Abstract
Purpose
To evaluate outcomes of the Boston Type 1 keratoprosthesis (KPro) and associated incidence of glaucoma.
Design
Retrospective cohort study.
Participants
All patients who underwent KPro surgery at one institution from 2003-2009 with at least 3 months follow-up.
Methods
Preoperative visual acuity, diagnosis, history of glaucoma, intraoperative and postoperative parameters were recorded. Statistical analysis was performed to identify factors that may influence increase in intraocular pressure (IOP) and glaucoma development or progression after surgery.
Main Outcome Measures
Best corrected visual acuity (BCVA), IOP, postoperative medical and surgical treatments for glaucoma, and KPro retention and complications.
Results
Thirty-six KPro procedures were performed in 30 eyes of 29 patients with mean (± SD) follow-up of 17±19 months (range 3-67 months). The main indication for KPro implantation was corneal graft failure (77%). Primary KPros were performed in 23% of eyes for limbal stem cell deficiency secondary to chemical burns and aniridia, and for herpetic disease. Median preoperative BCVA was hand motions with an overall improvement to 20/330 (range 20/20 to hand motions) at nine months postoperatively; mean BCVA was 20/600 (range 20/40 to NLP) at last follow-up. Twenty eyes (67%) had a preoperative history of glaucoma with eight of those eyes (40%) having undergone prior glaucoma surgery. Twenty-one eyes (70%) underwent concomitant glaucoma surgery. Postoperative increased IOP (22 mmHg or higher) was noted in 15 eyes (50%) while definite glaucoma development or progression was noted in 7 of those 15 eyes (23% of total eyes). Mean BCVA at last follow-up in eyes with glaucoma development or progression was 3/200 compared to 20/563 in the remaining 23 eyes. Six patients (20%) required repeat KPro implantation, and retroprosthetic membranes developed in 23 eyes (77%). No patient developed vitritis or infectious endophthalmitis.
Conclusions
The Boston Type 1 KPro is an effective option for management of eyes with poor prognosis for primary or repeat penetrating keratoplasty. Visual potential is limited by preoperative comorbidities; however, glaucoma development or progression of pre-existing glaucoma is a significant cause of postoperative visual loss. Rigorous perioperative management of elevated IOP is essential for long-term success of KPro surgery.
Keywords: Boston Type 1 Keratoprosthesis, Glaucoma, Keratoplasty
The Boston Type I keratoprosthesis (KPro), approved for use by the FDA in 1992, was developed for patients who are poor candidates for traditional full-thickness corneal transplantation because of a high risk for graft failure or rejection. The indications for KPro implantation include recurrent corneal graft failure, herpetic disease and limbal stem cell deficiency secondary to chemical burns and aniridia.
Initial KPro implantation was associated with poor outcomes related to implant extrusion, corneal necrosis and endophthalmitis.1 As modifications in the implant design and postoperative management have evolved, the retention rates of the Boston Type I KPro have dramatically improved, making the procedure an increasingly accepted alternative to standard keratoplasty in eyes with an unacceptably high risk of corneal graft failure.2 With a reduction in acute complications, the long term visual outcomes after KPro surgery are still limited by chronic diseases, such as glaucoma.
The purpose of this study was to review the clinical experience at one institution using the Boston Type I KPro and to evaluate the development and progression of glaucoma following KPro surgery in these patients.
Methods
A retrospective chart review was conducted of all KPro surgeries performed between October 2003 and June 2009 at the W.K. Kellogg Eye Center, University of Michigan by a single surgeon (S.I.M.) with a minimum follow-up of 3 months. Patients with less than 3 month follow-up were excluded to improve assessment of subacute and chronic change in intraocular pressure after surgery. Study approval was granted by the Institutional Review Board.
The Boston Type I Keratoprosthesis, obtained from the Massachusetts Eye and Ear Infirmary (Boston, MA), was used in all eyes. Inclusion criteria for surgery included vision worse or equal to 20/200, a reasonable blink or tear film, a history of two or more penetrating keratoplasty failures or poor candidacy for keratolimbal allograft surgery, no evidence of significant retinal disease or end-stage optic nerve cupping, and intact nasal light projection. Patients with less than three months of follow up were excluded from the study.
