Abstract
The NR2A:NR2B subunit ratio of the NMDA receptors is widely known to increase in the brain from postnatal development to sexual maturity and to aging, yet its impact on memory function remains speculative. We have generated forebrain-specific NR2A overexpression transgenic mice and show that these mice had normal basic behaviors and short-term memory, but exhibited broad long-term memory deficits as revealed by several behavioral paradigms. Surprisingly, increased NR2A expression did not affect 1-Hz-induced long-term depression (LTD) or 100 Hz-induced long-term potentiation (LTP) in the CA1 region of the hippocampus, but selectively abolished LTD responses in the 3–5 Hz frequency range. Our results demonstrate that the increased NR2A:NR2B ratio is a critical genetic factor in constraining long-term memory in the adult brain. We postulate that LTD-like process underlies post-learning information sculpting, a novel and essential consolidation step in transforming new information into long-term memory.
The N-methyl-d-aspartate receptor (NMDAR) is widely known to be the synaptic coincidence detector essential for controlling synaptic plasticity1,2,3 and gating memory formation4,5,6,7,8,9,10,11,12. Together with the NR1 core subunit, the NR2A and NR2B subunits form the diheteromeric or triheteromeric complex of the NMDA receptor in the forebrain regions13,14,15,16. Depending on ages or states of animals, dynamic changes in NR2A and NR2B can lead to the different mixture of NR1/NR2A, NR1/NR2B, and NR1/NR2A/NR2B receptors in the forebrain16,17. There is a higher amount of NR2B expression in postnatal and juvenile brains, but NR2A gradually becomes more prevalent in adulthood and advanced ages14,15,18,19,20,21,22. Based on distinct biophysical properties of the NR2A and NR2B (such as longer channel opening duration with the NR2B subunit than the NR2A, etc.), it has been hypothesized that an increased NR2A:NR2B ratio in the sexually matured and/or aged brains may represent a major genetic factor underlying the age-dependent, gradual constraint on memory functions in comparison to that of juvenile or younger brains9,17,21,22,23. However, it is difficult to test this NR2A:NR2B ratio hypothesis by directly comparing the young animals with the aged animals because there are significant differences in expression of many other genes between those two age groups. Moreover, the levels of NR2A or NR2B expression in the cortex and hippocampus can also be dynamically modulated by individual experiences (i.e. enriched environment, or social interactions).
A series of genetic studies have shown that global knockout of NR2A resulted in lesser CA1 long-term potentiation, a moderate deficiency in spatial reference memory and fear memory (but see4, reporting normal spatial reference memory), and/or significant spatial working memory deficit. This suggests that the presence of NR2B in NR2A−/− mice largely preserves LTP5,24 and most long-term memories. On the other hand, genetic deletion of NR2B in the forebrain- or hippocampus-specific knockout of NR2B results in more profound memory deficits and impaired LTP3,25. These experiments, by examining the extreme ends of the NR2A:NR2B ratio spectrum (without NR2A or NR2B), have provided fundamental insights into the roles of the pure NR2A- or NR2B-containing NMDA receptor population under the given test conditions.
The initial evidence for the concept that an increased NR2A:NR2B ratio may reduce synaptic plasticity and memory function in adulthood came from our NR2B transgenic experiments. We previously showed that genetic overexpression of NR2B in the mouse forebrain can lead to larger hippocampal long-term potentiation (10–100 Hz range, without affecting LTD) and enhanced learning and memory function as tested in seven different memory tasks9,20,22,26,27,28. Similar memory and LTP enhancement was also observed in NR2B overexpression transgenic rats, pointing to the conserved beneficial effects of NR2B in multiple animal species29. Thus, these NR2B overexpression experiments, along with other studies21,23, have provided important, but circumstantial evidence that the increased NR2A:NR2B ratio can be detrimental to greater synaptic plasticity and memory function in the older brains. In this study, we have directly tested this hypothesis and investigated the effects of increased NR2A:NR2B ratio in the adult mouse forebrain on synaptic plasticity and learning behaviors by producing CaMKII promoter-driven NR2A transgenic mice. We combined hippocampal slice electrophysiology and behavioral paradigms to investigate how such overexpression may alter synaptic plasticity and cognition. We show that the high NR2A amount in the forebrain principal excitatory neurons can selectively affect long-term memory formation. But surprisingly, instead of the predicted smaller LTP in the CA1 region of the NR2A transgenic mice, we found that NR2A overexpression selectively abolished 3–5 Hz frequency-induced LTD in the CA3-CA1 synapses without affecting 100 Hz LTP or 1 Hz LTD. This suggests to us a novel step by which long-term memory consolidation engages LTD-like process to sculpt, crystallize, and incorporate newly acquired information into long-term knowledge in the brain.
Results
Production and basic characterization of Tg-NR2A transgenic mice
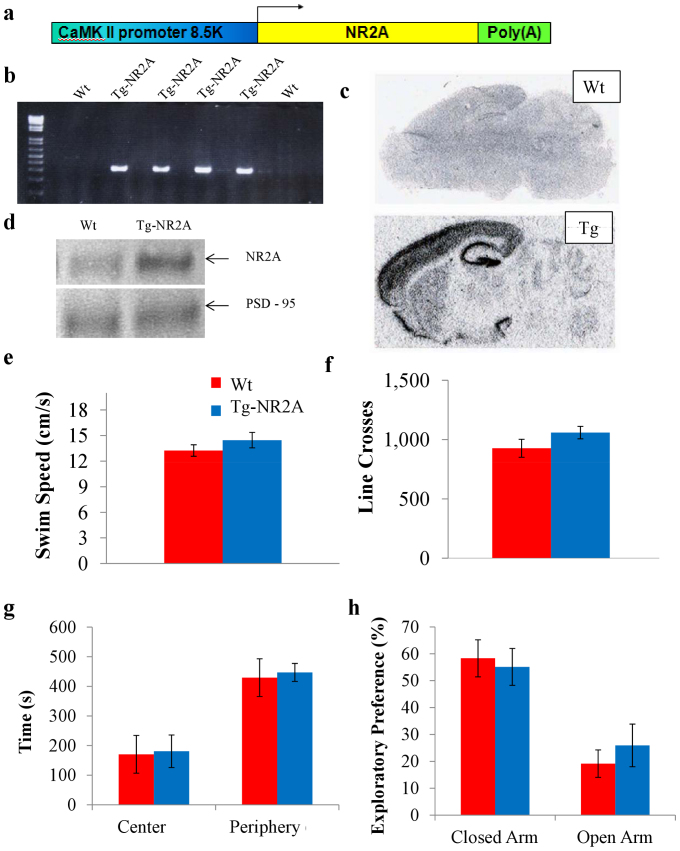
We used a CaM-kinase-II (CaMKII) promoter9,30,31 to construct NR2A subunit overexpression vector (Fig. 1A) and produced a forebrain-specific NR2A overexpression transgenic mouse line. We confirmed the transgene integration into genome by both Southern blot analysis (not shown) and PCR using SV40 Poly(A) probes (Fig. 1B). Next, we performed a series of in situ hybridization experiments to visualize the overexpression pattern of the NR2A in the forebrain regions (Figure 1C). The transgene expression was highly enriched in the cortex, striatum, and hippocampus, but not in the hind brain regions such as the cerebellum (Fig. 1C). Western blot analysis of the forebrain tissue also confirmed enhanced NR2A protein expression over the wild type animals (Fig. 1D) in these regions as well, while the expression of the NR2A in the cerebellum remained unchanged. Our light microscopy examination did not find any noticeable structural abnormalities in the transgenic brains. These biochemical and histological data confirm that these transgenic animals express as expected in the mouse forebrain areas.
Figure 1. Construction and basic characterizations of Tg-NR2A animals.
