Abstract
Stored product mites commonly occur in agricultural work environments and sometimes in homes in significant numbers. They are a source of allergens that sensitize and induce allergic reactions. This may include atopic dermatitis. The purpose of this investigation was to determine if the common species of storage mites are the sources of molecules that influence the function of human dermal microvascular endothelial cells that regulate the trafficking of inflammatory and immune cells into the dermis during allergic reactions and other skin diseases. Human dermal microvascular endothelial cells were challenged with varying doses of extracts of the storage mites Acarus siro L., Chortoglyphus arcuatus (Troupeau), Lepidoglyphus destructor (Schrank), or Tyrophagus putrescentiae (Schrank) and the secretion of cytokines and expression of adhesion molecules were measured. The role of endotoxin and protein in inducing these responses was evaluated. These stored product mite extracts induced secretion of interleukin-6, interleukin-8, monocyte chemotactic protein-1, and granulocyte/monocyte colony stimulating factor. Some of these effects were induced by protein present in the extracts, some were induced by endotoxin, and some were induced by other substances. C. arcuatus and T. putrescentiae extracts also down-regulated tumor necrosis factor α-induced vascular cell adhesion molecule-1 expression. Stored product mite extracts contain an assortment of molecules, including endotoxins and proteins, which modulate the expression of cell adhesion molecules and the secretion of cytokines by microvascular endothelial cells. These modulating properties varied among mite species indicating that each mite species has a unique set of molecules that is responsible for its activity.
Keywords: cell adhesion molecule, cytokine, endothelial cell, endotoxin, stored product mite
Allergy to the astigmatid stored product mites is common worldwide. Storage mites commonly occur in large densities in stored grains, hay, straw, and formulated livestock and companion animal feed but they also are frequently found in house dust. Thus, predisposed individuals may contact allergens from these mites in occupational settings as well as in their homes and develop sensitivities and allergic reactions to them. Unlike the phylogenetically related house dust mites that mostly feed on desquamated human skin scales in their natural environment (homes and nests of birds and mammals), stored product mites mainly feed on plant material and fungi. The common species found in agricultural settings and in homes are Tyrophagus putrescentiae (Schrank), Acarus siro L., Lepidoglyphus destructor (Schrank), Glycyphagus domesticus (De Geer), and Chortoglyphus arcuatus (Troupeau).
Stored product mites and products from them (saliva, feces, molting, and other body fluids) may contact the skin of humans in both occupational settings and in homes. The epithelial barrier of the skin may be disrupted by enzymes from mites, washing, soaps and detergents, rubbing, abrasions, chemicals, genetic skin diseases, bacteria, and fungi. This may allow molecules from these mites to penetrate the skin and reach the dermis. In addition, molecules in mite extracts cross the epidermal barrier during patch, skin prick, and intradermal skin testing.
Extracts of both house dust and storage mites contain a wide array of both allergenic and nonallergenic molecules including enzymes (Morgan and Arlian 2006; Thomas et al. 2007, 2010). Mite enzymes and their enzymatic properties may enhance penetration through the epidermis and dermis (Cork et al. 2006). For example, the house dust mite allergen, Der p 1 is a protease that increases the permeability in epithelial cells by opening tight junctions (Winton et al. 1998; Wan et al. 1999, 2000). Molecules from these mites may directly cause atopic dermatitis (AD) or inflammation of the skin and thus influence the course of allergic and nonallergic skin diseases.
Molecules from house dust and storage mites that penetrate the skin can contact keratinocytes, fibroblasts, endothelial cells of the microvasculature, various leukocytes, and antigen presenting cells of the skin. These cells may respond to these molecules by a modification of the secretion of cytokines and chemokines and the expression of cell adhesion molecules. We have shown that molecules in house dust mite extracts induce secretion of interleukin-1 receptor antagonist (IL-1ra) and growth-related oncoprotein-α (GROα) by normal epidermal keratinocytes and IL-6, IL-8, monocyte chemotactic protein-1 (MCP-1), and vascular endothelial growth factor (VEGF) from normal dermal fibroblasts (Arlian et al. 2008).
