Abstract
Rationale
Alteration of dopamine neurotransmission in the prefrontal cortex, especially hypofunction of dopamine D1 receptors, contributes to psychotic symptoms and cognitive deficit in schizophrenia. D1 receptors signal through the cAMP/PKA second messenger cascade, which is modulated by phosphodiesterase (PDE) enzymes that hydrolyze and inactivate cyclic nucleotides. Though several PDEs are expressed in cortical neurons, the PDE4 enzyme family (PDE4A-D) has been implicated in the control of cognitive function. The best studied isoform, PDE4B, interacts with a schizophrenia susceptibility factor, disrupted in schizophrenia 1 (DISC1).
Objectives
We explore the control of mouse frontal cortex dopamine D1 receptor signaling and associated behavior by PDE4.
Results
Inhibition of PDE4 by rolipram induced activation of cAMP/PKA signaling in cortical slices and in vivo, leading to the phosphorylation of DARPP-32 and other postsynaptic and presynaptic PKA-substrates. Rolipram also enhanced DARPP-32 phosphorylation invoked by D1 receptor activation. Immunohistochemical studies demonstrated PDE4A, PDE4B and PDE4D expression in DARPP-32-positive neurons in layer VI of frontal cortex, most likely in D1 receptor-positive, glutamatergic corticothalamic pyramidal neurons. Furthermore, the ability of rolipram treatment to improve the performance of mice in a sensorimotor gating test was DARPP-32-dependent.
Conclusions
PDE4, which is co-expressed with DARPP-32 in D1 receptor-positive cortical pyramidal neurons in layer VI, modulates the level of D1 receptor signaling and DARPP-32 phosphorylation in the frontal cortex, likely influencing cognitive function. These biochemical and behavioral actions of PDE4 inhibitors may contribute to the hypothesized antipsychotic actions of this class of compounds.
Keywords: PDE4, DARPP-32, PKA, frontal cortex, prepulse inhibition, rolipram
Introduction
Dopamine neurotransmission in the prefrontal cortex (PFC) contributes to emotional state and cognition. As an integral component of the mesolimbic system, the PFC receives dopaminergic projections from the ventral tegmental area (VTA), and activation of cortical dopamine D1 receptors improves cognition (Ehrman et al. 2006; Goldman-Rakic et al. 2004). Dopamine D1 receptor expression is reduced in the PFC of drug-naive and drug-free schizophrenic patients, and the reduction correlates with the severity of the negative symptoms and cognitive impairment that are core features of the disease (Okubo et al. 1997; Tamminga 2006). Thus, dopamine D1 receptor signaling in the PFC serves as a therapeutic target for the treatment of negative symptoms and cognitive impairment common in schizophrenic patients.
Activation of Gs-coupled dopamine D1 receptors causes an increase in cAMP synthesis, while the subsequent hydrolysis of cAMP is mediated by phosphodiesterases (PDEs). PDEs are subdivided into eleven families, encoded by 21 genes (Conti and Beavo 2007). PDEs representing at least three enzyme families, namely PDE1B, PDE2A and PDE4 (A, B and D subtypes), are expressed in cortical neurons (Cherry and Davis 1999; Hebb et al. 2004; Menniti et al. 2006; Noyama and Maekawa 2003; Zhu et al. 2001).
PDE4 is a cAMP-specific PDE with high affinity for cAMP (Km 1-10 μM) (Beavo 1995; Bender and Beavo 2006). The PDE4 family is encoded by four genes (PDE4A-PDE4D), and each isoform has multiple variants (Houslay and Adams 2003; McCahill et al. 2008). Each PDE4 variant has a modular structure consisting of a variant-specific N-terminal domain, regulatory domains termed upstream conserved region 1 (UCR1) and UCR2, and a catalytic domain. The phosphorylation of UCR1 by PKA disrupts the inhibitory interaction of UCR2 with the catalytic domain and activates PDE4 activity (McCahill et al. 2008), leading to the downregulation of cAMP/PKA signaling. The N-terminal domain and UCR1/2 interact with PDE4 variant-specific binding proteins including A-kinase anchor proteins (AKAP) and disrupted in schizophrenia 1 (DISC1), leading to the compartmentalization of cAMP signaling in cells (Houslay 2010; McCahill et al. 2008).
PDE4 enzymes have been implicated as therapeutic targets for schizophrenia (Millar et al. 2007; Siuciak 2008) and depression (Zhang 2009) as well as asthma and chronic obstructive pulmonary disease (Houslay et al. 2005; Huang and Mancini 2006). The involvement of PDE4B in the molecular mechanisms of schizophrenia is supported by its interaction with DISC1 (Clapcote et al. 2007; Millar et al. 2005; Murdoch et al. 2007), which is a genetic susceptibility factor for schizophrenia (Chubb et al. 2008), and by the association of PDE4B polymorphisms with schizophrenia (Fatemi et al. 2008; Numata et al. 2008; Pickard et al. 2007). Modulation of PDE4 activity has been implicated in the cognitive and sensorimotor gating deficits seen in schizophrenic patients. Sensorimotor gating deficits in schizophrenia, for example, can be modeled in animals in a cross-species measure of sensorimotor function called prepulse inhibition (PPI) (Braff et al. 2001). Interestingly, inhibition of PDE4 by a selective inhibitor, rolipram, can reverse the disruption of PPI in mice caused by psychotomimetic agents such as amphetamine, suggesting antipsychotic effects of PDE4 inhibitors (Kanes et al. 2007; Tamminga 2006).
Many of the actions of dopamine are mediated through signal transduction pathways that involve DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa) (Greengard et al. 1999; Svenningsson et al. 2004). When DARPP-32 is phosphorylated by cAMP-dependent protein kinase (PKA) on Thr34, it is converted into a potent inhibitor of protein phosphatase-1, and thereby controls the phosphorylation state and activity of many downstream physiological effectors. These pathways have been most well characterized in the striatum. For example, we have recently reported that PDE4 inhibition by rolipram induces a small increase in cAMP/PKA/DARPP-32 signaling in striatal medium spiny neurons, in addition to the large increase in cAMP/PKA signaling at presynaptic dopaminergic terminals (Nishi et al. 2008). DARPP-32 is also abundant in certain cortical neurons. DARPP-32 in frontal cortex has been implicated in regulation of synaptic plasticity between hippocampal and PFC neurons (Hotte et al. 2007), D1 receptor-mediated cognition (Hotte et al. 2006), and sensorimotor gating (Svenningsson et al. 2003). Dysregulation in DARPP-32 signaling has also been implicated in the dysfunction of PFC in schizophrenic patients (Albert et al. 2002).
Here, we investigated the role of PDE4 in the regulation of dopamine D1 receptor/PKA/DARPP-32 signaling in cortical neurons and in sensorimotor gating processes that affect cognitive function. We found that the PDE4 inhibitor, rolipram, enhances the dopamine D1 receptor/PKA/DARPP-32 signaling cascade in cortical neurons, and that DARPP-32 plays a critical role in the process of sensorimotor gating and its regulation by the PDE4 inhibitor.
Materials and Methods
Preparation and incubation of neostriatal slices
Male C57BL/6N mice at 6-8 weeks old were purchased from Japan SLC (Shizuoka, Japan). All mice used in this study were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the U.S. National Institutes of Health, and the specific protocols were approved by the Institutional Animal Care and Use Committee of Kurume University School of Medicine. C57BL/6N mice were sacrificed by decapitation. The brains were rapidly removed and placed in ice-cold, oxygenated Krebs-HCO3− buffer (124 mM NaCl, 4 mM KCl, 26 mM NaHCO3, 1.5 mM CaCl2, 1.25 mM KH2PO4, 1.5 mM MgSO4 and 10 mM D-glucose, pH 7.4). Coronal slices (350 μm) were prepared from the brain between 1.6 and 2.8 mm anterior to bregma using a vibrating blade microtome, VT1000S (Leica Microsystems, Nussloch, Germany). Frontal cortices were dissected from the slices in ice-cold Krebs-HCO3− buffer. Each slice was placed in a polypropylene incubation tube with 2 ml fresh Krebs-HCO3− buffer containing adenosine deaminase (10 μg/ml). The slices were preincubated at 30°C under constant oxygenation with 95 % O2/5% CO2 for 60 min. The buffer was replaced with fresh Krebs-HCO3− buffer after 30 min of preincubation. Adenosine deaminase was included during the first 30 min of preincubation to counter the increase in adenosine levels during slice preparations and minimize the variability among slices. Slices were treated with drugs as specified in each experiment. Drugs were obtained from the following sources: rolipram from Tocris Cookson (Bristol, UK); SKF81297 and SCH23390 from Sigma-Aldrich (St. Louis, MO). After drug treatment, slices were transferred to Eppendorf tubes, frozen on dry ice, and stored at −80°C until assayed.
