Abstract
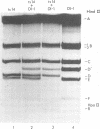
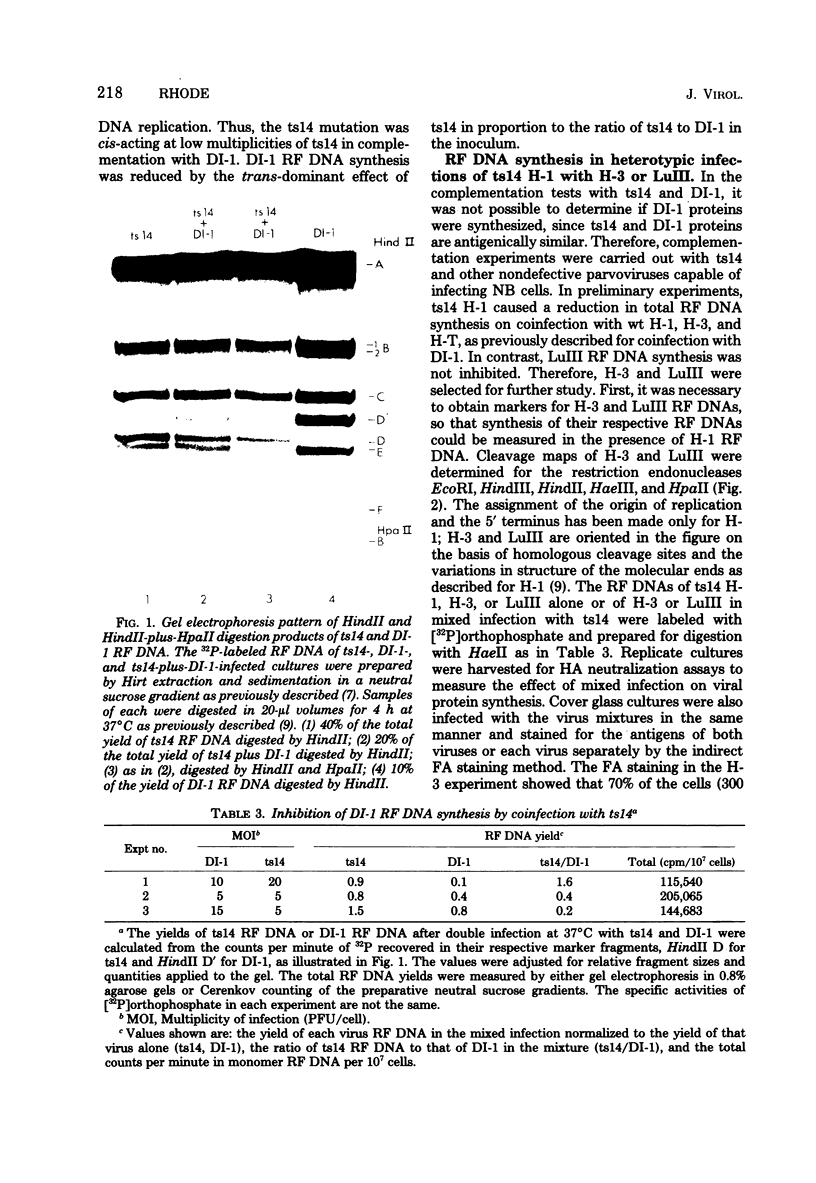
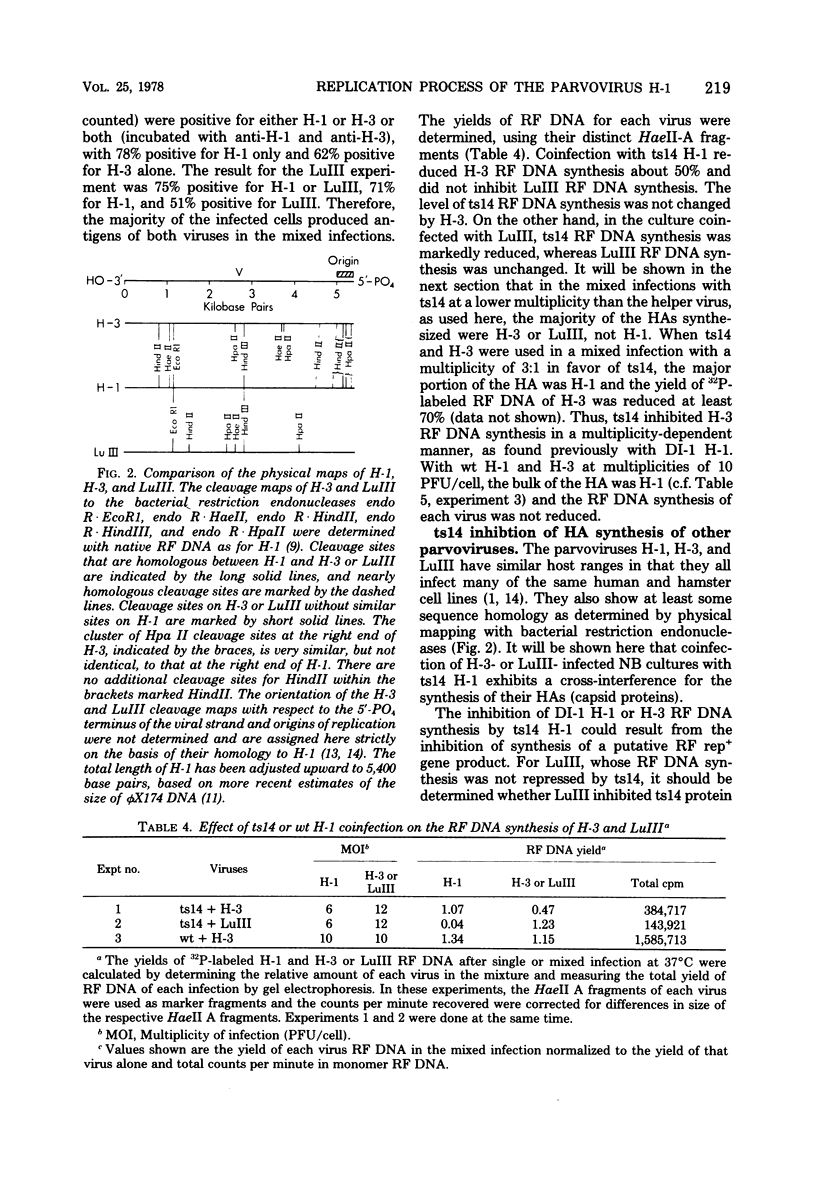
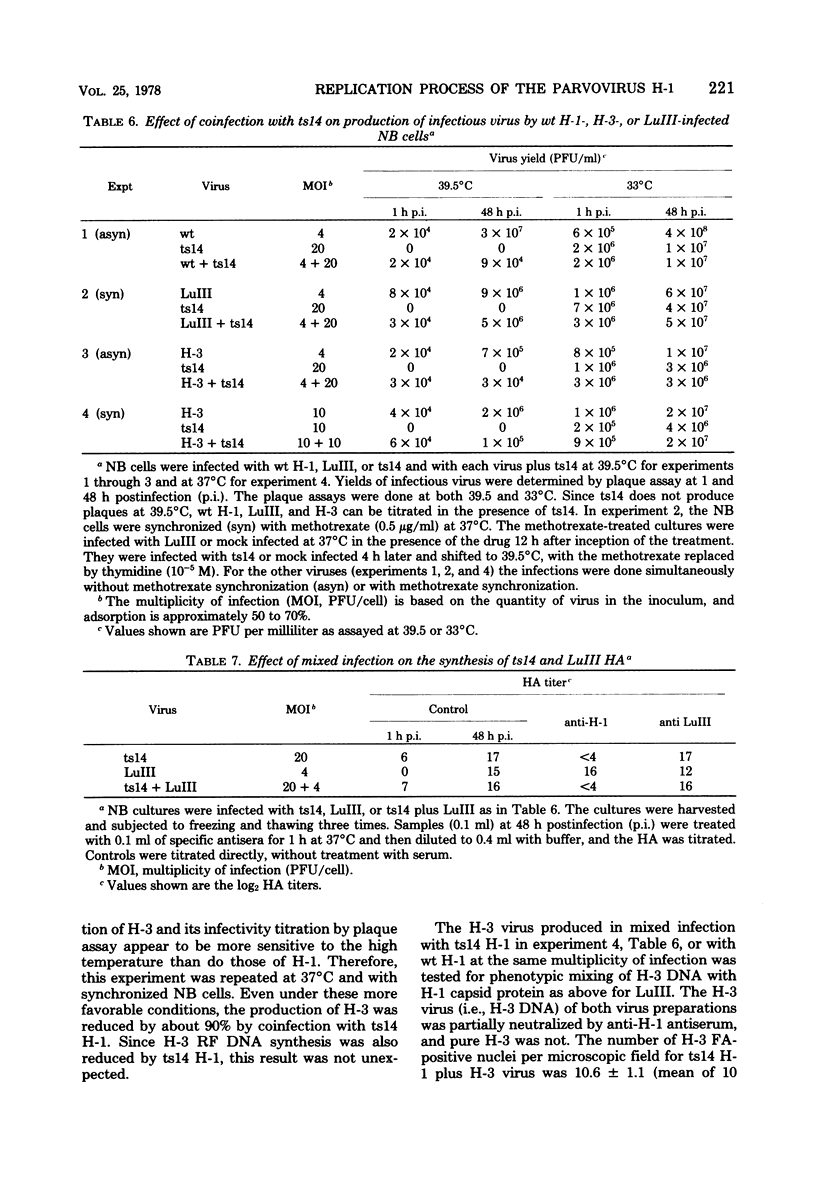
A temperature-sensitive mutant of H-1, ts14, that is partially defective in replicative-form (RF) DNA synthesis has been isolated. ts14 H-1 is characterized by a decrease in plaque-forming ability and production of infectious virus at the restrictive temperature of 39.5 degrees C. RF DNA synthesis of ts14 is reduced to 3 to 7% of that of wild-type H-1 at either the restrictive or the permissive temperature. A complementation analysis of RF synthesis of ts14 and a viable defective H-1 virus, DI-1, or wild-type H-3 indicates that the defective RF DNA synthesis of ts14 is cis-acting. ts14, unlike wild-type H-1, causes a multiplicity-dependent inhibition of DI-1 or H-3, but not LuIII, RF DNA synthesis. Mixed infections of cells with two parvoviruses also exhibited a cross-interference for viral protein synthesis that was multiplicity dependent, ts14 inhibited infectious virus production of H-1 or H-3, but not LuIII. LuIII-or H-3-pseudotype particles were produced by coinfection with H-1. H-3 and H-1 showed similar interactions with ts14, and