Likewise, extracts of some storage mite species can modulate cytokine secretion by epidermal keratinocytes and dermal fibroblasts (Arlian et al. 2008). A. siro and C. arcuatus extracts can reduce the constitutive secretion of IL-1ra, transforming growth factor-α(TGFα), and GROα by keratinocytes. Dermal fibroblasts stimulated with A. siro, C. arcuatus, and L. destructor increased secretion of IL-6, IL-8, and VEGF. C. arcuatus and L. destructor extracts also induced secretion of MCP-1. In contrast, A. siro and L. destructor inhibited the LPS-induced secretion of MCP-1 by fibroblasts. Likewise, low doses of extracts of these three mite species inhibited fibroblast secretion of macrophage colony-stimulating factor (M-CSF) in both the absence and presence of lipopolysaccharide (LPS).
Dermal endothelial cells of the microvasculature play a key role in inflammation and immune responses in the skin by regulating the extravasation of inflammatory and immune cells into the dermis. Expression of cell adhesion molecules and secretion of cytokines mediate the process. Extracts of the house dust mites, Dermatophagoides farinae Hughes, D. pteronyssinus (Trouessart), and Euroglyphus maynei (Cooreman) stimulate human dermal endothelial cells of the microvasculature to express intercellular and vascular cell adhesion molecules (ICAM-1 and VCAM-1) and E-selectin and to secrete IL-6, IL-8, MCP-1, and granulocyte/macrophage-colony stimulating factor (GM-CSF) (Arlian et al. 2009). The influence of stored product mites on dermal endothelial cells is unknown. The purpose of this study was to investigate the influence of storage mite extracts on the function of endothelial cells of the microvasculature of the skin.
Materials and Methods
Stored Product Mites and Extracts
The stored product mites A. siro (AS), C. arcuatus (CA), L. destructor (LD), and T. putrescentiae (TP) were all originally collected from livestock feed at a local dairy and pure cultures of each species were developed. All cultures were maintained in the laboratory at room temperature. A. siro was grown on a 9:1 (vol:vol) mixture of rodent chow (Teklad Rodent Diet 8640, Harlan Laboratories, Indianapolis, IN) and bakers’ yeast (Fleischmann’s 2192 active dry yeast, Chester-field, MO) at 75% relative humidity (RH) (Yella et al. 2011, Avula–Poola et al. 2012). C. arcuatus and L. destructor were both grown on TetraMin Tropical Flakes fish food (Tetra Holding, Inc., Blacksburg, VA) at 75% (L. destructor) or 85% (C. arcuatus) RH. T. putrescentiae was cultured at 75% RH on whole dried egg (BioServ, Frenchtown, NJ) mixed 9:1 (vol:vol) with bakers’ yeast (Avula–Poola et al. 2012). Spent culture (containing all life stages of mites, exoskeletons, eggs, egg casings, fecal material, and unconsumed food) of each species was collected, frozen to kill live mites, and lyophilized.
Dried mite material (≈5 g) was placed into a 50 ml centrifuge tube and suspended in eight volumes of saturated sodium chloride. Samples were centrifuged for 15 min at 1,200 × g and the top layer (containing mostly mite bodies) was transferred to a 45 μm sieve where it was washed with 100 volumes of MilliQ water. Washed mites were then extracted into endotoxin-free water (Lonza, Walkersville, MD) for 48 h at 43°C. The suspensions were ground in a TenBroeck homogenizer and the resultant homogenates centrifuged to remove insoluble material. The supernatants were filter-sterilized (0.22 μm) and stored in sterile vials at 4°C until use. The protein concentration of each extract was determined using the Bradford Protein Assay with bovine serum albumin standard (Bradford 1976). The endotoxin concentration of each extract was determined using a Limulus Amebocyte Lysate QCL-1000 assay kit (Lonza) according to manufacturer’s directions.
Reagents
Lipopolysaccharide (LPS), polymyxin B (PmB), and TMB ELISA Substrate were purchased from Sigma–Aldrich (St. Louis, MO). Horseradish peroxidase-conjugated streptavidin (HRP-SA) and goat anti-mouse Ig were purchased from Thermo–Fisher (Pittsburgh, PA). Biotinylated monoclonal antibodies directed against human ICAM-1 and VCAM-1 as well as recombinant TNF-α were purchased from eBioscience (San Diego, CA). The biotinylated monoclonal antibody directed against E-selectin was purchased from R&D Systems (Minneapolis, MN) as were all cytokine assay kits (Duo-Set ELISA kits) that were used according to the manufacturer’s directions.