Frozen tissue samples were sonicated in a solution of boiling 1% sodium dodecyl sulfate (SDS) containing 50 mM sodium fluoride, then boiled for an additional 10 min. Small aliquots of the homogenate were retained for protein determination by the BCA protein assay method (Pierce, Rockford, IL). Equal amounts of protein (200 μg) were separated by SDS/polyacrylamide gel electrophoresis (10% polyacrylamide gels), and transferred to nitrocellulose membranes (0.2 μm) (Schleicher and Schuell, Keene, NH).
Preparation of cortical tissues for analysis of protein phosphorylation in vivo
Mice were injected intraperitoneally (i.p.) with rolipram (10 mg/kg), or with drug-free vehicle (controls, 5 ml/kg body weight). The vehicle for rolipram contained (final concentration) 5% DMSO + 5% Tween-20 + 15% polyethylene glycol 400 + 75% water. Mice were sacrificed 15 min after injection by focused microwave cranial irradiation (4.5 -5.0 kW for 1.3 s) using a small animal microwave (Muromachi Kikai, Tokyo, Japan), which inactivates protein kinases and phosphatases to preserve in vivo levels of protein phosphorylation (O’Callaghan and Sriram 2004). Frontal cortices were dissected from each mouse brain, frozen in liquid nitrogen, and stored at −80°C until assayed.
Frozen samples of brain tissue were processed as described for slices. Equal amounts of protein (15-30 μg) were loaded on 10% polyacrylamide BIS-Tris gels (Bio-Rad, Hercules, CA), separated by electrophoresis, and transferred to nitrocellulose membranes (0.2 μM) (Schleicher and Schuell).
Immunoblotting
The membranes were immunoblotted using phosphorylation state-specific antibodies raised against phospho-peptides mainly expressed in postsynaptic neurons [phospho-Thr34 DARPP-32, a site phosphorylated by PKA (CC500; 1:500 dilution); phospho-Thr75 DARPP-32, the site phosphorylated by Cdk5 (1:2,000 dilution) [Bibb, 1999 #423]; phospho-Ser845 GluR1, a site phosphorylated by PKA (p1160-845; 1:250 dilution) (PhosphoSolutions, Aurora, CO); phospho-Ser831 GluR1, a site phosphorylated by PKC (1:1,000 dilution) (Millipore, Billerica, MA); phospho-Ser897 NR1, a site phosphorylated by PKA (1:500 dilution) (Millipore); phospho-Thr202/Tyr204 ERK (1:2,000 dilution) (New England BioLabs, Beverly, MA); phospho-Ser133 PDE4, a site phosphorylated by PKA (1:5,000 dilution] and mainly expressed in presynaptic neurons [phospho-Ser40 tyrosine hydroxylase, a site phosphorylated by PKA (AB5935; 1:500 dilution) (Chemicon, Temecula, CA); phospho-Ser9 synapsin I, a site phosphorylated by PKA and CaMKI (RU440, 1:6,000 dilution); phospho-Ser549 synapsin I, a site phosphorylated by MAP kinase (G555, 1:5,000 dilution; #2311, 1:1,000 dilution, Cell Signaling Technology, Danvers, MA); phospho-Ser603 synapsin I, a site phosphorylated by CaMKII and IV (1:10,000 dilution) (Millipore)]. Monoclonal antibody generated against DARPP-32 (C24-5a; 1:7,500 dilution), which is not phosphorylation state-specific, was used to determine the total amount of DARPP-32. The rabbit polyclonal phospho-Ser133 PDE4 antibody was raised against a conserved PDE4 phosphopeptide encompassing the PKA site in the UCR1 region of long PDE4 isoforms (MacKenzie et al. 2002; Sette and Conti 1996) and affinity purified using the same phosphopeptide (D.R. Benavides, K. Hayashi, and J. Bibb, manuscript in preparation).
The membranes were incubated with goat anti-mouse or rabbit Alexa 680-linked IgG (1:5,000 dilution) (Molecular Probes, Eugene, OR) or goat anti-mouse or rabbit IRDyeTM800-linked IgG (1:5,000 dilution) (Rockland Immunochemicals, Gilbertsville, PA). Fluorescence at infrared wavelengths was detected by the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE), and quantified using Odyssey software. In an individual experiment, samples from control and drug-treated slices or mice were analyzed on the same immunoblot. For each experiment, values were normalized to values obtained from control samples. Normalized data from multiple experiments were averaged and statistical analysis was carried out as described in the figure legends.
Immunohistochemistry
Under deep anesthesia induced with sodium pentobarbital, male C57BL/6N mice or GAD67-GFP knock-in mice (Tamamaki et al. 2003) at 6-8 weeks old were perfused rapidly through the left ventricle with 50 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) at room temperature. Serial coronal sections 50 μm in thickness were cut with a vibrating microtome, VT1000S (Leica Microsystems). Sections were processed for immunohistochemistry with the use of the free-floating method, as described (Fukuda et al. 1996). Sections were incubated with a rabbit anti-PDE4A antibody (sc-25811; 1:200) (Santa Cruz Biotechnology), a rabbit anti-PDE4B antibody (sc-25812; 1:200) (Santa Cruz Biotechnology), a rabbit anti-PDE4C antibody (sc-25813; 1:200) (Santa Cruz Biotechnology), a rabbit anti-PDE4D antibody (sc-25814; 1:200) (Santa Cruz Biotechnology) or a mouse anti-DARPP-32 antibody (C24-5a; 1:5,000 dilution) at 20°C for 7 days. Antibody binding was visualized with a fluorescein isothiocyanate (FITC)-conjugated donkey anti-mouse IgG (1:100; Jackson ImmunoResearch, West Grove, PA) and a rhodamine red-conjugated donkey anti-mouse or rabbit IgG (1:100; Jackson ImmunoResearch). Sections were mounted in Vectashield (Vector Laboratories, Burlingame, CA), and fluorescence of FITC, rhodamine-red and GFP in the sections was observed under optimal sensitivity conditions with a confocal laser-scanning microscope, LSM 5 PASCAL (Zeiss, Oberkochen, Germany).
Prepulse inhibition (PPI) test
Startle response and PPI behavioral tests were conducted using male DARPP-32 knockout and wild-type mice at 6-10 weeks-old using SR-LAB (San Diego Instruments, Inc., San Diego, CA). DARPP-32 knockout and wild-type mice were generated from the offspring of homozygous mutant and wild-type mating pairs, respectively (Fienberg et al. 1998). These mice have been backcrossed and maintained on a C57BL/6 background for more than 10 generations. For all sessions a 5 min acclimation period in the startle chamber, during which time only background noise (65 dB) was presented, preceded the testing session. The testing session began with five 40 ms startle pulses of 120 dB in an effort to make subsequently measured startle responses less variable. Startle responses were measured to 40 ms pulses of 70, 85, 90, 95, 100, 105, 110, and 115 dB, presented each 5 times in a pseudo-random order with an intertrial interval randomized from 10 to 20 s. PPI trials consisted of 20 ms white noise acoustic prepulse of 69, 73, 77 and 81 dB, followed 100 ms after the cessation of the prepulse by a 40 ms acoustic startle pulse of 120 dB. Five trials at each prepulse intensity level along with 10 startle only trials (i.e. trials with no prepulse) were presented in a pseudo-random order. Maximum startle amplitude of each trial was calculated from startle responses recorded for 60 ms after the onset of the startle pulse, and the data were averaged within each trial type. The first 5 startle-alone trials were not included in the prepulse inhibition calculation. Rolipram was dissolved in 5% DMSO + 0.5% Tween 20 + 1.5% polyethylene glycol 400 + 93% water (final concentration). Rolipram or vehicle was injected intraperitoneally 15 min prior to the PPI session at a dose of 3.0 mg/kg, with mice remaining in a holding cage during the injection-session interim. PPI was calculated as percentage score for each acoustic prepulse trial-type according to the following formula: [(startle pulse trial–PPI trial) / startle pulse trial]×100.