(A) Construct design for transgene vector for the Tg-NR2A mouse line. (B) PCR results for the transgenic animal line using an SV-40 primer with DNA isolated from tail biopsy. Columns labeled with “Tg” represent the positive transgene detection, columns labeled “Wt” indicate the wildtype littermates. (C) In situ hybridization with the NR2A probe. A significant increase in the amount of the NR2A subunit is present in the forebrain regions. (D) Western blot of the cortex confirms the significant increase of NR2A in the forebrain of the transgenic animal in comparison to the wildtype forebrain. (E) The Tg-NR2A animals and their wild-type littermates were found to have similar swimming speeds (Wt: n = 10, 13.25 ± 0.67 vs Tg: n = 10, 14.46 ± 0.90 cm/s). (F) The Wt and Tg-NR2A animals had similar number of line crosses in the open field paradigm (Wt: n = 6, 927.17 ± 75.63 vs. Tg: n = 9, 1,059 ± 52.33, t-test p = 0.22). (G) Both the Wt and the Tg mice preferred the periphery of the open field (Wt: 447.04 ± 33.03 s vs 429.41 ± 63.75 s) to the center of the open field (Wt: 170.60 ± 63.75 s vs. Tg: 180.91 ± 54.97 s). (H) Elevated Plus Maze: Both the Tg-NR2A and the Wt animals preferred the closed arms (Tg: 55.14 6.86% vs Wt: 58.33 ± 6.89%) to the open arms (Tg: 25.94 ± 7.94% vs Wt: 19.15 ± 5.11%).
The NR2A transgenic mice can mate and grow normally. They had similar body size and weight (30.88 ± 0.71 g, n = 12) in comparing to their wild-type littermates (30.62 ± 1.20 g, n = 13; t-test, p = 0.87). Throughout the handling and behavioral experiments, we observed no seizures or convulsions in the transgenic mice. Both genotypes of mice can swim normally and had the similar swimming speeds (Fig. 1E; (Tg: n = 10, 14.46 ± 0.90 cm/sec vs. Wt: n = 10, 13.25 ± 0.67 cm/sec). To assess the locomotor activity patterns, we use the open field paradigm and found that the transgenic mice were indistinguishable from the wild-type mice (Fig. 1F, Line crossed in Tg mice: 1,059 ± 52.33, n = 9; and in Wt: 927.17 ± 75.63, n = 6, t-test, p = 0.22), indicating comparable locomotor activity. Moreover, there is no difference in the time distribution in the center area and periphery area between the transgenic and wild-type controls (Fig. 1G. Center area: Tg, 180.91 ± 54.97 s vs. Wt, 170.60 ± 63.75s. Periphery area: Tg, 447.04 ± 30.33 s vs. Wt, 429.41 ± 63.75 s). To evaluate anxiety levels, we examined the mice using the elevated-plus maze paradigm. Once again, there was no difference between the genotypes (Fig. 1H). Both types of mice preferred to spend more time in the closed arm (Tg: 55.14 ± 6.86% vs. Wt: 58.33 ± 6.89%) than the open arm (Tg: 25.94 ± 7.94% vs. Wt: 19.15 ± 5.11%). These basic behavioral assays suggest that the transgenic NR2A mice are indistinguishable to their counterpart littermates in basic behaviors.
Selective deficits in long-term but not short-term novel object recognition
To assess the effect of NR2A overexpression on recognition memory, we tested the animals in a novel object recognition task. The Tg-NR2A animals exhibit similar exploratory behavior and motivation as their wild-type littermates in the training phase of our novel object recognition test spending approximately equal time with each identical object (Wt: n = 10, 48.57 ± 2.94%; Tg: n = 10, 50.15 ± 0.93%, Fig. 2A). As expected, the wild type mice showed a strong preference for the novel object at the one hour retention test. Similarly, we found that the Tg-NR2A transgenic mice also showed good 1-hr recognition memory, spending comparable amount of time in exploring the novel object than the familiar object (Wt: n = 10, 61.46 ± 1.54%, Tg: n = 10, 57.80 ± 3.53%, Fig. 2A). This indicates that the transgenic mice can learn, remember, and retrieve the memory of the older object on the short-term time scale at the similar level in comparison to that of the wild-type mice.
Figure 2. Impaired long-term novel Object Recognition.
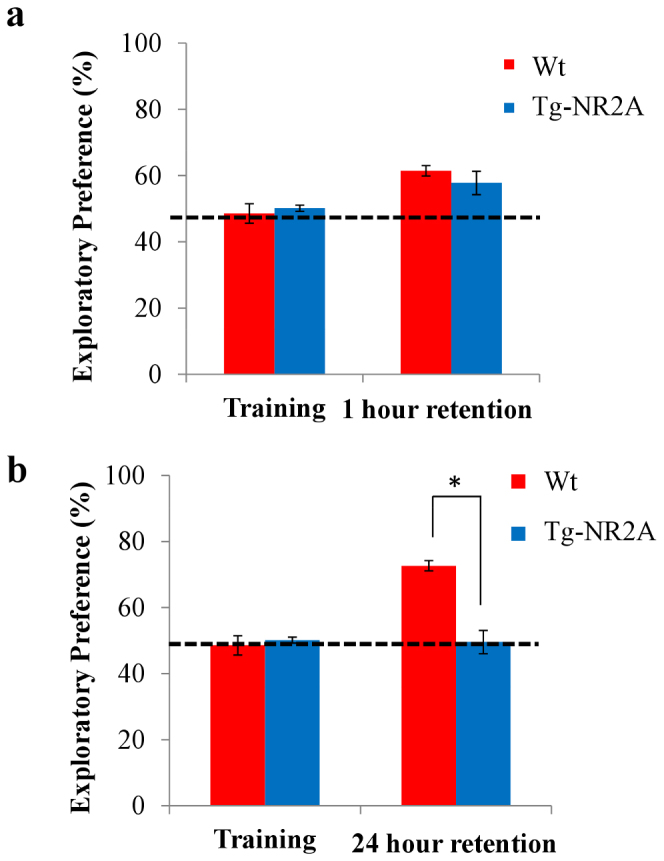
(A) During training both groups of animals spent approximately equal time exploring both objects (wt: n = 10, 48.57 ± 2.94%; NR2A: n = 10, 50.15 ± 0.93%). During the one hour retention test the tg-NR2A animals and their wild-type littermates spent approximately equal time investigating the novel object (wt: n = 10, 61.46 ± 1.55%; NR2A: n = 10, 57.80 ± 3.53%). (B) In the 24 hour recall session the tg-NR2A showed no preference for the novel object (NR2A: n = 10 49.59 ± 4.00%) whereas the wild-type animals show significantly more interest in the novel object (wt: n = 10, 72.67 ± 5.74). * p = 0.015.
Using another group of mice, we measured the 1-day novel object recognition to assess the long-term recognition memory of the tg-NR2A mice. The wild-type mice show robust 1-day memory performances, whereas the Tg-NR2A exhibited no preference at all (wt: n = 10, 72.67 ± 5.74%; NR2A: n = 10, 49.59 ± 4.0%, Fig. 2B). These results indicate that while the Tg-NR2A animals were able to retain object recognition memory for a short amount of time, they are impaired in long-term object recognition memory, unable to distinguish between the novel object and the familiar object after 24 hours of the initial learning.
Selective deficits in long-term but not short-term fear conditioning memories
To test the associative learning in our transgenic mouse line, we used two associative learning paradigms, namely contextual fear conditioning and cued fear conditioning. Contextual fear conditioning is a hippocampal-dependent associative learning process that requires the memory of the context in which an adverse event occurs to be remembered32,33,34,35. During the training phase, both the wild-type mice and the NR2A transgenic mice showed similar freezing responses (Wt: n = 10, 16.33 ± 5.24%; Tg: n = 14, 12.67 ± 3.86%, Fig. 3A) indicating a normal fear response. At the one hour recall, the animals are placed back into the identical operant chamber to assess their contextual memory. The Tg-NR2A animals demonstrated similar contextual fear memory at the one hour recall as the wild type animals (Wt: n = 10, 35.1 ± 8.15%, Tg n = 10, 33.68 ± 6.58%, Fig. 3A) demonstrating that tg-NR2A mice have normal short-term contextual emotional memory recall.
Figure 3. Impaired long-term contextual Fear Conditioning.