Adult Human Microvascular Endothelial Cells
Adult human microvascular endothelial cells of dermal origin (HMVEC-D) were purchased from Lonza. Cells were cultured in Clonetics endothelial cell basal medium-2 supplemented with EGM-2-MV growth factors (Lonza) at 37°C in 5–7% CO2. Cells were plated at 5,000 cells/well and grown for 24–48 h (to 80% confluency) in 96-well culture plates (Corning, Corning, NY) for adhesion marker and cytokine studies. All cells used were between passages 8 and 11.
Stimulation of HMVEC-D Cells
HMVEC-D cells were stimulated with mite extracts alone (at 0, 25, 50, and 100 μg of protein/ml), or mite extracts plus TNFα (4 ng/ml) for 6, 12, or 24 h. In addition, to detect if the stimulatory effect of a mite extract was because of the presence of LPS in the extract, polymyxin B (50 μg/ml for 18 h at 4°C) was used to inactivate LPS in the extracts before stimulation of the HMVEC-D cells (Cardoso et al. 2007, Arlian et al. 2009). In some experiments, mite extracts were boiled for 5 min before use and denatured precipitated protein was removed by centrifugation. At the conclusion of each stimulation experiment, culture media was removed and frozen at −80°C for subsequent cytokine assays.
The expression of cellular adhesion molecules was determined as previously described (Elder et al. 2006, Arlian et al. 2009). Briefly, plates were blocked, allowed to react with biotinylated monoclonal antibody (ICAM-1, VCAM-1, or E-selectin), and detected with HRP-SA followed by TMB ELISA substrate. Reactions were stopped with addition of 1 M sulfuric acid. Absorbance was measured at 450 nm using a BioTek EL800X microplate reader.
Data Analysis
All experiments were performed at least twice with triplicate wells for each individual test. The data presented here are the average ± SEM for triplicate wells from representative experiments.
Results
Endotoxin Control
Endotoxin concentrations of mite extracts varied among species (Table 1). A. siro extract had the highest concentration of endotoxin and T. putrescentiae the lowest. The endotoxin concentration (per milligram of protein) in A. siro extract was almost 50 times greater than that of T. putrescentiae and more than twice that of C. arcuatus and L. destructor extracts. Therefore, because mite extracts contained endotoxin, as a control, endothelial cells were also stimulated with LPS alone.
Table 1.
Endotoxin content of the storage mite extracts
| Mite extract | EU per 100 μg protein |
|---|---|
| Acarus siro | 1,040 |
| Chortoglyphus arcuatus | 450 |
| Lepidoglyphus destructor | 480 |
| Tyrophagus putrescentiae | 21 |
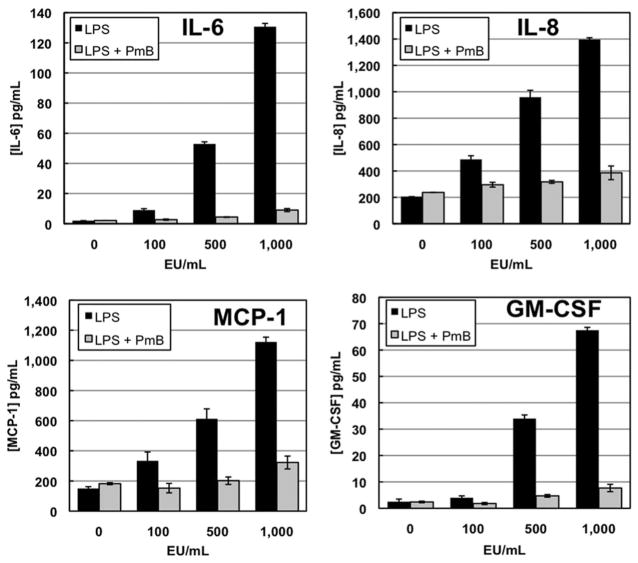
LPS in the absence of mite extracts stimulated endothelial cells to produce IL-6, IL-8, MCP-1, and GM-CSF in a dose-dependent fashion (Fig. 1). LPS activates cells by coupling with TLRs (toll-like receptors), mostly TLR4. Polymyxin B (PmB) is a cationic antibiotic that binds to LPS and prevents it from activating TLRs. In controls using only LPS at a concentration of 1,000 EU/ml (essentially equivalent to that found in A. siro extract used at 100 μg protein per ml), PmB was able to eliminate >85% of the LPS-induced cytokine secretion indicating that PmB preincubation was effective in eliminating the majority of the cytokine secretion elicited by the endogenous endotoxin in the mite extracts.
