Abstract
The dual-functioning antioxidant enzyme peroxiredoxin VI (Prdx6) detoxifies lipid peroxides particularly in biological membranes and its peroxidase function is activated by glutathione S-transferase pi (GSTP). The GSTP gene is polymorphic in humans, with the wild type GSTP1-1A (Ile105, Ala114) and three variants: GSTP1-1B (Ile105Val, Ala114), GSTP1-1C (Ile105Val, Ala114Val) and GSTP1-1D (Ile105, Ala114Val). The focus of the present study is to determine the influence of these polymorphisms on Prdx6 peroxidase function. Using extracellular generation of OH-radicals and fluorescent (DPPP dye) detection, we found a fast (~300 s) onset of lipid peroxidation in membranes of MCF-7 cells transfected with a catalytically inactive Y7F mutant of GSTP1-1 and either GSTP1-1B or GSTP1-1D. However, this effect was not detected in cells expressing either GSTP1-1A or GSTP1-1C. Imaging of DPPP labeled MCF-7 cells showed fluorescence localized in the plasma membrane, but intensity was substantially diminished in the GSTP1-1A and GSTP1-1C expressing cells. Moreover, in the Y7F mutant of GSTP1-1A, GSTP1-1B and GST1-1D expressing cells *OH generation resulted (after 36 h) in plasma membrane-permeability-related cell death, while GSTP1-1A and GSTP1-1C expressing cells had significantly better survival. We used FRET analyses to measure in vitro binding of purified GSTP1-1 allelic variant proteins to purified recombinant Prdx6. The affinities for Prdx6 binding to GSH-loaded GSTP1-1s mirrored their observed peroxidase activities (using phospholipid hydroperoxide as a substrate): GSTP1-1A > GSTP1-1C (KD= 51.0 vs 57.0nM) or corresponded with inactivation: GSTP1-1B (GSTP1-1D) (KD= 101.0 (94.0)nM, respectively). “In-silico” modeling of the GSTP1-1 – Prdx6 heterodimer revealed that the sites of GSTP1-1 polymorphism (Ile105 and Ala114) are in close proximity to the binding interface. Thus, there is a hierarchy of effectiveness for polymorphic variants of GSTP1-1 to regulate Prdx6 peroxidase function, a feature that may influence human population susceptibilities to oxidant stress.
Keywords: allelic variants, peroxiredoxin VI (Prdx6) peroxidase function, glutathione, glutathione S-transferase, lipid peroxidation, protein-protein interactions
Introduction
Peroxiredoxin VI (Prdx6) is a dual-functioning antioxidant enzyme that acts to protect biological membranes against damage caused by lipid peroxidation [1, 2]. The enzyme displays glutathione peroxidase [3] and calcium-independent PLA2 [4, 5] activities with specificities for phospholipid hydroperoxides providing physiological protection following cell exposure to oxidative stress. The glutathione peroxidase activity of Prdx6 is regulated by initial heterodimerization with, and subsequent S-glutathionylation by, glutathione-loaded GSTP [3, 6, 7]. One distinctive function of GSTP is its ability to form a redox-complex with a target protein with subsequent delivery of bound and activated glutathione (thiolate anion) for S-glutathionylation of target proteins [8]. In the case of Prdx6, the catalytic Cys47 is buried in the hydrophobic globular core region of the protein. Following reduction of peroxides, this cysteine becomes oxidized to a sulfenate and GSTP participates in overcoming the accessibility barrier for GSH to reach this hydrophobic region and reactivate Prdx6.
Glutathione (GSH) S-transferases (GST’s) are multifunctional isoenzymes that can detoxify xenobiotics and endogenous metabolites primarily by catalyzing their conjugation with GSH [9, 10]. GSTP1-1 is over-expressed in tumors and in cell lines selected for resistance to certain anticancer agents and has been shown to be polymorphic [11], a characteristic that imbues potential selective catalytic properties on the individual isozymes. The genetic polymorphisms in the GSTP1 gene arise from nucleotide transitions that change codon 105 from Ile to Val and codon 114 from Ala to Val, generating four GSTP1 alleles: wild-type GSTP1-1A (Ile105/Ala114), GSTP1-1B (Val105/Ala114), GSTP1-1C (Val105/Val114), and GSTP1-1D (Ile105/Val114) [12, 13]. The Ile105→Val105 and Ala114→Val114 substitutions do not alter glutathione-binding affinity, but cause a steric change at the substrate-binding site of the enzyme [13, 14]. The GSTP variants have different catalytic rates for the formation of thioether conjugates between GSH and some small molecule electrophiles [12, 14]. Altered conformation of the substrate-binding site(s) may contribute to final substrate specificities. The hydrophobicity and size of residue 114 could serve as an important determinant of the substrate specificity of each of the GSTP1 isozymes [15]. On the other hand, since GSTP1-1D, bearing Ile105 and Val114, has enzyme activity toward CDNB comparable to GSTP1-1A, Val105 may circumvent the influence of Val114 [13]. There are some examples where distinct polymorphisms influence response to specific anticancer drugs. For example, GSTP1-1A has a role in the acquisition of Cisplatin resistance reportedly through enhancing the formation of platinum–glutathione conjugates [16]. There are also some indications of epidemiological correlations for GSTP isotype expression with etiological aspects of endometrial [17], bladder and testicular cancers [18]. Our present report sought to determine if polymorphic variants of GSTP1 have general impact on response to oxidative stress by directly influencing peroxidase functions of Prdx6.
Materials and Methods
Reagents
PBS (pH=7.4, Invitrogen, CA), RPMI 1640 (Mesdiatech Inc., Manassas, VA), FBS (Atlas Biologicals, Fort Collins, CO), DPPP (Diphenyl-1pyrenylphosphine, Dojindo, Japan), Glutathione (reduced) and Glutathione (oxidized) (Sigma/Aldrich), Glutathione reductase (GR, From baker Yeast, Sigma), β-nicotinamide adenine dinucleotide phosphate reduced tetrasodium salt (NAD(P)H, Sigma), 1-hexadecyl-3-(trifluoroethyl)-sn-glycero-2-phosphomethanol lithium (MJ33, Sigma), ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 3,3′,3″-Phosphanetriyltripropanoic acid (TCEP), ascorbic acid (sodium salt, ASC) and CuSO4 and 3-amino 1,2,4-triazole (all from Sigma) were used. Purified E. coli-expressed Prdx6 (>95% purity) was from ProSpec–Tany TechnoGene Ltd., Ness Ziona, Israel. Alexa®546 N-succinimidyl ester and QSY®35 acetic acid, succinimidyl ester were from Invitrogen (Carlsbad, CA).
Tissue culture
MCF-7 cells were purchased from ATCC and were cultured in RPMI-1540 media with addition of 10% of FCS in humidified incubator at 37°C in atmosphere with 5% of CO2. The rationale for using MCF7 for our studies is that the GSTP gene is transcriptionally silenced; therefore levels of GSTP are essentially undetectable.