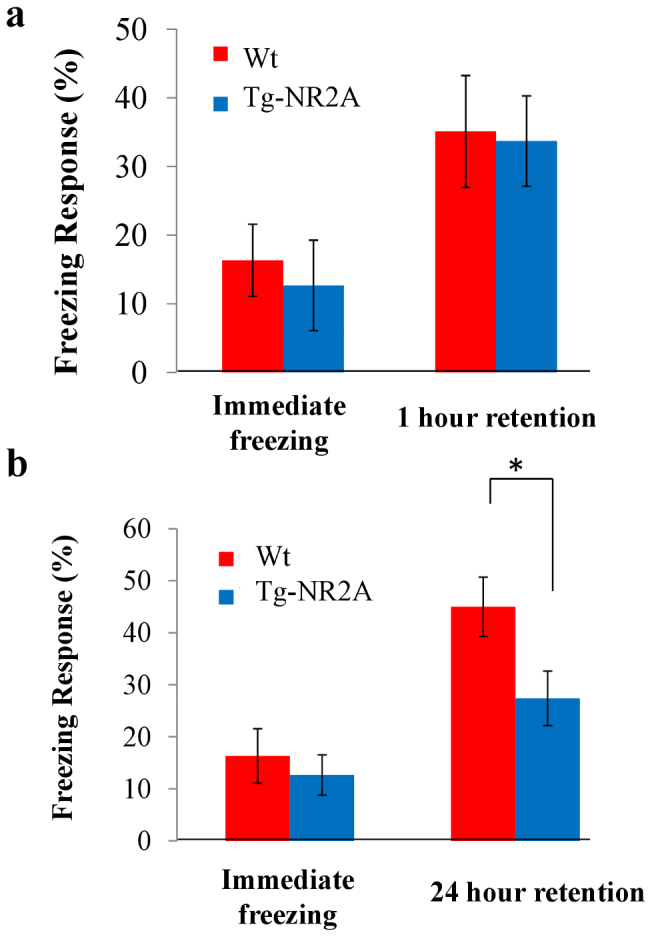
(A) There were no differences found in the immediate freezing responses of either group of animals (wt: n = 10, 16.33 ± 5.24%; NR2A: n = 10, 13.33 ± 6.30%). At the 1 hour recall session, both animals demonstrated similar freezing indicated no differences in fear memory at one hour (wt: n = 10, 35.1 ± 8.15%; NR2A: n = 10, 33.68 ± 6.58%). (B) During the 24 hour contextual recall session the wild-type animal has a significantly higher freezing response than the tg-NR2A animals (wt: n = 10, 45.57 ± 5.70%; NR2A: n = 10, 27.41 ± 5.24%).
For the 24 hour retention test, we used a separate group of animals for fear conditioning training. They were placed back into the identical operant chamber and observed for freezing 24-hr after learning. We found that the Tg-NR2A animals showed significantly less contextual freezing than the wild type mice (Wt: n = 10, 45.00 ± 5.70%; NR2A: n = 14, 27.41 ± 5.24%, Fig. 3B). This suggests that the Tg-NR2A are impaired in long-term contextual fear memory.
Cued fear conditioning pairs a conditioned stimulus (CS), a tone, co-terminated with an unconditioned stimulus (US), a mild shock. Learning of this paradigm is known to be independent of the hippocampus34. During the pre-tone time period, both groups of animals exhibited little freezing (Wt: 2.22 ± 1.00%; NR2A: 3.61 ± 2.00%, Fig. 4A), reflecting a similar exploratory response to a novel environment. Upon cue, the Tg-NR2A animals exhibited strong but similar cued freezing responses at the one hour recall testing in comparison to that of wild-type littermates (wt: n = 10, 68.69 ± 5.3%; NR2A: n = 10, 67.71 ± 4.6%, Fig. 4A).
Figure 4. Impaired long-term cued Fear Conditioning.
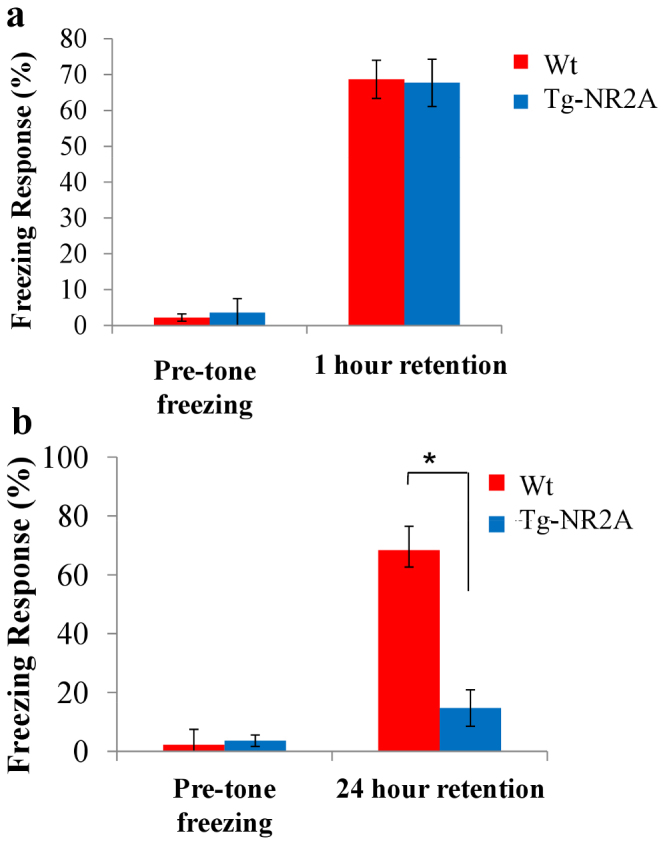
(A) In the cued fear conditioning training, neither group showed a significant freezing response to the chamber before the tone was presented (wt: n = 10 2.22 ± 1.00%; NR2A n = 10, 3.61 ± 2.00%). At the one hour recall session both the tg-NR2A and their wild-type littermates exhibited similar freezing responses to the tone (wt: n = 10, 68.69 ± 6.05%, NR2A: n = 10, 67.71 ± 4.60%). (B) In a separate cohort of animals at the 24 hour cued recall test the wild-type animals exhibited significantly more freezing than the tg-NR2A animals (wt: 68.33 ± 6.04%; NR2A 14.72 ± 6.1%; ** p = 2.96 × 10−6).
Using another batch of mice, we examined how transgenic mice may perform with long-term memory retention. Under this condition, the NR2A transgenic animals exhibited significantly less freezing (n = 10, 14.72 ± 6.18%, Fig. 5b) than their wild-type littermates (n = 10, 68.33 ± 6.04%) at the 24 hour cued recall test. Similar immediate freezing by the transgenic animals during the learning as well as during 1-hr recall tests collectively suggest that the transgenic mice had the same ability to learn, remember, recall, and behaviorally express fear memories, yet the transgenic animals were unable to retain the association of the context or tone to the foot shock for a longer period of time. These fear conditioning experiments revealed that the mutant mice are deficient in turning both short-term contextual and cued fear memories into long-term memories regardless of whether the fear conditioning occurred in hippocampal-dependent or hippocampal independent manner.
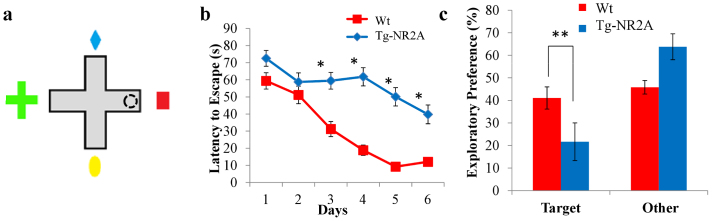
Figure 5. Impaired performance in spatial Plus Maze.
(A) Experimental set up of the water plus maze. The dotted circle in the east arm indicates the location of the fixed hidden platform. The starting position for each of the trials was the north, west or south arm. (B) In the hidden platform paradigm the wild-type were able to find the platform significantly faster than the Tg-NR2A animals on days 3–6. (C) During the transfer tests on day 7 the tg-NR2A animals spent significantly less time in the target arm than the wild-type animals (wt: 12.04 ± 1.67s, NR2A: 39.78 ± 5.5 s).