Patients were evaluated preoperatively by a member of the glaucoma service faculty (J.S.W., J.D.S., S.E.M., D.J) to assess need for preoperative or concomitant glaucoma surgery, which included endoscopic or transscleral diode cyclophotocoagulation, Ahmed FP-7 glaucoma drainage device (New World Medical, Rancho Cucamonga, CA) implantation, and 350 mm2 Baerveldt glaucoma drainage device (Advanced Medical Optics Inc, Santa Ana, CA) implantation.
All phakic patients underwent extracapsular cataract extraction at the time of the KPro procedure. Pars plana vitrectomy was performed when the glaucoma drainage device was placed in the pars plana. Aphakic KPros were used in eyes with crystalline lens extraction, anterior chamber intraocular lens implant removal and preoperative aphakia. Pseudophakic KPros were used in eyes with stable, previously implanted posterior chamber intraocular lenses.
KPro implantation was performed using a standard technique.3 A Kontur soft contact lens (Kontur Contact Lens, Richmond, CA) was placed either at the end of surgery or on the first postoperative day. Postoperatively, all patients were started on prednisolone acetate 1%, topical vancomycin (25mg/mL), and either topical moxifloxacin 0.5% or gatifloxacin 0.3% four times a day each in the operated eye. The vancomycin was continued for at least 6 months postoperatively. Topical or oral glaucoma medications were added or additional glaucoma surgery was performed based on clinical findings of increased intraocular pressure (IOP) by digital palpation (when compared to the IOP in the contralateral eye which was assessed by Goldmann applanation), optic nerve head appearance, or worsening visual fields (Humphrey [Carl Zeiss Ophthalmic Systems, Dublin, CA] or Goldmann).
Data collected regarding preoperative characteristics included demographics, indication for surgery, previous ocular surgeries, preoperative ocular and medical comorbidities such as glaucoma, diabetes mellitus and hypertension, smoking status, best-corrected visual acuity, IOP, and if available, optic nerve status. Intraoperative characteristics recorded included surgery time, type of keratoprosthesis (aphakic vs. pseudophakic and threadless vs. threaded), donor and host trephine size, front and back plate size, concomitant surgeries at time of KPro implantation, and intraoperative complications. Postoperative data included best-corrected visual acuity, tactile IOP, optic nerve appearance, retinal evaluation, topical and oral medications, anatomic retention of the KPro, and postoperative surgeries or procedures. When IOP was recorded as a range in the chart (e.g., 10-15 mm Hg), the median value of the range was used for statistical analysis. Data were collected postoperatively at intervals of 1 day, 1 week, 1 month, 3 months, 6 months, 9 months, 1 year, and then annually.
Elevated IOP was defined as 22 mmHg or higher, when measured by digital palpation, which required the addition of topical or oral glaucoma therapy. Glaucoma development or progression of pre-existing disease was defined as elevated IOP associated with glaucomatous optic nerve head changes, glaucomatous visual field changes, or need for subsequent glaucoma surgical intervention.
Descriptive and comparative analyses of the data were performed using SAS 9.2 statistical software (SAS Institute, Cary, NC). Comparisons of mean values made use of the independent, two-tailed t-test, and comparisons of categorical data made use of Fisher’s exact test. For all analyses, p-value ≤ to 0.05 was considered statistically significant.
Results
Thirty-three eyes of 32 patients underwent Boston Type I KPro surgery between October 2003 and June 2009. Three of these eyes were excluded from the analysis in this study due to follow-up of less than three months. Mean (± SD) follow-up was 17 ± 19 months (range: 3-67 months). There were 16 males (55%) and 13 females (45%). The KPros were implanted in 11 right eyes (37%) and 19 left eyes (63%). The mean age at surgery was 59 years (range: 21-88 years; SD: 20 years). Six eyes (20%) underwent repeat KPro implantation.
Twenty-three eyes (77%) had a history of failed penetrating keratoplasty (PKP) with an average of 2.2 prior PKPs per eye (range 1-5; SD: 1.2). Seven eyes (23%) underwent KPro surgery without prior PKP. Four of these eyes had chemical burns, two had aniridia, and one had a history of herpetic keratitis. (Table 1)
Table 1.
