Abstract
The success of an invasive species in a new region depends on its interactions with ecologically similar resident species. Invasions by disease vector mosquitoes are important as they may have ecological and epidemiological consequences. Potential interactions of a recent invasive mosquito, Aedes japonicus Theobald, with resident species in Virginia were evaluated by sampling larvae from containers and trapping adults. Distinct species compositions were observed for artificial containers and rock pools, with Ae. albopictus most abundant in the former and Ae. japonicus in the latter. However, these two species were found to co-occur in 21.2% of containers sampled. Among the six mosquito species most common in containers from May through September, 2006, only interspecific associations of Ae.japonicus with Aedesalbopictus(Skuse) and Aedestriseriatus(Say) were significant, and both were negative. In addition to differences in habitat preference, mean crowding estimates suggest that interspecific repulsion may contribute to the significant negative associations observed between these species. High relative abundances of late instars and pupae of Ae. japonicus seem to provide this species with a mechanism of evading competition with Ae. albopictus, facilitating their coexistence in artificial containers. Although annual fluctuations were observed, trends in adult populations over a 6-yr period provide no evidence of declines. In summary, this survey of diverse container types and all life stages provided only limited evidence for competitive displacements or reductions of resident container species by Ae. japonicus, as observed elsewhere in its invasive range.
Keywords: invasive, mosquito, container-inhabiting, interspecific interaction
Biological invasions are occurring at an alarming frequency worldwide, the impacts of which threaten biodiversity, ecosystem functioning, resource availability, national economies, and human health (Simberloff 1996, Pimentel et al. 2005, Vilà et al. 2010). Historically, some of the most important biological invasions have involved disease vectors, particularly mosquitoes (Diptera: Culicidae), a trend that has continued at an increasing rate over the past century (Lounibos 2002, Juliano and Lounibos 2005). The most recent successful invasions of mosquitoes have resulted from human transport of immature stages, especially of species that occupy container habitats (Lounibos 2002). Competition, predation, and parasitism among immature stages have been shown to play important roles in structuring mosquito communities of container habitats. In addition to their effects on resident species or ecosystems, invasive mosquitoes are of particular concern because they may also impact human or vertebrate animal health (Juliano and Lounibos 2005).
Native to Japan, Korea, Taiwan, and China (Tanaka et al. 1979), Aedes(Finlaya) japonicus Theobald (Diptera: Culicidae) is one of the most recently recognized mosquito species to invade the United States. Initially collected in the United States in 1998 (Peyton et al. 1999, Andreadis et al. 2001), Ae. japonicus has since become established throughout the northeastern United States (Scott 2003) and has expanded west of the Mississippi River (Reeves and Korecki 2004, Gallitano et al. 2005, Dunphy et al. 2009, Neitzel et al. 2009). Separate establishments of Ae. japonicus have occurred in Oregon, WA (Roppo et al. 2004, Irish and Pierce 2008) and Hawaii (Larish and Savage 2005). Distinct genetic profiles of Ae. japonicus collected from various sites in Japan and throughout its range in the United States suggest multiple introductions of the species in the United States (Fonseca et al. 2001, 2010) via international transport of used tires (Peyton et al. 1999, Lounibos 2002).
In both its native range and the United States, Ae. japonicus larvae primarily inhabit a wide variety of natural (treeholes, rock pools) and artificial containers (buckets, tires, and birdbaths), with rock pools being the preferred habitat (Tanaka et al. 1979, Andreadis et al. 2001, Scott 2003). Ae. japonicus are known to co-occur with numerous resident species in natural and artificial containers in North America (Andreadis et al. 2001, Scott et al. 2001, Oliver et al. 2003, Thielman and Hunter 2006, Andreadis and Wolfe 2010). The likelihood of Ae. japonicus colonizing a new region depends on its ability to contend with new environmental conditions and compete with resident species that occupy a similar ecological niche. In the United States, Ae. japonicus is most likely to co-occur with the indigenous rock hole mosquito, Aedes atropalpus (Coquillett), the Eastern treehole mosquito, Aedes triseriatus (Say) and its sibling species, Aedes hendersoni Cockerell, as well as the established invader Aedes albopictus (Skuse). Experimental results suggest that Ae. japonicus larvae are effective competitors in both natural and artificial containers (Armistead et al. 2008a, b; Alto 2011), and larval surveillance suggests that its presence in these habitats may contribute to population reductions of several species native to northeastern United States (Andreadis and Wolfe 2010).
In addition to ecological effects on other species, Ae. japonicus may pose public health risks as a vector. In the laboratory Ae. japonicus has demonstrated the ability to transmit Japanese encephalitis (Sucharit et al. 1989, Takashima and Rosen 1989) and Getah (Takashima and Hashimoto 1985) viruses, although it is not considered an important vector of either virus in its native range. Ae. japonicus is also a competent laboratory vector of eastern equine encephalitis (Sardelis et al. 2002a), LaCrosse (Sardelis et al. 2002b), St. Louis encephalitis (Sardelis et al. 2003), chikungunya, dengue (Schaffner et al. 2011), and West Nile viruses (WNV) (Sardelis and Turrell 2001, Turell et al. 2001). However, in the United States only WNV has been detected in wild-caught females (Werner 2001, White et al. 2001, Scott 2003, Centers for Disease Control and Prevention [CDC] 2009). The tendency of Ae. japonicus to feed primarily on mammalian hosts, including humans (Miyagi 1971, Scott 2003, Apperson et al. 2002, Molaei et al. 2009) illustrates the vector potential for this species. As of yet, however, reports of Ae. japonicus as a major nuisance species, especially when compared with Ae. albopictus, appear to be limited (Schaffner et al. 2011). Involvement of newly established Ae. japonicus in existing disease transmission cycles has the potential to alter the nature of an existing public health threat by changing the transmission rate because of differences in vector competence (Juliano and Lounibos 2005).
To assess potential interactions of Ae. japonicus in mosquito container communities and identify ecological processes that may be operating as a result of the invasion of this species, field surveys of larvae and adults were conducted in an area where this species is known to occur. Sampling of natural and artificial larval habitats was used to confirm and quantify co-occurrences and potential interactions of Ae.japonicus with resident mosquitoes. Routine trapping of adults over a 6-yr period was used to assess trends in abundance of the species of interest. The current study especially focuses on potential interactions among native and invasive Aedes spp. to evaluate the potential for competition resulting from their co-occurrence.
Materials and Methods
Study Site
A broad survey of immature and adult container-dwelling mosquitoes was conducted in Fair-fax County, VA (latitude 38° 50′: N, longitude 77° 7′: W), a suburb of Washington, DC.