Deficits in spatial reference memory
To examine the effect of NR2A overexpression on spatial memory, we further tested the spatial reference memory of the Tg-NR2A animals using a hidden platform water plus-maze test. This version of the water maze requires the animal to actively seek the platform to escape the water (Fig. 5A). The hidden platform location is at the end of the east arm. It is generally considered that this spatial plus maze is an easier task as the animal has considerably fewer options than the conventional Morris water maze10. The platform was submerged in one of the four arms of the cross maze and remained in the same location throughout the length of the experiment10. There were four trials per day, with each arm (North, west or south arm) being the starting location of at least one trial each day. The mutant mice exhibited indistinguishable swimming ability and swimming speed in comparison to that of the wild-type mice (Fig. 1E). The wild-type animals (n = 11) learned the task over the trials, spending approximately 59.37 ± 4.77 s on day one searching for the platform. They steadily improved to 12.04 ± 1.67 s on day six (Fig. 5B). In contrast, while the Tg-NR2A animals (n = 11) initially spent 72.53 ± 4.62 s searching for the hidden platform on day one, they were much slower in acquiring this task, and only improved their escape latency to 39.78 ± 5.0 s by day six (Fig. 5B). This indicates that the Tg-NR2A animals had difficulty in remembering the arm where the hidden platform was located using the distal spatial cues. To further confirm the spatial memory formation, we also performed a transfer test in which the platform was removed from the maze at the end of the day six training session. The mouse was placed in the maze and allowed to explore the platform for 90 seconds. The time in the target and other arm was measured and calculated. The wild-type animals spent significantly more time in the target arm than the transgenic animals (wt: n = 11, 41.08 ± 4.92%; NR2A, n = 11, 21.67 ± 8.35%, Fig. 5C). The difference in the probe test confirmed that the transgenic mice were impaired in spatial reference memory function.
Selective deficits in 3–5 Hz induced LTD but not in 1 Hz LTD, 100 Hz LTP, and long-term depotentiation
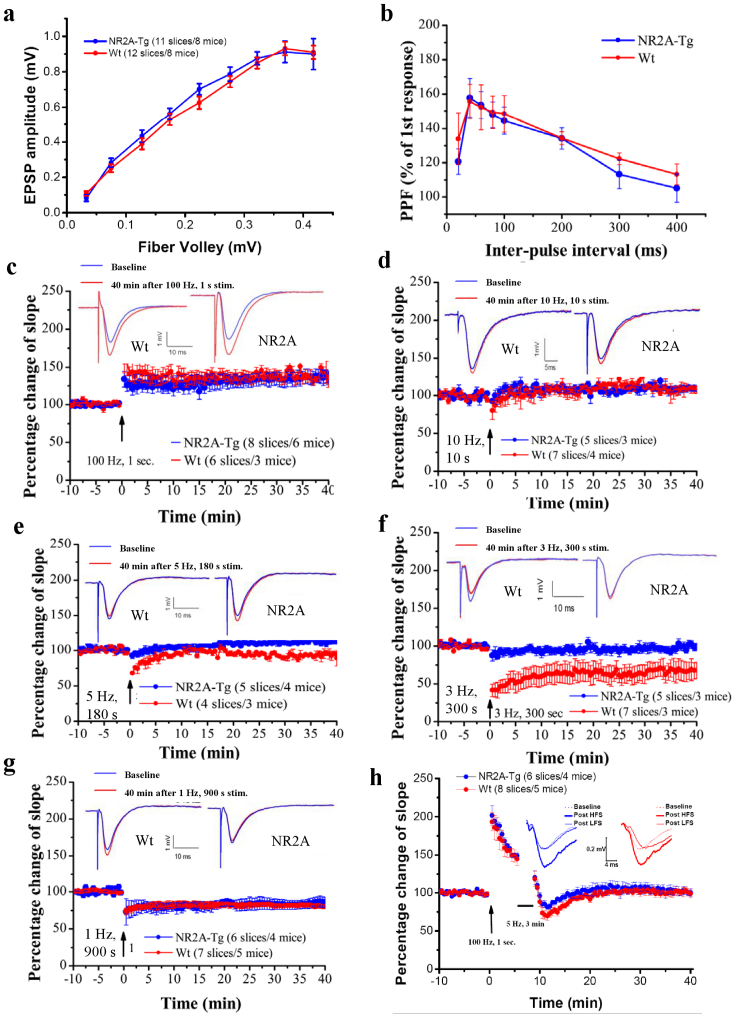
To investigate the effects of NR2A overexpression on electrophysiological properties in the brain, we used hippocampal slice recordings8,9,22 in the Schaffer collateral-CA1 path from adult (4–6 months old) transgenic mice and aged matched wild-type littermates. Wild-type and transgenic mice showed no significant differences in input-output properties, and there were no significant differences found in paired-pulse (Fig. 6A) facilitation (PPF, Fig. 6B), indicating that presynaptic function is unchanged in the Tg-NR2A animals.
Figure 6. Electrophysiology of hippocampal slices.
(A) There were no significant differences in the basal synaptic transmission as seen in the CA3-CA1 input-output curve. (B) The paired-pulse facilitation was unchanged between the Wt and the Tg animals indicating that the presynaptic function is unchanged. (C) A 100 Hz frequency for 1 s was able to produce similar LTP in Tg-NR2A animals and their wild-type littermates (wt: n = 6/3, NR2A: n = 8/6). (D) High frequency stimulation of 10 Hz for 10 s produced similar LTP in both the tg-NR2A animals and their wild-type littermates (wt: n = 7/4, NR2A: n = 5/3). (E) A 5 Hz stimulation for 180 s was also unable to produce LTD in the transgenic animals, also it was still able to produce LTD in the wild-type animals (wt: n = 4/3, NR2A: n = 5/4). (F) A 3 Hz stimulation for 300 s was unable to produce LTD in the transgenic animals, whereas the same stimulation was able to produce LTD in wild-type animals (wt: n = 7/3, NR2A: n = 5/3). (G) Low frequency stimulation (1 Hz for 900 s) induced similar LTD in both the wild-type and tg-NR2A animals (wt: n = 7/5, NR2A: n = 6/4). (H) There was no change seen in the depotentiation of the transgenic NR2A hippocampal slices.
To examine the effects of NR2A expression on in vitro synaptic plasticity, we applied tetanic stimulation at high frequencies. We found that the hippocampal slices from the tg-NR2A animals show normal 100 Hz-induced long-term potentiation (LTP) as compared to the hippocampal slices from their wild-type littermates (100 Hz for 1 s: Wt: n = 6 slices/3 mice, 135.2 ± 7.6%; Tg: n = 8 slices/6 mice, 131.4 ± 11.6%, Fig. 6C). Similarly, there is no significant change in EPSP slope in response to 10 Hz stimulation and no difference was observed between the genotypes. (10 Hz for 1.5 min: Wt: n = 7 slices/4 mice, 103.2 ± 13.0%; Tg: n = 5 slices/3 mice, 109.7 ± 3.7%; (Fig. 6D).
To investigate the effects of NR2A overexpression on LTD in the adult hippocampal slices (4–6 month old), we used three LTD frequency protocols, 5 Hz for 3 min, 3 Hz for 5 min, and 1 Hz for 15 min. Interestingly, at the 5 Hz stimulation, the transgenic hippocampal slices (n = 5 slices/4 mice, 113.6 ± 4.6%) show no LTD, while in the wild-type hippocampal slices (n = 4 slices/3 mice, 94.4 ± 1.8%) LTD was reliably induced (Fig. 6E). Moreover, at the 3 Hz low-frequency stimulation the transgenic slice potentials (n = 5 slices/3 mice, 99.2 ± 4.7%) show complete lack of synaptic depression in comparison to the wild type slice potentials (n = 7 slices/3 mice, 70.3 ± 11.3%, Fig. 6F). Surprisingly, at the 1 Hz low-stimulation frequency protocol, we found that there was no difference between the transgenic slices (n = 6 slices/4 mice, 86.1 ± 6.85%) and wild-type slices (n = 7 slices/5 mice, 82.4 ± 1.6%, Fig. 6G). These results revealed a selective plasticity deficit in the transgenic NR2A slices at the 3–5 Hz frequency range.
Finally, we also performed the long-term depotentiation experiments. We first evoked LTP with 100 Hz and that was followed by a low-frequency stimulus (LFS) of 5 Hz for 3 min to produce depotentiation. Our results did not reveal any differences in this form of long-term depotentiation (Fig. 6H, 6 slices/4 Tg mice, 8 slices/5 wt mice).
