Abstract
The distribution of red-spotted grouper nervous necrosis virus (RGNNV) antigens was examined by immunohistochemistry in the nervous and non-nervous organs of juvenile European seabass (Dicentrarchus labrax) during the course of an intramuscular infection. Histological changes resulting from the infection were evaluated from 3 days to 2 months post-infection. The specific antibody response was also studied 2 months post-challenge. Viral proteins were present throughout the experimental period in the retina (inner nuclear layer, ganglion layer, outer limiting membrane, and outer plexiform layer), brain (cerebellum and tectum opticum), and liver (hepatocytes and endothelial cells). These proteins were also observed in the renal tubular cells, white pulp of spleen, and in fibroblasts and cartilage of caudal fin. This is the first report of RGNNV proteins appearing in these organs, where the immunostaining was only detected at certain sampling times after the onset of mortality. Brain and retina of virus-exposed fish showed high levels of vacuolation, while accumulation of fat vacuoles was observed in the liver. RGNNV infection also induced a specific antibody response as measured by an ELISA. In summary, this is the first study demonstrating the presence of viral proteins in cells of caudal fin, kidney and spleen of European seabass.
Keywords: ELISA, European seabass, histopathology, immunohistochemistry, RGNNV
Introduction
European seabass (Dicentrarchus labrax) is one of the most economically important fish species in the marine Mediterranean aquaculture. One of the main threats for culturing this fish is the occurrence of outbreaks of viral nervous necrosis (VNN) disease, also known as viral encephalopathy and retinopathy. This disease can also affect other marine fish species with high economic importance worldwide such as striped jack (Pseudocaranx dentex), Atlantic halibut (Hippoglossus hippoglossus), turbot (Scophthalmus maximus) and groupers. The impact of VNN disease is mainly observed during the larval and juvenile stages [6,11,13,20,21,26,36]. Affected fish display abnormal behavior and visual dysfunction caused by damages in the central nervous system and retina, such as vacuolation and necrosis within brain and spinal cord, as well as the presence of granular layers in retinal tissue [8].
The causative agent of VNN disease is a non-enveloped icosahedral virus belonging to the Nodaviridae family, Betanodavirus genus. The viral genome is composed of two single-stranded positive-sense RNA segments lacking a poly(A) tail. RNA1 encodes an RNA-dependent RNA polymerase whereas the capsid protein is encoded by RNA2. In addition, RNA3, a subgenomic transcript of RNA1, encodes the B2 protein, which has a suppressor function in post-transcriptional gene silencing [18]. Fish nodaviruses are grouped into four genotypes based on the nucleotide sequence of the variable region (T4) within the viral capsid gene: striped jack nervous necrosis virus, tiger puffer nervous necrosis virus, barfin flounder nervous necrosis virus, and red-spotted grouper nervous necrosis virus (RGNNV) [29]. More recently, Johansen et al. [20] proposed a turbot nodavirus genotype. The RGNNV genotype has been frequently isolated during outbreaks affecting European seabass in the Mediterranean aquaculture [8,10].
The present work is part of a comprehensive study on RGNNV pathogenesis in juvenile European seabass in which the temporal appearance of viral genome and proteins in fish tissues has been observed by absolute real-time PCR, in situ hybridization (ISH), viral titration, immunohistochemistry (IHC) and histopathology [22]. Specific antibody production has also been identified using an ELISA. Our group recently discovered the presence of viral genome and particles in nervous and non-nervous organs of European seabass [22]. In that study, increases in the number of copies of both viral RNA segments were found by absolute real-time PCR in brain, eyes, pooled internal organs, and caudal fin during the course of the experiment. In comparison, ISH was shown to have lower sensitivity for detecting the RGNNV genome in these tissues. The present work completes this body of information by using IHC to study viral protein distribution during the course of the same infection. In addition, histopathological analyses and quantification of anti-RGNNV antibodies have also been performed. Although several studies on nodavirus distribution in tissues of European seabass have been performed, most of them have been conducted in larvae and were focused on virus detection only in nervous tissues [14,25,30,35].
IHC is a useful method to evaluate tissue distribution of viruses, and can detect nodavirus infections with low prevalence even when typical histological damages (i.e., vacuolation) have not yet developed [21]. In addition, this technique shows the target cells for virus replication within each organ and reveals associations with pathological changes. For these reasons, IHC is a valuable tool not only for viral diagnosis but also for pathogenesis studies.
The aim of the present study was to determine the tissue distribution of RGNNV antigens during the course of an experimental infection of juvenile European seabass. In addition, histopathological damages resulting from the infection were evaluated. The levels of specific anti-RGNNV antibodies in the serum of these fish were also measured.
Materials and Methods
Virus and cell culture
The E-11 cell line (ECACC Cat. No. 01110916; European Collection of Cell Cultures, UK), developed by Iwamoto et al. [19], was cultured at 25℃ in Leibovitz (L-15) medium (Invitrogen, USA) supplemented with 1% penicillin-streptomycin (Invitrogen, USA) and 5% fetal bovine serum (FBS; Lonza, Swiss) until semi-confluent prior to virus inoculation. An RGNNV isolate (ERV378/102-5/04), kindly provided by Dr. G. Bovo (Instituto Zooprofilattico Sperimentale delle Venezie, Italy) was propagated following standard protocols at 25℃ in the E-11 cell line. Inoculated cells were maintained in L-15 medium supplemented with 1% penicillin-streptomycin and 2% FBS. Viral titers were determined according to the final dilution causing cytopathic effects in the 50% of the inoculated wells (TCID50 method) [12].