Preoperative corneal diagnoses of eyes undergoing Boston Type 1 KPro implantation.
| Preoperative diagnosis | Number of eyes (N = 30) | Percentage of eyes |
|---|---|---|
| Failed corneal transplant | 23 | 77% |
| Bullous keratopathy | 5 | 17% |
| Herpetic keratitis | 5 | 17% |
| Aniridia | 4 | 13% |
| Chemical burn | 3 | 10% |
| Fuchs’ dystrophy | 2 | 7% |
| Other * (Mooren ulcer, acanthamoeba keratitis, fungal keratitis, chronic uveitis) | 4 | 13% |
| No prior corneal transplantation | 7 | 23% |
| Chemical burn | 4 | 13% |
| Aniridia | 2 | 7% |
| Herpetic keratitis | 1 | 3% |
The median preoperative best-corrected visual acuity was hand motions (range: 20/200 to light perception). Three eyes (10%) were light perception with projection, 16 eyes (53%) were hand motions, 8 eyes (27%) were count fingers, one eye (3%) was 20/400 and two eyes (7%) were 20/200.
Preoperatively, 20 eyes (67%) had a previous diagnosis of glaucoma with eight of the 20 eyes (40%) having undergone incisional glaucoma surgery prior to KPro implantation, including three with glaucoma drainage devices, three with transscleral diode cyclophotocoagulation, and two with a trabeculectomy. Prior to KPro implantation, the average number of glaucoma medications (topical or oral) in these 20 patients was 2.4 (range 0-5; SD 1.1). The mean preoperative IOP for all 30 patients was 15.8 mmHg (range 5-35; SD 6.8). The mean IOP for eyes with a previous diagnosis of glaucoma was 17.0 mmHg (range 5-35; SD 7.5), and for eyes without a diagnosis of glaucoma, it was 13.5 mmHg (range 7-21; SD 4.4). The mean cup to disc ratio was 0.55 (range 0.4 to 0.9; SD 0.24) in the four patients that could be assessed preoperatively.
Of the 30 eyes that underwent KPro implantation, 22 (73%) received an aphakic KPro and 8 (27%) received a pseudophakic KPro. Twenty-one (70%) eyes underwent concomitant glaucoma surgery, which included glaucoma drainage device implantation in 14 eyes (six Ahmed and eight Baerveldt glaucoma drainage devices) and endocyclophotocoagulation in seven eyes. Seven patients (23%) underwent concomitant cataract extraction and six patients (20%) required pseudophakic intraocular lens removal. Three of the 14 eyes (21%) with concomitant glaucoma drainage device implantation also underwent concomitant pars plana vitrectomy in order to facilitate placement of the glaucoma drainage device tube into the vitreous cavity. Three of the 11 eyes (27%) that had concomitant glaucoma drainage device implantation with insertion of the tube into the anterior chamber were noted to have occluded tubes postoperatively necessitated subsequent pars plana vitrectomy and repositioning of the tube into the vitreous cavity.
The mean best corrected visual acuity (BCVA) postoperatively was 20/510 (range: 20/20 to no light perception, N=30) at three months, 20/550 (range: 20/20 to no light perception, N=23) at 6 months, 20/330 at nine months (range 20/20 to hand motions, N=18), and 20/390 at one year (range 20/40 to light perception, N=16). The BCVA at last follow-up for all patients was 20/600 (range: 20/40 to no light perception). When comparing preoperative BCVA to final BCVA, vision improved in 19 out of 30 eyes (63%) and was unchanged in seven eyes (23%). Vision worsened in four eyes (13%) due to glaucoma in three eyes, two of which became no light perception, and retinal detachment in one eye. (Figure 1)
Figure 1.