Fig.1.
Cytokine secretion by endothelial cells in response to endotoxin (LPS) addition is abrogated by pretreatment with Polymyxin B (PmB) for 18 h at 4°C. Cells were challenged for 12 h with LPS alone or in the presence of PmB.
A. siro
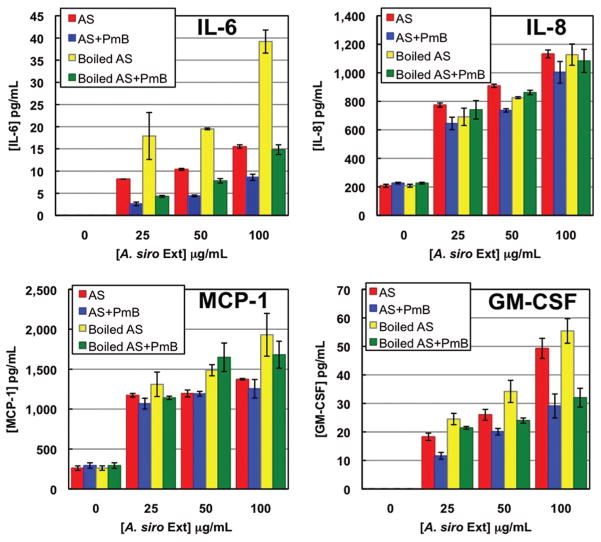
A. siro extract induced the secretion of GM-CSF, IL-6, IL-8, and MCP-1 in both a time- and dose-dependent manner (Fig. 2; data not shown). In all cases, costimulation with TNFα resulted in an additive increase in secretion. Therefore, the mite extract did not maximally stimulate the cells to secrete or release these cytokines. VEGF was secreted constitutively and its secretion was unaffected by mite, TNFα, or LPS addition (data not shown).
Fig. 2.
Cytokine secretion by endothelial cells in response to varying doses of A. siro extract. Cells were challenged for 12 h with untreated extract alone, extract that was boiled for 5 min, or extract pretreated with Polymyxin B (PmB). (Online figure in color.)
A. siro extract contains a very high level of endogenous endotoxin. Preincubation of A. siro extract with PmB before cell challenge had no effect on the levels of mite extract-induced IL-8 or MCP-1 secretion. PmB treatment did reduce the extract-induced GM-CSF secretion by 40% and IL-6 by 58% (Fig. 2).
The contribution of protein components to the extract-induced cytokine secretions was assessed by boiling the extracts before cell challenge to denature and inactivate proteins. Boiling of the A. siro extract did not alter the levels of IL-8, MCP-1, or GM-CSF secreted, suggesting that these cytokines were not secreted in response to mite proteins. Interestingly, boiling this extract before cell challenge doubled the amount of IL-6 secreted and this effect was reversed when boiled extract was also preincubated with PmB. Taken together, these data suggest that A. siro extract contains an LPS-binding protein that is denatured by boiling thereby releasing LPS that further stimulates IL-6 secretion. A. siro extract also stimulated the expression of the cell adhesion molecules E-selectin, VCAM-1, and ICAM-1. Costimulation with TNFα enhanced this expression (data not shown).
C. arcuatus and L. destructor
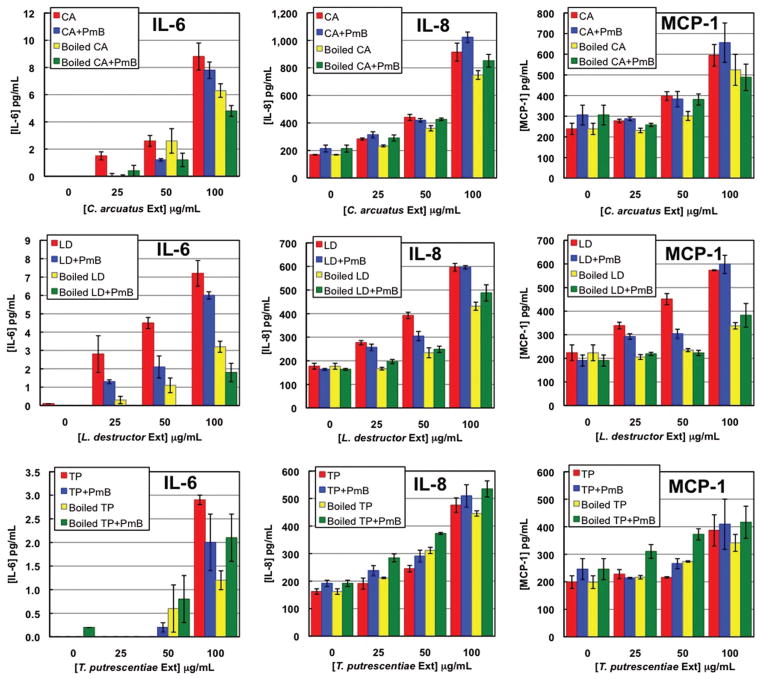
Both C. arcuatus and L. destructor extracts elicited time- and dose-dependent secretion of IL-6, IL-8, and MCP-1 and these levels were increased by TNFα costimulation (data not shown). In the absence of TNFα, neither of these mite extracts induced secretion of measurable amounts of GM-CSF.