Larval Collections
A county-wide field survey of containers was conducted from May through September 2006. Sampling was predominantly limited to public or county property; however, containers located at private residences were sampled when responding to nuisance mosquito complaint calls to the Fairfax County Health Department. A few tree holes and ornamental bromeliads were sampled at different times, but rock pools of the Potomac River (and tributaries) were the only natural containers yielding many observations. Containers were only sampled once, and the entire aquatic contents of each were removed, volume determined, and sieved through a mesh fine enough to retain first instars. Samples were transported to the laboratory in 18-ounce (532.32 ml) Whirl-Pak bags (Nasco, Fort Atkinson, WI). Wet containers without mosquitoes were noted. The GPS coordinates of each site, sun exposure (none, partial, or full), and container type were recorded.
In the laboratory, fourth instars from each container were identified to species and counted. All other immatures were counted, sorted and separated by stage, placed in separate 21 × 12 cm cylindrical growth chambers (BioQuip Products, Inc., Rancho Dominguez, CA) containing 400 ml of tap water and food (3:1 mix of ground rabbit chow and brewer’s yeast), and reared to fourth instar for identification. Predators, such as Toxorhynchitesrutilus (Coquillett), were identified and removed. Mosquito pupae were identified at adult emergence. Mortality at all immature stages was noted.
Data Analysis
Three metrics were used to assess overall mosquito community structure: Kendall’s co-efficient of rank correlation, tau (τ) (Ghent 1963); C8 coefficient of association (Hurlbert 1969); and mean crowding (Lloyd 1976, Rathcke 1976). Significant similarity between the ranked abundance of the six most common species collected (Ae. albopictus, Ae. japonicus, Culex pipiens, Culex restuans Theobald, Ae. triseriatus, and Ae. hendersoni) from small versus large artificial containers, and rock pools versus tires and artificial containers was determined using Kendall’s τ. Associations between co-occurring species were quantified from presence-absence data using the C8 coefficient of association, which ranges from 1 to −1. Positive associations between species may reflect a common habitat preference or interspecific attraction, while negative associations may result from different habitat preferences or interspecific repulsion.
Significance of positive and negative association (C8 values) was assessed with a corrected χ2 formula (Pielou 1977) or Fisher exact test in cases with cell totals less than or equal to five. Indices of mean intra-and interspecific crowding per unit resource (container fluid volume), were also calculated. Interspecific mean crowding of species x by y per unit resource a was quantified as Σ(xiyi/ai)/Σxi, and intraspecific mean crowding by the same formula but substituting (xi – 1) for yi
A subset of analyses was conducted to analyze habitat preferences and population performance of Ae. Albopictus and Ae.japonicus. Specifically, the effects of container type (n = 2), sun exposure (n = 3) and interaction of container type X sun exposure on collection frequency, mean crowding, and average instar number were determined. Because of limited samples in May and September, the effects of month were excluded. The effects of these variables on collection frequency was determined using the CATMOD procedure (SAS Institute 1989) with subsequent maximum likelihood contrasts, while two-way ANOVAs were used to assess their effects on average instar number, intra-, and interspecific mean crowding for each species. Finally, a Kolmogorov–Smirnov two-sample test was used to compare the distributions of average instar number, with pupae designated as the number five, for Ae. japonicus and Ae. albopictus.
Adult Collections
Adult mosquitoes were collected with carbon dioxide (CO2)-baited CDC light traps (John Hock Co., Gainesville, FL) and gravid traps (Reiter 1987) (BioQuip Products, Inc., Rancho Dominguez, CA) set at the same 70 preselected sites located throughout Fairfax County each May through October (epidemiological weeks 20 – 40) from 2004 to 2009. Gravid traps were baited with a fermented mixture of water, freshly mowed grass clippings, hay, and Brewer’s yeast. One of each trap type was set weekly at each site. Traps were set in the early afternoon and retrieved the following morning. All adult female mosquitoes collected were sorted, counted and identified to species. Efforts were made to distinguish between the morphologically similar Ae. hendersoni and Ae. triseriatus; however, it is likely that data presented here reflect a mixed sample of both species. The mean number of adult female Ae. albopictus, Ae. japonicus, and Ae. triseriatus per trap night was calculated for each week.
Results
Natural and Artificial Containers
During the study period, 191 containers (131 artificial and 60 natural) were sampled for the presence of larvae and pupae, of which 134 were positive for mosquitoes. These included rock pools, tires, flowerpot saucers, tarpaulins, drainpipes, French drains, birdbaths, cemetery vases, trash cans, drums, and other small miscellaneous artificial containers. The following 10 species were collected, in order of frequency of occurrence: Ae. albopictus, Ae. japonicus, Culex pipiens, Cx. restuans, Ae. triseriatus, Ae. hendersoni, Anopheles punctipennis Say, Tx. rutilus, Ae. atropalpus, and Orthopodomyia signifera (Coquillett). Ae. atropalpus and An. punctipenniswere collected only from rock pools, the former on only four occasions, while Tx. rutilus and Or. signifera were collected only from artificial containers. These four species have not been included in any subsequent analyses because of limited sample size.
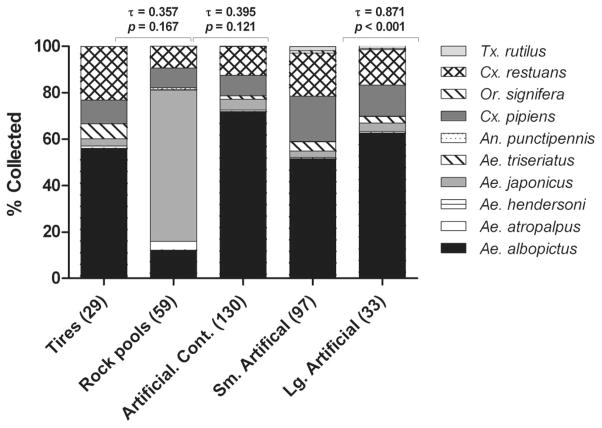
Kendall’s coefficient of rank correlation (τ) was positive for all three habitat comparisons, but the rank abundance of species was only significantly similar for small versus large artificial containers (Fig. 1). Ae. japonicus was the most abundant species collected from rock pools, while Ae. albopictus was the most abundant in artificial containers (Figs. 1 and 2). These species were also the most productive in these habitats, as measured by the total number of pupae collected per container type (Table 1). Greater numbers of Cx. pipiens, Cx. restuans, and Ae. triseriatus pupae were recovered from artificial containers; however, Cx. pipiens was more productive in larger habitats while the opposite was found for Ae.triseriatus(Table 1). No difference in productivity was observed for Ae. albopictus or Cx. restuans collected from artificial containers.