Discussion
In the present study, we have tested a long-standing notion that an increased NR2A:NR2B ratio in sexually matured and/or aged brains is a major genetic factor underlying the developmental and age-dependent constraint on memory functions17,21,22,23,29. Our transgenic approach to increase NR2A expression in the forebrain allowed us to drive the population distribution of NR2A:NR2B ratio more toward the sexually matured or older brain state. This overcomes the complication if one simply compared the young wild-type animals with older wild-type animals because those two age groups may also have significant differences in expression of many other genes and other environmental factors. Our integrated analyses of the NR2A transgenic mice not only demonstrates the effects of increased NR2A:NR2B ratio on reducing long-term memory function, but also suggests that such a decline is associated with the abolished 3–5 Hz LTD type plasticity responses.
Overexpression of NR2A selectively affected 3–5 Hz CA1 LTD but not on 1 Hz-induced LTD or 100 Hz-induced LTP is surprising. This selective effect on 3–5 Hz frequency range would be hard to predict based on the available knowledge in the literature1,5,36,37,38,39,40. Pharmacological experiments suggest that both NR2A antagonist (NVP-AAM077, or NVP) and NR2B antagonist (Ro25-6981, or Ro) can reduce LTP37,39,40,41. Induction of LTD was inhibited by NVP in concentration-dependent manner, whereas blockers of NR2B-containing NMDARs had no significant effect39,40; but see36. Our previous studies show that overexpression of NR2B in the forebrain excitatory neurons result in superior learning and memory9,20,22,26 and leads to enhanced 10–100 Hz LTP with no effect on LTD in either transgenic mice9,22 or in transgenic rats29. In combining these results, our present study further shows that the altered ratio in NR2A:NR2B can produce continuous tuning of the LTD-LTP response curve as well as its amplitude. Such dynamic changes can also affect the percentage of the NR1/NR2A/NR2B triheterotetramers, the majority form in the adulthood, at CA3-CA1 synapses16. Since NR2B decreases significantly over sexual maturity and aging, this change can make the NR2B subunit the rate-limiting factor for bring the NMDA complex to synapses. This means that the increased production of NR2A may eventually overwhelm the NR2B, thus depriving the synapses with NR1/NR2B diheterotetramers. That is, an increase in NR2A would lead to the elevated NR1/NR2A diheterotetramers, and mixed with NR1/NR2A/NR2B triheterotetramers in the adult or aged hippocampus. This could explain some of the controversies in the literature on the NR2A and NR2B antagonists' effects on LTP or LTD.
The observed relation between abolished 3-Hz range LTD and long-term memory deficits is very interesting and represents another surprising aspect of this study. The NMDA receptor is generally known to be essential for learning7,12,17,42, memory consolidation8,43,44, and long-term preservation of remote memories6. The traditional views have been focused primarily on LTP-like mechanism for long-term memory formation17,45,46. By comparison, whether and how LTD may play a role in learning and memory is much less clear47,48,49,50,51,52. The potential role of LTD in memory consolidation has been corroborated by a recent study reporting that i.p. injection of NR2A antagonist NVP blocked in vivo LTD and also impaired one-day high intensity training version of the Morris water maze53. However, the specificity of NVP has been questioned37,38. Another report shows that when NVP was administered via intrahippocampal injected, it also blocked in vivo LTP39. Thus, it is not clear what to account for such discrepancy on the drugs' effects on LTP vs. LTD in vivo. It is possible that mixed diheterotetramer and triheterotetramer complex in the hippocampus or other brain regions may contribute such observations. Another recent study demonstrated that NVP reduced spatial earning in mice perhaps via down-regulating neurogenesis54. Other studies also reported that NVP affected the behavioral expression in addition to memory function55. In the present study, our genetic experiments demonstrated that NR2A overexpression in the excitatory neurons selectively abolished 3–5 Hz-induced LTD at CA3-CA1 synapses without affecting 1 Hz LTD, 100 Hz LTP, or long-term depotentiation. We would like point out that our present study only examined one form of LTP in the CA1-region during the early phase of LTP. Thus, it might be possible that other induction LTP-protocols as well as LTP-maintenance in both the CA1 and other brain regions might be affected by overexpression of NR2A-receptors as well. This needs to examine in future experiments.
Nonetheless, this loss of 3–5 Hz frequency plasticity in the NR2A transgenic mice offered us a unique opportunity to analyze possible functions on learning and memory within such context: We found that the mutant mice exhibited specific impairment in long-term memory but not in short-term memory tests of the same tasks, suggesting that the consolidation process, not recall, is compromised. Furthermore, these consolidation deficits were consistently observed in several tasks, namely, novel object recognition, contextual fear conditioning, cued fear conditioning, and spatial plus-water maze. Thus, the correlation between in abolished 3–5 Hz LTD responses and selective impairment LTM raises the fundamental question regarding the role of LTD in brain function in general49,50, and more specifically, how and why converting or consolidating short-term memory into long-lasting memory may require the LTD-like process (at this frequency range)?
Here, we postulate a possibly conceptual framework for memory consolidation. In this novel “LTD-memory trace sculpting” framework, LTD, with its full low-frequency range, is essential for the memory circuits to perform the “memory trace sculpting”. In other words, memory consolidation is not merely to reinforce the potentiated synapses (i.e. via LTP-like process) by repeating the learning patterns/sequences during the post-learning period, but also to sculpt and transform the newly acquired information by modifying and incorporating them into the existing knowledge network, a process engaging LTD in the memory circuits. The post-learning information pruning, sculpting, and crystalizing process requires LTD-like process via removing less relevant or less important information from learning, or disconnect contradictory knowledge already existed in long-term memory systems. Is there any possible indicator or evidence for such memory pattern sculpting during memory consolidation?
Our large-scale in vivo recording experiments show that real-time memory traces in the brain during the post-learning period not only routinely undergo spontaneous, but also differential reverberations56,57,58. More intriguingly, we also observed unexplained, new types of real-time memory traces during post-learning stage, which are different from the typical patterns evoked by learning stimuli59. The emergent new ensemble patterns provide circumstantial evidence for our interpretation which would also fit well the memory transformation theory60,61.
This “LTD-memory trace sculpting” process may occur in at least two possible manners: The first scenario is that the LTD-like process occurs on the learning-activated cells, but only at those non-activated synapses. This heterosynaptic information-sculpting mechanism would enable the activated neurons to select, shape, alter, and crystallize the learning-acquired synaptic information for long-term pattern encoding. The major outcome of this sort of information sculpting is to reduce firing of the learning-activated neurons during the basal states or to other non-relevant inputs, thereby enabling the cells with great robustness and further pruning or modifying response selectivity during subsequent learning trials or recall. This cellular ‘information-sculpting' during memory consolidation via LTD-like process is also consistent with the in vitro data showing that LTP and LTD can occur at spatially segregated compartments in the same cells62,63,64. More importantly, heterosynaptic induction of either LTD or LTP can lead to late-associative interactions, reinforcing each other by the synaptic “cross-tagging”65,66. Therefore, the cross-tagging mechanism can form a cellular basis for achieving LTD-mediated heterosynaptic information sculpting necessary for memory consolidation and memory transformation.
The second scenario is that the LTD-like memory trace sculpting process occurs more broadly among general populations which were not necessarily activated by specific learning stimuli per se (i.e. via secondary interneuron actions). This ‘network-level sculpting' process may be further useful to achieve robust overall signal-to-noise ratio within the scale of the network by lowering the general background firing. Indeed, a broad effect of LTD responses on the “local network” has been observed in rat hippocampus upon exploring novel space or environment48,67,68,69 as well as in the nucleus accumbens after cocaine use70. Such network-level effects may be mediated by neurotransmitters such as Ach, norepinephrine, or dopamine71,72,73. It will be of interest to test and distinguish the cellular- vs. network-level sculpting scenarios using in vivo neural recording techniques57,59. It is noteworthy to point out that the cellular sculpting and network sculpting processes are not necessarily exclusive to each other, but rather can work together for achieving efficient memory trace sculpting during long-term memory consolidation.