Expression of GSTP1-1 allelic variants in MCF-7 cells
Exponentially growing MCF-7 cells were transfected using GenJet™ (“In Vitro DNA Transfection Reagent for MCF-7 Cells (Ver. II), SignaGen laboratories, Ijamsville, MD) with pCMV-Tag2a-Flag (KanR and NeoR) empty or GSTP1-1A encoding vectors. The transfection efficiency was ~60% as detected with GFP expression and flow-cytometric analysis. The site-directed mutagenesis of the latter was performed using the following primers (Integrated DNA Technologies, Inc., Coralville, IA, USA) for PCR amplification:
5′-CCT CCG CTG CAA ATA CGT CTC CCT CAT CTA CAC C-3′ (Ile105Val),
5′-ACA CCA ACT ATG AGG TGG GCA AGG ATG AC-3′ (Ala114Val),
5′-ACC ATC CTGCGT CAC CT -3′ (Tyr7Phe)
and QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer recommendations.
E. coli expression and purification of wild type and allelic variants of GSTP1-1
His-Tagged GSTP1-1A (WT), the inactive Y7F mutant and GSTP1-1B, 1-1C, 1-1D were expressed in E. coli and purified up to ~85% of homogeneity with Ni-column ion-exchanged chromatography [12, 19].
Immunoblot detection of GSTP 1-1 allelic variants, GSTM, PHGPx(GPx4) and Prdx6 in MCF-7 cell lysates
SDS PAGE was performed using 12% Tris-Glycine 1.5mM/15 well precast gels, XCell SureLock™ Electrophoresis Cells and Novex Protein analysis solutions (all from Invitrogen, CA). Electrophoretically resolved proteins were transferred onto PVDF membranes (Bio-Rad; Hercules, CA). Non-specific binding was blocked by incubating membranes in TBST (Tris-buffered saline with 0.1% Tween-20) containing 5% dry non-fat milk or 5% bovine serum albumin Fraction V. Specific polyclonal rabbit anti-Prdx6 (1:4,000 dilutions, Strategic Biosolutions, Ramona, CA), polyclonal rabbit anti-GSTM5 (1:1,000 dilution, Abnova, Walnut, CA), polyclonal rabbit anti-GPx4 (1:500 dilution, Cayman chemical, Ann Arbor, MI), mouse monoclonal anti-GAPDH (1:2,000 dilution, Abcam, Cambrige, MA) and anti-GSTP1 (1:500 dilution, MBL, MA) antibodies were incubated with membranes for two hours at room temperature or overnight at 4°C. Membranes after washing with PBS (3 times) were incubated with secondary HRP-conjugated or green (or red) chromophore-conjugated antibodies (Li-COR biosciences, Lincoln, NE) for one to two hours at room temperature. The blots with HRP-conjugated secondary antibodies were developed with ECL (GE Healthcare Bio-Sciences Corp.). Recombinant purified human Prdx6 (PRDX6, ProSpec- Tany TechnoGene Ltd., Israel) and GSTP1-1 were used as standards. The blots were analyzed using ChemiDoc XRS Imager and Quantity One software (both from BioRad). The blots with green (and red) fluorescent secondary antibodies were imaged and quantified with dual color IR-excited fluorescent imager Odyssey CLx using Image Studio 1.0.9 software (all from Li-COR, NE).
Quantification of relative amounts of Prdx6, GSTP1, GSTM and GPx4
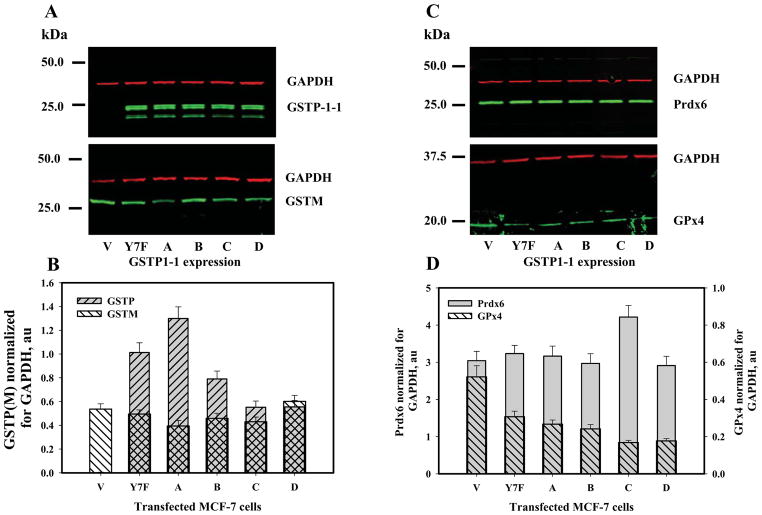
SDS-PAGE of control and transfected MCF-7 cells was performed under reducing conditions (4mM TCEP in the loading buffer). Proteins were electrophoretically resolved, transferred to membranes and subject to immunodetection with appropriate primary and fluorescently-labeled secondary antibodies. The blots with simultaneous detection of GAPDH (red fluorescence, monoclonal antibody) and Prdx6 (or GPx4, GSTP1, GSTM) (green fluorescence, polyclonal antibody) were visualized on an Odyssey CLx imager and quantified using Image Studio 1.0.9 software. Relative amounts of the GSTP1 allelic variants, GSTM, Prdx6 and GPx4 were calculated after normalization with GAPDH. Quantitative results are presented as mean±SE for 3 independent experiments in Fig. 2.
Fig. 2.
Prdx6, GSTP1, GSTM, GPx4 expression in WT and GSTP1-1-transfected MCF-7 cells. Panels A and C: fluorescent imaging (Odyssey CLx) of proteins using immunodetection with green (polyclonal) and red (GAPDH, monoclonal) secondary antibodies after electrophoretic resolution of MCF-7 lysate proteins under reducing conditions (25μg of total protein per lane, 2mM TCEP). Panels B and D: quantification of specified protein expression by normalization of the corresponding bands fluorescent signal to those of GAPDH on the same blot (GPx4 expression corresponds with a right Y-axe of panel D). The representative blots of three independent experiments are shown in panels A and C. Mean ±SE for 3 independent experiments are presented in panels B and D.
Fluorescent analysis of Prdx6 peroxidase activity
We used a standard GSH reductase/GSH/NADPH-coupled assay [1] with either PLPCOOH or H2O2 as substrate to study Prdx6 peroxidase activity in lysates of WT and GSTP1-1 allelic variant-transfected MCF-7 cells. Fluorescent (Em 460 nm, Ex 340 nm) detection of the kinetics of NADPH oxidation to NADP+ resulted in measurable fluorescence decrease, using a QM-4 fluorimeter (PTI, Piscatway, NJ).