Fig. 1.
Mosquito species composition of container habitats expressed as percentage of total mosquitoes collected in Fairfax County, VA, from May through September 2006. The number of each type of container sample is provided on the x-axis as (n). Kendall’s coefficient of rank correlation (τ) to test similarity of compositions and associated P values for each habitat comparison are indicated above the histogram bars.
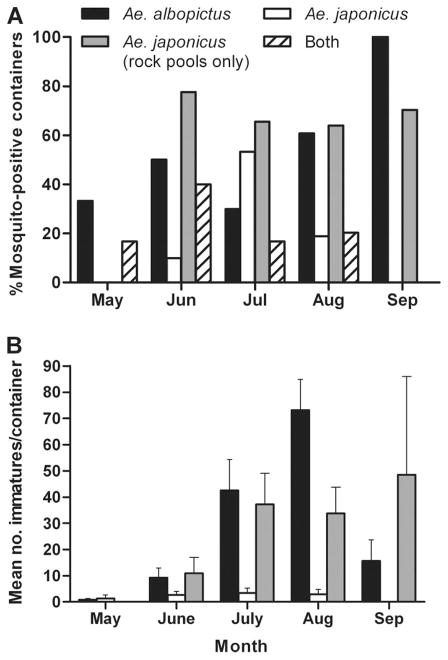
Fig. 2.
Seasonal occurrences of Ae. albopictus and Ae. japonicus collected from artificial containers May through September, 2006. (A) Proportion of mosquito-positive containers containing Ae. albopictus, Ae. japonicus, Ae. japonicus from rock pools collections only, or both species, by month. (B) Monthly abundance (mean number of mosquitoes collected per container) of Ae. albopictus and Ae. japonicus (±SE) from 91 mosquito-positive artificial containers.
Table 1.
Productivity as measured by the total no. of pupae sampled for the most abundant species collected in Fairfax County, VA, in 2006 by container type
| Container type | Total no. pupae collected
|
|||||
|---|---|---|---|---|---|---|
| No. containers | Ae. albopictus | Ae. japonicus | Ae. triseriatus | Cx. pipiens | Cx. restuans | |
| Artificial | 130 | 412 | 27 | 18 | 71 | 163 |
| Small | 97 | 191 | 1 | 17 | 4 | 80 |
| Large | 33 | 221 | 26 | 1 | 67 | 83 |
| Tires | 59 | 52 | 22 | 9 | 3 | 92 |
| Rock pools | 59 | 13 | 129 | 4 | 5 | 11 |
Using the C8 index of association, significant positive associations included those between Cx. pipiens and Cx. restuans, Ae. albopictus and Ae. triseriatus, Ae. albopictus, and Ae. hendersoni, and Ae. hendersoni and Ae. triseriatus (Table 2). Ae. japonicus was found to have significant negative associations with Ae. triseriatus and Ae. albopictus. The exclusion of rock pools had no effect on the statistical significance of any associations (Table 2). It should be noted that a non-significant coefficient of association of −0.04 was calculated for Ae.atropalpus and Ae.japonicus when only rock pools were included.
Table 2.
Coefficients of association (C8) for the most abundant species in 191 artificial and natural container samples from Fairfax County, VA, in 2006
| Species | Coefficient of association (C8)
|
||||
|---|---|---|---|---|---|
| Cx. restuans | Cx. pipiens | Ae. triseriatus | Ae. japonicus | Ae. hendersoni | |
| Ae. albopictus | −0.056 | −0.015 | 0.067** | −0.238** (−0.207**) | 0.037* |
| Ae. hendersoni | 0 | −0.272 | 0.232* | 0 (0) | |
| Ae. japonicus | 0.075 (0.105) | 0.042 (0.133) | −0.048** (0) | ||
| Ae. triseriatus | −0.122 | −0.140 | |||
| Cx. pipiens | 0.638** | ||||
Parentheses enclose C8 values that exclude rock pools.
P < 0.01,
P < 0.001 by χ2; all other interspecific associations are nonsignificant.
Calculations of mean intra- and interspecific crowding for the six most abundant mosquito species collected showed that Ae. japonicus, Ae. triseriatus, and Cx. pipiens encountered a higher density of Ae. albopictus than any other species or conspecifics, while Ae. hendersoni most frequently encountered a higher density of Ae. triseriatus (Table 3). Ae. albopictus and Cx. restuans both encountered a higher density of conspecifics than any other mosquito species. Based on these data, further analyses were performed to investigate the co-occurrences of Ae. japonicus with Ae. albopictus. The limited number of Ae. triseriatus sampled and low frequency of its collection prohibited further analyses of co-occurrences of this species with Ae. japonicus.
Table 3.
Intra- and interspecific mean crowding of the most abundant mosquito species collected from artificial and natural containers in Fairfax County, VA, in 2006
| Species, x | Mean crowdinga (density of species y encountered by species x per liter vol)
|
|||||
|---|---|---|---|---|---|---|
| Species, y
| ||||||
| Ae. albopictus | Ae. hendersoni | Ae. japonicus | Ae. triseriatus | Cx. pipiens | Cx. restuans | |
| Ae. albopictus | 395.80 | 3.013 | 53.01 | 31.59 | 351.88 | 57.37 |
| Ae. hendersoni | 40.73 | 32.49 | 18.06 | 56.27 | 1.77 | 5.74 |
| Ae. japonicus | 139.3 | 9.03 | 127.56 | 12.85 | 51.56 | 83.06 |
| Ae. triseriatus | 136.7 | 11.90 | 19.21 | 34.47 | 25.93 | 39.26 |
| Cx. pipiens | 1, 098.43 | 2.48 | 37.56 | 12.96 | 126.98 | 52.33 |
| Cx. restuans | 20.16 | 2.51 | 57.41 | 4.33 | 34.05 | 116.60 |
Interspecific mean crowding was determined from only those containers in which both species co-occurred.
