Abstract
Objectives
To investigate the effect of intellectual and physical activity on biomarkers of Alzheimer’s disease (AD) pathophysiology and cognition in a non-demented elderly population. The biomarkers evaluated were brain Aβ-amyloid load via PIB-PET, neuronal dysfunction via FDG-PET and neurodegeneration via Structural-MRI.
Methods
We studied 515 non-demented (428 cognitively normal and 87 MCI) participants in the population based Mayo Clinic Study of Aging who completed a 3T MRI, PET scans, APOE genotype, had lifestyle activity measures and cognition data available. The imaging measures computed were global PiB-PET uptake; global FDG-PET and MRI based hippocampal volume. We consolidated activity variables into lifetime intellectual, current intellectual and current physical activities. We used a global cognitive Z-score as a measure of cognition. We applied two independent methods – partial correlation analysis adjusted for age and gender and path analysis using structural equations to evaluate the associations between lifestyle activities, imaging biomarkers and global cognition.
Results
None of the lifestyle variables correlated with the biomarkers and the path associations between lifestyle variables and biomarkers were not significant (p>0.05). On the other hand, all the biomarkers were correlated with global cognitive Z-score (p<0.05) and the path associations between (lifetime and current) intellectual activities and global Z-score were significant (p<0.01).
Interpretation
Intellectual and physical activity lifestyle factors were not associated with AD biomarkers but intellectual lifestyle factors explained variability in the cognitive performance in this non-demented population. This study provides evidence that lifestyle activities may delay the onset of dementia but do not significantly influence the expression of AD pathophysiology.
Keywords: Alzheimer’s disease, Imaging biomarkers, Lifestyle Activities
INTRODUCTION
Intellectual and physical activities are increasingly viewed as possible preventive strategies for AD. Components of both lifetime and current intellectual activities – education 1, 2, occupation 3 and cognitive activities 4–9 -as well as physical activity 10, 11 have been hypothesized to protect against AD dementia.
Most studies in this field have only considered the effect of lifestyle activities on cognitive performance or incidence of dementia as outcome measures. However AD pathophysiology (AD-P) commonly exists in non-demented elderly subjects. An unanswered question therefore is: do lifestyle activities affect the underlying AD-P? In this paper, we study the effect of intellectual and physical activity measures on AD-P and cognition by asking – 1) do lifestyle choices influence the degree of AD-P as measured by AD imaging biomarkers and 2) are lifestyle choices related to better global cognition.
The three imaging biomarkers that we use as surrogates of each of the major AD pathologies are: a) Pittsburgh compound B positron emission tomography (PIB-PET) imaging as a biomarker of cerebral amyloidosis - high PIB-PET uptake in the brain reflects deposition of Aβ plaques; b) 18fluorodeoxyglucose PET imaging (FDG-PET) as a biomarker of neuronal dysfunction and injury – low FDG-PET levels in the brain indicates low cerebral metabolic uptake of glucose and c) hippocampal volume as measured by structural MRI as an indicator of neurodegeneration due to AD.
The Mayo Clinic Study of Aging (MCSA) - a population based sample of non-demented elderly with information on both lifestyle activity measures as well as imaging measures provides us with an excellent opportunity to investigate these questions in an unbiased fashion among subjects randomly selected from the population.
METHODS
Selection of Participants
Study subjects were participants in the Mayo Clinic Study of Aging (MCSA), an epidemiological study of the prevalence, incidence, and risk factors for Mild Cognitive Impairment (MCI) and dementia among Olmsted County residents ages 70–90 years on October 1, 2004. We included all 515 non-demented MCSA (428 cognitively normal and 87 MCI) patients that completed all three imaging studies (3T MRI, FDG-PET, PiB-PET), APOE genotype and had intellectual and physical activity variables as well as cognition data available. Details about the design of the MCSA have previously been published12, 13. All subjects gave written informed consent and the study was approved by the Mayo Institutional Review board (IRB).
