative complications related to an abnormal pulmonary function
Table 6 summarizes the details of complication types in both the normal and abnormal pulmonary function groups. In terms of surgical complications, anastomosis leakage (4.1% vs. 0.5%, P = 0.011) and wound complication (4.1% vs. 0.9%, P = 0.039) were found to be significantly more common in patients with an abnormal pulmonary function. For systemic complications, respiratory complications (6.1% vs. 1.1%, P = 0.006) were found to be significantly more common in patients with an abnormal pulmonary function. As far as respiratory complications were concerned, there were two cases of pleural effusion and four cases of pneumonia in patients with an abnormal pulmonary function, and all were associated with local complications, such as, abdominal bleeding, anastomosis leakage, or a wound complication.
Table 6.
Comparisons of complication types in the two study groups
Values are presented as number (%).
DISCUSSION
Preoperative pulmonary function testing is often ordered before major surgery to screen patients for an abnormal pulmonary function [2]. However, unlike a general acceptance of preoperative lung spirometry as an effective tool for predicting operative risk in the field of thoracic surgery, its role, in terms of predicting postoperative morbidity, is somewhat controversial in the field of major abdominal surgery [6]. Some studies have even demonstrated that clinical findings are more predictive of postoperative respiratory complications than pulmonary function test results [12,13]. However, a comprehensive review of pulmonary function testing in the context of abdominal surgery by Lawrence et al. [8] revealed significant methodological flaws associated with all the previously performed studies, and thus, it was recommended that the efficacy of pulmonary function testing for abdominal surgery should be reexamined. In the present study, we found that preoperative lung spirometry could effectively predict the risk of postoperative surgical complications, as well as systemic complications in patients undergoing gastric cancer surgery.
Several authors have investigated the impact of underlying comorbidities on surgical outcomes after gastric cancer surgery, and it is generally accepted that the presence of comorbidity is a major determinant of postoperative morbidity after gastrectomy [14-16]. Regarding the pulmonary disease, previous studies have arrived at different conclusions about its impact on surgical outcomes. In a large multicenter based study on laparoscopic gastrectomy, Kim et al. [16] investigated the operative risk presented by different comorbidity types and found that pulmonary disease was significantly associated with postoperative morbidity. On the other hand, several other studies have concluded that pulmonary disease did not significantly increase the risk of postoperative complications after gastric cancer surgery [14,15]. Because pulmonary disease is seldom clinically diagnosed unless overt respiratory symptoms develop, preoperative screening of pulmonary disease simply based on history taking or previous medical history might have inconsistent predictive value for postoperative morbidity. In fact, in the present study, of 97 patients with an abnormal pulmonary function suggested by lung spirometry, 60 (61.9%) had no previous medical history of pulmonary disease, which suggests that conventional clinical assessments significantly underestimate pulmonary abnormalities. Therefore, we consider that preoperative screening using lung spirometry is likely to be more useful than conventional preoperative assessment, in terms of evaluating pulmonary abnormality and predicting postoperative complications.
Tissue hypoxia, poor nutritional status, and systemic inflammatory reactions, which are common in patients with pulmonary disease, are widely held responsible to lead to an increase in the risk of surgical complications by inhibiting the wound healing process after gastrointestinal surgery [4]. However, no study has been undertaken to identify as which surgical complications after gastric cancer surgery are related to preoperative abnormal pulmonary function. In the present study, we identified that wound complications and anastomosis leakage were significantly increased in an abnormal pulmonary group. In addition, gastric stasis tended to be more frequent among those with an abnormal pulmonary function (P = 0.075). Similarly, a large study conducted on identification of the impact of comorbidity on postoperative complications after laparoscopic gastrectomy, revealed a significant increase in wound complications and intestinal obstruction in pulmonary disease [16]. Our study does stimulate questions and the role of hypoxia by an abnormal pulmonary function on anastomosis healing, and this needs to be further investigated by carrying out animal or laboratory study in the future.