Experimental challenge and samplings
Juvenile European seabass (average weight, 10 g; age, 6~7 months) from an aquaculture centre located in the South-West of the Iberian Peninsula were distributed into three tanks: 1) control non-infected animals (n = 250), 2) infected animals used for sampling (n = 250), and 3) infected animals used for cumulative mortality determination (n = 100, no sampling was conducted for this group). Water temperature was maintained between 21 and 24℃ over the course of the trial. Fish in the challenge and control groups were intramuscularly injected with RGNNV (106 TCID50/fish) or L-15 medium (0.1 mL), respectively. Mortality was recorded daily and the dead fish were frozen at -80℃ until virological testing. To confirm that the virus was the causal infectious agent, virological test were performed, involving the inoculation of pooled brain and eye homogenates of dead fish on E-11 cells, and the subsequent confirmation for the RGNNV presence by a previously reported RT-PCR [23]. This RT-PCR protocol amplifies a 292-pb fragment within the T4 region, located in the RNA2 segment, using the primers Noda-Fwr1 and Noda-Rev2 [23]. Tissue homogenization was performed in L-15 medium (20%, w/v) using a T10 basic Ultra-Turrax (IKA, Germany) disperser following the procedure previously described [1].
Two apparently healthy fish from the challenged (tank No. 2) and control (tank No. 1) groups were randomly collected and sacrificed, with an overdose of anesthetic (MS-222; Sigma, USA), at 3, 10, 17, 24, and 31 days post-infection (PI) and 2 months PI. These animals were dissected, and the brain, eyes, liver, spleen, kidney, and caudal fin were fixed in 4% buffered formalin (Merck, Germany) at room temperature for 24 h. The organs were then washed with distilled water for 3 h, and maintained in 70% ethanol at room temperature until use.
In addition, blood samples were collected 24 days and 2 months PI from the caudal vein of challenged and control fish (15 animals per time and group). Samples from five animals were pooled in order to obtain sufficient volume for anti-RGNNV antibody quantification. After clotting at 4℃ overnight, sera were collected by two consecutive rounds of centrifugation (400 × g at 4℃) for 15 min, and stored at -20℃ until the ELISA was performed to measure anti-RGNNV antibody levels. Three samples, each containing sera from five fish, were analyzed at each sampling time.
IHC
Organs were processed with the Shandon Excelsior ES Tissue Processor (Thermo Scientific, USA) according to the manufacturer's instructions, embedded into paraffin blocks using the Leica Histoembedder (Leica Microsystems, Germany), and 5-µm tissue sections were mounted onto slides. Sectioning was performed using the microtome RM2035 (Leica Microsystems, Germany) and Leica Disposable Microtome Blades (Leica Microsystems, Germany). The blade was carefully disinfected with ethanol between each sample. After deparaffinization and rehydrating in a graded series of ethanol, endogenous peroxidase activity was blocked by incubating at 37℃ for 1 h with a solution composed of 0.36% (w/v) β-D-glucose, 0.01% (w/v) glucose oxidase, and 0.0013% (w/v) sodium azide in PBS. Afterwards, the sections were rinsed twice with PBS for 3 min, and non-specific binding sites were blocked by incubating with normal goat serum at 1 : 10 dilution in PBS (Sigma, USA) for 30 min at room temperature (ca. 20℃). The sections were then incubated at room temperature with an anti-RGNNV monoclonal antibody (1 : 5 dilution in PBS) for 1 h (P09; Aquatic Diagnostics Ltd, UK), and the resulting antigen-antibody complex was detected using a 1 : 100 dilution of a goat anti-mouse IgG-biotin conjugate (Sigma, USA) for 1 h at room temperature (ca. 20℃). For colorimetric detection, the tissues were incubated at room temperature with 1 : 100 dilution of streptavidin-horseradish peroxidase (HRP; Sigma, USA) in PBS for 1 h. The reaction was visualized using a Vector VIP substrate kit for peroxidase (Vector Laboratories, USA). The sections were finally counter-stained with methyl green (Vector laboratories, USA) at 60℃ for 3 min, dehydrated, and mounted with Pertex mounting medium (Leica Microsystems, Germany). Antibody labeling appeared as dark purple staining in the target cells. Staining specificity in every organ analyzed was evaluated by performing IHC without the primary antibody.
Histopathology
Paraffin-embedded organs from challenged animals were examined for histological changes and compared to organs from non-exposed fish. After deparaffinization and rehydration, 5-µm tissue sections were stained with hematoxylin-eosin following standard histology protocols [32]. Sections were observed using an optical microscope (E800; Nikon, Japan).
Anti-RGNNV antibody quantification
Quantification of anti-RGNNV antibodies was conducted by an indirect ELISA using 96-well polystyrene plates (Immulon 4HBX; Thermo Scientific, USA) coated with 0.4 mg of lysates from RGNNV-infected and non-infected (control) E-11 cells as previously described by Grove et al. [15]. Negative controls (PBS instead of fish sera) and positive controls (anti-RGNNV monoclonal antibodies instead of fish sera) were included in each ELISA plate. After coating, the plates were washed three times with 1× low salt wash (LSW) buffer (2.4 g/L Trizma base, 22.2 g/L NaCl, 0.1 g/L merthiolate, and 0.5 mL/L Tween 20), and non-specific binding sites were blocked with 3% skimmed powder milk (Sigma, USA) in 1× LSW buffer during 2 h at room temperature (ca. 20℃). Afterwards, the plates were washed three times with 1× LSW buffer, and 100 µL of double-diluted tested pooled sera (from 1 : 32 to 1 : 262,144, in PBS) were added. Pooled samples were analyzed in triplicate. An overnight incubation was carried out at 4℃. Afterwards, plates were washed five times with 1× high salt wash (HSW) buffer (2.4 g/L Trizma base, 29.2 g/L NaCl, 0.1 g/L merthiolate, and 1 mL/L Tween 20), and each well was subsequently incubated with 100 µL of an anti-European seabass IgM monoclonal antibody (F01; Aquatic Diagnostics, UK) (1 : 33 dilution of the reconstituted antibody in 1% BSA in PBS) for 1 h at room temperature following the manufacturer's instructions. After washing five times with 1× HSW buffer, 100 µL of HRP-conjugated goat anti-mouse IgG (1 : 4,000 dilution in 1% BSA in 1× LSW buffer; Sigma, USA) were added and the plates were incubated at room temperature for 1 h. After washing five times with 1× HSW buffer, the reactions were finally visualized by adding 3,3',5,5'-tetramethylbenzidine (50 µL/well) (Sigma, USA).