Co-occurrences of Ae.japonicus and Ae.albopictus
Ae. albopictus and Ae. japonicus were identified from 68.9 and 43.9%, respectively, of mosquito-positive containers sampled during the study, and co-occurred together in 21.2% of these containers. Both species were collected most frequently from June through August (Fig. 2A). Ae.albopictus was consistently present in higher abundance than Ae. japonicus in all months except May (Fig. 2B), even in July when Ae. japonicus was found in a greater percentage of containers. Container type was found to have a significant effect on collection frequency of both Ae. albopictus and Ae.japonicus (Table 4), with the former occurring predominantly in artificial containers and the latter in rock pools. A significant effect of sun exposure on collection frequency was found only for Ae. japonicus, with maximum likelihood contrasts suggesting a preference for fully or partly shaded containers (full shade versus full sun, P = 0.0102; partial vs. full sun, P = 0.0007). The ML ANOVA showed no significant container type X sun exposure interactions for either species.
Table 4.
ML ANOVA for the effects of container type and sun exposure on the occurrence of Ae. albopictus and Ae. japonicus larvae in Fairfax County, VA, from May through Sept. 2006
| Species | Source | df | χ2 | P |
|---|---|---|---|---|
| Ae. albopictus | Intercept | 1 | 2.84 | 0.0919 |
| Container type | 1 | 31.31 | 30.0001 | |
| Sun exposure | 2 | 1.06 | 0.5877 | |
| Container type X sun exposure | 2 | 5.89 | 0.0525 | |
| Likelihood ratio | 0 | |||
| Ae. japonicus | Intercept | 1 | 14.31 | 0.0002 |
| Container type | 1 | 7.32 | 0.0068 | |
| Sun exposure | 2 | 11.46 | 0.0033 | |
| Container type X sun exposure | 2 | 5.77 | 0.0559 | |
| Likelihood ratio | 0 |
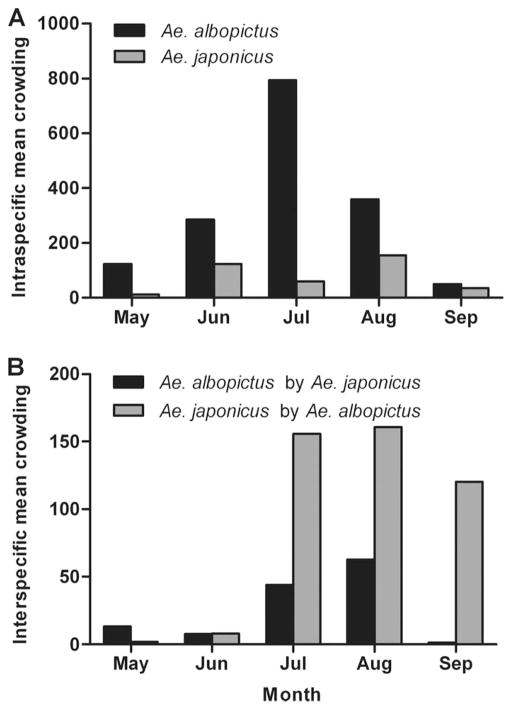
Intraspecific mean crowding of both Ae. albopictus and Ae. japonicus followed similar trends throughout the study period, however Ae. japonicus encountered the highest density of conspecifics 1 mo before that of Ae. albopictus (Fig. 3). A similar pattern of intraspecific mean crowding was observed when only Ae. japonicus recovered from rock pools were considered for analysis (data no shown). Interspecific mean crowding of Ae.japonicus by Ae.albopictus was greater than that of Ae. albopictus by Ae. japonicus in all months except May, and was highest for both species in August (Fig. 3). No significant effects of container type, sun exposure, or container type X sun exposure on intraspecific mean crowding were observed for Ae. albopictus (F5, 75 = 0.55; P = 0.7397) or Ae. japonicus (F4, 60 = 0.55; P = 0.702). These variables were not found to have significant effects on interspecific mean crowding of Ae. japonicus by Ae. albopictus (F4, 23 = 0.65; P = 0.6308) or Ae. albopictus by Ae. japonicus (F4, 23 = 1.41; P = 0.262) either.
Fig. 3.
Crowding experienced by Ae. albopictus and Ae. japonicus in artificial containers sampled from May through September 2006. (A) Intraspecific mean crowding (density of conspecifics encountered per unit resource) experienced by Ae. albopictus and Ae. japonicus by month. (B) Interspecific mean crowding of Ae.albopictus by Ae.japonicus and Ae. japonicus by Ae. albopictus.
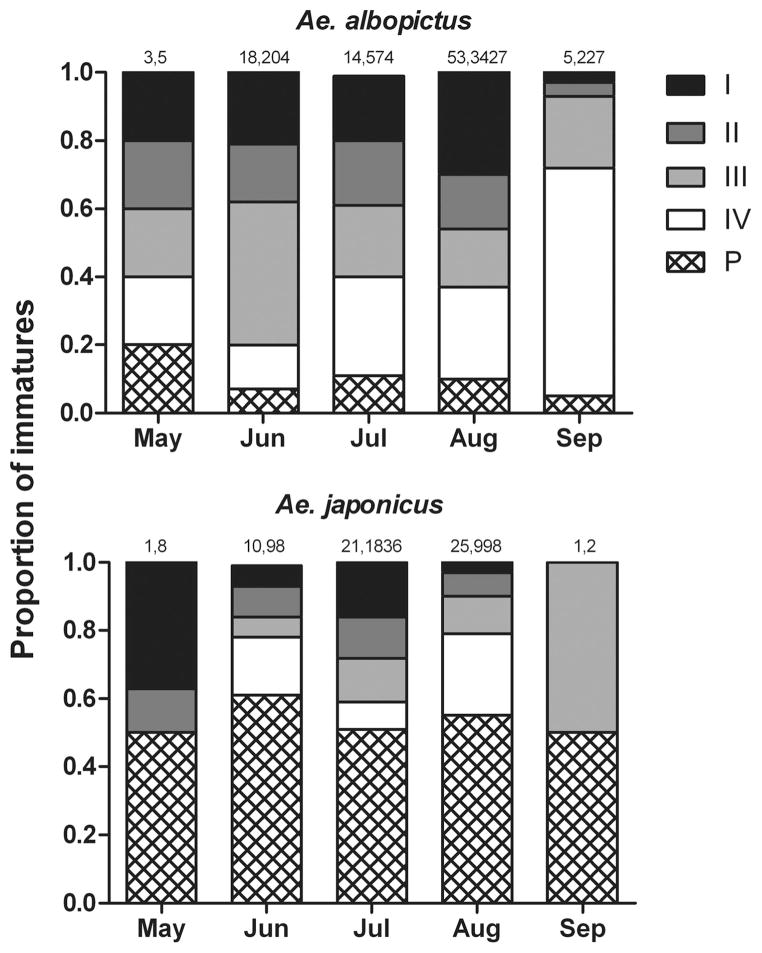
The average instar number, or age, of Ae. albopictus larvae from all collections during the study was 2.77, while that of Ae.japonicus was 3.86. When considering only those containers in which both species occurred, average instar numbers were similar (2.8 vs. 3.3). Neither container type nor sun exposure was found to have a significant effect on the average instar number of Ae. albopictus (F5, 85 = 0.73; P = 0.6019) or Ae. japonicus(F5, 52 = 1.59; P = 0.1788). The average instar number of Ae. japonicus larvae was consistently greater than that of Ae. albopictus, with pupae comprising the greatest proportion of Ae. japonicus sampled in each month of the study (Fig. 4). A comparison of the overall instar distributions of the two species from May through September by a Kolmogorov–Smirnov two-sample test indicated a significant difference (D = 18.130; P < 0.001).