In the present study, we classified abnormal pulmonary function using a spirometric airway limitation classification system to diagnose COPD [9], but we found no significant differences in the incidences of local or systemic complications with respect to the airway limitation severity. Spirometric cut-off points (a postbronchodilator FEV1/FVC ratio of <0.70 or an FEV1 of <80, 50, or 30% of predicted) provide a straightforward means of achieving a clinical diagnosis and of classifying abnormal pulmonary function [17]. However, these spirometric classifications alone do not reflect symptom severity, disease progression, or prognosis in patients with pulmonary disease [18], and thus, we think perioperative management aimed at optimizing lung function should be considered for any patient with abnormal spirometric findings, regardless of the severity of airway limitation.
Other common comorbidities, such as, cardiovascular, endocrine, and hepatic disease, are likely to be more common in patients with an abnormal pulmonary function, and could contribute to increased postoperative morbidity. Thus, the predictive value of lung spirometry should be evaluated by adjusting for the operative risks associated with these comorbidities. In the present study, no significant differences were observed between the normal and abnormal pulmonary function groups with respect to the incidence of other comorbidities. In addition, comorbidities, such as cardiovascular, endocrine, hepatic, and renal diseases, were not found to significantly increase the risk of postoperative morbidity as per the univariate analysis. However, the impact of comorbidities other than pulmonary disease on surgical outcomes needs to be further evaluated in terms of large studies in which accurate preoperative assessment is implemented.
In the present study, most of the respiratory complications of patients with an abnormal pulmonary function followed surgical problems and so might be the consequence of factors other than the pulmonary status of the patients. Based on the present results, it is difficult to clearly conclude whether increasing incidence of pulmonary complications is directly related to an abnormal pulmonary function or just a consequence of increased surgical complications. However, it is apparent that patients with an abnormal pulmonary function tend to be more vulnerable to subsequent pulmonary problem when compared to normal patients. Therefore, patients with an abnormal pulmonary function should be carefully monitored with emphasis on the development of subsequent pulmonary problems when they develop local complications.
In the present study, the abnormal pulmonary function group received a greater extent of open surgery than the normal group (64.9% vs. 51.7%, P = 0.018), which might be thought to contribute to the greater postoperative morbidity observed in patients with an abnormal pulmonary function. However, our multivariate analysis was adjusted for operative approach, and it revealed that lung spirometry independently predicts postoperative morbidity regardless of operative approaches.
The present study is limited by the fact that the cohort was confined to patients that underwent gastric cancer surgery, and thus, we are unable to comment on the ability of preoperative pulmonary function testing to predict outcomes after other forms of abdominal surgery. In addition, we have to admit that we cannot provide useful information in the present study about what measures should be undertaken to reduce the operative risk in patients with an abnormal pulmonary function. Actually, we tried to find any controllable surgeon factors, such as operative approaches, operating time, or lymph node dissection, which may be expected to change the risk of complications in patients with an abnormal pulmonary function. Unfortunately, however, our data showed no significant differences in postoperative morbidity according to any surgical factors in patients with an abnormal pulmonary function. Actually, proper perioperative interventions for optimizing lung function would be of essence to reduce the operative risk than surgical factors itself. In the present study, every individual patient with an abnormal pulmonary function was given some of the preventive measure, such as postoperative oxygen supplementation, incentive spirometry, or bronchodilator therapy. Therefore, the proper risk reducing strategies and its efficacy need to be further evaluated in the form of prospective clinical trial.