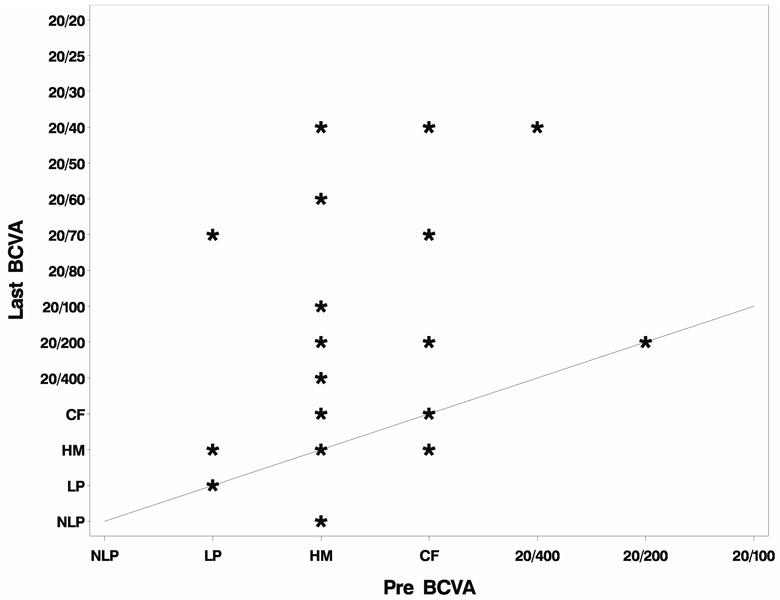
Preoperative and postoperative visual acuity with Boston Type 1 Keratoprosthesis implantation.
Postoperative elevation in IOP (22 mmHg or higher) which required the addition of topical or oral glaucoma medications was noted in 15 eyes (50%). These 15 eyes had a mean preoperative IOP of 13.7 mmHg, a mean postoperative IOP of 29.5 mmHg (range 22.5-35; SD 4.0) prior to initiation of treatment, and a mean postoperative IOP at last follow-up of 9.5 mmHg after addition of glaucoma therapy. Here was a trend toward an increase in the number of glaucoma medications postoperatively compared to preoperatively (1.8 postoperative when compared to 1.1 preoperative). In the 15 eyes that did not develop elevated IOP, the mean preoperative IOP was 18.0 mmHg and the mean postoperative IOP at last follow-up was 12.8 mmHg. There was also a trend towards a decrease in the number of glaucoma medications postoperatively as compared to preoperatively (0.7 postoperative when compared to 2.1 preoperative). In the 15 eyes that developed postoperative elevated IOP, 14 (93%) had no prior glaucoma surgery and one had prior transscleral diode cyclophotocoagulation, whereas 7 (47%) of the 15 eyes that did not have elevated IOP postoperatively had glaucoma surgery prior to KPro implantation (p=0.04). (Table 2)
Table 2.
Descriptive statistics by event status of IOP increase (n=30)
| No IOP increase (n=15) | IOP increase (n=15) | No Glaucoma development/progression(n=23) | Glaucoma development/progression (n=7) | |||
|---|---|---|---|---|---|---|
| Continuous Variables | Mean (SD) | Mean (SD) | P-value* | Mean (SD) | Mean (SD) | P-value* |
|
| ||||||
| Number of glaucoma meds, preoperatively | 2.07 (1.4) | 1.13 (1.4) | 0.0780 | 1.61 (1.4) | 1.57 (1.7) | 0.9538 |
| Number of glaucoma meds, postoperatively | 0.73 (1.1) | 1.80 (0.8) | 0.0047 | 1.17 (1.2) | 1.57 (0.8) | 0.4036 |
| Preoperative IOP | 13.67 (5.9) | 18.00 (7.1) | 0.0798 | 14.26 (5.4) | 21.00 (8.7) | 0.0185 |
| Final IOP | 9.50 (3.2) | 12.83 (4.4) | 0.0246 | 10.8 (3.9) | 12.50 (5.0) | 0.3391 |
| Categorical Variables | Frequency (Column %) | Frequency (Column %) | P-value** | Frequency (Column %) | Frequency (Column %) | P-value** |
|
|
||||||
| Glaucoma History | ||||||
| No | 3 (20.0) | 7 (46.7) | 0.2451 | 8 (34.8) | 2 (28.6) | 1.0000 |
| Yes | 12 (80.0) | 8 (53.3) | 15 (65.2) | 5 (71.4) | ||
| Glaucoma Surgery (Preoperative) | ||||||
| No | 8 (53.3) | 14 (93.3) | 0.0352 | 16 (69.6) | 6 (85.7) | 0.6378 |
| Yes | 7 (46.7) | 1 (6.7) | 7 (30.4) | 1 (14.3) | ||
| Concomitant Glaucoma surgery with KPro implant | ||||||
| None | 7 (46.7) | 2 (13.3) | 0.1404 | 7 (30.4) | 2 (28.6) | 0.4985 |
| Ahmed | 2 (13.3) | 4 (26.7) | 6 (26.1) | 0 (0.0) | ||
| Baerveldt | 2 (13.3) | 6 (40.0) | 5 (21.7) | 3 (42.9) | ||
| Endoscopic cyclophotocoagulation | 4 (26.7) | 3 (20.0) | 5 (21.7) | 2 (28.6) | ||
| Glaucoma Surgery (Postoperative) | ||||||
| No | 15 (100.0) | 7 (46.7) | 0.0022 | 21 (91.3) | 1 (14.3) | 0.0003 |
| Yes | 0 (0.0) | 8 (53.3) | 2 (8.7) | 6 (85.71) | ||
t-test
Fisher’s exact test
Glaucoma development or progression was noted in seven eyes (23% of total eyes) of the 15 eyes that had postoperative elevated IOP. Glaucoma developed in two of 10 eyes without a previous history of glaucoma (20%) and progressed in five of the 20 eyes with a previous diagnosis of glaucoma (25%). Of the seven eyes with glaucoma development or progression, only one had glaucoma surgery prior to KPro implantation. Five of these seven eyes, including the one with prior glaucoma surgery, underwent concomitant glaucoma surgery. Postoperatively, six of these seven