Fig. 4.
Monthly instar distributions of Ae. albopictus and Ae. japonicus from May through September 2006 in Fairfax, VA. Total numbers of species-positive containers sampled (n1) and larvae collected (n2) per month are indicated above each histogram bar (n1, n2).
Adult Surveillance
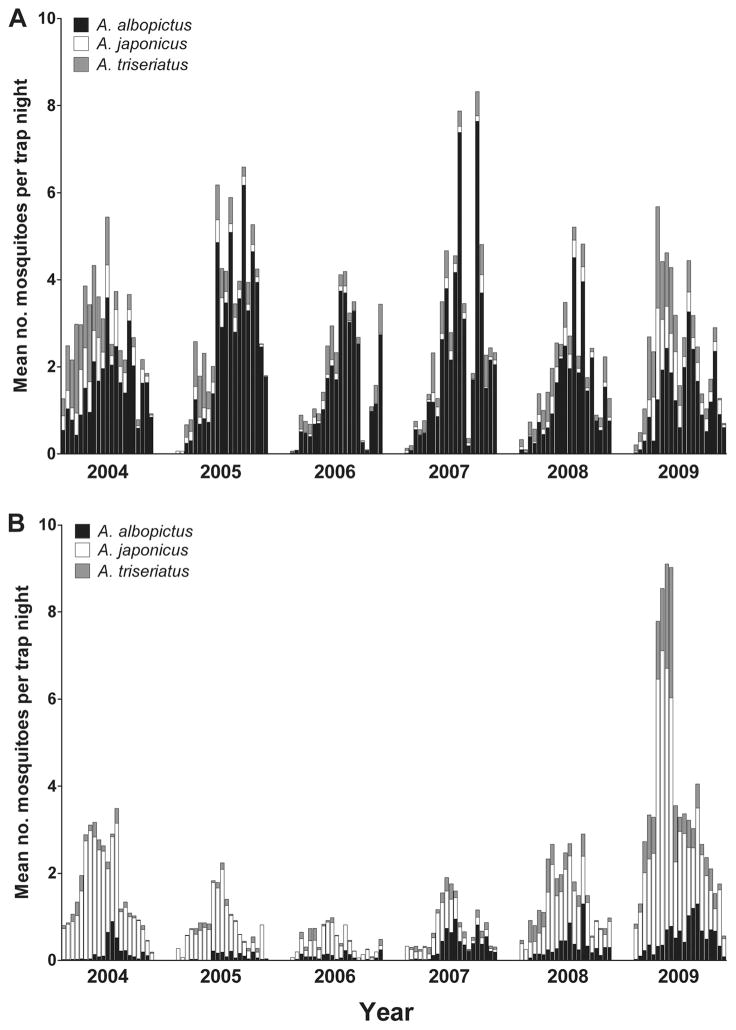
Weekly collections of adult mosquitoes demonstrated that Ae. albopictus was consistently collected in greater abundance in CO2-baited CDC light traps than gravid traps, while the opposite was found for Ae. japonicus (Fig. 5). Fluctuations in trapping abundance were observed for both species from 2004 to 2009, although these changes were most pronounced for Ae.japonicus, especially in gravid trap collections, which showed a marked decline for this species from 2004 to 2006 followed by an increase from 2007 to 2009. Similar trends were observed from gravid trap collections of Ae. albopictus, albeit to a lesser extent, while CDC light trap collections suggest that abundance was consistent across study years.
Fig. 5.
Weekly abundance of Ae. albopictus, Ae. japonicus, and Ae. triseriatus calculated as the mean number of female mosquitoes collected per trap night in (A) CO2-baited light traps and (B) gravid traps (bottom) from epidemiological week 20–40 (May–September), 2004–2009, in Fairfax County, VA.
Discussion
Invasion success of a non-native species in a new region is dependent on its interactions with ecologically similar resident species. A field survey of natural and artificial containers was conducted to assess container-inhabiting mosquito community structure and glean insight into potential species interactions after the invasion of Ae. japonicus in Virginia. The mosquito species occupying the containers sampled during this study were consistent with previously published reports from the eastern United States, and the significant positive associations observed (Table 2) were not unexpected in container habitats as co-occurrences of these species in container habitats are well documented (e.g., Andreadis et al. 2001, Hribar et al. 2001, Yee 2008).
Distinct species compositions based on ranked abundances were observed for artificial containers and rock pools, with Ae. albopictus most abundant in the former and Ae. japonicus in the latter (Fig. 1). However, both species were frequently found to co-occur in containers sampled throughout the study period (Fig. 2). Data indicate a preference of Ae. japonicus for shaded habitats, particularly rock pools, while Ae. albopictus was more frequently collected from artificial containers (Fig. 1; Table 4). However, development and performance of neither species appeared to be significantly impacted by container type or sun exposure, as measured by average instar number and intraspecific mean crowding.
Differences in habitat preference likely contribute to the significant negative association between Ae. albopictus and Ae. japonicus (Table 2); however, this finding persisted even when rock pools were excluded from analyses, suggesting that interspecific repulsion may also be occurring. Mean crowding estimates provide some evidence for this, indicating that intraspecific competition is likely more important in constraining population growth of Ae. albopictus than competition with Ae. japonicus, while interactions with Ae. albopictus may be just as important as those with conspecifics for Ae. japonicus (Table 3). This notion is supported by recent field competition experiments in which higher survivorship, shorter developmental time, and higher population growth of Ae. albopictus under conditions of interspecific larval competition gave this species a competitive advantage over Ae. japonicus, suggesting negative effects on growth and development of Ae. japonicus in habitats where this species co-occurs with Ae. albopictus (Armistead et al. 2008a). However, an investigation of behavior in the presence of predators suggests that more high-risk behaviors exhibited by Ae. albopictus may promote coexistence with Ae. japonicus (Kesavaraju et al. 2011). It is also possible that selection for interspecific avoidance occurred before invasion, given that Ae. japonicus is sympatric with Ae. albopictus throughout most of its native range.