The optical density (OD) of each well was measured at 450 nm using an ELISA Whittaker Microplate Reader 2001 (Anthos Labtec, Austria). The resulting OD values were normalized by subtracting the OD of the negative control wells. Based on a report by Skinner et al. [34], the mean OD value of the negative controls multiplied by three was the cut-off threshold used in the present study. The highest serum dilution above this value was the anti-RGNNV antibody endpoint titer for that sample.
Results
Clinical observations
RGNNV infection resulting from viral intramuscular (IM) injection induced fish mortality between 7 and 29 days PI. External signs of the disease were dark skin pigmentation, reduced feeding, hyperactivity, and erratic swimming patterns. Internally, the swim bladder was expanded and liver mass was reduced. Cumulative mortality at the end of the experiment in tank No. 3, in which no sampling was conducted, was 37%. No mortality was recorded among the uninfected fish. Analyses of dead fish by cell culture inoculation demonstrated the presence of RGNNV, which was subsequently confirmed by a previously described specific RT-PCR protocol (data not shown).
RGNNV localization by IHC
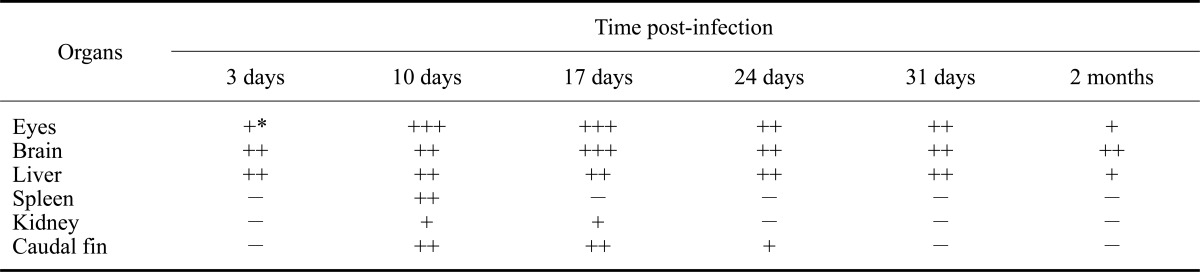
IHC revealed the presence of stained cells in tissue sections from infected fish prior to the appearance of the typical external VNN symptoms, which occurred 5 days PI. In contrast, no labeling was observed in tissues from the uninfected fish at any time (Figs. 1 and 2). Staining intensity and the number of labeled cells indicated the degree of infection in each tissue (Table 1). Staining was evident in the retina, brain, and liver throughout the entire experimental period (from 3 days to 2 months PI). In the remaining tissues, viral antigens were only detected after the onset of mortality (7 days PI; Table 1). Thus, at 10 days PI all non-nervous tissues analyzed (liver, kidney, spleen, and caudal fin) were positive for RGNNV. On the other hand, the spleen was negative 17 days PI, and the caudal fin and liver were the only non-nervous tissues showing immunostaining 24 days PI. Specifically, viral antigens were present in tubular epithelial cells of the kidney, hepatocytes, endothelial cells of the liver (Figs. 1B and D), white pulp of the spleen (Fig. 1F), and fibroblasts located in the epidermis and cartilage of the caudal fin (Fig. 1H).
Fig. 1.
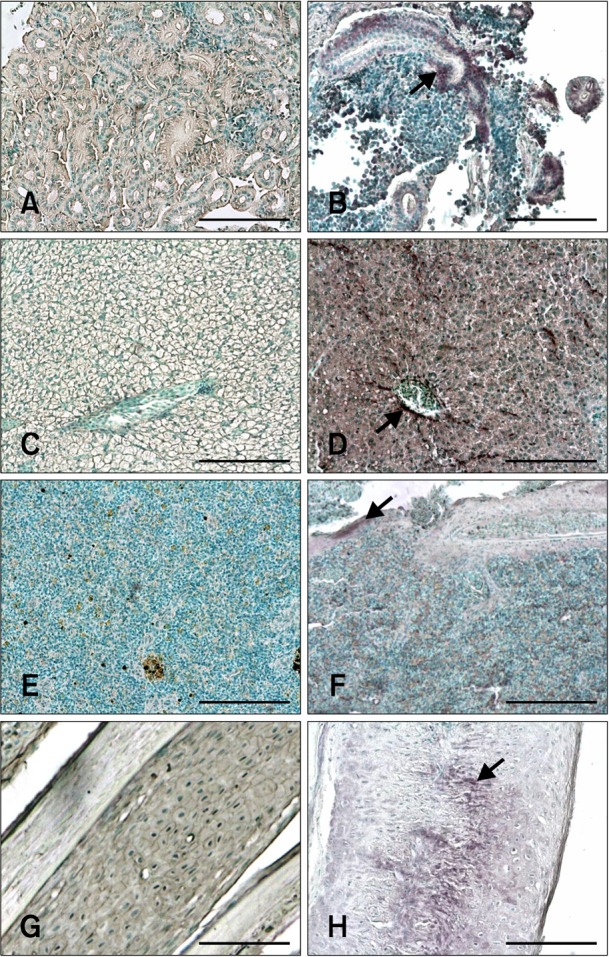
Immunohistochemistry (IHC) signals in sections of non-nervous tissues. Positive cells contain dark purple signals (arrows). (A) Kidney from non-infected fish (negative control). (B) Kidney sampled 17 days post-infection (PI) showing red-spotted grouper nervous necrosis virus (RGNNV) antigens in tubular epithelial cells. (C) Liver section from non-infected fish (negative control). (D) Liver section from infected fish 3 days PI. Staining was observed in hepatocytes and endothelial cells. (E) Spleen section from non-infected fish (negative control). (F) Positive signal in white pulp of the spleen on day 10 PI. (G) Caudal fin section from non-infected fish (negative control). (H) Positive cartilage cells in the caudal fin 17 days PI. Scale bars = 100 µm.