H-3 DNA was more homologous to H-1 than was LuIII by comparative physical mapping studies. The results suggest that ts14 is a mutant with a defect in a regulatory sequence of its DNA that influence RF DNA replication.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hallauer C., Siegl G., Kronauer G. Parvoviruses as contaminants of permanent human cell lines. 3. Biological properties of the isolated viruses. Arch Gesamte Virusforsch. 1972;38(4):366–382. doi: 10.1007/BF01262827. [DOI] [PubMed] [Google Scholar]
- Kongsvik J. R., Gierthy J. F., Rhode S. L., 3rd Replication process of the parvovirus H-1. IV. H-1-specific proteins synthesized in synchronized human NB kidney cells. J Virol. 1974 Dec;14(6):1600–1603. doi: 10.1128/jvi.14.6.1600-1603.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledinko N. Plaque assay of the effects of cytosine arabinoside and 5-iodo-2'-deoxyuridine on the synthesis of H-I virus particles. Nature. 1967 Jun 24;214(5095):1346–1347. doi: 10.1038/2141346a0. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1 V. Isolation and characterization of temperature-sensitive H-1 mutants. J Virol. 1976 Feb;17(2):659–667. doi: 10.1128/jvi.17.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. II. Isolation and characterization of H-1 replicative form DNA. J Virol. 1974 Feb;13(2):400–410. doi: 10.1128/jvi.13.2.400-410.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. III. Factors affecting H-1 RF DNA synthesis. J Virol. 1974 Oct;14(4):791–801. doi: 10.1128/jvi.14.4.791-801.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. IX. Physical mapping studies of the H-1 genome. J Virol. 1977 May;22(2):446–458. doi: 10.1128/jvi.22.2.446-458.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. VI. Characterization of a replication terminus of H-1 replicative-form DNA. J Virol. 1977 Feb;21(2):694–712. doi: 10.1128/jvi.21.2.694-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Replication process of the parvovirus H-1. VII. Electron microscopy of replicative-form DNA synthesis. J Virol. 1977 Feb;21(2):713–723. doi: 10.1128/jvi.21.2.713-723.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Replication process of the parvovirus H-1. VIII. Partial denaturation mapping and localization of the replication origin of H-1 replicative-form DNA with electron microscopy. J Virol. 1977 Feb;21(2):724–731. doi: 10.1128/jvi.21.2.724-731.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]