The persistence of Ae. japonicus in artificial containers in the presence to Ae. albopictus may also be facilitated by differences in seasonality and instar distributions. High relative abundances of pupae and older instars of Ae. japonicus (Fig. 4) and higher interspecific mean crowding of Ae. japonicus on Ae. albopictus than that of Ae. albopictus on Ae. japonicus early in the season (Fig. 3) suggest this species gets a head start over Ae. albopictus (Fig. 4). The earlier appearance of Ae. japonicus in the season my stem from this species ability to overwinter in the larval stage, an observation that has been made by others in its native range (LaCasse and Yamaguti 1950), New Jersey (Scott 2003), and North Carolina (B. Harrison, personal communication), or may simply be explained by earlier hatching of overwintering eggs. Regardless, it would appear to enable Ae. japonicus to avoid Ae. albopictus and escape competitive effects when the two species co-occur early in the season. However, it is unclear if Ae. japonicus is afforded a competitive advantage over Ae. albopictus when the former has a head start. The importance of cohort structure in density-dependent interspecific competition between these two species needs to be explored experimentally to address this question further.
Observations made here on differences in habitat preference and seasonality suggest that the invasion of Ae. japonicus in Virginia will ultimately promote its coexistence with Ae. albopictus. Population trends gleaned from broad-based surveillance of adult females of these species suggest that this is the case in Fairfax, VA. Although populations of Ae. japonicus appeared to be on the decline during the first years of surveillance (2004 –2006), a subsequent rebound was observed from 2007 to 2009. These observations are likely attributable to annual variations in environmental conditions (i.e., temperature and rainfall) and do not reflect a local decline, or suggest an eventual extinction, of Ae. japonicus populations after the introduction and initial expansion of this species throughout the area. The varying attractiveness of CO2-baited CDC light traps and gravid traps to the target species has been well characterized, and collections using these methods clearly reflect different population subsets (host vs. oviposition seeking); however, the size, duration, and consistency of these regular adult collections should sufficiently overcome these inherent biases to provide an accurate depiction of population trends.
The current study focused primarily on populations of Ae. albopictus and Ae. japonicus, however interesting relationships between other container-inhabiting species were observed. Ae. triseriatus and Cx. pipiens were found to encounter a greater density of Ae. albopictus than any other species or conspecifics (Table 3), suggesting that interspecific interactions are likely to affect mosquito and population performance of these species. This is supported by multiple studies in which Ae. albopictus was found to be competitively superior to both Ae. triseriatus (Livdahl and Willey 1991, Novak et al. 1993, Teng and Apperson 2000) and Cx. pipiens (Costanzo et al. 2005). The frequency of collection and abundance of Ae.triseriatus larvae were low during this study, although adult mosquito surveillance suggests that this is a reflection of sampling methods, particularly a paucity of tree hole collections, and does not represent a true decline in Ae. triseriatus populations. An evaluation of co-occurrences between Ae. triseriatus and Ae. japonicus was not possible, although mean crowding estimates indicate that interactions with Ae. albopictus are more likely important for both of these species than interactions with each other (Table 3). These findings are consistent with competition experiments that have shown only minimal evidence for competitive asymmetry between Ae. japonicus and Ae. triseriatus (Alto 2011). However, the decline or displacement of Ae. triseriatus in container habitats associated with an increased prevalence of Ae.japonicus has been observed in multiple larval surveys (Joy and Sullivan 2005, Burger and Davis 2008, Andreadis and Wolfe 2010), and warrants further investigation.
Finally, the successful establishment of Ae. japonicus in Fairfax County, VA, appears to be associated with a population decline and potential displacement of Ae. atropalpus in local rock pools. The limited collection of this native rock pool mosquito while surveying these habitats is cause for concern as this species was once abundant throughout the area; in fact its type locality given by Coquillett (1902) is nearby Plummer’s Island in Montgomery County, MD. The decline of Ae. atropalpus in rock pools has been observed in Connecticut (Andreadis and Wolfe 2010) and North Carolina (B. Byrd, personal communication), and this species is absent from New Jersey rock pools, albeit common in tires (Scott et al. 2001). It is important to note that two major flooding events caused by heavy rains and a hurricane occurred during the larval habitat portion of this study and limited rock pool collections in late June and early July, and again in early September. Although no conclusions can be drawn from the limited co-occurrence of these species during this study, it is possible that interspecific larval resource competition with Ae. japonicus in rock pools may have had negative effects on populations of Ae. atropalpus in areas where these two species co-occur. These findings are supported by recent laboratory competition experiments in which Ae. atropalpus was found to be more sensitive to larval densities than Ae. japonicus (Armistead et al. 2008b). However, the opposite has also been demonstrated (Hardstone et al. 2012). Differences in overwintering strategies (eggs vs. larvae) or later hatching of diapausing eggs than Ae. japonicus may have played a role in the seeming decline of Ae. atropalpus in this region.
In conclusion, the invasion success of Ae. japonicus in Fairfax, VA, appears to be associated with both its preference for rock pools and its early seasonal appearance. These data suggest that Ae. japonicus is likely to persist in both rock pools and artificial containers, even in the presence of Ae. albopictus. Although it is unclear when Ae. atropalpus populations began to decline in local rock pools, there is some evidence to suggest that it may have resulted from interactions with Ae. japonicus. In contrast, Ae. japonicus appears to be able to overcome the superior competitive ability of Ae.albopictus through differences in seasonality, specifically with higher relative abundances of older instars early in the season. While this study focused predominantly on the potential inter-specific interactions of Ae. japonicus with resident mosquito species in artificial containers and rock pools, other factors, particularly predators, may be influencing the structure of these communities and should be investigated. Furthermore, the tolerance of these species to varying environmental conditions, intraguild predation, or differences in foraging behavior may be important in their interspecific interactions.
Acknowledgments
We thank Fairfax County for permission to conduct field work on their land and the Fairfax Department of Health for use of laboratory facilities and especially the Disease Carrying Insects Program staff for their support in the field; and B. Byrd, B. Harrison, G. O’Meara, and J. Scott for their discussions on the ecology of Ae. japonicus and Ae. atropalpus. Special thanks to B. Alto, G. O’Meara, and two anonymous reviewers for their reviews of earlier versions of this manuscript. This work was supported by funds from National Institutes of Health grant (AI)-044793.