These two mite extracts contained similar levels of endotoxin (450–480 EU/100 μg protein) that were about half that in A. siro extract. At the highest doses of extract, PmB pretreatment of the extract did not appreciably reduce the secretion of the three cytokines indicating that endogenous endotoxin was not responsible for their production (Fig. 3). When C. arcuatus extract was boiled, the amounts of IL-6 and IL-8 secreted were moderately reduced (28 and 22%, respectively), while MCP-1 levels were not significantly changed. In response to boiled L. destructor extract, endothelial cells secreted 55% less IL-6, 39% less IL-8, and 67% less MCP-1.
Fig.3.
Cytokine secretion by endothelial cells in response to varying doses of C. arcuatus, L. destructor, and T. putrescentiae extracts. Cells were challenged for 12 h with untreated extract alone, extract that was boiled for 5 min, or extract pretreated with Polymyxin B (PmB). (Online figure in color.)
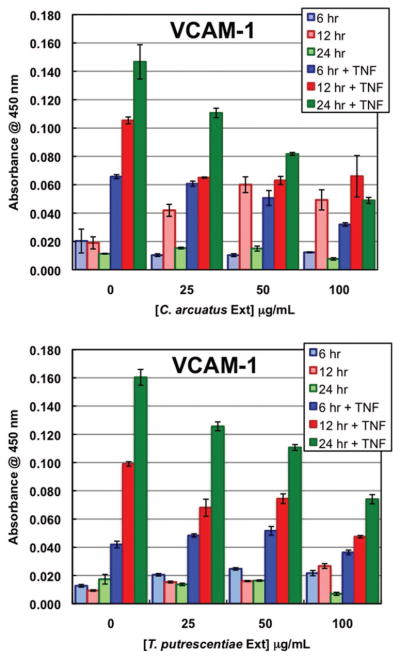
Neither of these mite extracts was able to induce the expression of E-selectin, ICAM-1, or VCAM-1. L. destructor had no effect on TNFα-induced adhesion molecule expression while C. arcuatus extract did decrease TNFα-induced VCAM-1 expression on the endothelial cell surfaces (Fig. 4). This indicates that C. arcuatus, but not L. destructor, is the source of molecules that inhibited VCAM-1 expression.
Fig. 4.
VCAM-1 expression by endothelial cells in response to varying doses of C. arcuatus and T. putrescentiae extracts. Cells were challenged for 6, 12, or 24 h with extract alone or along with TNFα. (Online figure in color.)
T. putrescentiae
IL-8 and MCP-1 were secreted in response to increasing doses of T. putrescentiae extract; the concentrations of these cytokines in the culture supernatants rose over time and this was amplified when TNFα was added as a co-stimulant (Fig. 3; data not shown). Detectable amounts of IL-6 were only produced when the highest dose (100 μg/ml) of T. putrescentiae was used and no GM-CSF was detected without TNFα stimulation.
T. putrescentiae extract had very little endogenous endotoxin (21 EU/100 μg protein) and PmB treatment did not alter the cytokine secretion indicating that endotoxin was not responsible for eliciting these responses (Fig. 3). Boiling also had no effect on IL-8 or MCP-1 secretion; its effect on IL-6 secretion is difficult to assess because the levels of this cytokine in the culture supernatant were so low.
T. putrescentiae extract was unable to stimulate adhesion molecule expression. However, like C. arcuatus extract, this extract was able to decrease TNFα-induced VCAM-1 expression (Fig. 4).