An alternative argument in addition to the above two proposed mechanisms may be that 3–5 Hz LTD occurred at the activated synapses of the learning-activated cells. However, this type of depotentiation mechanism has been suggested to be involved in reversal learning or tasks testing behavioral flexibility74, where the acquired information relationship needs to be reversed or disassociated from trial to trial. Also, long-term memories are likely distributed among various cortical circuits beyond the hippocampus or limbic system32,33,34,75,76,77,78,79. We would like to suggest that the proposed cellular and network LTD-memory trace sculpting mechanism(s) may not be limited to the hippocampus, but also applicable to other cortex and limbic memory circuits. Testing this hypothesis will require both in vitro and in vivo physiological experiments in the future on different brain subregions. Interestingly, existing evidence already indicates that amygdala neurons produce both NMDAR-dependent LTP and LTD80 which may engage in differential activation of NR2A or NR2B subunits81,82,83. However, it is conceivable that the complexity of the NR2A and NR2B ratio distribution among different subregions may result in different or unique physiological effects upon increased NR2A overexpression82,84. As such, the ratio of endogenous NR2B over NR2A subunits, for instance, in the central amygdala vs. the lateral amygdala84 can provide a different base line for the NR2A overexpression effects in the transgenic brain. Individual variations of the NR2A:NR2B ratios in healthy brains may also lead to different LTP/LTD capacities, thereby different long-term memory performances.
Finally, our finding that increased NR2A can be detrimental to long-term memory or low-frequency range synaptic plasticity may potentially offer a genetic explanation for age-dependent constraint on certain aspects of long-term memory functions. As we all have experienced, long-term memory skills, such as foreign language learning skills after sexual maturity, exhibit subtle but critical decline (i.e. inability to get rid of native accent in contrast to learning a perfect second language without accent if starting before the onset of sexual maturity). Such changes are believed to be genetically based, and can benefit from LTD-like synaptic-weakening and pruning mechanism in the overlapping circuits. Although this particular example can be difficult to be examined in animal models, it can provide a clue as to how LTD-like process may be crucial for fine-tuning long-term memory function. Increased NR2A:NR2B ratio is likely one of the major genetic factors underlying age-dependent constraint on plasticity and long-term memory. It may also point to the often ignored aspects when seeking the treatment of brain disorders in humans such as Alzheimer's disease which are accompanied by increased NR2A85,86,87,88. In addition, the NR2A:NR2B ratio adjustment strategy may need to be fully considered in the future treatment for patients who carry heterozygous NR2A or NR2B mutations which are linked to mental retardation and/or epilepsy in humans88. For normal elders, it might be useful to explore strategies in modifying the NR2A:NR2B ratio by enriched experience or dietary supplements20,22,89.
Future work may include the efforts to further understand how increased NR2A:NR2B ratio acts as a major genetic factor underlying age-dependent constraint on plasticity and long-term memory functions as early as the beginning of onset sexual maturity. This period is correlated with reduction in certain long-term memory skills, such as reduced ability in learning foreign languages without native accent in humans or song-learning in birds. Such changes are believed to be genetically based, and could benefit from LTD-like synaptic-weakening and pruning mechanism in the overlapping circuits.
In conclusion, we have tested the NR2A:NR2B ratio hypothesis on learning and memory, and demonstrated that increased NR2A expression did not affect short-term memory but can constrain long-term memory function. The selective reduction in long-term memory function is associated with abnormally compressed LTD responses in the 3–5 Hz frequency range without affecting 1 Hz LTD or 100 Hz LTP. Based on this unexpected finding, we present the “LTD-memory traces sculpting” hypothesis that LTD-like process actively engages in post-learning cellular- and network-level information sculpting, an essential step in transforming and consolidating long-term memory traces in the brain.
Methods
Transgenic animal production
The founding line of transgenic animals was produced by pronuclear injection of a linearized NR2A transgene vector pJT-NR2A into C57B/6 zygotes similar to previously described9,12. Animals were then intercrossed with B6C57 for at least 8 generations. The genotypes of the Tg-NR2A transgenic mice were determined by PCR analysis of a tail biopsy. The transgene was detected using the SV40 poly(A) sequence, as previously described9,22,29. Southern blotting was used to confirm the transgene integration in to the transgenic mouse line. Western blotting of the forebrain regions (cortex and hippocampus) was visualized with a polyclonal anti-NR2A (Upstate/Millipore). For in situ hybridizations, brains from both the tg-NR2A animal and their wild-type littermates were isolated and 20 μm sections were prepared using a cryostat. The slices were hybridized to the [α35S] oligonucleotide probe which hybridized to the untranslated artificial intron region in the transgene similarly to the previously described9.
Hippocampal-slice recordings
Hippocampal recordings procedures were the same as previously described9,22,90. Briefly, transverse slices of the hippocampus, obtained from (4–6 month old) NR2A transgenic mice and wild-type littermates, were rapidly prepared and placed into a submergible chamber for at least 2 hours prior to experimentation. The slices were subfused with artificial cerebral-spinal fluid consisting of 124 mM NaCl, 4.4 mM KCl, 2.0 mM CaCl2, 1.0 mM MgSO4, 25 mM NaCHO3, 1.0 mM Na2HPO4 and 10 mM glucose, and bubbled with 95% O2 and 5% CO2. A bipolar twisted insulated Ni-Cad wire was placed in Stratum Radiatum of CA1 region to deliver electro-stimuli. Extracellular field potentials were recorded with a glass microelectrode (3–7 MΩ, filled with ACSF) in the Stratum Radiatum of CA1 also. Test response elicited at 0.02 Hz. Current intensity (0.5–1.2 mA) which produced 30% of maximal response was used for studies of PPF and synaptic plasticity at different frequencies. For depotentiation experiments, the stimulus to produce LTP was 100 Hz for 1 sec. This was followed by a low-frequency stimulus (LFS) of 5 Hz for 3 min to produce depotentiation91. Data are presented as mean ± SEM. One way ANOVA followed by Donnett if applicable compared to wild-type controls were used for statistical analysis.
Behavioral testing
Animals were maintained in a 12 hr light/dark cycle in a temperature and humidity controlled environment. Adult NR2A transgenic mice and their wild-type littermates, aged 3–4 months were obtained and used for all behavioral testing. Separate cohorts of animals were used for each testing paradigm and each recall time point. All testing procedures were conducted in soundproofed, specialized behavior rooms. All procedures were approved by the Institutional Animal Care and Use Committee of the Georgia Health Sciences University and closely adhere to the animal standards of care of the National Institutes of Health.
Open field paradigm
Transgenic NR2A animals and their wild-type littermates are individually placed into a 20 in × 20 in × 10 in white Plexiglas box with a 2 in grid at the bottom. The animal is allowed to explore for ten minutes. The number of times the animal crosses the horizontal or vertical lines is counted as the number of line crosses. The periphery of the open field was considered to be the first four inches along the wall, while the center of the open field was the square inside this area10.
Elevated plus maze
The elevated plus maze consisted of a black Plexiglas “plus” approximately 24 in above the floor, with each arm measuring 30 cm in length. Two opposite arms were left open, with the other two arms being enclosed on three sides. The ambient room lighting was 75 lux. The amount of time the animals spend within the enclosed arms was recorded, as while as the amount of time the animal spent in the open arms10.
Novel object recognition
Behavioral paradigm was the same as previously described9,90. Animals were individually habituated to the open field testing environment. Due the training session the animals were placed in the open field apparatus with two identical objects and the ratio of the time each animal spent exploring the two identical objects was measured to determine the exploratory preference. At the describe time the animals were placed back in the open field apparatus with one familiar and one novel object, the time each animal spend with each object was measured to determine the recognition memory. Each group of animals was subjected to only one retention test.
Fear conditioning
Animals underwent testing as previously described8,90. To test the cued-fear associative memory in the tg-NR2A and wild-type mice, animals are trained by being placed in an operant testing chamber (10 in × 10 in × 15 in). The conditioned stimulus (CS) used was a 85 dB tone at 2,800 Hz for 30 seconds, followed by a continuous scrambled foot shock at 0.75 mA (the unconditioned stimulus, US) during the last 2 seconds of the CS. The animal remained in the chamber for another 30 s to measure immediate freezing responses. After 1 hour or 24 hours, the mice were placed into a novel chamber for three minutes, followed immediately by the CS tone for 3 minutes and the freezing responses were recorded. Freezing was judged as the complete immobility of the animal, except for respiratory movements. To test the contextual memory of the animals, 1 hour or 24 hours after the training, the mice were placed back into the shock chamber and monitored for 3 min (contextual conditioning). Separate cohorts of animals were used for each recall session and no animal underwent two recall sessions.