Results
Effect of a PDE4 inhibitor, rolipram, on DARPP-32 phosphorylation in mouse cortical slices
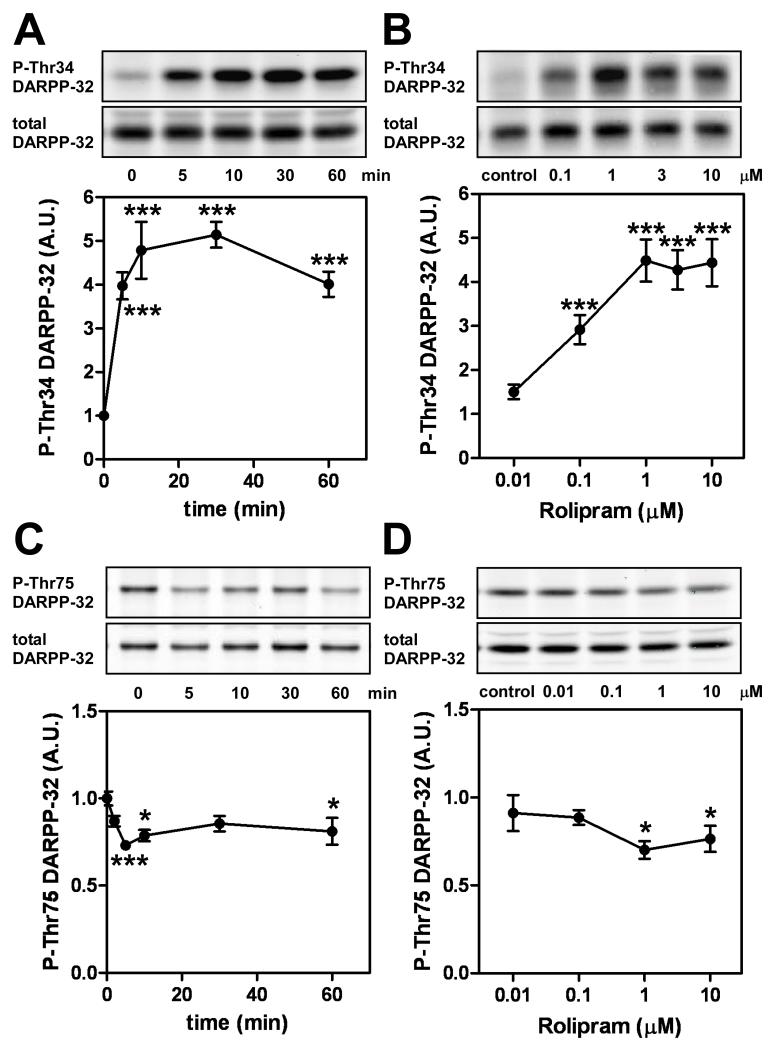
The role of PDE4 in D1 receptor/PKA/DARPP-32 signaling in cortical neurons was investigated using a PDE4 selective inhibitor, rolipram (Houslay et al. 2005; Huang and Mancini 2006). The expression of DARPP-32 in the cortex is ~10% of that in the striatum (Ouimet et al. 1984; Walaas and Greengard 1984). Nonetheless, DARPP-32 phosphorylation at Thr34 and Thr75 was reliably detected in untreated mouse cortical slices. Treatment of cortical slices with rolipram (1 μM) increased the level of phospho-Thr34 DARPP-32 (PKA-site), by ~5-fold [F(4,19) = 39.23, p < 0.001] (Fig. 1A). A maximal increase in DARPP-32 Thr34 phosphorylation was observed at 10-60 min of incubation. DARPP-32 Thr34 phosphorylation at 60 min of incubation was maximally increased by a 1 μM concentration of rolipram, with a half maximal effect at ~0.1 μM [F(5,35) = 26.50, p < 0.001] (Fig. 1B). Based on these observations, rolipram was used at 1 μM in other studies using cortical slices. Treatment with rolipram (1 μM) decreased the level of phospho-Thr75 DARPP-32 (Cdk5-site) to 70% of control, presumably by activation of protein phosphatase 2A associated with phosphorylation of B56δ regulatory subunit by PKA (Ahn et al. 2007). The rolipram-induced decrease in DARPP-32 Thr75 phosphorylation occurred after 5 min of incubation and was sustained for at least 60 min [F(5,24) = 6.110, p < 0.001] (Fig. 1C). Significant decreases in DARPP-32 Thr75 phosphorylation were induced by 1 μM or higher rolipram treatments [F(4,32) = 3.870, p < 0.05] (Fig. 1D). Treatment of cortical slices with papaverine (10 μM), an inhibitor of PDE10A, had no effect on DARPP-32 phosphorylation at Thr34 or Thr75 (data not shown), an observation consistent with the low level of PDE10A expression reported in rodent cortex (Seeger et al. 2003).
Fig. 1. Effects of a PDE4 inhibitor, rolipram, on DARPP-32 phosphorylation at Thr34 and Thr75 in cortical slices.
Time-course and dose-response effects for rolipram on phospho-Thr34 (A, B) and phospho-Thr75 (C, D) DARPP-32 are shown. Mouse cortical slices were incubated with rolipram (1 μM) for the indicated times (A, C) or with the indicated concentrations of rolipram for 60 min (B) or 5 min (D). Typical immunoblots for phospho-Thr34, phospho-Thr75 and total DARPP-32 are shown with quantitation. Data represent means ± SEM for 4-8 experiments. *p < 0.05, ***p < 0.001 compared with untreated slices; one-way ANOVA followed by Newman-Keuls test
Effect of rolipram on postsynaptic PKA-dependent signaling: phosphorylation of GluR1, NR1, PDE4 and ERK2
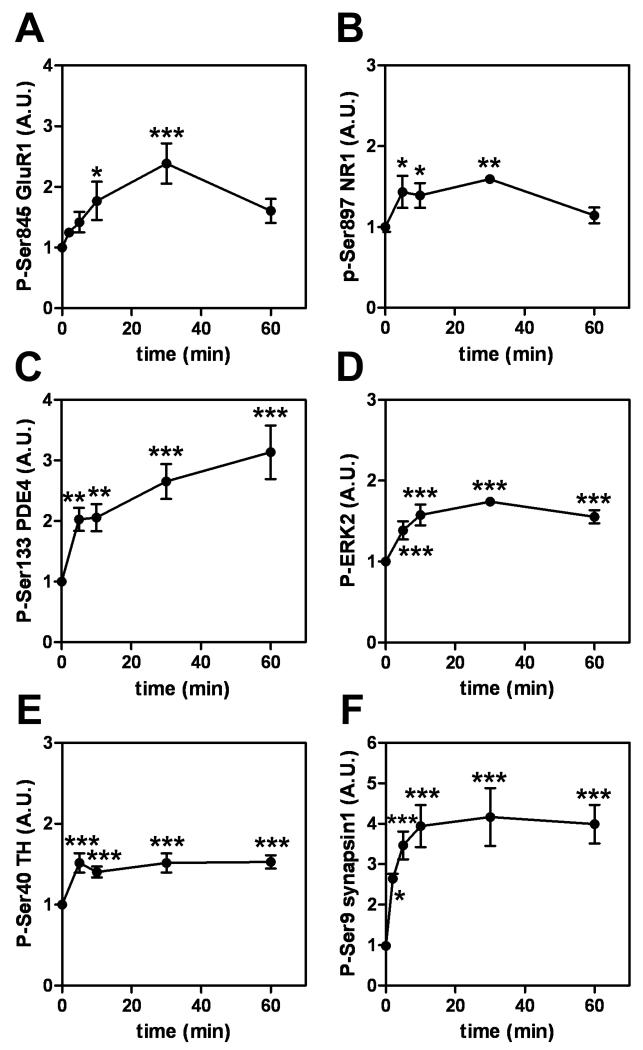
Glutamate receptors mediate excitatory synaptic transmission in the cortex, and the phosphorylation of AMPA and NMDA receptors by PKA enhances their function (Chen and Roche 2007; Roche et al. 1996). Therefore, we examined the effect of rolipram on the phosphorylation-states of the AMPA receptor, GluR1, at Ser845 and the NMDA receptor, NR1, at Ser897 in cortical slices. Slices were treated with a concentration of rolipram (1 μM) that induced maximal effects on DARPP-32 phosphorylation. Phosphorylation of GluR1 at Ser845 [F(5,54) = 7.234, p < 0.001] and NR1 at Ser897 [F(4,21) = 6.724, p < 0.01] was maximally increased after 30 min of incubation with rolipram (Fig. 2A, B). PDE4 long isoforms containing UCR1 are also a substrate for PKA (MacKenzie et al. 2002; Sette and Conti 1996). Phosphorylation of PDE4 by PKA at a residue (Ser133) within UCR1 reduces autoinhibition and increases enzyme activity (Sette and Conti 1996). Rolipram treatment (1 μM) significantly increased the level of phospho-Ser133 PDE4, especially at the migrating position of PDE4B1, within 5 min of incubation, with a level 3-fold over baseline at 60 min [F(4,25) = 15.80, p < 0.001] (Fig. 2C). The effect of rolipram on ERK, a downstream target of PKA signaling, was also examined. Treatment with rolipram (1 μM) increased the level of phospho-Thr202/Tyr204 ERK2 by ~2-fold within 10 min of incubation [F(4,19) = 19.23, p < 0.001] (Fig. 2D). Collectively, the results suggest that inhibition of PDE4 by rolipram induces activation of cAMP/PKA signaling in cortical neurons, leading to the phosphorylation of a wide-spectrum of substrates for PKA or PKA-dependent signaling; GluR1, NR1, PDE4 and ERK as well as DARPP-32.