Fig. 2.
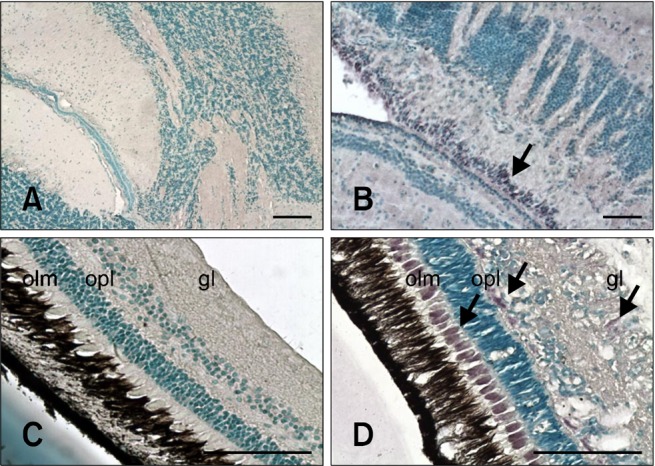
Sections of nervous tissues evaluated with IHC. Positive cells contain dark purple signals (arrows). (A) Brain from non-infected fish (negative control). (B) Brain from inoculated fish 24 days PI. Staining is in the area between the tectum opticum and cerebellum. (C) Retina from non-infected fish (negative control). (D) Retina from inoculated fish 17 days PI. Stained cells are in the outer limiting membrane (olm), outer plexiform layer (opl), and ganglion layer (gl). Scale bars = 100 µm.
Table 1.
Distribution of red-spotted grouper nervous necrosis virus (RGNNV) antigenic proteins detected by immunohistochemistry (IHC) in organs of experimentally infected European seabass
*Staining intensity: (-) no signal, (+) weakly positive, (++) moderately positive, (+++) strongly positive.
When examining nervous tissues, cells in the brain showed a constant level of immunostaining throughout the experiment. In contrast, increased intensity of the IHC signal was recorded in retina as the mortality period was approached. The staining intensity then decreased to levels similar to those obtained in brain at the end of the mortality period (Table 1). The only parts of the brain that could be examined and compared among all sampled animals were the cerebellum and tectum opticum (TO). Immunolabeling was first observed in the peripheral zone of the cerebellum (i.e., the only signal observed in brain 3 days PI). Viral antigens in TO of animals sampled from 10 days PI onwards were also observed. At 17 and 24 days PI, IHC signals were predominately located in the area between the cerebellum and TO (Fig. 2B).
In retina, the ganglion layer was the only area that was stained throughout the experiment. Additionally, viral antigens also appeared in the inner nuclear layer 3 days PI as well as in the outer limiting membrane and outer plexiform layer 17 days PI (Fig. 2D). The outer plexiform layer and inner nuclear layer also exhibited IHC signals 2 months PI.
Histopathology
Characteristic VNN lesions, such as vacuolation, were only observed in the brain (cerebellum and TO) along with retina where degeneration was always more evident than in brain. In the cerebellum and TO, vacuolation was only observed at 3, 10, 17, and 24 days PI, coinciding with the maximum level of mortality (Fig. 3B). In retina, vacuoles appeared in the outer and inner plexiform, inner nuclear, and ganglion layers up to 10 days PI. At 17 days PI, these lesions were also observed in the outer nuclear layer (Fig. 3D). However, vacuolation was noted only in the outer plexiform, inner plexiform, and inner nuclear layers 24 days PI. The only histological alteration found in internal organs was accumulation of fat vacuoles in the liver of challenged fish 10, 17, and 24 days PI (Fig. 3F).
Fig. 3.