Intact and transiently transfected MCF-7 cells were washed 3 times with ice-cold PBS and lysed with standard buffer (Invitrogen) and a plastic scraper. Cell lysates were centrifuged (15.4*103×g, 15 min at 4°C) and supernatants collected. After measuring protein (Bradford, Bio-Rad) supernatants aliquots were frozen (−20°C) and used for subsequent experiments.
For in vitro controls E. Coli-expressed and purified GSTP1-1 allelic variant protein (2.5μg) was incubated with reaction buffer containing 1mM GSH, 25 μM NADPH and GR [1] for 5 min at room temperature in a quartz cuvette under constant stirring in the dark. After an addition of purified recombinant Prdx6 (2.5μg) (ProSpec–Tany TechnoGene Ltd., Israel) samples were incubated for an additional 5 min under the same conditions in the dark, and a recording of the NADPH emission was started 2 min before and 10 min after an indicated peroxide substrate addition. Aliquots (~50μg of total protein) of control or transiently transfected cell lysates were used instead of purified proteins for analysis of peroxidase activity under similar conditions.
PLPCOOH was freshly prepared using a lipoxygenase-mediated oxidation of PLPC (Sigma) with purification using C18 Sep-pack micro-columns (Waters, MA) [1]. To show specificity of Prdx6 peroxidase activity we used a specific competitive Prdx6 inhibitor MJ33 [4]. To exclude any (essentially trivial) catalase-mediated H2O2 degradation (in experiments with H2O2 as a substrate) in cell lysates we pre-incubated our samples with 3-Amino-1, 2, 4-triazole (250 μM, 3-AT, Sigma).
The peroxidase activities of Prdx6/GSTP1-1 in vitro and in MCF-7 cell lysates are presented in Table 1 as mean±SD for 3 independent experiments.
Table 1.
Peroxidase activities of Prdx6 in vitro and in MCF-7 cell lysates.
| # | Sample | Substrate | Activity, μmol/min/mg protein |
|---|---|---|---|
| 1 | Purified Prdx6 and GSTP-1-1A (or C) | H2O2 | 4.35±0.40 (4.28±0.40) |
| 2 | Purified Prdx6 and GSTP-1-1A (or C) + MJ33 | H2O2 | 4.30±0.35 (4.25±0.40) |
| 3 | Purified Prdx6 and GSTP-1-1B (or D) | H2O2 | ND |
| 4 | Purified Prdx6 and Y7F mutant of GSTP-1-1 | H2O2 | ND |
| 5 | Lysate of MCF-7 cell with Y7F mutant of GSTP-1-1A expression + AT | H2O2 | 0.65±0.07 |
| 6 | Lysate of MCF-7 cell with GSTP-1-1A (or C) expression + AT | H2O2 | 1.0±0.08 (0.89±0.09) |
| 7 | Lysate of MCF-7 cell with GSTP-1-1A (or C) expression + AT + MJ33 | H2O2 | 0.98±0.07 (0.90±0.08) |
| 8 | Lysate of MCF-7 cell with GSTP-1-1B (or D) expression + AT | H2O2 | 0.67±0.07 (0.61±0.05) |
| 10 | Purified Prdx6 and GSTP-1-1A (or C) | PLPCOOH | 5.0±0.45 (4.94±0.48) |
| 11 | Purified Prdx6 and GSTP-1-1A (or C) + MJ33 | PLPCOOH | ND |
| 12 | Purified Prdx6 and GSTP-1-1B (or D) | PLPCOOH | ND |
| 13 | Purified Prdx6 and Y7F mutant of GSTP-1-1 | PLPCOOH | ND |
| 14 | Lysate of MCF-7 cell with Y7F mutant of GSTP-1-1A expression | PLPCOOH | 0.21±0.04 |
| 15 | Lysate of MCF-7 cell with GSTP-1-1A (or C) expression + AT | PLPCOOH | 0.63±0.07 (0.57±0.05) |
| 16 | Lysate of MCF-7 cell with GSTP-1-1A (or C) expression + MJ33 | PLPCOOH | 0.20±0.04 (0.18±0.04) |
| 17 | Lysate of MCF-7 cell with GSTP-1-1B (or D) expression | PLPCOOH | 0.19±0.02 (0.12±0.03) |
| 18 | Lysate of MCF-7 cell with GSTP-1-1A (or C) expression without AT | PLPCOOH | 0.65±0.07 (0.60±0.05) |
All experiments were performed with 1mM GSH. Activity of cell lysate was calculated for 50μg of total protein. Data represent mean ±SD for 3 independent experiments. ND = not detected
Prdx6 binding to GSTP1-1
Purified Prdx6 protein was incubated with 5-fold excess of succinimidyl ester of Alexa 546 Fluor ® (Alexa546) carboxylic acid (Invitrogen) and GSTP1-1 allelic variant purified proteins were incubated with 5-fold excess of succinimidyl ester of QSY® 35 (QSY35) acetic acid (Invitrogen) in 0.1 M sodium bicarbonate, pH=8.3, at room temperature, overnight, under constant agitation to label primary amines. The reaction mixture was processed using a BioSpin 6 (BioRad) size-exclusion spin micro-column to remove excess unreacted dyes and exchange reaction buffers to 10mM PB (pH 7.4). Spectroscopic analysis of Alexa546 Fluor or QSY35 label incorporation showed ~2.0mol of each dye per 1mol of Prdx6 or GSTP1-1 allelic variant monomer, respectively.
All labeled allelic variants were pre-incubated with 1mM GSH before any titration experiments.
The fluorescent analysis of the FRET-based (R0=25 Å for Alexa546/QSY35 couple) protein binding was performed using the QM-4 spectrofluorometer (PTI, Birmingham, NJ). The titrations of Prdx6 and GSTP1-1 were performed using 2.0nM of Alexa546-labeled Prdx6 and indicated concentrations of the QSY35-labeled GSTP1-1 proteins in 10×10×40 mm quartz cuvette in a fluorimeter sample holder under constant stirring at room temperature. The emission spectra of Alexa546-labeled Prdx6 were recorded and integrated (Ex.=546 nm) before any titration experiment. The indicated amounts of QSY35-labeled GSTP1-1 allelic variants were added to Alexa546-labeled Prdx6 in solution. After incubation for 5 min, emission spectra of Alexa546 were recorded. The integral of the latter was subtracted from initial Alexa546 spectra and the results were normalized for integral of initial spectra. The Y-axis represents the decrease of Alexa546 emission, which is proportional to those amounts of GSTP1-QSY35 bound to Prdx6. The X-axis represents the total amount of added labeled GSTP1-1. Experimental data were analyzed using standard SigmaPlot pharmacological applications (model of “One binding site with saturation”, Ver. 10.0, Systat Software, Inc.,Chicago, IL) and fit with the hyperbolic equation:
| (1) |
where: X – is a concentration of total GSTP1-1 allelic variant labeled with QSY35; Y – representing the amount of GSTP1-1 allelic variant bound to Alexa546-labeled Prdx6; KD is an apparent equilibrium dissociation constant; and Bmax is maximal number of high affinity binding sites on Prdx6 for each GSTP1-1 allelic variant.