Fig. 2. Effect of rolipram on the phosphorylation of GluR1, NR1, PDE4, ERK2, TH and synapsin I in cortical slices.
Cortical slices were treated with rolipram (1 μM) for the indicated times. Quantitation of phosphorylation of GluR1 at Ser845 (the PKA-site) (A), NR1 at Ser897 (the PKA-site) (B), PDE4 at Ser133 (the PKA-site) (C), ERK2 at Thr202/Tyr204 (the MEK-site) (D), TH at Ser40 (the PKA-site) (E), and synapsin I at Ser9 (the PKA/CaMKI-site) (F) is shown. Data represent means ± SEM for 4-15 experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with untreated slices; one-way ANOVA followed by Newman-Keuls test
Effect of rolipram on presynaptic PKA-dependent signaling: phosphorylation of tyrosine hydroxylase (TH) and synapsin I
We also examined the effect of rolipram on the phosphorylation of PKA substrates that are enriched in presynaptic nerve terminals in cortex, namely TH and synapsin I. Phosphorylation of TH, the rate limiting enzyme in dopamine biosynthesis which is abundant in catecholaminergic nerve terminals, at Ser40 is known to play an important role in the synthesis of dopamine (Dunkley et al. 2004; Harada et al. 1996). Synapsin I, which is widely distributed in nerve terminals in the brain is phosphorylated at Ser9, affecting the release of neurotransmitters (Fiumara et al. 2004). Treatment of cortical slices with rolipram (1 μM) resulted in a small but significant increase in the level of phospho-Ser40 TH within 5 min of incubation [F(4,63) = 13.09, p < 0.001] (Fig. 2E). Rolipram (1 μM) increased synapsin I Ser9 phosphorylation maximally by 4-fold within 10 min of incubation [F(5,84) = 16.09, p < 0.001] (Fig. 2F).
Rolipram enhances dopamine D1 receptor signaling in cortical neurons
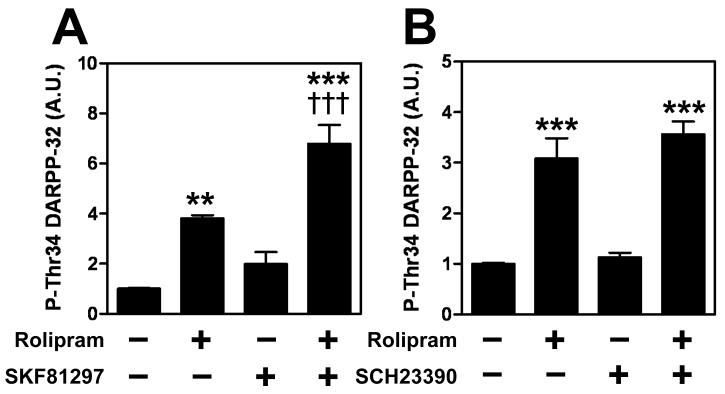
The prefrontal cortex is involved in cognitive processes, and activation of dopamine D1 receptors can improve cognitive function (Chudasama and Robbins 2004; Fletcher et al. 2005; Sawaguchi and Goldman-Rakic 1991). We examined the ability of rolipram to modify DARPP-32 Thr34 phosphorylation stimulated by a dopamine D1 receptor agonist, SKF81297 (1 μM) (Fig. 3A). Treatment of cortical slices with SKF81297 alone increased the level of phospho-Thr34 DARPP-32 by ~2-fold [F(3,14) = 23.34, p < 0.001]. The effect of SKF81297 on DARPP-32 Thr34 phosphorylation was enhanced by rolipram. The results indicate that PDE4 activity may modulate the impact of dopamine D1 receptor activity on cAMP/PKA signaling in cortical neurons.
Fig. 3. Effect of rolipram on dopamine D1 receptor/DARPP-32 signaling in cortical slices.
Cortical slices were treated with (A) rolipram (1 μM) and/or a dopamine D1 receptor agonist, SKF81297 (1 μM), or (B) rolipram (1 μM) and/or a dopamine D1 receptor antagonist, SCH23390 (1 μM), for 10 min. Quantitation of phospho-Thr34 DARPP-32 is shown. Data represent means ± SEM for 4 experiments. ***p < 0.001 compared with control; †††p < 0.001 compared with rolipram alone; one-way ANOVA followed by Newman-Keuls test
To evaluate the contribution of dopamine D1 receptor signaling to the rolipram-induced increase in DARPP-32 Thr34 phosphorylation, the effect of rolipram was examined in the presence of a dopamine D1 receptor antagonist, SCH23390 (Fig. 3B). Treatment of cortical slices with SCH23390 (1 μM) had no effect on either the basal or rolipram-stimulated levels of phospho-Thr34 DARPP-32 [F(3,20) = 25.45, p < 0.001]. To determine neurotransmitter receptors which couple to cAMP/PKA signaling and are tonically active in cortical neurons, we examined the effects of a non-selective β-adrenergic antagonist (propranolol), an adenosine A2A receptor antagonist (ZM241385), a 5-HT4 receptor antagonist (GR113808), and a 5-HT6 receptor antagonist (SB258585) on the rolipram-induced increase in DARPP-32 Thr34 phosphorylation, but none of these treatments attenuated the increase (data not shown).
Effects of rolipram on postsynaptic and presynaptic PKA-dependent signaling in vivo
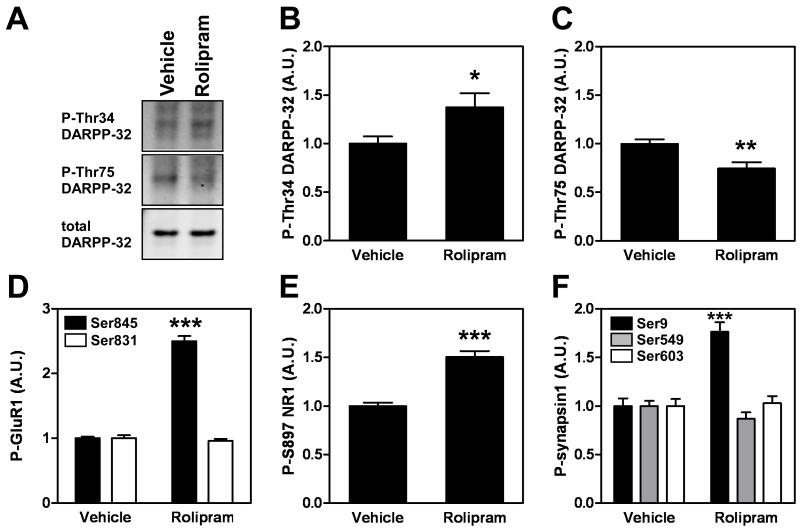
We next examined the role of PDE4 in intact animals. Consistent with the results from slices, rolipram caused an increase in phospho-Thr34 DARPP-32 (~1.5-fold, Fig. 4A, B) and a decrease in phospho-Thr75 DARPP-32 (72 ± 4% of control, Fig. 4C) in the frontal cortex 15 min after a single injection (10 mg/kg, i.p). This treatment also increased the phosphorylation of other postsynaptic and presynaptic PKA substrates; GluR1 at Ser845, NR1 at Ser897, and synapsin I at Ser9 (Fig. 4D-F). In contrast, the phosphorylation sites for other kinases such as GluR1 at Ser831 (PKC-site) and synapsin I at Ser549 (MAPK-site) or Ser603 (CaMKII- and IV-site) were not affected by rolipram. These results indicate that PDE4 plays a significant role in the regulation of cAMP/PKA signaling in cortical neurons under in vivo conditions.
Fig. 4. Effect of rolipram on the phosphorylation of DARPP-32, GluR1, NR1 and synapsin I in the frontal cortex in vivo.