eyes required further surgical intervention. This included a Baerveldt drainage device implantation in an eye that had no prior or concomitant glaucoma surgery, transscleral diode cyclophotocoagulation in two eyes, and pars plana vitrectomy with tube revision to the pars plana in three eyes. The mean IOP at last follow-up in the seven eyes that developed glaucoma or had glaucomatous progression was 21.0 mmHg compared to 14.3 mmHg in eyes that did not develop glaucoma. (Table 2) The mean visual acuity at last follow-up of those that had glaucoma development or progression was 3/200 when compared to 20/563 in the remaining 23 eyes (p=0.51). All seven eyes were noted to have glaucomatous optic nerve head changes during their postoperative course. The mean cup to disc ratio in these seven eyes was 0.83 (range 0.5 to 1.0; SD 0.19) at either the one month or three months visit and 0.93 (range 0.7 to 1.0; SD 0.11) at one year postoperatively. There was no statistically significant difference in preoperative corneal diagnoses between the eyes that developed glaucoma or an elevation in IOP when compared to eyes without elevation in IOP or glaucoma development or progression.
Complications
No significant intraoperative complications occurred. In one eye, a limited suprachoroidal hemorrhage developed postoperatively within a week of surgery and spontaneously cleared over the next 2 months. Two patients (7%) required retinal detachment repair, and no patients developed vitritis or infectious endophthalmitis. Six patients (20%) required repeat KPro implantation. KPros without titanium locking rings had been placed in the first two eyes and were subsequently replaced within one to four months in both cases due to dislocation. Corneal stromal necrosis developed in four eyes (13%), requiring replacement of the KPros four to 12 months after initial surgery. All 4 patients had inflammatory corneal disease with a history of chemical burn (3/4) and Mooren’s ulcer (1/4). Retroprosthetic membranes (RPM) developed postoperatively in 23 eyes (77%). Fourteen of these 23 eyes (61%) underwent Nd:YAG laser membranotomy, and one (4%) underwent pars plana pupillary membranectomy. The following cases illustrate development and progression of glaucoma after Boston Type I KPro surgery.
Case 1
N.M. was a 25-year-old female with an ocular history significant for aniridia and poorly controlled secondary glaucoma of both eyes. At presentation, her visual acuity was hand motions in the right eye and 20/200 in the left eye. On exam, she had significant bilateral corneal neovascularization with stromal haze and scarring. Her IOP was 31 mmHg in both eyes despite treatment with topical latanoprost 0.05%, brimonidine 0.2%, and timolol 0.5%, as well as oral methazolamide 100 mg BID. There was no view of the fundus of the left eye, and visual fields could not be obtained due to her poor vision. In January 2009, she underwent combined KPro with Baerveldt glaucoma drainage device implantation in the right eye. During the Baerveldt portion of the surgery, fenestration slits were created in the tube to allow for some immediate pressure-lowering effect. On postoperative day one, her acuity remained hand motions and her IOP was 20-25 mmHg. All of her preoperative glaucoma medications were continued. Four days later, she was seen emergently for pain and decreased vision. At that time, her vision was no light perception with an IOP in the 30’s. At this point, the occluding stent suture of the Baerveldt implant was pulled to open the tube, and this ultimately lowered the IOP to 15-20 mmHg. Despite this, her vision remained no light perception.