To show the effects of Prdx6 fluorescent labeling on the specificity of its binding to GSTP1-1 competition experiments were performed. At each point of Prdx6-Alexa546 (2.0nM) titration the emission spectra of Alexa546 was recorded before and after 5 min incubation with an indicated amount of GSTP1-1-QSY®35. After that the same amount (2.0nM) of intact unlabeled Prdx6 was added and after 5 min of incubation (RT), the emission spectra of Alexa®546 were recorded. In parallel experiments Prdx6-Alexa®546 (2.0nM) was incubated with unlabeled protein (2.0nM) and emission spectra recorded. Both emission spectra were integrated and used for normalization of the effect of GSTP1-1-QSY35 addition according to the formula:
| (2) |
where: ∫INT - corresponds to integration of a labeled Prdx6 or of a labeled Prdx6 after incubation with intact protein emission spectra, and ∫GSTP1-1 – corresponds to integration of emission spectra after GSTP1-1-QSY35 addition to labeled protein or to dilution of Prdx6-GSTP1-1 complex with intact Prdx6. All spectra were corrected for trivial dilution effect. Our results indicate ~2 times decreased binding of GSTP1-1 to labeled Prdx6 in the presence of an equal amount of intact Prdx6 (not shown). To show the effects of GSTP1-1 allelic variant fluorescent labeling on the specificity of its binding to Prdx6 we used a “back titration” method with intact unlabelled proteins. The methodology of both controls is described in great details in our recent publication [20]. Our results show minimal effect of fluorescent labeling on affinity of GSTP1-1 binding with Prdx6.
To show competition between allelic variants of GSTP1-1 for the same binding sites on Prdx6 we pre-incubated 2.0nM of labeled Prdx6 with 250nM of intact unlabeled GSTP1-1A (saturating concentration) for 5 min and then titrated with indicated amounts of each of the other allelic variants GSTP1-1B(or GSTP1-1C, GSTP1-1D) labeled with QSY35. The emission spectra of Alexa546 after each addition of labeled allelic variant was recorded, integrated, subtracted from the spectra before titration and normalized for the spectral integration of the latter. To account for the role of GSH on Prdx6-GSTP1 binding, titration of Alexa546-labeled Prdx6 with QSY35-labeled GSTP1-1A without pre-incubation with GSH was performed.
The final results of Bmax and KD are presented in Table 2 as mean ±SD for 5 independent experiments.
Table 2.
Binding parameters of GSTP1-1 allelic variants to Prdx6.
| Binding partners | Bmax, au | KD, nM |
|---|---|---|
| GSTP1-1A – Prdx6* | 0.25 ± 0.03 | 317.00 ± 29.30 |
| GSTP1-1A – Prdx6 | 0.26 ± 0.01 | 51.00 ± 4.71 |
| GSTP1-1B – Prdx6 | 0.25 ± 0.01 | 101.00 ± 5.43 |
| GSTP1-1C – Prdx6 | 0.26 ± 0.01 | 57.17 ± 3.27 |
| GSTP1-1D – Prdx6 | 0.29 ± 0.01 | 94.00 ± 4.10 |
| GSTP1-1B – Prdx6** | 0.03 ± 0.002 | 98.40 ± 10.14 |
| GSTP1-1C – Prdx6** | 0.04 ± 0.003 | 56.10 ± 3.25 |
| GSTP1-1D – Prdx6** | 0.024 ± 0.001 | 87.70 ± 5.32 |
Data are mean ± SD for 5 independent experiments.
without pre-incubation of GSTP with GSH
after pre-incubation with 250nM GSTP1-1A
Fluorescent detection of lipid peroxidation in MCF-7 cells
Intact MCF-7 cells were grown to ~70% confluence attached to the 12 × 25mm plastic slides (Aclar, Electron Microscopy Sciences, Hatfield, PA) in RPMI-1540 media (with 10% of FCS) in a humidified incubator at 37°C in an atmosphere with 5% of CO2. These cells were transfected with empty vector (vector), Y7F mutant of GSTP1-1A, GSTP1-1A, GSTP1-1B, GSTP1-1C and GSTP1-1D using procedures described above and harvested at >90% confluence. The transfection efficiency was ~60%. The slides with control and transfected cells were labeled with DPPP (5μM final concentration, 45 min at 37°C) in complete media, washed 3 times with PBS (pH=7.4) and placed in a quartz cuvette (10×10 mm, on diagonal) with 3 ml of PBS (pH=7.4). The DPPP emission (Ex.=352 nm, Em=380 nm) was recorded ~1min before and ~10min after an addition of 0.2mM ascorbic acid (sodium salt, pH=7.4) and 10μM CuSO4 (to generate OH* outside the cells, [1]) in an SM-4 fluorimeter (PTI) at 37°C under constant gentle stirring in real time kinetics. After each kinetic experiment the cells were placed in a Petri dish with fresh media for 36 h and then detached from the slide with trypsin and their viability determined using trypan blue exclusion and counting in a Cellometer™ Auto T4 (Nexcelom, Biosciences, Lawrence, MA). Similar cells and treatments were used for the imaging of DPPP fluorescence with a Radiance 2000 confocal microscope (Bio-Rad, Hercules, CA, USA). The fluorescent and CIT images of particular cells on slides (with similar DPPP-labeling) were acquired before and ~10 min after ascorbic acid-Cu2+ addition and processed using standard confocal microscope software. The traces of DPPP emission kinetics and the corresponding images are representative of 3 independent experiments.
Statistical treatment of experimental data
Experimental data were processed using SigmaStat 10.0 (Systat, Chicago, IL) standard software and where relevant presented as mean ±SD for the number of independent experiments indicated. Statistical relevance of differences was determined using ANOVA.