Mice were injected with vehicle or rolipram (10 mg/kg). Frontal cortex was dissected 15 min later and analyzed for the phosphorylation of DARPP-32 at Thr34 (B) and Thr75 (C), GluR1 at Ser845 and Ser831 (D), NR1 at Ser897 (E), and synapsin I at Ser9, Ser549 and Ser603 (F). Typical immunoblots for detection of phospho-Thr34, phospho-Thr75 and total DARPP-32 are shown in (A). Data represent means ± SEM for 12 experiments. *p <0.05, **p < 0.01, ***p < 0.001 compared with vehicle treated mice; Student’s t-test
Expression of PDE4 subtypes in DARPP-32-positive cortical neurons
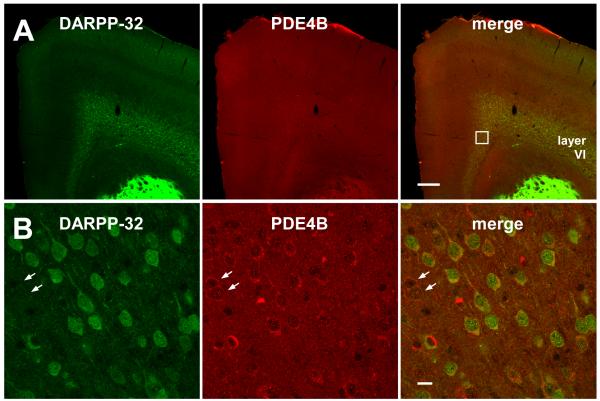
Considering the relationship between DARPP-32 and PDE4 revealed by our pharmacological studies and the importance of PDE4B in schizophrenia (Millar et al. 2005; Murdoch et al. 2007; Numata et al. 2008; Pickard et al. 2007), the histological expression patterns of DARPP-32 and PDE4B were first analyzed in mouse cortex. Strong immunostaining for DARPP-32 in the caudate putamen, nucleus accumbens and olfactory tubercle occurred under conditions in which it was much more weakly detected in the cortex (Fig. 5A and 6A). Immunoreactivity for DARPP-32 has been previously reported in rat and monkey cortex, mainly in layer VI, with sparse staining in layer II-III (Ouimet 1991; Ouimet et al. 1992; Ouimet et al. 1984). In agreement, DARPP-32-positive neurons were mainly detected in layer VI of the mouse frontal cortex. A uniform distribution of PDE4B was observed in the frontal cortex, and the expression of PDE4B was detected in all DARPP-32-positive neurons in the cingulate cortex (Fig. 5B). In addition, some PDE4B immunoreactivity was also detected in DARPP-32-negative neurons (Fig. 5B, arrows).
Fig. 5. Expression of PDE4B in the cortex.
Double immunostaining of mouse brain tissues (A) and cortical tissues (cingulate cortex, area 1) (B) with DARPP-32 and PDE4B antibodies. High magnification pictures in cingulate cortex, area 1 (B) correspond to the boxed area in the merged picture of brain tissues (A). Arrows indicate neurons expressing PDE4B, but not DARPP-32. Scale bars, 250 μm for A and 10 μm for B
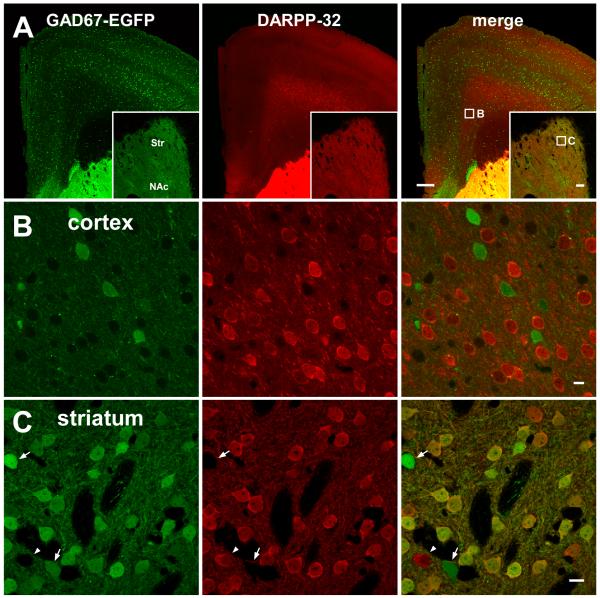
Fig. 6. Expression of DARPP-32 in cortical and striatal tissues from GAD67-GFP knock-in mice.
Immunostaining of brain tissues (A), cortical tissues (B), and striatal tissues (C) from GAD67-GFP knock-in mice with a DARPP-32 antibody. Fluorescence of GFP and rhodamine-red for the DARPP-32 antibody was detected. In brain tissues (A), striatal, but not cortical, signals for GAD-EGFP and DARPP-32 are saturated, and therefore images of striatum (Str) and nucleus accumbens (NAc) obtained with reduced sensitivity conditions are shown (A, insets). High magnification pictures in cingulate cortex, area 1 (B) and dorsal striatum (C) correspond to the boxed areas in the merged picture of brain tissues (A and inset). Arrows indicate DARPP-32-negative/GAD67-EGFP-positive neurons, and an arrowhead indicates DARPP-32-positive/GAD67-EGFP-negative neurons. Scale bars, 250 μm for A, 100 μm for A inset and 10 μm for B and C
We next analyzed the expression of other PDE4 subtypes (PDE4A, PDE4C and PDE4D) in DARPP-32-positive neurons in the cingulate cortex. PDE4A staining was observed homogeneously in layer VI of the mouse frontal cortex, and PDE4A was weakly expressed in DARPP-32-positive neurons (Online Resource 1A). PDE4C staining was observed in limited cells, but not in DARPP-32-positive neurons (Online Resource 1B). PDE4D staining was diffuse in layer VI of the mouse frontal cortex (Online Resource 1C). DARPP-32 positive neurons expressed PDE4D more densely in neuropil than in soma (Online Resource 1D). These immunohistochemical findings support our biochemical data that DARPP-32 phosphorylation is under the control of PDE4 in cortical slices
Expression of DARPP-32 in GAD67-GFP knock-in mouse brain
We further analyzed the cell types of DARPP-32-positive cortical neurons. Excitatory glutamatergic cells that project distally and GABAergic inhibitory cells that innervate locally comprise the two major classes of cortical neurons. To distinguish these, we analyzed the brains of mice that express the fluorescent marker EGFP in GABAergic neurons throughout mouse brain, including cerebral cortex (Tamamaki et al. 2003). In these mice, EGFP expression is under control of the endogenous promoter for the major GABA biosynthetic enzyme, GAD67. In our hands, EGFP was expressed intensely in the striatum and weakly in the cortex (Fig. 6A). As in wild-type mice, DARPP-32 immunoreactivity was detected in deep layers (layer VI) of the cingulate cortex in GAD67-GFP knock-in mice. Interestingly, DARPP-32 expression did not overlap with GAD67-EGFP fluorescence in layer VI (Fig. 6B), or any other regions of the frontal cortex. These data suggest that DARPP-32-positive neurons in layer VI of the cingulate cortex, including a sub-population that co-express PDE4B, are non-GABAergic. These neurons likely represent dopaminoceptive, glutamatergic neurons that project to dorsal thalamus (Ouimet 1991).
In contrast to cortex, almost all DARPP-32-positive striatal neurons were GAD67-positive (Fig. 6C), which is consistent with previous reports that DARPP-32 is selectively enriched in GABAergic medium spiny neurons (Bateup et al. 2008; Ouimet et al. 1992; Ouimet et al. 1984; Walaas and Greengard 1984). DARPP-32-negative/GAD67-EGFP-positive neurons, which are likely GABAergic interneurons (Tamamaki et al. 2003), and DARPP-32-positive/GAD67-EGFP-negative neurons, were also detected. The identity of the observed DARPP-32-positive/GAD67-EGFP-negative neurons in the striatum is currently unknown.