In conclusions, preoperative pulmonary function testing using lung spirometry was found to be effective for the screening of an abnormal pulmonary function and for predicting postoperative surgical complications after gastric cancer surgery. More specifically, abnormal pulmonary function was found to be significantly related to wound problems and anastomosis leakage. The proper evaluation of operative risk would undoubtedly improve the quality of perioperative management and encourage tailored care. Finally, proper strategies to reduce the postoperative morbidity in patients with an abnormal pulmonary function should be evaluated in the clinical trials.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Maehara Y, Emi Y, Tomisaki S, Oshiro T, Kakeji Y, Ichiyoshi Y, et al. Age-related characteristics of gastric carcinoma in young and elderly patients. Cancer. 1996;77:1774–1780. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1774::AID-CNCR3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 2.Neragi-Miandoab S, Wayne M, Cioroiu M, Zank LM, Mills C. Preoperative evaluation and a risk assessment in patients undergoing abdominal surgery. Surg Today. 2010;40:108–113. doi: 10.1007/s00595-009-3996-7. [DOI] [PubMed] [Google Scholar]
- 3.Kanat F, Golcuk A, Teke T, Golcuk M. Risk factors for postoperative pulmonary complications in upper abdominal surgery. ANZ J Surg. 2007;77:135–141. doi: 10.1111/j.1445-2197.2006.03993.x. [DOI] [PubMed] [Google Scholar]
- 4.Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med. 2005;26:142–153. doi: 10.1055/s-2005-869535. [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Snow V, Fitterman N, Hornbake ER, Lawrence VA, Smetana GW, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144:575–580. doi: 10.7326/0003-4819-144-8-200604180-00008. [DOI] [PubMed] [Google Scholar]
- 6.Smetana GW. Preoperative pulmonary evaluation. N Engl J Med. 1999;340:937–944. doi: 10.1056/NEJM199903253401207. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence VA, Dhanda R, Hilsenbeck SG, Page CP. Risk of pulmonary complications after elective abdominal surgery. Chest. 1996;110:744–750. doi: 10.1378/chest.110.3.744. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence VA, Page CP, Harris GD. Preoperative spirometry before abdominal operations. A critical appraisal of its predictive value. Arch Intern Med. 1989;149:280–285. [PubMed] [Google Scholar]
- 9.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250:177–186. doi: 10.1097/SLA.0b013e3181afde41. [DOI] [PubMed] [Google Scholar]
- 10.Global Initiative for Chronic Obstructive Lung Disease. 2006 Revision: GOLD report, global strategy for diagnosis, management, and prevention of COPD [Internet] [place unknown]: Global Initiative for Chronic Obstructive Lung Disease; 2006. [cited 2012. Mar 12]. Available from: http://www.goldcopd.org/Guidelines/guidelines-globalstrategy-for-diagnosis-management-2006.html. [Google Scholar]
- 11.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K, Lawrence VA, Theroux JF, Tuley MR, Hilsenbeck S. Postoperative complications after thoracic and major abdominal surgery in patients with and without obstructive lung disease. Chest. 1993;104:1445–1451. doi: 10.1378/chest.104.5.1445. [DOI] [PubMed] [Google Scholar]
- 13.Williams-Russo P, Charlson ME, MacKenzie CR, Gold JP, Shires GT. Predicting postoperative pulmonary complications. Is it a real problem? Arch Intern Med. 1992;152:1209–1213. [PubMed] [Google Scholar]
- 14.Hwang SH, Park do J, Jee YS, Kim HH, Lee HJ, Yang HK, et al. Risk factors for operative complications in elderly patients during laparoscopy-assisted gastrectomy. J Am Coll Surg. 2009;208:186–192. doi: 10.1016/j.jamcollsurg.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Jeong SH, Ahn HS, Yoo MW, Cho JJ, Lee HJ, Kim HH, et al. Increased morbidity rates in patients with heart disease or chronic liver disease following radical gastric surgery. J Surg Oncol. 2010;101:200–204. doi: 10.1002/jso.21467. [DOI] [PubMed] [Google Scholar]
- 16.Kim W, Song KY, Lee HJ, Han SU, Hyung WJ, Cho GS. The impact of comorbidity on surgical outcomes in laparoscopy-assisted distal gastrectomy: a retrospective analysis of multicenter results. Ann Surg. 2008;248:793–799. doi: 10.1097/SLA.0b013e3181887516. [DOI] [PubMed] [Google Scholar]
- 17.Celli BR, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 18.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]