Results
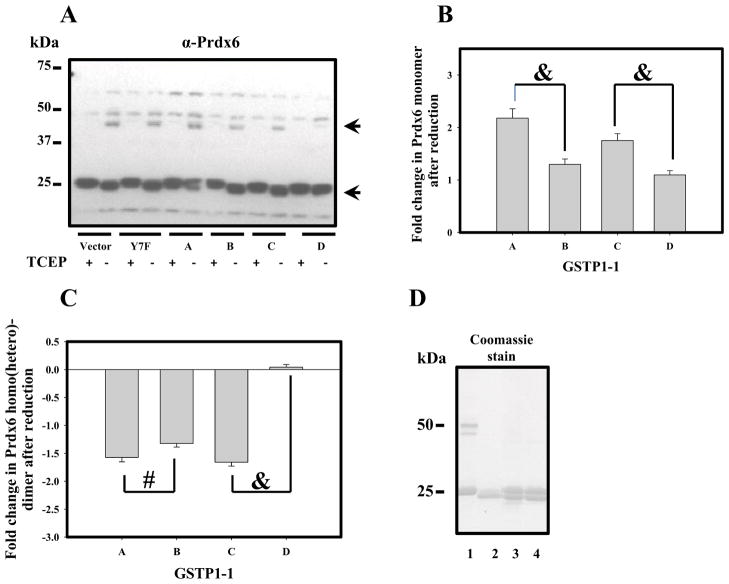
Unlike most tumor cell lines, MCF-7 cells have intrinsically low expression levels of GSTP1-1 [3]. Prdx6 expression in these cells was measureable and was unaffected by forced expression of various GSTP1-1 allelic variants (Fig. 1, panel A, Fig. 2, panels C, D). Our data confirm both barely detectable levels of GSTP1 and appropriate expression levels of GSTP1-1 following selection of pertinent clones of transfectants (WT transfected with empty vector (V), Y7F mutant and allelic variants; Fig. 1, panel B, Fig. 2, panels A, B). GSTM was detected in MCF-7 cells, but was unaffected by expression of GSTP1-1 allelic variants (Fig. 2, panel B). In contrast, levels of PhGPx(GPx4) expression were decreased by expression of GSTP1-1 allelic variants (Fig. 2, panel D). The redox state of Prdx6 is regulated through its hetero-dimerization with GSTP1 that serves to deliver activated GSH (GS−) as a source of electrons for reduction of its catalytic cysteine sulfenate. Consequently, disulfide-based homo/hetero-dimerization of Prdx6 correlates with protein reduction (activation). We used TCEP to reduce the disulfide of Prdx6 homo/hetero-dimerization (Fig. 1, panel A, lower and upper arrows, respecively). Quantification of Prdx6 monomer/dimer patterns (from Fig. 1, panel A) was influenced by the GSTP1-1 variant expression. For example, either GSTP1-1A or GSTP1-1C was more effective at forming the heterodimer with Prdx6 than either GSTP1-1B or 1-1D (Fig. 1, panels C and D). This would be expected to result in better reduction (activation) of Prdx6 by either GSTP1-1A or GSTP1-1C. Expression and purification of his-tagged GSTP variants in E. coli provided a source of essentially homogenous proteins, which were used for subsequent in vitro experiments (Fig. 1, panel E).
Fig. 1.
Prdx6 detection and redox state evaluation in WT and GSTP1-1-trasfected MCF-7 cells. Panel A: Immunoblot detection of Prdx6 in MCF-7 cell lysates (50μg total protein) under oxidized or reduced (2mM TCEP) conditions. Upper arrow indicates homo/hetero-dimer and lower arrow - monomeric Prdx6. Panels B and C: quantification of Prdx6 monomer (panel A, lower arrow) and of Prdx6 homo/hetero-dimer (panel A, upper arrow) content before and after reduction with TCEP. Data represent MEAN±SD for 3 independent experiments (“&” represents p≤ 0.001, and # represents p≤ 0.05). Panel D: SDS PAGE of: commercial Prdx6 (lane 1, 2μg); E. Coli expressed and purified GSTP1-1A (lane 2, 2μg), and Prdx6 mixtures with E.Coli expressed and purified GSTP1-1B or 1-1C (lanes 3 and 4, respectively; 2μg of each protein).
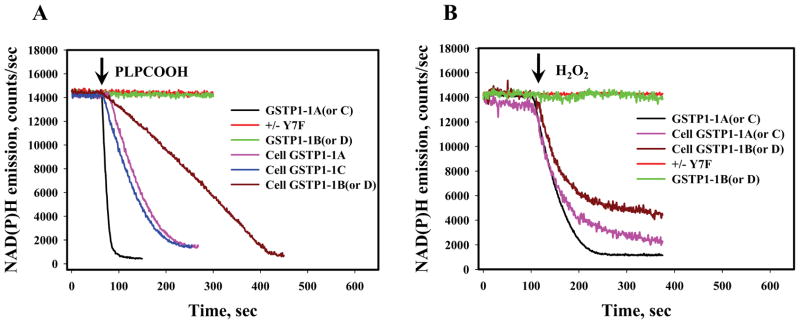
GSTP1-1 catalyzed reduction of Prdx6 enhances its peroxidase activity. Using standard NADPH/GR/GSH- coupled assays we used cell lysates from the transiently-transfected cells to measure the influence of GSTP1 allelic variation on peroxidase activity. Specificity for detection of Prdx6 activity was provided by selection of 1-palmitoyl-2-linolenoyl-sn-glycerol-3-phosphorylcholine hydroperoxide (PLPCOOH) as a substrate. Catalytically inactive purified Y7F mutant of GSTP1-1A was the negative control and did not facilitate PLPCOOH reduction (Fig. 3, panel A). For the variants, the hierarchy for reduction of PLPCOOH was: GSTP1-1A >GSTP1-1C >GSTP1-1B >GSTP1-1D (all were pre-loaded with GSH for all in vitro experiments). When PLPCOOH was used as a substrate, pre-incubation of the cell lysates (or in vitro proteins) with MJ33 (a PLPCOOH binding to Prdx6 inhibitor at 100μM) abolished peroxidase activity (Table 1).
Fig. 3.
Peroxidase activity of Prdx6 in MCF-7 cell lysates and in vitro. Panel A: NADPH fluorescence traces for MCF-7 cell lysates (~50μg of total protein) transiently expressing GSTP1-1 allelic variants (Cell GSTP1-1A (or GSTP1-1B, GSTP1-1C, GSTP1-1D)) or for in vitro analysis using recombinant Prdx6 (ProSpec–Tany TechnoGene Ltd., 2.5 μg) and GSTP1-1 E. Coli expressed and purified proteins (GSTP1-1A, GSTP1-1B, GSTP1-1C, GSTP1-1D) and catalytically inactive mutant Y7F of GSTP1-1A, 2.5μg, respectively) after 50μM PLPCOOH addition (arrow). Panel B: similar to panel A experiments after addition of 100μM H2O2 (arrow). For experiments with MCF-7 cell lysates a pretreatment with 250 μM of AT was performed. The traces are representative of three independent experiments.
For general Prdx6-mediated peroxidase activity H2O2 was also used as a substrate (Fig. 3, panel B). To eliminate the possible effects of catalase, cell lysates were pre-incubated with 100μM 3-amino 1,2,4-triazole (AT). Our results mirrored those for PLPCOOH: GSTP1-1A > GSTP1-1C >GSTP1-1B >GSTP1-1D (with no activity for GSTP1-1A Y7F mutant). Of note, the addition of MJ33 as a specific Prdx6 inhibitor only impacted reduction of PLPCOOH and not H2O2. All data for peroxidase activities of Prdx6 for the cell lysates and in vitro experiments are summarized in Table 1.