Effect of a PDE4 inhibitor on prepulse inhibition in wild-type and DARPP-32 knockout mice
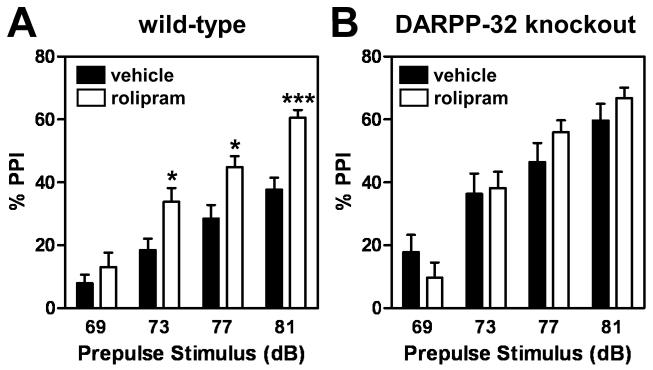
The inhibition of PDE4 by rolipram has been reported to increase PPI, suggesting PDE4 inhibition as a potential antipsychotic strategy (Kanes et al. 2007). To investigate whether DARPP-32 is involved in the machinery by which rolipram improves sensorimotor gating, we tested the effect of acute treatment with rolipram on PPI in wild-type and DARPP-32 knockout mice. Startle responses to acoustic pulse intensities from 70 to 115 dB were similar in wild-type and DARPP-32 knockout mice (data not shown). Startle responses to acoustic pulse at 120 dB, obtained during PPI trials, were not affected by rolipram (3 mg/kg, i.p.) either in wild-type or DARPP-32 knockout mice (data not shown). However, DARPP-32 knockout mice showed a significantly higher level of prepulse inhibition compared to control mice [genotype effect, F(1,99) = 7.865, p < 0.01], in agreement with a previous report on DARPP-32 knockout mice (Svenningsson et al. 2003) (Fig. 7). As expected from a previous report (Kanes et al. 2007), administration of rolipram to wild-type mice at 3.0 mg/kg (i.p.) [drug effect, F(1,96) = 10.52, p < 0.01] (Fig. 7A) as well as at a higher dose (10 mg/kg, i.p.) (data not shown) increased PPI. Interestingly, in DARPP-32 knockout mice, the ability of rolipram to increase PPI was not observed [drug effect, F(1,87) = 0.1591, p = 0.6929] (Fig. 7B). These results suggest that DARPP-32 plays a critical role in the process of sensorimotor gating and its regulation by the PDE4 inhibitor.
Fig. 7. Effect of rolipram on prepulse inhibition (PPI) of the startle response in wild-type (A) and DARPP-32 knockout (B) mice.
Mice were injected intraperitoneally with vehicle or rolipram (3.0 mg/kg) and tested 15 min later. The effect of stimulus intensity on % PPI is shown. Data represent means ± SEM for 15-19 mice in each experimental group. * p < 0.05, ***p < 0.001 compared with vehicle-treated mice; two-way repeated measures ANOVA followed by Bonferroni test
Discussion
Here we show that PDE4 activity regulates dopamine D1 receptor/cAMP/PKA signaling in mouse frontal cortex. The inhibition of PDE4 in acute cortical slices and in vivo by rolipram increases D1 receptor/cAMP/PKA signaling, leading to the phosphorylation of DARPP-32 at Thr34, GluR1 at Ser845, and other postsynaptic and presynaptic PKA-substrates. Immunohistochemical data support a direct influence of PDE4A, PDE4B and PDE4D on DARPP-32 signaling, as both proteins are co-expressed in a sub-population of cortical pyramidal neurons. Furthermore, the inhibition of PDE4 by rolipram in mice improves sensorimotor gating (PPI) in a DARPP-32-dependent manner. Thus, the PDE4 inhibitor, rolipram, regulates biochemical and behavioral outcomes of increased dopamine D1 receptor signaling in the frontal cortex, which may, in part, contribute to its reported antipsychotic actions.
Co-expression of DARPP-32 and PDE4B in cortical neurons
DARPP-32 is expressed prominently in layer VI and sparsely in layer II-III in the rat and monkey cortex (Ouimet 1991; Ouimet et al. 1992; Ouimet et al. 1984). DARPP-32-immunoreactive neurons in layer VI show morphological features of pyramidal neurons (Berger et al. 1990; Ouimet et al. 1984) that project to the thalamus (Ouimet 1991). These observations strongly suggest that DARPP-32 is expressed in glutamatergic, corticothalamic pyramidal neurons in the cortex. In the present study, DARPP-32 was mainly detected in layer VI of mouse frontal cortex. Immunohistochemical staining of cortex from GAD67-GFP knock-in mice, in which EGFP expression is driven in GABAergic neurons by activation of the GAD67 promoter (Tamamaki et al. 2003), revealed that DARPP-32 was exclusively expressed in EGFP-negative, non-GABAergic neurons. In contrast, as anticipated, DARPP-32 was highly co-expressed with EGFP in GABAergic medium spiny neurons in the striatum. Thus, our data in mice are consistent with the known distribution of DARPP-32 in other species and support the conclusion that DARPP-32 is most likely expressed in glutamatergic pyramidal neurons in layer VI of frontal cortex.
PDE4B has been implicated in schizophrenia by virtue of its interaction with the disrupted in schizophrenia 1 (DISC1) protein (Millar et al. 2005; Murdoch et al. 2007). Mutations in DISC1 have been linked to genetic susceptibility for schizophrenia (Chubb et al. 2008; Clapcote et al. 2007). Several single nucleotide polymorphisms (SNPs) in PDE4B are also associated with an altered genetic risk for schizophrenia (Fatemi et al. 2008; Numata et al. 2008; Pickard et al. 2007). Our immunohistochemical studies reveal the expression of PDE4B as well as PDE4A and PDE4D in DARPP-32-positive pyramidal neurons in layer VI of mouse frontal cortex. The pyramidal neurons are also known to express D1 receptors (Bergson et al. 1995; Goldman-Rakic et al. 1990). A study using in situ hybridization and retrograde labeling in rats demonstrated that D1 receptor mRNA is highly abundant in pyramidal neurons in layer VI of medial prefrontal cortex, and that corticothalamic neurons, mainly present in layer VI, contain D1 receptor mRNA (Gaspar et al. 1995). It is highly likely that PDE4 isoforms are co-expressed with dopamine D1 receptors and DARPP-32 in corticothalamic pyramidal neurons in layer VI of frontal cortex.
Enhancement of D1 receptor/cAMP/PKA/DARPP-32 signaling by PDE4 inhibitor
PDE4 is a cAMP-specific PDE with high affinity for cAMP (Km 1-10 μM) (Bender and Beavo 2006), and is expressed in DARPP-32-positive cortical neurons. Castro et al. (Castro et al. 2010) recently reported that the PDE4 inhibitor rolipram increases cAMP levels and PKA activity under conditions of β-adrenoceptor stimulation in the soma and dendrites of cortical pyramidal neurons. The biochemical pathways utilized by dopamine have been less thoroughly studied in frontal cortex, compared with striatum. Our study in cortical tissues demonstrates that the inhibition of PDE4 by rolipram activates cAMP/PKA signaling, leading to the phosphorylation of DARPP-32 at Thr34 under basal and D1 receptor-stimulated conditions. Thus, PDE4 is required for the regulation of dopaminergic neurotransmission especially mediated through D1 receptors in the frontal cortex.
It is known that PDE4 adopts two distinct conformational states with different rolipram affinities; the low affinity (IC50 0.1-1.0 μM) and the high affinity (IC50 1-50 nM) rolipram-binding states (Houslay and Adams 2003; Souness and Rao 1997). Rolipram increased DARPP-32 Thr34 phosphorylation with the IC50 value of 100 nM, suggesting that a large proportion of PDE4 is in a high affinity conformational state in the frontal cortex. The factors which regulate the affinity states of PDE4 in the frontal cortex, such as UCR2 capping (Burgin et al. 2010), PKA phosphorylation (Alvarez et al. 1995; Hoffmann et al. 1998) and interaction with specific binding proteins (Burgin et al. 2010; McPhee et al. 1999), need to be identified in future studies.
Role of DARPP-32 in the enhancement of PPI by the PDE4 inhibitor
Pharmacological inhibition of PDE4 by a selective inhibitor, rolipram, or genetic deletion of PDE4B in mice reveals that PDE4, especially PDE4B, affects behaviors that are dependent upon dopaminergic activity, including locomotor activity (e.g., amphetamine-induced locomotor activity), cognition (e.g., conditioned avoidance responding) and sensorimotor gating (e.g., PPI) (Kanes et al. 2007; Siuciak 2008; Siuciak et al. 2007; Siuciak et al. 2008). Patients with schizophrenia exhibit sensorimotor gating deficits (Braff et al. 2001; Swerdlow et al. 2006). PPI of the acoustic startle response is the most appropriate test for sensorimotor gating currently available both in experimental animals and humans (Geyer et al. 2001). In agreement with previous reports (Kanes et al. 2007), we observed that the inhibition of PDE4 by rolipram increased PPI in wild-type mice with a C57BL/6J background, supporting the notion that inhibition of PDE4 activity may serve as an effective antipsychotic strategy (Nishi and Snyder 2010). We also found an increase in PPI in DARPP-32 knockout mice compared to wild-type mice, as was previously suggested (Svenningsson et al. 2003). Importantly, rolipram failed to increase PPI over these higher basal values in DARPP-32 knockout mice. These results suggest that DARPP-32 is involved in the process of sensorimotor gating and its regulation by the PDE4 inhibitor.