Spatial memory
To test the spatial memory of the transgenic animals, we used a modified Morris water maze paradigm, the water-filled cross maze, each of the four arms (N, S, E and W) measures 30 cm and was constructed of thick-walled, clear Plexiglass. The maze was surrounded by a black curtain with 4 distal spatial cues hung on it such that one cue was at the end of each of the four arms, N, S, E and W. The water was maintained at 25°C and made opaque with titanium oxide. For the landmark task, a white rectangle was placed directly over the distal end of the arm in which the platform was located, directly above the platform. Each animal was placed into one of the remaining arms and allowed to search for the platform for 90 seconds. At the end of 90 seconds if the animal was unable to find the platform it was guided to the platform by the experimenter and allowed to remain there for 10 seconds. The platform and landmark were relocated to a different arm for each trial. Each animal underwent 4 trials/day for two days10. To investigate the spatial memory of the Tg-NR2A animals, we also tested them in the hidden platform cross maze. In this paradigm the platform is submerged so that it is not visible to the animal, the landmark is removed, and the platform remains in the same position for all trials. Each animal was placed in the water and allowed to explore the four arms for 90 seconds in search of the fixed hidden escape platform. If, at the conclusion of the trial, the animal was unable to find the hidden platform, it was guided to the platform by the experimenter and allowed to stay for 10 seconds. Each animal underwent 4 trials/day for six days. During the transfer test the platform was removed from the water and the animal was allowed to search for 90 seconds. The time in each arm was recorded. For the landmark platform test, the protocol was the same as described except that the platform was moved for each block of trials. The landmark was a white rectangle that was placed over the distal end of the arm in which the platform was located.
Data analysis
All results are expressed as mean ± SEM. Behavioral data were analyzed by either one-way or two way ANOVA followed by post hoc Dunnett's test or a Student's T-test to determine significance. Differences were considered statistically significant if p < 0.05.
Author Contributions
Conceived and designed the experiments: Z.C. and J.Z.T. Performed the experiments: Z.C., R.F., S.J., H.W., Y.D., X.C. Analyzed the data: Z.C., R.F., S.J., H.W., Y.D., X.C. and J.Z.T. Wrote the paper: S.J., Z.C., R.F. and J.Z.T.
Acknowledgments
We thank Shuqin Zhang and Fenying Huang for technical assistance. This work was supported by funds from NIMH, NIA, and Georgia Research Alliance (all to JZT) and from National Natural Science Fund of China (NO30670682 and NO31070993).
References
- Malenka R. C. & Nicoll R. A. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci 16, 521–527 (1993). [DOI] [PubMed] [Google Scholar]
- Bear M. F. & Malenka R. C. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 4, 389–399 (1994). [DOI] [PubMed] [Google Scholar]
- Sprengel R. et al. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell 92, 279–289 (1998). [DOI] [PubMed] [Google Scholar]
- Bannerman D. M. et al. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J Neurosci 28, 3623–3630 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama Y. et al. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor epsilon1 subunit. J Neurosci 18, 6704–6712 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z. et al. Inducible and reversible NR1 knockout reveals crucial role of the NMDA receptor in preserving remote memories in the brain. Neuron 41, 781–793 (2004). [DOI] [PubMed] [Google Scholar]
- Rampon C. & Tsien J. Z. Genetic analysis of learning behavior-induced structural plasticity. Hippocampus 10, 605–609 (2000). [DOI] [PubMed] [Google Scholar]
- Shimizu E., Tang Y. P., Rampon C. & Tsien J. Z. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science 290, 1170–1174 (2000). [DOI] [PubMed] [Google Scholar]
- Tang Y. P. et al. Genetic enhancement of learning and memory in mice. Nature 401, 63–69 (1999). [DOI] [PubMed] [Google Scholar]
- Wang L. P. et al. NMDA receptors in dopaminergic neurons are crucial for habit learning. Neuron 72, 1055–1066 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman J. L. et al. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem 15, 50–54 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien J. Z., Huerta P. T. & Tonegawa S. The essential role of hippocampal CA1 NMDA receptor–dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338 (1996). [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Axel R. & Shneider N. A. Alternative splicing generates functionally distinct N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A 89, 8552–8556 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H., Burnashev N., Laurie D. J., Sakmann B. & Seeburg P. H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540 (1994). [DOI] [PubMed] [Google Scholar]
- Monyer H. et al. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256, 1217–1221 (1992). [DOI] [PubMed] [Google Scholar]
- Rauner C. & Kohr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem 286, 7558–7566 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien J. Z. Linking Hebb's coincidence-detection to memory formation. Curr Opin Neurobiol 10, 266–273 (2000). [DOI] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 357, 686–689 (1992). [DOI] [PubMed] [Google Scholar]
- Sheng M., Cummings J., Roldan L. A., Jan Y. N. & Jan L. Y. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368, 144–147 (1994). [DOI] [PubMed] [Google Scholar]
- Tang Y. P., Wang H., Feng R., Kyin M. & Tsien J. Z. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology 41, 779–790 (2001). [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Mesches M. H., Alvarez E., Bickford P. C. & Browning M. D. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J Neurosci 22, 3628–3637 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. et al. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci 25, 1815–1822 (2007). [DOI] [PubMed] [Google Scholar]
- Zhao X. et al. The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience 162, 933–945 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K. et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature 373, 151–155 (1995). [DOI] [PubMed] [Google Scholar]
- von Engelhardt J. et al. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron 60, 846–860 (2008). [DOI] [PubMed] [Google Scholar]
- Jacobs S. A. & Tsien J. Z. Genetic overexpression of NR2B subunit enhances social recognition memory for different strains and species. PLoS One 7, e36387 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Shimizu E. & Tsien J. Z. Do ‘smart’ mice feel more pain, or are they just better learners? Nat Neurosci 4, 453–454 (2001). [DOI] [PubMed] [Google Scholar]
- Cui Y. et al. Forebrain NR2B overexpression facilitating the prefrontal cortex long-term potentiation and enhancing working memory function in mice. PLoS One 6, e20312 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. et al. Genetic enhancement of memory and long-term potentiation but not CA1 long-term depression in NR2B transgenic rats. PLoS One 4, e7486 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M. et al. Control of memory formation through regulated expression of a CaMKII transgene. Science 274, 1678–1683 (1996). [DOI] [PubMed] [Google Scholar]
- Tsien J. Z. et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell 87, 1317–1326 (1996). [DOI] [PubMed] [Google Scholar]
- Biedenkapp J. C. & Rudy J. W. Context preexposure prevents forgetting of a contextual fear memory: implication for regional changes in brain activation patterns associated with recent and remote memory tests. Learn Mem 14, 200–203 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behav Neurosci 100, 814–824 (1986). [DOI] [PubMed] [Google Scholar]
- Kim J. J. & Jung M. W. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev 30, 188–202 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. G. & LeDoux J. E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106, 274–285 (1992). [DOI] [PubMed] [Google Scholar]
- Liu L. et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304, 1021–1024 (2004). [DOI] [PubMed] [Google Scholar]
- Berberich S. et al. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci 25, 6907–6910 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf C. et al. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci 25, 8386–8390 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. J., Russell K. I., Wang Y. T. & Christie B. R. Contribution of NR2A and NR2B NMDA subunits to bidirectional synaptic plasticity in the hippocampus in vivo. Hippocampus 16, 907–915 (2006). [DOI] [PubMed] [Google Scholar]
- Morishita W. et al. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology 52, 71–76 (2007). [DOI] [PubMed] [Google Scholar]
- Bartlett T. E. et al. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology 52, 60–70 (2007). [DOI] [PubMed] [Google Scholar]