Discussion
Previous studies have shown that extracts of storage mites influence the secretion of cytokines and chemokines by epidermal keratinocytes and dermal fibroblasts of the skin (Arlian et al. 2008). This study demonstrated that extracts from stored product mites stimulated skin endothelial cells to secrete cytokines and express cell adhesion molecules but this ability varied among species. In addition, C. arcuatus and T. putrescentiae extracts contained molecules that inhibited VCAM-1 expression.
These mite extracts contained many mite-derived molecules as well as varied amounts of bacterial endotoxins (LPS). Endotoxin alone in these extracts could stimulate cells to secrete some cytokines, presumably by coupling to toll-like receptors (TLRs) on the cell surface. TLRs play a critical role in the innate immune response to endotoxins, peptidogylcans, and lipoproteins by serving as pattern-recognition receptors (Sabroe et al. 2002). Endothelial cells express TLR2 and TLR4 on their cell membranes (Faure et al. 2000, Henneke and Golenbock 2002, Fitzner et al. 2008). TLR4 is activated by endotoxins (LPS). Therefore, to differentiate between LPS stimulation and stimulation by other molecules in the extracts, we treated the extracts with polymyxin B that binds the LPS and prevents it from activating TLRs. Elimination of the effects of endogenous LPS by polymyxin B pretreatment did not reduce the ability of any of the mite extracts to stimulate IL-8 or MCP-1 cytokine secretion, suggesting that these activities were not elicited by LPS in the extracts. Conversely, PmB pre-treatment of A. siro extract resulted in a partial reduction in the ability of this extract to stimulate IL-6 and GM-CSF secretion (58 and 40%, respectively), indicating that some, but not all, of the production of these cytokines was induced by endotoxin in this extract. Thus, overall, most of the stimulating effects of these extracts cannot be attributed to the endotoxin present in them.
These mite extracts contained varied amounts of endotoxins. The source of these endotoxins is as yet unknown. These endotoxins could be from bacteria that grow on the media in the mite cultures or, as for house dust mites, they could be from endosymbiont bacteria in the bodies of the mites (Valerio et al. 2005). The mites used in this study were cultured on different media but the mites harvested from the cultures were thoroughly washed with and the extracts prepared in endotoxin-free water. This suggests that the endotoxins came from endosymbiont bacteria in the mite bodies. Bacterial DNA has been detected in whole body preparations of A. siro, C. arcuatus, D. farinae, E. maynei, L. destructor, and T. putrescentiae (Valerio et al. 2009). The endosymbiont bacteria may vary in species and/or quantity among the species of storage mites. In this respect, the relationship is similar to that for the house dust mites D. farinae and D. pteronyssinus. D. farinae contains high levels of endotoxins compared with D. pteronyssinus, even when both are grown on the same media and climatic conditions (Trivedi et al. 2003, Arlian et al. 2009, Avula–Poola et al. 2012). However, the endotoxin levels of our storage mite extracts were generally less than that of comparable house dust mite extracts (D. farinae, D. pteronyssinus, and E. maynei) (Arlian et al. 2009). This might suggest that because LPS can stimulate, there could have been a relationship between the stimulating ability of the extracts and the level of endotoxins. This was not the case. In most cases the stimulating ability of the storage mite extracts was independent of that induced by the LPS. For example, IL-8 and MCP-1 secretions were induced by all four mite extracts that contained various levels of endotoxin but these secretions were not effected by PmB preincubation demonstrating that LPS was not responsible for eliciting these cytokines. This suggests that other molecules from the mites are the inducers of these cytokines and the effects of the extracts are mediated through other receptors and signaling pathways. These molecules may include proteins, bioamines (e.g., dopamine), lipid mediators such as prostaglandins, carbohydrates, and ions such as Ca2+and Mg2+. Many bioactive molecules are found in the saliva of parasitic insects and ticks (Karim et al. 2011, Oliveira et al. 2011, Simo et al. 2011). For example, the saliva of ticks (metastigmatid mites) contains adenosine and prostaglandin E2 that can modulate cytokine secretion by dendritic cells (Oliveira et al. 2011). Dopamine is present in the salivary glands of ticks (Simo et al. 2011).