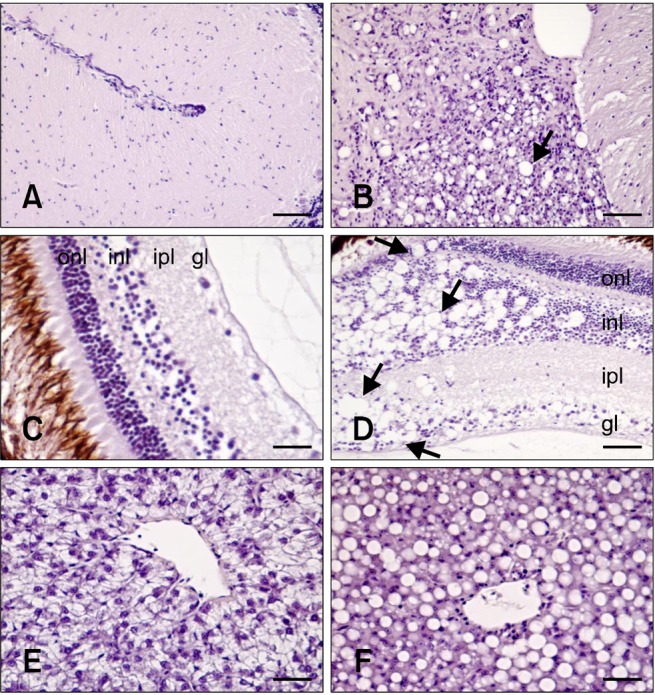
Histological damage caused by RGNNV infection. (A) Brain from non-infected fish (negative control). (B) Brain from infected fish. Vacuolation was observed in the cerebellum (arrow). (C) Retina from non-infected fish (negative control). (D) Retina from infected fish. Vacuoles (arrows) were found in the outer nuclear layer (onl), inner nuclear layer (inl), inner plexiform layer (ipl), and ganglion layer (gl). (E) Liver from non-infected fish (negative control). (F) Accumulation of fat vacuoles in hepatocytes from challenged fish. All tissues shown were sampled 17 days PI. H&E stain. Scale bars = 50 µm (B, C, and D), and 25 µm (A, E, and F).
Antibody response against RGNNV infection
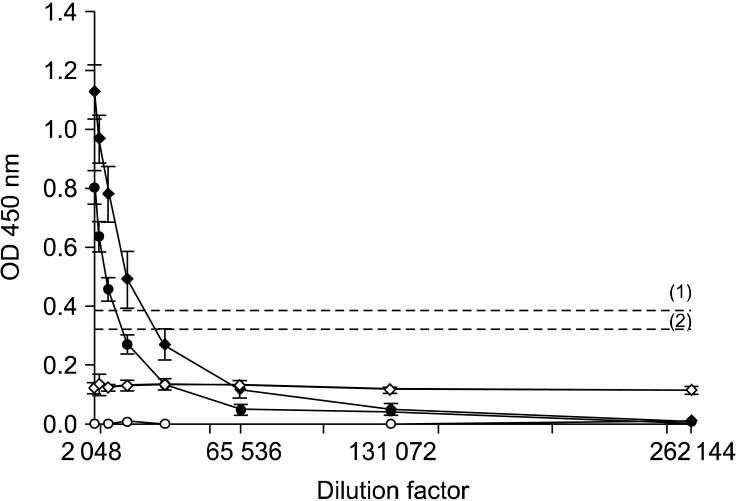
Three samples consisting of pooled sera from five different mocked-injected or challenged fish were screened for the presence of anti-RGNNV antibodies by an ELISA. A measurable level of specific anti-RGNNV antibodies was recorded only in challenged animals. The results indicated that intense anti-RGNNV antibody production was initiated as soon as 24 days PI when the titer was 1 : 16,384. At 2 months PI, the antibody titer was 1 : 8,192 (Fig. 4).
Fig. 4.
The mean optical density (OD) values from the ELISA analysis of three replicates of three samples composed of pooled sera from five fish. Titration of anti-RGNNV antibodies in sera obtained from challenged animals 24 days (♦) and 2 months (•) PI. Sera collected from control fish 24 days (◊) and 2 months (○) PI were also tested. (1) and (2) are the thresholds for samples collected 24 days and 2 months PI, respectively.
Discussion
In the present study, we have described the distribution of nodavirus antigens in nervous and non-nervous tissues of juvenile European seabass during the course of an experimental viral infection together with histological changes and a specific antibody response. Fish were exposed to RGNNV (106 TCID50) by IM injection, which is an artificial method of infecting fish comparable to bath challenge. We chose to use IM injection based on three premises: 1) this method is a good way to control the amount of virus to which animals are exposed; 2) mortality caused by nodavirus after IM inoculation is significantly higher than that recorded after bath challenge as previously demonstrated [30,35]; and 3) IM injection is the only inoculation-based method for inducing nodavirus infection in some fish species such as seabream [2]. The specimens analyzed in our study showed typical external signs of the nodavirus infection. Mortalities occurred between 7 and 29 days PI, and the virus was re-isolated from all the dead fish we evaluated, thus fulfilling River's postulates [31]. The cumulative mortality after 31 days was 37%, which is similar to percentages previously reported in European seabass after IM inoculation ranging from 2 to 50% [5,35,37].
According to the IHC results, the brain, retina, and liver were the first sites in which RGNNV was detected (3 days PI). Later, viral proteins were also present in spleen, kidney, and caudal fin. The non-nervous organ with the highest persistence of viral antigens was the liver in which staining was observed at all sampling times (up to 2 months PI). Virus detection in endothelial cells of the liver may support epitheliotrophism of nodavirus suggested in earlier studies [9,21,25,27,28]. Previously, Lopez-Jimena et al. [22] detected the viral genome by ISH during the course of the same infection, resulting in belated virus detection compared to IHC (i.e., in the brain and eyes) or even lack of detection in organs found to be positive by IHC (i.e., caudal fin and spleen). Although it is important to emphasize that IHC and ISH yield different types of information, based on the results obtained in the present study IHC should be recommended over ISH as an in situ diagnostic tool.