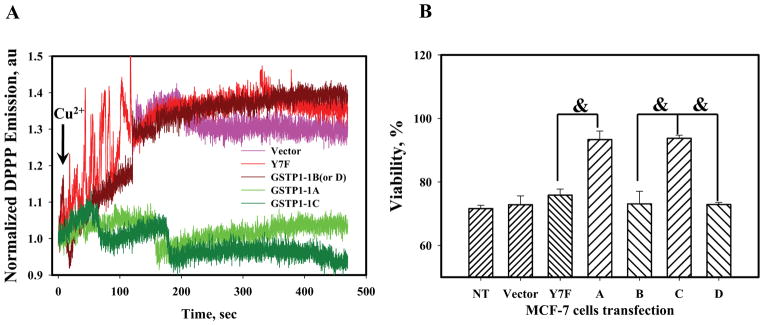
For quantification of plasma membrane associated lipid peroxidation, MCF-7 cells transfected with GSTP1-1 allelic variants were labeled with DPPP, which specifically accumulates in the lipid bilayers of plasma membranes and becomes fluorescent after oxidation by lipid hydroperoxides [1]. After extracellular chemical generation of *OH (Cu2+-ASC reaction, ~100μM), kinetic analyses (Fig. 4, panel A) showed that expression of GSTP1-1A and GSTP1-1C quantitatively suppressed lipid peroxidation in the plasma membranes of MCF-7 cells, while expression of Y7F mutant of GSTP1-1A, GSTP1-1B, and GSTP1-1D showed a rapid (~300sec) accumulation of lipid hydroperoxides. In addition, 36 hr incubation of MCF-7 cells transfected with GSTP1-1 allelic variants after *OH exposure in fresh media compromised the viability of cells transfected with GSTP1-1B, GSTP1-1D and Y7F mutant of GSTP1-1A compared to cells transfected with GSTP1-1A or GSTP1-1C (Fig. 4, panel B).
Fig. 4.
The OH-radical initiated lipid peroxidation in MCF-7 cells expressing GSTP1-1 allelic variants. Panel A: real-time kinetics of DPPP fluorescence in MCF-7 cells after Cu2+/Asc addition (arrow). Traces are representative of three independent experiments. Panel B: viability of the same MCF-7 cells 36h after Cu2+/Asc treatments. Data represent mean ±SE for 3 independent experiments, & - represents p≤ 0.001.
Confocal imaging of WT and transiently transfected MCF-7 cells (labeled with DPPP) showed substantial DPPP-associated fluorescence localized primarily (10 min after Cu2+-ASC addition) in plasma membranes of GSTP1-1B, GSTP1-1D and Y7F mutant of GSTP1-1 expressing cells (Fig. 5). Consistent with the kinetic results (Fig. 4, panel A) only minimal DPPP fluorescence was detected in the plasma membranes of GSTP1-1A and GSTP1-1C transfected cells (Fig. 5).
Fig. 5.
Confocal imaging of lipid peroxidation in MCF-7 cells (Fig. 4) expressing GSTP1-1 allelic variants. Images are fluorescent and DCI of DPPP-labeled MCF-7 cells 10 min after treatment with Cu2+/Asc. The images are representative of at least three independent experiments. Scale bar is 10nm.
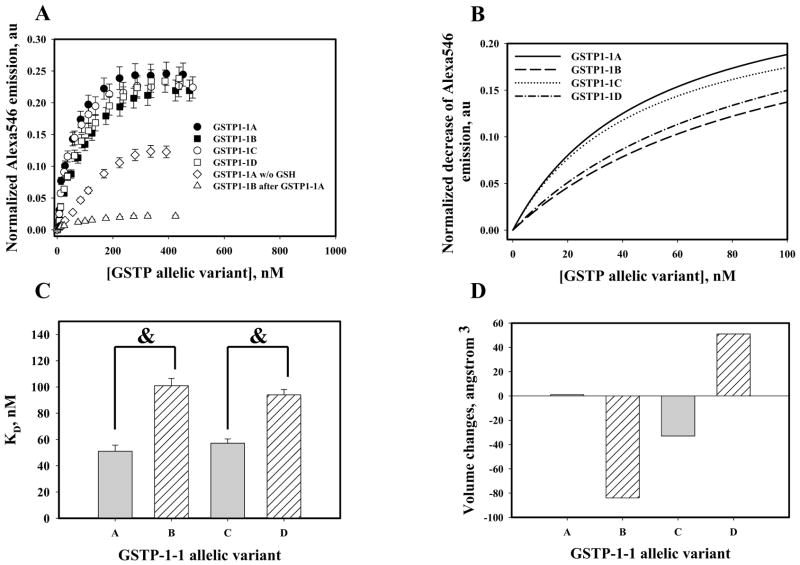
Activation of Prdx6 by hetero-dimerization with GSTP requires effective binding interactions of the participating proteins. To determine the affinity of binding for the proteins forming the heterodimer, purified recombinant Prdx6 and GSTP1-1 (A, B, C, D or Y7F mutant) were used for fluorescent labeling and FRET-based analyses. Our data showed similar high affinities of GSTP1-1A and GSTP1-1C for Prdx6 (Fig. 6, Table 2). Binding affinities for GSTP1-1B and GSTP1-1D for Prdx6 were statistically significantly lower (Fig. 6, panel C). Fig. 6, panel D presents actual volume (amino acids 105 and 114) changes in the vicinity of the allelic variant GSTP1-1 close contact site with Prdx6 (Fig. 7). Our data show small changes for GST1-1C as compared to those for GSTP1-1B or GSTP1-1D. Pre-incubation of Prdx6 with GSTP1-1A before titration with either GSTP1-1B (or GSTP1-1C, GSTP1-1D) indicated essentially the same binding sites for each of the GTTP-1-1 allelic variants. Only maximal numbers of binding sites (Bmax) but not binding affinities (KD) were altered. The absence of GSH in the binding mixture produced extensive decreases in binding affinity values, indicating that GSH loading of GSTP1-1 plays a crucial role in stabilizing of the Prdx6-GSTP1 complex (Table 2).
Fig. 6.
Binding of Prdx6 with recombinant purified GSTP1-1 allelic variants in vitro. Panel A: titration of 2.0nM Alexa®546-labeled Prdx6 with QSY®35-labelled and GSH-loaded GSTP1-1 allelic variants; effects of labeled Prdx6 pre-incubation with 250nM unlabeled GSTP1-1A; and omission of GSTP1-1 pre-incubation with GSH. Panel B: Hyperbolic fit of experimental data (see Materials and Methods) for initial titrations of GSTP1 allelic variants. Panel C: statistical differences in affinity (KD) of GSTP1 allelic variants for Prdx6. Data represent mean ±SE for 5 independent experiments, &-represents p≤ 0.001. Panel D: Volume of polymorphic amino acids for GSTP1-1allelic variants. Total volume (cubic angstroms) is calculated as a function of variable (105 and 114) amino acid specific volumes comparison with GSTP1-1A variant.