References Cited
- Alto BW. Interspecific larval competition between invasive Aedesjaponicus and native Aedestriseriatus(Diptera: Culicidae) and adult longevity. J Med Entomol. 2011;48:232–242. doi: 10.1603/me09252. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Munstermann LE, Wolfe RJ, Florin DA. Discovery, distribution, and abundance of the newly introduced mosquito Aedes japonicus (Diptera: Culicidae) in Connecticut, USA. J Med Entomol. 2001;38:774–779. doi: 10.1603/0022-2585-38.6.774. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Wolfe RJ. Evidence for reduction of native mosquitoes with increased expansion of invasive Ochlerotatus japonicus japonicus (Diptera: Culicidae) in the northeastern United States. J Med Entomol. 2010;47:43–52. doi: 10.1603/033.047.0106. [DOI] [PubMed] [Google Scholar]
- Apperson CH, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, Nasci RS. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- Armistead JS, Arias JR, Nishimura N, Lounibos LP. Interspecific larval competition between Aedes albopictus and Aedes japonicus (Diptera: Culicidae) in northern Virginia. J Med Entomol. 2008a;45:629– 636. doi: 10.1603/0022-2585(2008)45[629:ilcbaa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead JS, Nishimura N, Escher RL, Lounibos LP. Larval competition between Aedesjaponicus and Aedes atropalpus (Diptera: Culicidae) in simulated rock pools. J Vector Ecol. 2008b;33:238–246. doi: 10.3376/1081-1710-33.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger JF, Davis H. Discovery of Ochlerotatus japonicus (Theobald) in southern New Hampshire, U.S.A. and its subsequent increase in abundance in used tire casings. Entomol News. 2008;119:439– 444. [Google Scholar]
- Centers for Disease Control and Prevention. Mosquito species producing WNV positives by year. 2009 ( www.cdc.gov/ncidod/dvbid/westnile/mosquitospecies.htm)
- Coquillett DW. Three new species of Culex. Can Entomol. 1902;34:292–293. [Google Scholar]
- Costanzo KS, Mormann K, Juliano SA. Asymmetrical competition and patters of abundance of Aedes albopictus and Culexpipiens(Diptera: Culicidae) J Med Entomol. 2005;42:559–570. doi: 10.1093/jmedent/42.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy BM, Tucker BJ, Petersen MJ, Blitvich BJ, Bartholomay LC. Arrival and establishment of Aedes japonicus japonicus (Diptera: Culicidae) in Iowa. J Med Entomol. 2009;46:1282–1289. doi: 10.1603/033.046.0605. [DOI] [PubMed] [Google Scholar]
- Fonseca DM, Campbell S, Crans WJ, Mogi M, Miyagi I, Toma T, Bullians M, Andreadis TG, Berry RL, Pagac B, Sardelis MR, Wilkerson RC. Aedes (Finlaya) japonicus (Diptera: Culicidae), a newly recognized mosquito in the United States: analyses of genetic variation in the United States and putative source populations. J Med Entomol. 2001;38:135–146. doi: 10.1603/0022-2585-38.2.135. [DOI] [PubMed] [Google Scholar]
- Fonseca DM, Widdel AK, Hutchinson M, Spichiger SE, Kramer LD. Fine-scale spatial and temporal population genetics of Aedes japonicus, a new US mosquito, reveal multiple introductions. Mol Ecol. 2010;19:1559– 1572. doi: 10.1111/j.1365-294X.2010.04576.x. [DOI] [PubMed] [Google Scholar]
- Gallitano S, Blaustein L, Vonesh J. First occurrence of Ochlerotatus japonicus in Missouri. J Vector Ecol. 2005;30:347–348. [PubMed] [Google Scholar]
- Ghent AW. Kendall’s “tau” coefficient as an index of similarity in comparisons of plant or animal communities. Can Entomol. 1963;95:568–575. [Google Scholar]
- Hardstone MC, Andreadis TG. Weak larval competition between the invasive mosquito Aedesjaponicus japonicus (Diptera: Culicidae) and three resident container-inhabiting mosquitoes in the laboratory. J Med Entomol. 2012;49:277–285. doi: 10.1603/me11050. [DOI] [PubMed] [Google Scholar]
- Hribar LJ, Smith JM, Vlach JJ, Verna TN. Survey of container-breeding mosquitoes from the Florida Keys, Monroe County, Florida. J Am Mosq Control Assoc. 2001;17:245–248. [PubMed] [Google Scholar]
- Hurlbert SH. A coefficient of interspecific association. Ecology. 1969;50:1–9. [Google Scholar]
- Irish SR, Pierce CS. Update on the distribution of Ochlerotatus japonicus in Oregon and Washington. J Am Mosq Control Assoc. 2008;24:110–111. doi: 10.2987/5572.1. [DOI] [PubMed] [Google Scholar]
- Joy JE, Sullivan SN. Occurrence of tire-inhabiting larvae in different geographic regions of West Virginia. J Am Mosq Control Assoc. 2005;21:380–386. doi: 10.2987/8756-971X(2006)21[380:OOTIML]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Khan DF, Gaugler R. Behavioral differences of invasive container-dwelling mosquitoes to a native predator. J Med Entomol. 2011;48:526–532. doi: 10.1603/me10200. [DOI] [PubMed] [Google Scholar]
- LaCasse WJ, Yamaguti S. Mosquito Fauna of Japan and Korea. Parts I and II. Mosquito Survey data on Japan and their application in the control of mosquitoborne diseases. Off Surg. 1950 HQ 1 Corps APO 301. [Google Scholar]
- Larish LB, Savage HM. Introduction and establishment of Aedes (Finlaya) japonicus japonicus (Theobald) on the island of Hawaii: implications for arbovirus transmission. J Am Mosq Control Assoc. 2005;21:318–321. doi: 10.2987/8756-971X(2005)21[318:IAEOAF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Livdahl TP, Willey MS. Prospects for invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lloyd M. Mean crowding. J Anim Ecol. 1967;36:1–20. [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Miyagi I. Notes on the Aedes (Finlaya) chrysolineatus subgroup in Japan and Korea (Diptera: Culicidae) Trop Med. 1971;13:141–151. [Google Scholar]
- Molaeik G, Farajollahi A, Scott JJ, Gaugler R, Andreadis TG. Human bloodfeeding by the recently introduced mosquito, Aedesjaponicusjaponicus, and public health implications. J Am Mosq Control Assoc. 2009;25:210–214. doi: 10.2987/09-0012.1. [DOI] [PubMed] [Google Scholar]