The inhibition of PDE4 by rolipram increases dopamine D1 receptor signaling in cortical pyramidal neurons. The findings in DARPP-32 knockout mice suggest that the rolipram-induced upregulation of D1 receptor signaling and DARPP-32 phosphorylation is required for the increase in PPI. It has been reported that high dopaminergic tone, especially activation of D2 receptor signaling, disrupts PPI (Geyer et al. 2001; Geyer et al. 2002). However, the role of D1 receptor signaling in PPI is not clearly understood. There are several reports showing the disruption of PPI by D1 receptor activation (Doherty et al. 2008; Ralph and Caine 2005; Ralph-Williams et al. 2003; Tamminga 2006), but the disruptive effects of D1 receptors are not consistently observed and likely dependent on dopaminergic tone (Ralph et al. 2001; Ralph-Williams et al. 2002; Tanda et al. 2009).
The inhibitory function of the prepulse is modulated via neural circuitry involving limbic cortex, striatum, pallidum or pontine tegmentum (Swerdlow et al. 2001). Direct or indirect D1 receptor agonists may regulate the activity state of this circuitry, resulting in the alteration of PPI. We have previously reported that, in the striatum, rolipram increases dopamine synthesis and turnover at dopaminergic terminals, but the ability of rolipram to increase D1 receptor signaling in striatal neurons is limited (Nishi et al. 2008). In contrast, in the frontal cortex, rolipram induced a profound increase in D1 receptor signaling. These findings suggest that rolipram upregulates D1 receptor signaling more effectively in the frontal cortex than in the striatum, which may be important for the enhancement of PPI. This hypothesis is supported by the findings that selective depletion of dopamine or infusion of D1 or D2 receptor antagonists in medial prefrontal cortex decreases PPI (Swerdlow et al. 2001). Activation of D1 or D2 receptors in the frontal cortex seems to increase PPI, whereas activation of D1 or D2 receptors in the nucleus accumbens or striatum seems to decrease PPI (Doherty et al. 2008; Swerdlow et al. 2001). It is possible that in DARPP-32 knockout mice, downregulation of D1 or D2 receptor signaling in the striatum results in high basal PPI, and the attenuated action of rolipram to upregulate D1 receptor signaling in the frontal cortex may result in no modulation of PPI over high basal values.
In conclusion, the PDE4 inhibitor, rolipram, clearly increases dopamine D1 receptor signaling in pyramidal neurons of frontal cortex. This biochemical effect in frontal cortex is associated with the enhancement of cognitive function, as measured with PPI. The ability of PDE4 inhibitors to improve sensorimotor gating supports their potential utility for the treatment of psychiatric disorders.
Supplementary Material
Acknowledgement
This research was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (18300128 to A.N.) and grants from the U.S. National Institutes of Health to G.L.S. (MH067488), J.A.B. (MH79710, MH083711, and DA016672) and P.G. and A.C.N. (MH 090963 and DA10044), the Michael Stern Parkinson’s Research Foundation (to P.G.), and the Department of Defense (DOD/USAMRAA W81XWH-09-1-402 and W81XWH -10-1-0640 to P.G.). GLS was supported by funds from Intra-Cellular Therapies Inc. GLS receives additional support from the United States Army Medical Research and Materiel Command NETRP Program (DAMD 17-03-2-0019, W81XWH-05-1-0400 and W81XWH-06-C-0013 to Intra-Cellular Therapies Inc). The authors thank Yukako Terasaki, Keiko Fujisaki, Michiko Sakamoto, Minal Rana, Tiffany Tsui, and Tomonori Furukawa for excellent technical assistance.
Footnotes
No Conflict of Interest
References
- Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc Natl Acad Sci U S A. 2007;104:2979–84. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert KA, Hemmings HC, Jr., Adamo AI, Potkin SG, Akbarian S, Sandman CA, Cotman CW, Bunney WE, Jr., Greengard P. Evidence for decreased DARPP-32 in the prefrontal cortex of patients with schizophrenia. Arch Gen Psychiatry. 2002;59:705–12. doi: 10.1001/archpsyc.59.8.705. [DOI] [PubMed] [Google Scholar]
- Alvarez R, Sette C, Yang D, Eglen RM, Wilhelm R, Shelton ER, Conti M. Activation and selective inhibition of a cyclic AMP-specific phosphodiesterase, PDE-4D3. Mol Pharmacol. 1995;48:616–22. [PubMed] [Google Scholar]
- Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci. 2008;11:932–9. doi: 10.1038/nn.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. [Review] Physiol. Reviews. 1995;75:725–48. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Berger B, Febvret A, Greengard P, Goldman-Rakic P. DARPP-32, a phosphoprotein enriched in dopaminoceptive neurons bearing dopamine D1 receptor: Distribution in the cerebral cortex of the newborn and adult rhesus monkey. J. Comp. Neurol. 1990;299:327–348. doi: 10.1002/cne.902990306. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–36. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, Thorsteinsdottir M, Hrafnsdottir S, Hagen T, Kiselyov AS, Stewart LJ, Gurney ME. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. [DOI] [PubMed] [Google Scholar]
- Castro LR, Gervasi N, Guiot E, Cavellini L, Nikolaev VO, Paupardin-Tritsch D, Vincent P. Type 4 phosphodiesterase plays different integrating roles in different cellular domains in pyramidal cortical neurons. J Neurosci. 2010;30:6143–51. doi: 10.1523/JNEUROSCI.5851-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–8. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JA, Davis RL. Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J Comp Neurol. 1999;407:287–301. [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004;29:1628–36. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, Kaneda H, Shiroishi T, Houslay MD, Henkelman RM, Sled JG, Gondo Y, Porteous DJ, Roder JC. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Masten VL, Powell SB, Ralph RJ, Klamer D, Low MJ, Geyer MA. Contributions of dopamine D1, D2, and D3 receptor subtypes to the disruptive effects of cocaine on prepulse inhibition in mice. Neuropsychopharmacology. 2008;33:2648–56. doi: 10.1038/sj.npp.1301657. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem. 2004;91:1025–43. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- Ehrman LA, Williams MT, Schaefer TL, Gudelsky GA, Reed TM, Fienberg AA, Greengard P, Vorhees CV. Phosphodiesterase 1B differentially modulates the effects of methamphetamine on locomotor activity and spatial learning through DARPP32-dependent pathways: evidence from PDE1B-DARPP32 double-knockout mice. Genes Brain Behav. 2006;5:540–51. doi: 10.1111/j.1601-183X.2006.00209.x. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, King DP, Reutiman TJ, Folsom TD, Laurence JA, Lee S, Fan YT, Paciga SA, Conti M, Menniti FS. PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. Schizophr Res. 2008;101:36–49. doi: 10.1016/j.schres.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein P, Song W-J, Snyder GL, Nishi A, Cheramy A, O’Callaghan JP, Miller D, Cole D, Corbett R, Haile C, Cooper D, Onn S, Grace AA, Ouimet C, White FJ, Hyman SE, Surmeier DJ, Girault JA, Nestler E, Greengard P. DARPP-32, regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Fiumara F, Giovedi S, Menegon A, Milanese C, Merlo D, Montarolo PG, Valtorta F, Benfenati F, Ghirardi M. Phosphorylation by cAMP-dependent protein kinase is essential for synapsin-induced enhancement of neurotransmitter release in invertebrate neurons. J Cell Sci. 2004;117:5145–54. doi: 10.1242/jcs.01388. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Rizos Z, Lovic V, Kapur S. Sensitization to amphetamine, but not PCP, impairs attentional set shifting: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Psychopharmacology (Berl) 2005;183:190–200. doi: 10.1007/s00213-005-0157-6. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Aika Y, Heizmann CW, Kosaka T. Dense GABAergic input on somata of parvalbumin-immunoreactive GABAergic neurons in the hippocampus of the mouse. Neurosci Res. 1996;26:181–94. doi: 10.1016/s0168-0102(96)01102-9. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–63. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–54. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–53. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–38. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–47. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Harada K, Wu J, Haycock JW, Goldstein M. Regulation of L-DOPA biosynthesis by site-specific phosphorylation of tyrosine hydroxylase in AtT-20 cells expressing wild-type and serine 40-substituted enzyme. J Neurochem. 1996;67:629–35. doi: 10.1046/j.1471-4159.1996.67020629.x. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Robertson HA, Denovan-Wright EM. Striatal phosphodiesterase mRNA and protein levels are reduced in Huntington’s disease transgenic mice prior to the onset of motor symptoms. Neuroscience. 2004;123:967–81. doi: 10.1016/j.neuroscience.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Wilkinson IR, McCallum JF, Engels P, Houslay MD. cAMP-specific phosphodiesterase HSPDE4D3 mutants which mimic activation and changes in rolipram inhibition triggered by protein kinase A phosphorylation of Ser-54: generation of a molecular model. Biochem J. 1998;333(Pt 1):139–49. doi: 10.1042/bj3330139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotte M, Thuault S, Dineley KT, Hemmings HC, Jr., Nairn AC, Jay TM. Phosphorylation of CREB and DARPP-32 during late LTP at hippocampal to prefrontal cortex synapses in vivo. Synapse. 2007;61:24–8. doi: 10.1002/syn.20339. [DOI] [PubMed] [Google Scholar]