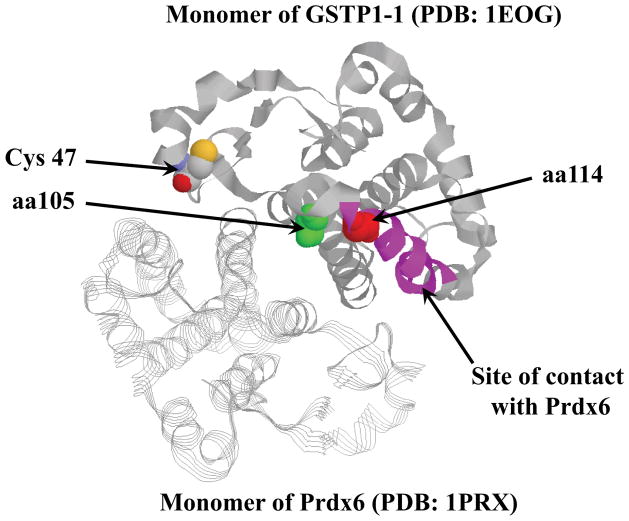
Fig. 7.
Structure of GSTP1-1A - Prdx6 hetero-dimer (PDB: 1EOG and PDB: 1PRX, respectively, ZDOCK 3.0.2). Monomer GSTP1-1 is represented as a “ribbon” and monomer Prdx6 - as a “strand”. Catalytic Cys47 is shown as “space-filled” in CPK. The positions of Ile105 (green) and Ala114 (red) are also “space-filled”. The site of closest contact with Prdx6 is represented in pink (6). Visualization of the crystal structure was performed using RasMol software (V2.7.5, http://www.bernstein-plus-sons.com/software/rasmol/doc/rasmol.html).
Discussion
Regulation of redox homeostasis is critical in the maintenance of normal cell functions, and both glutathione S-transferases and peroxiredoxins enzymes are important contributors to this process. There are numerous reports of aberrant expression patterns of GSTP linked with cancer and with altered response of tumor cells to a variety of anticancer drugs (reviewed [21]). The identification of single nucleotide polymorphisms in GSTP1-1 (Ile/Val 105, Ala/Val 114) has led to attempts to correlate their incidence with disease susceptibility and drug metabolism. For example, the GSTP1-1B allele was found to associate with bladder and testicular cancer and decreased GSTP1-1A was linked with prostate cancer, but associations between GSTP1-1 polymorphisms and colorectal or lung cancer have not been found [18, 22]. In a similar vein, increased expression of Prdx6 has been associated with malignancies of various organ sites including breast [23], and Prdx6 supports the growth, invasiveness and metastasis of breast cancer cells [24]. Moreover, the general peroxidase and PLA2 activities of Prdx6 promote growth and metastases of cancer cells [25, 26]. While epidemiological studies remain inconsistent, based on the protein interactions that regulate responses to ROS, activation of Prdx6 as a peroxidase initially depends on its heterodimerization with GSTP1-1 [2, 3, 6]. S-glutathionylation of target proteins is a function recently ascribed to GSTP1-1 [27] and Prdx6 is one of the enzymes where S-glutathionylation serves to enhance enzyme activity [8].
The in silico modeling (Fig. 7) and deletion studies suggest that the sites of closest contact between Prdx6 and the GSTP1-1 monomers is on the interface of their interaction at residues 115–124 for GSTP1-1 and 163–169 for Prdx6 [6]. Adding a GSTP1-1 synthetic peptide (aa 113–130) into the mixture of Prdx6 and GSTP1-1 results in a substantial inhibition of heterodimerization of these proteins (~80%, [6]). Amino acid 114 (Ala for (GSTP1-1A or GSTP1-1B) or Val for (GSTP1-1C or GSTP1-1D) is in this site of interaction and could be critical in stabilizing the complex. Another GSTP1-1 synthetic peptide (aa 98–112) also inhibits GSTP1-1 and Prdx6 heterodimerization by~25% [6]. This site contains amino acid 105 (Ile (GSTP1-1A and GSTP1-1D) or Val (GSTP1-1B and GSTP1-1C)), which also influence protein binding [6]. Such observations imply that each site of the GSTP1-1 allelic variation (aa 105 and 114) may be involved in its binding to Prdx6. Most likely the combination of differences in molecular volume (Ala – 69Å3; Val – 120Å3 and Ile – 204Å3) and hydrophobicity in the side-chains will influence the affinity of GSTP1-1 for Prdx6. Our present data show that affinities of GSTP1-1A (KD= 51nM) or GSTP1-1C (KD= 57nM) for Prdx6 are higher than those of GSTP1-1B (KD=101nM) or GSTP1-1D (KD= 94nM). The affinity of binding determines proximity between a catalytic Cys47-sulfenate of Prdx6 and activated GSH (thiolate) bound to the GSTP1-1 allelic variant and consequently, the efficiency with which the sulfenate at Cys47 is S-glutathionylated and reduced (i.e. activated) [6]. Our data show that the peroxidase activities of MCF-7 cell lysates transiently transfected with GSTP1-1A (or GSTP1-1C) were substantially higher than those of the same cells transfected with either GSTP1-1B (or GSTP1-1D). The catalytically inactive Y7F mutant of GSTP1-1A did not support activation of Prdx6 (Table 1) and furthermore peroxidase activity towards phospholipid hydroperoxide was abolished by the addition of specific Prdx6 inhibitor MJ33 [6].
The interface of the Prdx6-GSTP1-1 heterodimer is essentially similar to that of the GSTP1-1 homodimer (Fig. 7). E. coli expressed and purified GSTP1-1A has better catalytic efficiency and greater affinity for CDNB (Km=0.33±0.07mM) than GSTP1-1B (Km=1.15±0.07mM) [28]. Since the active unit of GSTP1-1 is a homodimer [6], this result may be explained by differences in interactions between monomeric subunits, similar to those for the Prdx6-GSTP1-1 heterodimer shown here. As a further corollary, in earlier studies the bulkiness of aa105 (Ile/Ala, Ile/Val, Ile and Ile/Trp) was shown to correlate negatively with GSTP1-1 kcat/Km values for anti-diol epoxides [29].
Thus, our data demonstrate that polymorphisms of GSTP1-1 differentially mediate activation of Prdx6 peroxidase activity providing a platform to imply that contingent upon their GSTP genotype, individuals will have significant differences in mounting an antioxidant response, particularly impacting protection of cell membranes against lipid peroxidation. Of particular interest, our results indicate that quantitative GSTP1-1 expression inversely correlates with GPx4 levels in MCF-7 cells. This could indicate the presence of a co-regulatory mechanism governing expression of Prdx6 and GPx4 in mounting an antioxidant response. This concept is presently under investigation. Polymorphic variants of GSTP will also potentially impact S-glutathionylation of other client proteins clusters [27]. Moreover, previous studies have suggested that GSTP1 polymorphisms are associated with cancer risk with frequencies of 0.65 (GSTP1-1A), 0.262 (GSTP1-1B) and 0.068 (GSTP1-1C) in Caucasian populations [22, 30]. Such differences will be of importance in the analysis of epidemiological data studying differences in population sensitivities to oxidant-stress involving procedures.