Intellectual and Physical Activity
The primary intellectual and physical activity variables of interest that were available in this study were: education, job-level score based on the primary occupation throughout life, current weekly cognitive activity and current weekly physical activity and exercise both averaged over the last 12 months. These lifestyle data were recorded for all subjects at the baseline or enrollment visit; however, for patients with multiple evaluations, data closest to the MRI/PET scan date were used in the analyses. The median time between the imaging assessments and lifestyle assessments was 1.5 years and for most of patients (except two) the imaging measurement was obtained at or after the lifestyle assessments. The details about the questionnaires used for recording and consolidating each individual measure are provided in the appendix and the actual questionnaires are provided in the supplemental material. In subjects with multiple assessments the concordance coefficients comparing the first and last assessments that were at least 15 months apart were found to be 0.68 for cognitive as well as physical activity indicating good reliability of the measures.”
Lifetime and Current Intellectual Activities
We evaluated two components of intellectual environment: i) lifelong intellectual learning assessed from years of education and occupation and ii) current ongoing intellectual activities from a self-report of cognitive activities in the previous 12 months. We found that each of the reliable cognitive lifestyle variables that were available – education, job-level score and current weekly cognitive activity were highly correlated with each other. Therefore we applied a principal component analysis (PCA) to produce uncorrelated information that segregates the effect of lifetime learning and current cognitive activity contained in the cognitive activity measures. PCA is a method used to extract information from correlated variables and to condense the information into smaller sets of uncorrelated variables. We found that the first two PCA components explained the majority (88%) of the variance in the data and thus only retained those. The first PCA (PC1) component loaded mainly on lifetime intellectual activity – education and job-level score with some contribution from cognitive activity score (PC1=0.67xeducation + 0.36xcognitive activity+ 0.65xjob-score). PC1 thus described intellectual activity throughout life, up to and including a part of the current intellectual activity consistent with the other lifetime measures. The second component (PC2) loaded mainly on current intellectual activity – i.e. current cognitive activity score (PC2=-0.17xEducation + 0.93xCognitive activity - 0.33xJob-score). PC2 to some extent contrasted current intellectual activity vs. lifetime intellectual activity, that is, it described current activity distinct from lifetime activity. We will refer to these as lifetime intellectual activity (PC1) and current intellectual activity (PC2), respectively.
Current Physical Activity
The current physical activity and exercise questionnaire were condensed into a single number which we refer to simply as physical activity.
Alzheimer’s disease Biomarkers
MRI acquisition and processing
All subjects were scanned on a 3T MRI and a 3D volumetric T1-MPRAGE was acquired and preprocessed as described previously 14. Freesurfer software (version 4.5.0) was used to obtain hippocampal volumes 15 and the volumes were adjusted for total intracranial volume.
PET acquisition and processing
Images were acquired with a PET/CT operating in 3-dimensional mode (septa removed). The complete details of PET acquisition were described previously 16. All PET quantitative image analysis including quality control were performed at the Mayo Clinic using the same fully automated image processing pipeline as described previously 17, 18. Statistics on image voxel values were extracted from automatically labeled cortical regions of interest using an in-house modification of the automated anatomic labeling atlas 19.
Global PIB-PET ratio measure
A global cortical PIB-PET retention ratio was computed by calculating the median uptake over voxels in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus regions of interest (ROIs) for each subject and dividing this by the median uptake over voxels in the cerebellar gray matter ROI of the atlas 20.
Global FDG-PET ratio measure
A global FDG PET retention summary was computed by averaging the left and right angular gyri, bilateral posterior cingulate and left middle/inferior temporal gyrus values for each subject as described previously 21 normalized by the pons uptake.