To assess the role of mite protein components in eliciting secretion of these cytokines, the mite extracts were also boiled to denature proteins in them. Cells stimulated with boiled extracts of C. arcuatus, L. destructor, or T. putrescentiae exhibited moderately (≈30–60%) reduced secretion of IL-6 but not of IL-8. This suggests that 1) different components of the extract are stimulating the cells to secrete these two cytokines; 2) IL-6 and IL-8 secretion are induced by different mechanisms; and, 3) in the case of C. arcuatus, L. destructor, and T. putrescentiae, the factor responsible for stimulating IL-6 secretion is likely a protein. Together, these results clearly show that stored product mites contain mite-derived stimulating molecules other than LPS, some of which are proteins.
Unlike the other storage mite extracts, boiling A. siro extract increased IL-6 secretion, rather than reducing it. This suggests that A. siro may contain an LPS-binding protein that binds to LPS thus decreasing the amount of IL-6 secreted in response to this extract. This protein is denatured by boiling and thus releases endotoxin that then stimulated additional IL-6 secretion.
Protease-activated receptors (PARs) are present on the surface of many cell types including endothelial cells and are activated by many proteases. House dust and storage mites are the sources of many cysteine and serine proteases, some of which are allergens (Thomas et al. 2007, 2010). The allergens Der p 1 (cysteine protease), Der p 3 (trypsin), Der p 6 (chymotrypsin), and Der p 9 (collagenolytic serine protease) can induce secretion of IL-6 and IL-8 by lung epithelial cells and this effect may be mediated by PARs (King et al. 1998, Sun et al. 2001, Reed and Kita 2004, Kauffman et al. 2006, Thomas et al. 2007). The role of mites in activating PARs on endothelial cells of the microvasculature of the skin has not been investigated. The fact that storage mite extracts induce IL-6 secretion that is diminished by boiling the extracts to denature protein suggests that proteases in these extracts could mediate some of their effects via PARs, as do house dust mites.
One other notable finding of this study was the presence of molecules able to down-modulate the TNFα-induced VCAM-1 expression in extracts of C. arcuatus and T. putrescentiae, but not in extracts of A. siro or L. destructor. We have previously demonstrated that extracts of the ectoparasitic mite Sarcoptesscabiei (De Geer) also down-regulated TNFα-induced VCAM-1 expression by endothelial cells (Elder et al. 2006). In the case of S. scabiei, we postulated that this is an adaptation that assists the parasite’s ability to survive in the host skin by depressing inflammation and the extravasation of leukocytes (Elder et al. 2006). In contrast, house dust mite extracts do not exert this effect on endothelial cell VCAM-1 expression and therefore appear not to possess these substances (Arlian et al. 2009). The implications of these observations remain unclear. However, they may suggest evolution of C. arcuatus and T. putrescentiae from a parasitic ancestral lineage.
In conclusion, this study demonstrated that stored product mite extracts contain an assortment of molecules, including endotoxins and proteins that modulate the expression of cell adhesion molecules and the secretion of cytokines by microvascular endothelial cells of the skin. These modulating properties varied among mite species indicating that each mite species has a unique set of molecules that is responsible for its activity.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health, National Institute of Allergy & Infectious Diseases (AI 017252). The authors thank DiAnn Vyszenski–Moher for technical assistance in mite culturing. Jennifer Graff Curp conducted preliminary experiments for this study.