Case 2
H.F. was a 74-year-old female with an ocular history significant for pseudophakic bullous keratopathy of the right eye status-post two failed full-thickness penetrating keratoplasties performed in 1999 and 2003. Because of her poor prognosis for a third PKP, a decision was made to implant a Boston Type I KPro. She had no preoperative history of glaucoma. On exam, her BCVA was count fingers. Her preoperative intraocular pressure was 13mmHg, and she had a healthy-appearing optic nerve with a cup/disc ratio of 0.4. She underwent uncomplicated KPro implantation with concomitant anterior chamber intraocular lens removal in December 2005. Postoperatively, her acuity improved to 20/25 three months after surgery and remained stable for over a year. Her IOP ranged between 15-20 mmHg, and her cup/disc ratio ranged between 0.4-0.5. On a routine follow-up visit in November 2006, she was noted to have an IOP between 20 to 25 mmHg. Her acuity remained 20/25 and cup/disc ratio was 0.5. She was started on timolol 0.5%. Three months later her vision had dropped to 20/70, the IOP was in the 30’s, and her optic nerve was noted to have significantly increased cupping. She underwent urgent pars plana vitrectomy with insertion of a Baerveldt glaucoma drainage device in February 2007. Postoperatively, her vision stabilized at 20/70 with IOP ranging between 10-15 mmHg on timolol 0.5%. At this point her cup/disc ratio was greater than 0.9. Despite IOP in the low teens with a functional Baerveldt drainage device and close follow-up by both the cornea and glaucoma services over the next year, her visual acuity dropped from 20/80 to hand motions by April 2008 as a result of glaucomatous progression. Her vision has remained unchanged as of January 2010.
Discussion
Our results indicate that patients undergoing KPro surgery with adequate control of IOP through either medical or surgical intervention during the perioperative period fared better than those without. In patients with a preoperative diagnosis of glaucoma and aggressive preoperative IOP control who underwent KPro implantation, the mean postoperative IOP was lower and there were fewer glaucoma medications added postoperatively. The eyes that had prior glaucoma surgery were less likely to experience glaucoma progression or elevated IOP after KPro implantation when compared to those that did not.
Glaucoma is already a prevalent disease in this patient population and is likely multifactorial in pathogenesis. In eyes with chemical burns, the prevalence of glaucoma has been reported to be between 22-55%. 4,5 In aniridic eyes, glaucoma incidence has been reported between 6-75% with a prevalence as high as 91%.5,6 In eyes that have undergone standard penetrating keratoplasty, the incidence of glaucoma has been reported to vary between 9-31% in the early postoperative period and from 18-35% in the late postoperative period.7
Glaucoma management is especially challenging in eyes that have undergone KPro implantation for several reasons. First, evaluations of the optic nerve and visual fields are usually difficult preoperatively due to corneal opacification. In addition, tonometers are inaccurate over a Boston KPro, and digital palpation is currently the closest available assesment of IOP.2 There is significant need for methods to be developed to accurately measure IOP after KPro implantation. Another challenge in monitoring these patients is that they often have comorbid eye diseases, including advanced glaucoma, retinal detachment, and macular ischemia, which limit their ability to perform visual field testing.