- Neitzel DF, Johnson KA, Brogren S, Kemperman MM. First collection records of Aedes japonicus in Minnesota. J Am Mosq Control Assoc. 2009;25:367–369. doi: 10.2987/09-0015.1. [DOI] [PubMed] [Google Scholar]
- Novak MG, Higley LG, Christianssen CA, Rowley WA. Evaluating larval competition between Aedes albopictus and A. triseriatus (Diptera: Culicidae) through replacement series. Environ Entomol. 1993;22:311– 318. [Google Scholar]
- Oliver J, Means RG, Howard JJ. Geographical distribution of Aedes japonicus in New York State. J Am Mosq Control Assoc. 2003;19:121–124. [PubMed] [Google Scholar]
- Peyton EL, Campbell SR, Candeletti TM, Romanowski M, Crans WJ. Aedes (Finlaya) japonicus japonicus (Theobald), a new introduction into the United States. J Am Mosq Control Assoc. 1999;15:238– 241. [PubMed] [Google Scholar]
- Pielou EC. Mathematical ecology. Wiley; New York, NY: 1977. [Google Scholar]
- Pimentel D, Zuniga R, Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ. 2005;52:273. [Google Scholar]
- Rathcke BJ. Competition and coexistence within a guild of herbivorous insects. Ecology. 1976;57:76– 87. [Google Scholar]
- Reeves WK, Korecki JA. Aedes japonicus japonicus (Theobald) (Diptera: Culicidae), a new invasive mosquito for Georgia and South Carolina. Proc Entomol Soc Wash. 2004;106:233–234. [Google Scholar]
- Reiter P. A revised version of the CDC gravid mosquito trap. J Am Mosq Control Assoc. 1987;3:325–327. [PubMed] [Google Scholar]
- Roppo MR, Lilya JL, Maloney FA, Sames WJ. First occurrence of Aedes japonicus in the state of Washington. J Am Mosq Control Assoc. 2004;20:83– 84. [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ. Aedes j. japonicus in Frederick County, Maryland: discovery, distribution, and vector competence for West Nile virus. J Am Mosq Control Assoc. 2001;17:137–141. [PubMed] [Google Scholar]
- Sardelis MR, Dohm JD, Pagac B, Andre RG, Turell MJ. Experimental transmission of eastern equine encephalitis virus by Aedes j. japonicus (Diptera: Culicidae) J Med Entomol. 2002a;39:480– 484. doi: 10.1603/0022-2585-39.3.480. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Andre RG. Laboratory transmission of LaCrosse virus by Aedes j. japonicus (Diptera: Culicidae) J Med Entomol. 2002b;39:635– 639. doi: 10.1603/0022-2585-39.4.635. [DOI] [PubMed] [Google Scholar]
- Sardelis MRM, Turell J, Andre RG. Experimental transmission of St. Louis encephalitis virus by Aedes j japonicus. J Am Mosq Control Assoc. 2003;19:159– 162. [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT user’s guide. SAS Institute; Cary, NC: 1989. [Google Scholar]
- Schaffner F, Vazeille M, Kaufmann C, Failloux A, Mathis A. Vector competence of Aedes japonicus for chikungunya and dengue viruses. Eur Mosq Bull. 2011;29:141–142. [Google Scholar]
- Scott JJ. PhD dissertation. Rutgers, The State University of New Jersey; New Brunswick, NJ: 2003. The ecology of the exotic mosquito Aedes (Finlaya) japonicus japonicus (Theobald 1901) (Diptera: Culicidae) and an examination of its role in the West Nile virus cycle in New Jersey. [Google Scholar]
- Scott JJ, Carle FL, Crans WJ. Aedesjaponicus collected from natural rock pools in New Jersey. J Am Mosq Control Assoc. 2001;17:91–92. [PubMed] [Google Scholar]
- Simberloff D. Impacts of introduced species in the United States. Consequences: Nat Implic Environ Change. 1996;2:13–22. [Google Scholar]
- Sucharit S, Surathin K, Shrestha SR. Vectors of Japanese encephalitis virus (JEV): species complexes of the vectors. Southeast Asian J Trop Med Public Health. 1989;20:611– 621. [PubMed] [Google Scholar]
- Takashima I, Hashimoto N. Getah virus in several species of mosquitoes. Trans R Soc Trop Med Hyg. 1985;79:546–550. doi: 10.1016/0035-9203(85)90091-4. [DOI] [PubMed] [Google Scholar]
- Takashima I, Rosen L. Horizontal and vertical transmission of Japanese encephalitis virus by Aedes japonicus (Diptera: Culicidae) J Med Entomol. 1989;26:454– 458. doi: 10.1093/jmedent/26.5.454. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Mizusawa K, Saugstad ES. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara Islands) and Korea (Diptera: Culicidae) Contrib Am Entomol Inst. 1979;16:1–987. [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition on response to temperatures. J Med Entomol. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Thielman A, Hunter FF. Establishment of Aedes japonicus (Diptera: Culicidae) in Ontario, Canada. J Med Entomol. 2006;43:138–142. doi: 10.1603/0022-2585(2006)043[0138:eoojdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Vilà M, Basnou C, Pysek P, Josefsson M, Genovesi P, Gollasch S, Nentwig W, Olenin S, Roques A, Roy D, Hulme PE DAISIE partners. How well do we understand the impacts of alien species on ecosystem services? A pan-European cross-taxa assessment. Front Ecol Environ. 2010;8:135–144. [Google Scholar]
- Werner BG. Arbovirus surveillance and testing in Massachusetts, 2001. Proceedings of the 47th Annual Meeting of the Northeastern Mosquito Control Association; December 3–5, 2001; Salem, MA. 2001. [Google Scholar]
- White DJ, Kramer LD, Backenson PB, Lukacik G, Johnson G, Oliver J, Howard JJ, Means RG, Eidson M, Gotham I, Kulasekerea V, Campbell S and the Arbo-virus Research Laboratory and the Statewide West Nile Virus Response Teams. Mosquito surveillance and polymerase chain reaction detection of West Nile virus, New York State. Emerg Infect Dis. 2001;7:643– 649. doi: 10.3201/eid0704.010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA. Tires as habitats for mosquitoes: a review of studies within the eastern United States. J Med Entomol. 2008;45:581–593. doi: 10.1603/0022-2585(2008)45[581:tahfma]2.0.co;2. [DOI] [PubMed] [Google Scholar]