Previous reports have shown that nodavirus is present in some non-nervous tissues of European seabass such as liver [9,25] and caudal fin [22,24]. However, previous detection of the virus in caudal fin was based on a PCR technique that cannot rule out the presence of the virus exclusively on the caudal fin surface. In the present study, immunolabeling was observed in fibroblastic cells of caudal fin, which demonstrates for the first time the presence of nodavirus inside this tissue. Ours is also the first report of nodavirus detection in the spleen and kidney of seabass. Lopez-Jimena et al. [22] detected RGNNV RNA and infectious particles in the internal organs of European seabass. However, in that study liver, spleen, and kidney were processed as a pool and, therefore, the authors could not establish which of the organs were positive for nodavirus. The presence of viral proteins in these organs does not necessarily mean that they are involved in virus replication since viral proteins could have been transported there as immune complexes by host defense mechanisms [17].
The pattern of presence or absence of viral proteins in non-nervous tissues described in this study concurs with the detection of infectious particles in the same organs reported by Lopez-Jimena et al. [22]. These authors did not detect viral particles in caudal fin 31 days or 2 months PI, or in pooled internal organs 2 months PI, which are the sampling times when the viral proteins were not observed by IHC in these organs in the present study (except for a weak signal in liver 2 months PI). According to these authors, internal organs and caudal fin of seabass do not support productive RGNNV infection, suggesting post-replication failure. IHC results from the present study support this idea, and may indicate a failure of viral protein synthesis.
Virus distribution we observed by IHC in nervous tissues (brain and retina) is similar to that previously reported [9,21,25,30,35]. Staining intensity as well as the number of cells presenting cytoplasmic staining may indicate that the virus first appears in brain, which showed stable labeling intensity, and then in retina, where a progressive increase in signal intensity was observed [30]. Previously, Lopez-Jimena et al. [22] also described a significant increase in the number of copies of both viral segments in the eyes (from 3 to 10 days PI), whereas the number of viral genome copies in brain was very high and constant from the first sampling time.
Brain and retina from the virus-exposed fish exhibited important histopathological lesions consistent with behavioral change observed in the affected animals. These lesions, consisting of significant vacuolation, have also been described in previous studies of VNN pathology in European seabass [3,35]. Animals sampled 2 months PI did not develop vacuolation in the brain or retina, although these tissues contained immunopositive cells. Previously, Johansen et al. [20] and Bovo et al. [7] described the same findings in other fish species subclinically infected with nodavirus. In the present study, fish could be considered subclinically infected 2 months PI since no external symptoms or mortalities were recorded at that time. Based on our results, IHC is recommended over histopathological methods in order to detect subclinically nodavirus-infected seabass populations.
No changes were detected in the kidney, spleen, or caudal fin of challenged animals. In contrast, accumulation of fat vacuoles was observed in the liver, which also displayed macroscopic alterations. However, fat vacuole accumulation in the liver may not have been directly virus-related. This could have been caused by changes in feeding behavior observed in the challenged animals, which could have resulted in physiological alterations. Histological damages in the liver (vacuolation and necrosis) have been previously observed after VNNV infection in Asian seabass [4].
Immune responses against RGNNV were evaluated by measuring the specific antibodies produced by infected fish using an ELISA. RGNNV infection induced the production of specific antibodies, which were detected 24 days and 2 months PI (the only sampling times for this experiment) in the infected fish with titers of 1 : 16,384 and 1 : 8,192, respectively. This finding is in concordance with those of Skliris and Richards [35], who suggested that antibody titers in seabass after a primary antigen challenge should peak 28 days PI. In addition, Scapigliati et al. [33] detected high levels of antibodies in RGNNV-challenged European seabass 43 days PI. The results from the present study are also consistent with those reported in other fish species infected with nodavirus. In Atlantic halibut intramuscularly infected, a significant increase in the titer of specific antibodies was observed from 18 days PI onwards with maximum titers observed on day 55 PI [16].
In summary, our investigation completes an integrated study of RGNNV pathogenesis in juvenile European seabass over the course of an experimental infection. Our findings demonstrated that the brain, retina, and liver are the tissues where viral antigens first appear, and where the virus has the highest persistence. In addition, these organs displayed viral-specific immunolabeling even when the typical histopathological lesions were not longer observed. This study demonstrated for the first time the presence of viral proteins in cells of the caudal fin, kidney, and spleen of European seabass. This study completes the knowledge on the RGNNV pathogenesis in juvenile seabass, which may be very useful to design diagnostic strategies for the early detection of nodavirus in seabass farms, which is essential to develop prophylactic measures.
Acknowledgments
This study was supported by the project AGL2007-63380 (Ministerio de Ciencia e Innovación), co-financed with FEDER funds (EU), and P09-RNM-4898 (Proyecto de Excelencia de la Junta de Andalucía). B. Lopez-Jimena was supported by a fellowship from INIA (Subprograma de Formación de Personal Investigador en Agroalimentación en Los Centros de Investigación INIA-CCAA, the Spanish government). C. Infante was supported by a post-doctoral research contract from the INIA-CCAA. Authors would like to thank Mrs. H. McEwan (Institute of Aquaculture, University of Stirling) for her technical support with the IHC and ELISA studies, and all personnel of Centro El Toruño for their assistance in handling and maintaining the fish.