Research Highlights.
GSTP1-1 allelic variants control plasma membrane lipid peroxidation in MCF-7 cells
GSTP1-1A (and -1C) activates peroxidase function of Prdx6 in MCF-7 cells and in vitro
GSTP1-1A (and -1C) protein have higher affinity to Prdx6 than GSTP1-1B (and -1D)
Acknowledgments
This work was supported by grants from the National Institutes of Health (CA08660, CA117259, NCRR P20RR024485 - COBRE in Oxidants, Redox Balance and Stress Signaling) and support from the South Carolina Centers of Excellence program and was conducted in a facility constructed with the support from the National Institutes of Health, Grant Number C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. Supported in part by the Drug Metabolism and Clinical Pharmacology shared Resource, Hollings Cancer Center, Medical University of South Carolina.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manevich Y, et al. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11599–604. doi: 10.1073/pnas.182384499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free radical biology & medicine. 2005;38(11):1422–32. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Manevich Y, Feinstein SI, Fisher AB. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(11):3780–5. doi: 10.1073/pnas.0400181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manevich Y, et al. Structure and phospholipase function of peroxiredoxin 6: identification of the catalytic triad and its role in phospholipid substrate binding. Journal of lipid research. 2007;48(10):2306–18. doi: 10.1194/jlr.M700299-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, et al. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A(2) activity. The Biochemical journal. 2009;419(3):669–79. doi: 10.1042/BJ20082061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ralat LA, et al. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry. 2006;45(2):360–72. doi: 10.1021/bi0520737. [DOI] [PubMed] [Google Scholar]
- 7.Ralat LA, et al. Characterization of the complex of glutathione S-transferase pi and 1-cysteine peroxiredoxin. Archives of biochemistry and biophysics. 2008;474(1):109–18. doi: 10.1016/j.abb.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend DM. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol Interv. 2007;7(6):313–24. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannervik B. The isoenzymes of glutathione transferase. Advances in enzymology and related areas of molecular biology. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- 10.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Critical reviews in biochemistry and molecular biology. 1995;30(6):445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 11.Pal A, et al. Catalytic efficiencies of allelic variants of human glutathione S-transferase Pi in the glutathione conjugation of alpha, beta-unsaturated aldehydes. Cancer letters. 2000;154(1):39–43. doi: 10.1016/s0304-3835(00)00390-6. [DOI] [PubMed] [Google Scholar]
- 12.Ali-Osman F, et al. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. The Journal of biological chemistry. 1997;272(15):10004–12. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- 13.Watson MA, et al. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19(2):275–80. doi: 10.1093/carcin/19.2.275. [DOI] [PubMed] [Google Scholar]
- 14.Zimniak P, et al. Naturally occurring human glutathione S-transferase GSTP1-1 isoforms with isoleucine and valine in position 104 differ in enzymic properties. European journal of biochemistry/FEBS. 1994;224(3):893–9. doi: 10.1111/j.1432-1033.1994.00893.x. [DOI] [PubMed] [Google Scholar]
- 15.Hu X, et al. Differential protection against benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide-induced DNA damage in HepG2 cells stably transfected with allelic variants of pi class human glutathione S-transferase. Cancer research. 1999;59(10):2358–62. [PubMed] [Google Scholar]
- 16.Goto S, et al. Overexpression of glutathione S-transferase pi enhances the adduct formation of cisplatin with glutathione in human cancer cells. Free radical research. 1999;31(6):549–58. doi: 10.1080/10715769900301121. [DOI] [PubMed] [Google Scholar]
- 17.Chan QK, et al. Single nucleotide polymorphism of pi-class glutathione s-transferase and susceptibility to endometrial carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11(8):2981–5. doi: 10.1158/1078-0432.CCR-04-2038. [DOI] [PubMed] [Google Scholar]
- 18.Harries LW, et al. Identification of genetic polymorphisms at the glutathione S-transferase Pi locus and association with susceptibility to bladder, testicular and prostate cancer. Carcinogenesis. 1997;18(4):641–4. doi: 10.1093/carcin/18.4.641. [DOI] [PubMed] [Google Scholar]
- 19.Mannervik B, et al. Nomenclature for human glutathione transferases. The Biochemical journal. 1992;282(Pt 1):305–6. doi: 10.1042/bj2820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowers RR, et al. Sulfiredoxin Redox-Sensitive Interaction with S100A4 and Non-Muscle Myosin IIA Regulates Cancer Cell Motility. Biochemistry. 2012;51(39):7740–54. doi: 10.1021/bi301006w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tew KD, et al. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free radical biology & medicine. 2011;51(2):299–313. doi: 10.1016/j.freeradbiomed.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris MJ, et al. Polymorphism of the Pi class glutathione S-transferase in normal populations and cancer patients. Pharmacogenetics. 1998;8(1):27–31. doi: 10.1097/00008571-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Karihtala P, et al. Peroxiredoxins in breast carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9(9):3418–24. [PubMed] [Google Scholar]
- 24.Chang XZ, et al. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast cancer research: BCR. 2007;9(6):R76. doi: 10.1186/bcr1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho JN, et al. Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells. Molecular cancer therapeutics. 2010;9(4):825–32. doi: 10.1158/1535-7163.MCT-09-0904. [DOI] [PubMed] [Google Scholar]
- 26.Li DQ, et al. Identification of breast cancer metastasis-associated proteins in an isogenic tumor metastasis model using two-dimensional gel electrophoresis and liquid chromatography-ion trap-mass spectrometry. Proteomics. 2006;6(11):3352–68. doi: 10.1002/pmic.200500617. [DOI] [PubMed] [Google Scholar]
- 27.Townsend DM, et al. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2009;284(1):436–45. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodrich JM, Basu N. Variants of glutathione s-transferase pi 1 exhibit differential enzymatic activity and inhibition by heavy metals. Toxicology in vitro: an international journal published in association with BIBRA. 2012;26(4):630–5. doi: 10.1016/j.tiv.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundberg K, et al. Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1-1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinogenesis. 1998;19(3):433–6. doi: 10.1093/carcin/19.3.433. [DOI] [PubMed] [Google Scholar]
- 30.Townsend DM, et al. A glutathione S-transferase pi-activated prodrug causes kinase activation concurrent with S-glutathionylation of proteins. Mol Pharmacol. 2006;69(2):501–8. doi: 10.1124/mol.105.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]