- Hotte M, Thuault S, Lachaise F, Dineley KT, Hemmings HC, Nairn AC, Jay TM. D1 receptor modulation of memory retrieval performance is associated with changes in pCREB and pDARPP-32 in rat prefrontal cortex. Behav Brain Res. 2006;171:127–33. doi: 10.1016/j.bbr.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–19. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Huang Z, Mancini JA. Phosphodiesterase 4 inhibitors for the treatment of asthma and COPD. Curr Med Chem. 2006;13:3253–62. doi: 10.2174/092986706778773040. [DOI] [PubMed] [Google Scholar]
- Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP. Rolipram: a specific phosphodiesterase 4 inhibitor with potential antipsychotic activity. Neuroscience. 2007;144:239–46. doi: 10.1016/j.neuroscience.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie SJ, Baillie GS, McPhee I, MacKenzie C, Seamons R, McSorley T, Millen J, Beard MB, van Heeke G, Houslay MD. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in Upstream Conserved Region 1 (UCR1) Br J Pharmacol. 2002;136:421–33. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCahill AC, Huston E, Li X, Houslay MD. PDE4 associates with different scaffolding proteins: modulating interactions as treatment for certain diseases. Handb Exp Pharmacol. 2008:125–66. doi: 10.1007/978-3-540-72843-6_6. [DOI] [PubMed] [Google Scholar]
- McPhee I, Yarwood SJ, Scotland G, Huston E, Beard MB, Ross AH, Houslay ES, Houslay MD. Association with the SRC family tyrosyl kinase LYN triggers a conformational change in the catalytic region of human cAMP-specific phosphodiesterase HSPDE4A4B. Consequences for rolipram inhibition. J Biol Chem. 1999;274:11796–810. doi: 10.1074/jbc.274.17.11796. [DOI] [PubMed] [Google Scholar]
- Menniti FS, Faraci WS, Schmidt CJ. Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov. 2006;5:660–70. doi: 10.1038/nrd2058. [DOI] [PubMed] [Google Scholar]
- Millar JK, Mackie S, Clapcote SJ, Murdoch H, Pickard BS, Christie S, Muir WJ, Blackwood DH, Roder JC, Houslay MD, Porteous DJ. Disrupted in schizophrenia 1 and phosphodiesterase 4B: towards an understanding of psychiatric illness. J Physiol. 2007;584:401–5. doi: 10.1113/jphysiol.2007.140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, Thomson PA, Hill EV, Brandon NJ, Rain JC, Camargo LM, Whiting PJ, Houslay MD, Blackwood DH, Muir WJ, Porteous DJ. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–91. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, Porteous DJ, Millar JK, Houslay MD. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J Neurosci. 2007;27:9513–24. doi: 10.1523/JNEUROSCI.1493-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Miller DB, O’Callaghan JP, Bateup HS, Shuto T, Sotogaku N, Fukuda T, Heintz N, Greengard P, Snyder GL. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci. 2008;28:10460–71. doi: 10.1523/JNEUROSCI.2518-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Snyder GL. Advanced research on dopamine signaling to develop drugs for the treatment of mental disorders: biochemical and behavioral profiles of phosphodiesterase inhibition in dopaminergic neurotransmission. J Pharmacol Sci. 2010;114:6–16. doi: 10.1254/jphs.10r01fm. [DOI] [PubMed] [Google Scholar]
- Noyama K, Maekawa S. Localization of cyclic nucleotide phosphodiesterase 2 in the brain-derived Triton-insoluble low-density fraction (raft) Neurosci Res. 2003;45:141–8. doi: 10.1016/s0168-0102(02)00208-0. [DOI] [PubMed] [Google Scholar]
- Numata S, Ueno S, Iga J, Song H, Nakataki M, Tayoshi S, Sumitani S, Tomotake M, Itakura M, Sano A, Ohmori T. Positive association of the PDE4B (phosphodiesterase 4B) gene with schizophrenia in the Japanese population. J Psychiatr Res. 2008;43:7–12. doi: 10.1016/j.jpsychires.2008.01.013. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Sriram K. Focused microwave irradiation of the brain preserves in vivo protein phosphorylation: comparison with other methods of sacrifice and analysis of multiple phosphoproteins. J Neurosci Methods. 2004;135:159–68. doi: 10.1016/j.jneumeth.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–6. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- Ouimet CC. DARPP-32, a dopamine and cyclic AMP-regulated phosphoprotein, is present in corticothalamic neurons of the rat cingulate cortex. Brain Res. 1991;562:85–92. doi: 10.1016/0006-8993(91)91190-c. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, LaMantia AS, Goldman-Rakic P, Rakic P, Greengard P. Immunocytochemical localization of DARPP-32, a dopamine and cyclic-AMP-regulated phosphoprotein, in the primate brain. J Comp Neurol. 1992;323:209–18. doi: 10.1002/cne.903230206. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, Miller PE, Hemmings J, H C, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J. Neurosci. 1984;4:114–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard BS, Thomson PA, Christoforou A, Evans KL, Morris SW, Porteous DJ, Blackwood DH, Muir WJ. The PDE4B gene confers sex-specific protection against schizophrenia. Psychiatr Genet. 2007;17:129–33. doi: 10.1097/YPG.0b013e328014492b. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. J Pharmacol Exp Ther. 2005;312:733–41. doi: 10.1124/jpet.104.074468. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–13. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology. 2003;28:108–18. doi: 10.1038/sj.npp.1300017. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J Neurosci. 2002;22:9604–11. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–88. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–50. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Seeger TF, Bartlett B, Coskran TM, Culp JS, James LC, Krull DL, Lanfear J, Ryan AM, Schmidt CJ, Strick CA, Varghese AH, Williams RD, Wylie PG, Menniti FS. Immunohistochemical localization of PDE10A in the rat brain. Brain Res. 2003;985:113–26. doi: 10.1016/s0006-8993(03)02754-9. [DOI] [PubMed] [Google Scholar]
- Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem. 1996;271:16526–34. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- Siuciak JA. The role of phosphodiesterases in schizophrenia: therapeutic implications. CNS Drugs. 2008;22:983–93. doi: 10.2165/0023210-200822120-00002. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Martin AN. Antipsychotic profile of rolipram: efficacy in rats and reduced sensitivity in mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 2007;192:415–24. doi: 10.1007/s00213-007-0727-x. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Martin AN. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 2008;197:115–26. doi: 10.1007/s00213-007-1014-6. [DOI] [PubMed] [Google Scholar]
- Souness JE, Rao S. Proposal for pharmacologically distinct conformers of PDE4 cyclic AMP phosphodiesterases. Cell Signal. 1997;9:227–36. doi: 10.1016/s0898-6568(96)00173-8. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–96. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, McKinzie DL, Fienberg AA, Nomikos GG, Greengard P. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302:1412–5. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–35. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. The neurobiology of cognition in schizophrenia. J Clin Psychiatry. 2006;67(Suppl 9):9–13. discussion 36-42. [PubMed] [Google Scholar]
- Tanda K, Nishi A, Matsuo N, Nakanishi K, Yamasaki N, Sugimoto T, Toyama K, Takao K, Miyakawa T. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. I. Regional and cellular disrtribution in rat brain. J. Neurosci. 1984;4:84–98. doi: 10.1523/JNEUROSCI.04-01-00084.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des. 2009;15:1688–98. doi: 10.2174/138161209788168092. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mix E, Winblad B. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev. 2001;7:387–98. doi: 10.1111/j.1527-3458.2001.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.