References
- 1.Alonso MC, Cano I, Garcia-Rosado E, Castro D, Lamas J, Barja JL, Borrego JJ, Bergmann SM. Isolation of lymphocystis disease virus from sole, Solea senegalensis Kaup, and blackspot sea bream, Pagellus bogaraveo (Brünnich) J Fish Dis. 2005;28:221–228. doi: 10.1111/j.1365-2761.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 2.Aranguren R, Tafalla C, Novoa B, Figueras A. Experimental transmission of encephalopathy and retinopathy induced by nodavirus to sea bream, Sparus aurata L., using different infection models. J Fish Dis. 2002;25:317–324. [Google Scholar]
- 3.Athanassopoulou F, Billinis C, Psychas V, Karipoglou K. Viral encephalopathy and retinopathy of Dicentrarchus labrax (L.) farmed in fresh water in Greece. J Fish Dis. 2003;26:361–365. doi: 10.1046/j.1365-2761.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 4.Azad IS, Shekhar MS, Thirunavukkarasu AR, Jithendran KP. Viral nerve necrosis in hatchery-produced fry of Asian seabass Lates calcarifer: sequential microscopic analysis of histopathology. Dis Aquat Organ. 2006;73:123–130. doi: 10.3354/dao073123. [DOI] [PubMed] [Google Scholar]
- 5.Bigarré L, Baud M, Cabon J, Crenn K, Castric J. New PCR probes for detection and genotyping of piscine betanodaviruses. J Fish Dis. 2010;33:907–912. doi: 10.1111/j.1365-2761.2010.01188.x. [DOI] [PubMed] [Google Scholar]
- 6.Bloch B, Gravningen K, Larsen JL. Encephalomyelitis among turbot associated with a picornavirus-like agent. Dis Aquat Organ. 1991;10:65–70. [Google Scholar]
- 7.Bovo G, Gustinelli A, Quaglio F, Gobbo F, Panzarin V, Fusaro A, Mutinelli F, Caffara M, Fioravanti ML. Viral encephalopathy and retinopathy outbreak in freshwater fish farmed in Italy. Dis Aquat Organ. 2011;96:45–54. doi: 10.3354/dao02367. [DOI] [PubMed] [Google Scholar]
- 8.Bovo G, Nishizawa T, Maltese C, Borghesan F, Mutinelli F, Montesi F, De Mas S. Viral encephalopathy and retinopathy of farmed marine fish species in Italy. Virus Res. 1999;63:143–146. doi: 10.1016/s0168-1702(99)00068-4. [DOI] [PubMed] [Google Scholar]
- 9.Breuil G, Pépin JFP, Boscher S, Thiéry R. Experimental vertical transmission of nodavirus from broodfish to eggs and larvae of the sea bass, Dicentrarchus labrax (L.) J Fish Dis. 2002;25:697–702. [Google Scholar]
- 10.Chérif N, Thiéry R, Castric J, Biacchesi S, Brémont M, Thabti F, Limem L, Hammami S. Viral encephalopathy and retinopathy of Dicentrarchus labrax and Sparus aurata farmed in Tunisia. Vet Res Commun. 2009;33:345–353. doi: 10.1007/s11259-008-9182-3. [DOI] [PubMed] [Google Scholar]
- 11.Comps M, Pépin JF, Bonami JR. Purification and characterization of two fish encephalitis viruses (FEV) infecting Lates calcarifer and Dicentrarchus labrax. Aquaculture. 1994;123:1–10. [Google Scholar]
- 12.Cunningham CH. Quantal and enumerative titration of virus in cell cultures. In: Kruse PF Jr, Patterson MK Jr, editors. Tissue Culture: Methods and Applications. New York: Academic Press; 1973. pp. 527–532. [Google Scholar]
- 13.Curtis PA, Drawbridge M, Iwamoto T, Nakai T, Hedrick RP, Gendron AP. Nodavirus infection of juvenile white sea bass, Atractoscion nobilis, cultured in Southern California: first record of viral nervous necrosis (VNN) in North America. J Fish Dis. 2001;24:263–271. [Google Scholar]
- 14.Frerichs GN, Rodger HD, Peric Z. Cell culture isolation of piscine neuropathy nodavirus from juvenile sea bass, Dicentrarchus labrax. J Gen Virol. 1996;77:2067–2071. doi: 10.1099/0022-1317-77-9-2067. [DOI] [PubMed] [Google Scholar]
- 15.Grove S, Johansen R, Dannevig BH, Reitan LJ, Ranheim T. Experimental infection of Atlantic halibut Hippoglossus hippoglossus with nodavirus: tissue distribution and immune response. Dis Aquat Organ. 2003;53:211–221. doi: 10.3354/dao053211. [DOI] [PubMed] [Google Scholar]
- 16.Grove S, Johansen R, Reitan LJ, Press CM, Dannevig BH. Quantitative investigation of antigen and immune response in nervous and lymphoid tissues of Atlantic halibut (Hippoglossus hippoglossus) challenged with nodavirus. Fish Shellfish Immunol. 2006;21:525–539. doi: 10.1016/j.fsi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Húsgağ S, Grotmol S, Hjeltnes BK, Rødseth OM, Biering E. Immune response to a recombinant capsid protein of striped jack nervous necrosis virus (SJNNV) in turbot Scophthalmus maximus and Atlantic halibut Hippoglossus hippoglossus, and evaluation of a vaccine against SJNNV. Dis Aquat Organ. 2001;45:33–44. doi: 10.3354/dao045033. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto T, Mise K, Takeda A, Okinaka Y, Mori K, Arimoto M, Okuno T, Nakai T. Characterization of Striped jack nervous necrosis virus subgenomic RNA3 and biological activities of its encoded protein B2. J Gen Virol. 2005;86:2807–2816. doi: 10.1099/vir.0.80902-0. [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto T, Nakai T, Mori K, Arimoto M, Furusawa I. Cloning of the fish cell line SSN-1 for piscine nodaviruses. Dis Aquat Organ. 2000;43:81–89. doi: 10.3354/dao043081. [DOI] [PubMed] [Google Scholar]