- Davis S., Butcher S. P. & Morris R. G. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J Neurosci 12, 21–34 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Lindl K. A., Mei B., Zhang S. & Tsien J. Z. Requirement of NMDA receptor reactivation for consolidation and storage of nondeclarative taste memory revealed by inducible NR1 knockout. Eur J Neurosci 22, 755–763 (2005). [DOI] [PubMed] [Google Scholar]
- Wittenberg G. M., Sullivan M. R. & Tsien J. Z. Synaptic reentry reinforcement based network model for long-term memory consolidation. Hippocampus 12, 637–647 (2002). [DOI] [PubMed] [Google Scholar]
- Pastalkova E. et al. Storage of spatial information by the maintenance mechanism of LTP. Science 313, 1141–1144 (2006). [DOI] [PubMed] [Google Scholar]
- Wang H., Hu Y. & Tsien J. Z. Molecular and systems mechanisms of memory consolidation and storage. Prog Neurobiol 79, 123–135 (2006). [DOI] [PubMed] [Google Scholar]
- Etkin A. et al. A role in learning for SRF: deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron 50, 127–143 (2006). [DOI] [PubMed] [Google Scholar]
- Kemp A. & Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci 30, 111–118 (2007). [DOI] [PubMed] [Google Scholar]
- Bear M. F. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci 358, 649–655 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey P. V. & Bashir Z. I. Long-term depression: multiple forms and implications for brain function. Trends Neurosci 30, 176–184 (2007). [DOI] [PubMed] [Google Scholar]
- Braunewell K. H. & Manahan-Vaughan D. Long-term depression: a cellular basis for learning? Rev Neurosci 12, 121–140 (2001). [DOI] [PubMed] [Google Scholar]
- Brigman J. L. et al. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci 30, 4590–4600 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y. et al. Hippocampal long-term depression is required for the consolidation of spatial memory. Proc Natl Acad Sci U S A 107, 16697–16702 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M. et al. Reduced spatial learning in mice treated with NVP-AAM077 through down-regulating neurogenesis. Eur J Pharmacol 622, 37–44 (2009). [DOI] [PubMed] [Google Scholar]
- Walker D. L. & Davis M. Amygdala infusions of an NR2B-selective or an NR2A-preferring NMDA receptor antagonist differentially influence fear conditioning and expression in the fear-potentiated startle test. Learn Mem 15, 67–74 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. et al. Identification of network-level coding units for real-time representation of episodic experiences in the hippocampus. Proc Natl Acad Sci U S A 102, 6125–6130 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osan R., Chen G., Feng R. & Tsien J. Z. Differential consolidation and pattern reverberations within episodic cell assemblies in the mouse hippocampus. PLoS One 6, e16507 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien J. Z. The memory code. Researchers are closing in on the rules that the brain uses to lay down memories. Discovery of this memory code could lead to the design of smarter computers and robots and even to new ways to peer into the human mind. Sci Am 297, 52–59 (2007). [DOI] [PubMed] [Google Scholar]
- Chen G., Wang L. P. & Tsien J. Z. Neural population-level memory traces in the mouse hippocampus. PLoS One 4, e8256 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien J. Z. Real-time neural coding of memory. Prog Brain Res 165, 105–122 (2007). [DOI] [PubMed] [Google Scholar]
- Winocur G. & Moscovitch M. Memory transformation and systems consolidation. J Int Neuropsychol Soc 17, 766–780 (2011). [DOI] [PubMed] [Google Scholar]
- Sajikumar S., Navakkode S. & Frey J. U. Identification of compartment- and process-specific molecules required for "synaptic tagging" during long-term potentiation and long-term depression in hippocampal CA1. J Neurosci 27, 5068–5080 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez S., Ramachandran B. & Frey J. U. Properties of subsequent induction of long-term potentiation and/or depression in one synaptic input in apical dendrites of hippocampal CA1 neurons in vitro. Neuroscience 171, 712–720 (2010). [DOI] [PubMed] [Google Scholar]
- Parvez S., Ramachandran B. & Frey J. U. Functional differences between and across different regions of the apical branch of hippocampal CA1 dendrites with respect to long-term depression induction and synaptic cross-tagging. J Neurosci 30, 5118–5123 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajikumar S. & Frey J. U. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem 82, 12–25 (2004). [DOI] [PubMed] [Google Scholar]
- Frey S. & Frey J. U. ‘Synaptic tagging’ and ‘cross-tagging’ and related associative reinforcement processes of functional plasticity as the cellular basis for memory formation. Prog Brain Res 169, 117–143 (2008). [DOI] [PubMed] [Google Scholar]
- Tsumoto T. Long-term depression in cerebral cortex: a possible substrate of "forgetting" that should not be forgotten. Neurosci Res 16, 263–270 (1993). [DOI] [PubMed] [Google Scholar]
- Xu L., Anwyl R. & Rowan M. J. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature 394, 891–894 (1998). [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. & Braunewell K. H. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A 96, 8739–8744 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer J. A. & Malenka R. C. Synaptic plasticity and addiction. Nat Rev Neurosci 8, 844–858 (2007). [DOI] [PubMed] [Google Scholar]
- Giovannini M. G. et al. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience 106, 43–53 (2001). [DOI] [PubMed] [Google Scholar]
- Huerta P. T. & Lisman J. E. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron 15, 1053–1063 (1995). [DOI] [PubMed] [Google Scholar]
- Acquas E., Wilson C. & Fibiger H. C. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: effects of novelty, habituation, and fear. J Neurosci 16, 3089–3096 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Oliet S. H. & Malenka R. C. NMDA receptor-dependent and metabotropic glutamate receptor-dependent forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neurobiol Learn Mem 70, 62–72 (1998). [DOI] [PubMed] [Google Scholar]
- Brown M. W. & Xiang J. Z. Recognition memory: neuronal substrates of the judgement of prior occurrence. Prog Neurobiol 55, 149–189 (1998). [DOI] [PubMed] [Google Scholar]
- Thompson R. F. In search of memory traces. Annu Rev Psychol 56, 1–23 (2005). [DOI] [PubMed] [Google Scholar]
- Bangasser D. A., Waxler D. E., Santollo J. & Shors T. J. Trace conditioning and the hippocampus: the importance of contiguity. J Neurosci 26, 8702–8706 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M. & Mayford M. R. CaMKII activation in the entorhinal cortex disrupts previously encoded spatial memory. Neuron 50, 309–318 (2006). [DOI] [PubMed] [Google Scholar]
- Reijmers L. G., Perkins B. L., Matsuo N. & Mayford M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007). [DOI] [PubMed] [Google Scholar]
- Royer S. & Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience 115, 455–462 (2002). [DOI] [PubMed] [Google Scholar]
- Dalton G. L., Wu D. C., Wang Y. T., Floresco S. B. & Phillips A. G. NMDA GluN2A and GluN2B receptors play separate roles in the induction of LTP and LTD in the amygdala and in the acquisition and extinction of conditioned fear. Neuropharmacology 62, 797–806 (2012). [DOI] [PubMed] [Google Scholar]
- Miwa H., Fukaya M., Watabe A. M., Watanabe M. & Manabe T. Functional contributions of synaptically localized NR2B subunits of the NMDA receptor to synaptic transmission and long-term potentiation in the adult mouse CNS. J Physiol 586, 2539–2550 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T., Albrecht D. & Gebhardt C. Both NR2A and NR2B subunits of the NMDA receptor are critical for long-term potentiation and long-term depression in the lateral amygdala of horizontal slices of adult mice. Learn Mem 16, 395–405 (2009). [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M. & Sah P. Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J Neurosci 23, 6876–6883 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M. & Hardy J. The presenilins and Alzheimer's disease. Hum Mol Genet 6, 1639–1646 (1997). [DOI] [PubMed] [Google Scholar]
- Saura C. A. et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42, 23–36 (2004). [DOI] [PubMed] [Google Scholar]
- Shen J. & Kelleher R. J. 3rd. The presenilin hypothesis of Alzheimer's disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A 104, 403–409 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endele S. et al. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat Genet 42, 1021–1026 (2010). [DOI] [PubMed] [Google Scholar]
- Abumaria N. et al. Effects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdala. J Neurosci 31, 14871–14881 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. CaMKII activation state underlies synaptic labile phase of LTP and short-term memory formation. Curr Biol 18, 1546–1554 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon E. P. et al. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RI beta subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci U S A 92, 8851–8855 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]