References Cited
- Arlian LG, Elder BL, Morgan MS. House dust mite extracts activate cultured human dermal endothelial cells to express adhesion molecules and secrete cytokines. J Med Entomol. 2009;46:595–604. doi: 10.1603/033.046.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Peterson KT. House dust and storage mite extracts influence skin keratinocyte and fibroblast function. Int Arch Allergy Immunol. 2008;145:33–42. doi: 10.1159/000107464. [DOI] [PubMed] [Google Scholar]
- Avula-Poola S, Morgan MS, Arlian LG. Diet influences growth rates and allergen and endotoxin contents of cultured Dermatophagoides farinae and D. pteronyssinus house dust mites. Int Arch Allergy Immunol. 2012;159:226–234. doi: 10.1159/000336026. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cardoso LS, Araujo MI, Goes AM, Pacifico LG, Oliveira RR, Oliveira SC. Polymyxin B as inhibitor of LPS contamination of Schistosomamansoni recombinant proteins in human cytokine analysis. Microb Cell Fact. 2007;6:1. doi: 10.1186/1475-2859-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, Duff GW, Ward SJ, Tazi-Ahnini R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21. doi: 10.1016/j.jaci.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Elder BL, Arlian LG, Morgan MS. Sarcoptes scabiei (Acari: Sarcoptidae) mite extract modulates expression of cytokines and adhesion molecules by human dermal microvascular endothelial cells. J Med Entomol. 2006;43:910–915. doi: 10.1603/0022-2585(2006)43[910:ssasme]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- Fitzner N, Clauberg S, Essmann F, Liebmann J, Kolb-Bachofen V. Human skin endothelial cells can express all 10 TLR genes and respond to respective ligands. Clin Vaccine Immunol. 2008;15:138–146. doi: 10.1128/CVI.00257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneke P, Golenbock DT. Innate immune recognition of lipopolysaccharide by endothelial cells. Crit Care Med. 2002;30:S207–S213. doi: 10.1097/00003246-200205001-00006. [DOI] [PubMed] [Google Scholar]
- Karim S, Singh P, Ribeiro JM. A Deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS One. 2011;6:e28525. doi: 10.1371/journal.pone.0028525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman HF, Tamm M, Timmerman JA, Borger P. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin Mol Allergy. 2006;4:5. doi: 10.1186/1476-7961-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Brennan S, Thompson PJ, Stewart GA. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J Immunol. 1998;161:3645–3651. [PubMed] [Google Scholar]
- Morgan MS, Arlian LG. Enzymatic activity in extracts of allergy-causing astigmatid mites. J Med Entomol. 2006;43:1200–1207. doi: 10.1603/0022-2585(2006)43[1200:eaieoa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Oliveira CJ, Sa-Nunes A, Francischetti IM, Carregaro V, Anatriello E, Silva JS, Santos IK, Ribeiro JM, Ferreira BR. Deconstructing tick saliva: non-protein molecules with potent immunomodulatory properties. J Biol Chem. 2011;286:10960–10969. doi: 10.1074/jbc.M110.205047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Sabroe I, Parker LC, Wilson AG, Whyte MK, Dower SK. Toll-like receptors: their role in allergy and non-allergic inflammatory disease. Clin Exp Allergy. 2002;32:984–989. doi: 10.1046/j.1365-2745.2002.01451.x. [DOI] [PubMed] [Google Scholar]
- Simo L, Koci J, Zitnan D, Park Y. Evidence for D1 dopamine receptor activation by a paracrine signal of dopamine in tick salivary glands. PLoS One. 2011;6:e16158. doi: 10.1371/journal.pone.0016158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol. 2001;167:1014–1021. doi: 10.4049/jimmunol.167.2.1014. [DOI] [PubMed] [Google Scholar]
- Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med. 2010;16:321–328. doi: 10.1016/j.molmed.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Thomas WR, Heinrich TK, Smith WA, Hales BJ. Pyroglyphid house dust mite allergens. Protein Pept Lett. 2007;14:943–953. doi: 10.2174/092986607782541169. [DOI] [PubMed] [Google Scholar]
- Trivedi B, Valerio C, Slater JE. Endotoxin content of standardized allergen vaccines. J Allergy Clin Immunol. 2003;111:777–783. doi: 10.1067/mai.2003.1338. [DOI] [PubMed] [Google Scholar]
- Valerio C, Arlian LG, Slater JE. Bacterial DNA sequences isolated from standardized dust mite extracts and wild mites. J Allergy Clin Immunol. 2009;123:S216. [Google Scholar]
- Valerio CR, Murray P, Arlian LG, Slater JE. Bacterial 16S ribosomal DNA in house dust mite cultures. J Allergy Clin Immunol. 2005;116:1296–1300. doi: 10.1016/j.jaci.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Wan H, Winton HL, Soeller C, Gruenert DC, Thompson PJ, Cannell MB, Stewart GA, Garrod DR, Robinson C. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1. Clin Exp Allergy. 2000;30:685–698. doi: 10.1046/j.1365-2222.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, Robinson C. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton HL, Wan H, Cannell MB, Thompson PJ, Garrod DR, Stewart GA, Robinson C. Class specific inhibition of house dust mite proteinases which cleave cell adhesion, induce cell death and which increase the permeability of lung epithelium. Br J Pharmacol. 1998;124:1048–1059. doi: 10.1038/sj.bjp.0701905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yella L, Morgan MS, Arlian LG. Population growth and allergen accumulation of Dermatophagoides pteronyssinus cultured at 20 and 25 degrees C. Exp Appl Acarol. 2011;53:103–119. doi: 10.1007/s10493-010-9394-4. [DOI] [PubMed] [Google Scholar]