- 20.Johansen R, Grove S, Svendsen AK, Modahl I, Dannevig B. A sequential study of pathological findings in Atlantic halibut, Hippoglossus hippoglossus (L), throughout one year after an acute outbreak of viral encephalopathy and retinopathy. J Fish Dis. 2004;27:327–341. doi: 10.1111/j.1365-2761.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- 21.Le Breton A, Grisez L, Sweetman J, Ollevier F. Viral nervous necrosis (VNN) associated with mass mortalities in cage-reared sea bass, Dicentrarchus labrax (L.) J Fish Dis. 1997;20:145–151. [Google Scholar]
- 22.Lopez-Jimena B, Alonso MC, Thompson KD, Adams A, Infante C, Castro D, Borrego JJ, Garcia-Rosado E. Tissue distribution of red spotted grouper nervous necrosis virus (RGNNV) genome in experimentally infected juvenile European seabass (Dicentrarchus labrax) Vet Microbiol. 2011;154:86–95. doi: 10.1016/j.vetmic.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Jimena B, Cherif N, Garcia-Rosado E, Infante C, Cano I, Castro D, Hammami S, Borrego JJ, Alonso MC. A combined RT-PCR and dot-blot hybridization method reveals the coexistence of SJNNV and RGNNV betanodavirus genotypes in wild meagre (Argyrosomus regius) J Appl Microbiol. 2010;109:1361–1369. doi: 10.1111/j.1365-2672.2010.04759.x. [DOI] [PubMed] [Google Scholar]
- 24.Mazelet L, Dietrich J, Rolland JL. New RT-qPCR assay for viral nervous necrosis virus detection in sea bass, Dicentrarchus labrax (L.): application and limits for hatcheries sanitary control. Fish Shellfish Immunol. 2011;30:27–32. doi: 10.1016/j.fsi.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Mladineo I. The immunohistochemical study of nodavirus changes in larval, juvenile and adult sea bass tissue. J Appl Ichthyol. 2003;19:366–370. [Google Scholar]
- 26.Munday BL, Kwang J, Moody N. Betanodavirus infections of teleost fish: a review. J Fish Dis. 2002;25:127–142. [Google Scholar]
- 27.Nguyen HD, Mushiake K, Nakai T, Muroga K. Tissue distribution of striped jack nervous necrosis virus (SJNNV) in adult striped jack. Dis Aquat Organ. 1997;28:87–91. [Google Scholar]
- 28.Nguyen HD, Nakai T, Muroga K. Progression of striped jack nervous necrosis virus (SJNNV) infection in naturally and experimentally infected striped jack Pseudocaranx dentex larvae. Dis Aquat Organ. 1996;24:99–105. [Google Scholar]
- 29.Nishizawa T, Furuhashi M, Nagai T, Nakai T, Muroga K. Genomic classification of fish nodaviruses by molecular phylogenetic analysis of the coat protein gene. Appl Environ Microbiol. 1997;63:1633–1636. doi: 10.1128/aem.63.4.1633-1636.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Péducasse S, Castric J, Thiéry R, Jeffroy J, Le Ven A, Baudin Laurencin F. Comparative study of viral encephalopathy and retinopathy in juvenile sea bass Dicentrarchus labrax infected in different ways. Dis Aquat Organ. 1999;36:11–20. doi: 10.3354/dao036011. [DOI] [PubMed] [Google Scholar]
- 31.Rivers TM. Viruses and Koch's Postulates. J Bacteriol. 1937;33:1–12. doi: 10.1128/jb.33.1.1-12.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez-Gómez FJ, Sarasquete C, Muñoz-Cueto JA. A morphological study of the brain of Solea senegalensis. I. The telencephalon. Histol Histopathol. 2000;15:355–364. doi: 10.14670/HH-15.355. [DOI] [PubMed] [Google Scholar]
- 33.Scapigliati G, Buonocore F, Randelli E, Casani D, Meloni S, Zarletti G, Tiberi M, Pietretti D, Boschi I, Manchado M, Martin-Antonio B, Jimenez-Cantizano R, Bovo G, Borghesan F, Lorenzen N, Einer-Jensen K, Adams S, Thompson K, Alonso C, Bejar J, Cano I, Borrego JJ, Alvarez MC. Cellular and molecular immune responses of the sea bass (Dicentrarchus labrax) experimentally infected with betanodavirus. Fish Shellfish Immunol. 2010;28:303–311. doi: 10.1016/j.fsi.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Skinner LA, LaPatra SE, Adams A, Thompson KD, Balfry SK, McKinley RS, Schulte PM. Concurrent injection of a rhabdovirus-specific DNA vaccine with a polyvalent, oil-adjuvanted vaccine delays the specific anti-viral immune response in Atlantic salmon, Salmo salar L. Fish Shellfish Immunol. 2010;28:579–586. doi: 10.1016/j.fsi.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Skliris GP, Richards RH. Induction of nodavirus disease in seabass, Dicentrarchus labrax, using different infection models. Virus Res. 1999;63:85–93. doi: 10.1016/s0168-1702(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 36.Starkey WG, Ireland JH, Muir KF, Jenkins ME, Roy WJ, Richards RH, Ferguson HW. Nodavirus infection in Atlantic cod and Dover sole in the UK. Vet Rec. 2001;149:179–181. doi: 10.1136/vr.149.6.179. [DOI] [PubMed] [Google Scholar]
- 37.Thiery R, Peducasse S, Castric J, Le Ven A, Jeffroy J, Baudin Laurencin F. Experimental transmission of viral encephalopathy and retinopathy to juvenile sea bass (Dicentrarchus labrax) Bull Eur Assoc Fish Pathol. 1997;17:118–122. [Google Scholar]