According to our results, KPro implantation alone can increase the risk for glaucoma development or progression. We postulate that the mechanism of increased IOP in KPro eyes is multifactorial, including gradual closure of the angle from peripheral anterior synechiae, abrupt blockage of the angle from the KPro device itself, inflammation, bleeding and vitreous debris.8 Netland et al. reported performing a total iridectomy during KPro implantation in an attempt to prevent secondary angle closure glaucoma; however, the prevalence of glaucoma postoperatively was still 58% in that series, indicating that other mechanisms likely played a role in glaucoma development.1
One complication observed in our patient population that underwent combined surgery with a glaucoma drainage device implanted into the anterior chamber was unintentional occlusion of the tube, which required tube revision surgeries in five eyes. If the tube is placed in the anterior chamber, retaining a stable previously placed posterior chamber intraocular lens implant may help prevent vitreous from occluding the tube. Ciliary sulcus placement of a tube shunt is difficult due to presence of a unicameral chamber or anterior chamber crowding in pseudophakic eyes with the KPro implant design (anterior-posterior stem length of 2.7mm). Pars plana tube insertion into the vitreous cavity may be a viable alternative that can offer potential advantages over anterior chamber insertion. Though it requires prior or concurrent pars plana vitrectomy, a pars plana tube insertion can avoid occlusion secondary to anterior segment crowding or to vitreous and iris plugging. If pars plana vitrectomy and glaucoma drainage device placement are performed subsequent to KPro implantation, the vitrectomy also offers the ability to clear any vitreous opacities or retroprosthetic membranes that may be obstructing vision.9
Another challenge in management of patients undergoing KPro implantation is determining which eyes are at risk for an acute elevation of IOP, and which are at risk for late development of glaucoma, as exemplified in cases #1 and #2. Therefore, aggressive control of IOP is warranted including surgical intervention in all patients undergoing KPro surgery. Although larger non-valved glaucoma drainage devices may offer better long-term IOP control than valved devices, they require intentional occlusion for several weeks postoperatively in order to avoid early hypotony.10 Thus, patients may be at risk for glaucoma progression in the immediate postoperative period if their IOP cannot be controlled by medical management or venting slits while the tube is still intentionally occluded. Careful assessment of a patient’s glaucoma status preoperatively is imperative. Eyes at risk of developing vision loss from increased IOP during the immediate postoperative period may require use of a flow-restrictive device or placement of a glaucoma drainage device prior to KPro implantation. Endoscopic or transcleral diode cylcophotocoagulation may be preferred in the setting of conjunctival cicatricial disease or when previously placed glaucoma drainage devices are not adequately functioning.
A significant complication in our study that affected postoperative visual acuity results was retroprosthetic membrane formation. Twenty-three eyes (77%) were found to have retroprosthetic membranes, which is higher than previously reported rates of 26-65%.2, 11, 12, 13 This may be associated with predisposing chronic inflammatory conditions in these eyes. There were no cases of endophthalmitis or sterile vitritis compared to previous reports of 0-11% and 0-10% respectively. This may be due to the use of topical antibiotic therapy with both vancomycin and a fluoroquinolone for a period of at least six months. (Table 3)
Table 3.
Postoperative complications
| Postoperative Complications | Multicenter Study1 | UCLA11 | UC Davis12 | Wills Eye13 | U of Michigan |
|---|---|---|---|---|---|
| # of eyes | 136 | 50 | 30 | 36 | 30 |
| Age range | N/A | 21-95 yrs | 3-87 yrs | 24-93 yrs | 21-88 yrs |
| Mean follow-up | 8.5 mos | 18 mos | 19 mos | 16 mos | 17 mos |
| Retroprosthetic membrane | 26% | 44% | 43% | 65% | 77% |
| Corneal melt | <1% | 16% | 17% | 0% | 13% |
| Retinal detachment | 3.5% | 2% | 0% | 0% | 7% |
| Choroidal detachment | 3.5% | 2% | 7% | 3% | 7% |
| Endophthalmitis | 0% | 0% | 10% | 11% | 0% |
| Sterile vitritis | 5% | 10% | 10% | 0% | 0% |
| IOP increase | 15% | 18% | 27% | 38% | 50% |
| Glaucoma development/progression | 8% | 2% | 7% | 13.5% | 23% |
Although previously reported rates have been low (2-13.5%), our series indicates that postoperative glaucoma is a significant disease to contend with in this patient population. 2, 11, 12, 13(Table 3) Some of the limitations of our study include its retroprospective design, small sample size, limited follow-up for some eyes, reliance on digital palpation to measure IOP, and difficulty assessing optic nerve status and visual fields due to media opacities.
As implantation of the Boston Type I KPro becomes a more widely accepted alternative to standard keratoplasty surgery in high-risk patients due to improved outcomes and retention rates, glaucoma may be increasingly seen as a significant cause of visual loss including blindness. Aggressive perioperative management of glaucoma, including preoperative and concomitant glaucoma surgery, may help prevent the development or progression of glaucoma and improve visual outcomes in these challenging patients.
Acknowledgments
Supported in part by a departmental grant from Research to Prevent Blindness (RPB), the RPB Lew R. Wasserman Merit Award (Dr. D. Musch), and the National Eye Institute K23 Mentored Clinician Scientist Award (1K23EY019511-01, Dr. J. Stein).
The authors would like to acknowledge the contributions of Akiko Fukimoto, MD, Leslie M. Niziol, MS, Sasha Dutta, BS and Fareed Riyaz, BS.
Footnotes
The authors have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology. 1998 Apr;105(4):751–7. doi: 10.1016/S0161-6420(98)94034-9. [DOI] [PubMed] [Google Scholar]
- 2.Zerbe BL, Belin MW, MD, Ciolino JB. Results from the multicenter Boston type 1 keratoprosthesis study. Ophthalmology. 2006;113:1779–1784. doi: 10.1016/j.ophtha.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Dohlman CH, Abad JC, Dudenhoefer EJ, Graney JM. Keratoprosthesis: beyond corneal graft failure. In: Spaeth GL, editor. Ophthalmic Surgery: Principles and Practice. 3. Philadelphia: W.B. Saunders; 2002. pp. 199–207. [Google Scholar]
- 4.Tsai JH, Derby E, Holland EJ, Khatana AK. Incidence and prevalence of glaucoma in severe ocular surface disease. Cornea. 2006 Jun;25(5):530–2. doi: 10.1097/01.ico.0000220776.93852.d9. [DOI] [PubMed] [Google Scholar]
- 5.Kuckelkorn R, Kottek A, Reim M. Klin Monatsbl Augenheilkd. 2. Vol. 205. German: 1994. Aug, Intraocular complications after severe chemical burns--incidence and surgical treatment; pp. 86–92. [DOI] [PubMed] [Google Scholar]
- 6.Nelson LB, Spaeth GL, Nowinski, Margo CE, Jackson L. Aniridia. A review. Surv Ophthalmol. 1984;28:621–642. doi: 10.1016/0039-6257(84)90184-x. [DOI] [PubMed] [Google Scholar]
- 7.Ayyala RS. Penetrating keratoplasty and glaucoma. Surv Ophthalmol. 2000;45:91–105. doi: 10.1016/s0039-6257(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 8.Khan BF, Harissi-Dagher M, Khan DM, Dohlman CH. Advances in Boston keratoprosthesis: enhancing retention and prevention of infection and inflammation. Int Ophthalmol Clin. 2007;47(2):61–71. doi: 10.1097/IIO.0b013e318036bd8b. [DOI] [PubMed] [Google Scholar]
- 9.Ritterband DC, Shapiro D, Trubnik V, Marmor M, Meskin S, Seedor J, Liebmann JM, Tello C, Koplin R, Harizman N, Shabto U, Ritch R. Penetrating keratoplasty with pars plana glaucoma drainage devices. Cornea Glaucoma Implant Study Group (COGIS) Cornea. 2007 Oct;26(9):1060–6. doi: 10.1097/ICO.0b013e3181342835. [DOI] [PubMed] [Google Scholar]
- 10.Goulet RJ, 3rd, Phan AD, Cantor LB, WuDunn D. Efficacy of the Ahmed S2 glaucoma valve compared with the Baerveldt 250-mm2 glaucoma implant. Ophthalmology. 2008 Jul;115(7):1141–7. doi: 10.1016/j.ophtha.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology. 2009 Apr;116(4):640–51. doi: 10.1016/j.ophtha.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 12.Bradley JC, Hernandez EG, Schwab IR, Mannis MJ. Boston type 1 keratoprosthesis: the University of California Davis experience. Cornea. 2009 Apr;28(3):321–7. doi: 10.1097/ICO.0b013e31818b8bfa. [DOI] [PubMed] [Google Scholar]
- 13.Chew HF, Ayres BD, Hammersmith KM, Rapuano CJ, Laibson PR, Myers JS, Jin YP, Cohen EJ. Boston keratoprosthesis outcomes and complications. Cornea. 2009 Oct;28(9):989–96. doi: 10.1097/ICO.0b013e3181a186dc. [DOI] [PubMed] [Google Scholar]