Global Cognition Measure
The neuropsychological battery was constructed as previously described 12, 13. Domain specific measures are assessed from subsets of the Wechsler Adult Intelligence Scale-Revised (WAIS-R), Wechsler Memory Scale-Revised (WMS-R), Auditory Verbal Learning Test (AVLT), Trail Making Test (TMT), category fluency test, and Boston Naming Test (BNT). Four cognitive domains are assessed from nine tests: Executive (TMT: Part B, WAIS-R Digit Symbol); Language (BNT, category fluency); Memory (WMS-R Logical Memory-II (delayed recall), WMS-R Visual Reproduction-II (delayed recall), AVLT delayed recall); and Visuospatial performance (WAIS-R Picture Completion, WAIS-R Block Design). Individual test scores were first converted to z-scores using the mean and standard deviation from the MCSA 2004 enrollment cohort that consisted of non-demented subjects (n=1969). The individual z-scores were averaged to create 4 domain scores which were then also converted to z-scores. A global cognitive summary score was estimated from the average of the 4 domain z-scores and then converted to a z-score by subtracting the mean and dividing by the standard deviation. This global summary score was used to assess cognitive impairment in our subjects and as an outcome variable in this study.
Statistical Analysis
We conducted two types of analyses to investigate the association of lifestyle activities with AD biomarkers and cognition. The first analysis consisted of a partial correlation analysis to evaluate the direct associations among the lifestyle variables, AD biomarkers and cognition. In the second analysis, we used structural equation models to evaluate interdependent relationships among the three sets of variables: global cognition, lifestyle activities and AD biomarkers. The age and PIB variables had skewed distributions and were log transformed and negative log transformed respectively.
I. Correlation of Biomarkers and Cognition with Lifestyle Variables
We estimated the association between variables using partial Pearson correlations which we denote by “partial rs” and were adjusted for the effects of age and gender. Partial correlations were calculated using SAS version 9.2.
II. Structural Equation Models
We conducted path analyses using structural equations software to model the global cognitive Z-score as a function of lifestyle variables (lifetime intellectual activity, current intellectual activity, and current physical activity) and biomarkers (amyloid burden, hippocampal volume, and glucose metabolism). In addition we modeled the relationships between lifestyle variables and biomarkers. All models were adjusted for age, gender, APOE, and relationships among the biomarkers. The primary a priori model had age, gender, APOE, lifetime intellectual activity, current intellectual activity, and current physical activity specified as exogenous variables. Amyloid burden, hippocampal volume, and glucose metabolism formed an intermediate layer of endogenous variables, and the global cognitive Z-score was the final downstream endogenous variable. We first fit the full model, estimating all paths, and then investigated two reduced models suggested by the primary analysis. The first reduced model had all paths from lifestyle variables to biomarkers set to 0. The second additionally had paths from current physical activity set to 0. We assessed these models using chi-square goodness of fit tests and Bayesian Information Criteria (BIC). We set up one additional a priori model with the placement of the lifestyle variables and biomarkers exchanged, as an extreme sensitivity analysis of the effects of ordering. Since exchanging the variables made no difference in the significant pathways, results are not reported here. All analyses used maximum likelihood estimation in the R package sem 22. Plots were produced using Graphviz 23.
RESULTS
Subject Characteristics
The demographics, clinical summary, lifestyle variables and biomarker characteristics of the non-demented subjects used in this study are shown in Table 1. The population characteristics of the subjects in this study were found to be comparable to the overall MCSA study participants except that our study participants had a slightly higher proportion of males and had slightly higher physical activity scores and global cognitive performance.
Table 1.
Patient characteristics with the median [min, q1, q3, max] for the continuous variables. q1 and q3 indicate lower and upper quartiles.
| Study participants n = 515 | Study non-participants n = 2191 | MCSA non-demented participants n = 2706 | |
|---|---|---|---|
| No. of females (%) | 223 (43) | 1107 (51) | 1330 (49) |
| No. of MCI (%) | 87 (17) | 402 (18) | 489 (18) |
| Age, yrs. | 79 [70, 75, 83, 93] | 80 [70, 75, 84, 94] | 79 [70, 75, 84, 94] |
| Education, yrs. | 13 [7, 12, 16, 20] | 13 [0, 12, 16, 20] | 13 [0, 12, 16, 20] |
| No. of APOE ε4 carriers (%)* | 145 (28) | 550 (26) | 695 (26) |
| Short Test of mental status | 34 [22, 32, 36, 38] | 34 [17, 32, 36, 38] | 34 [17, 32, 36, 38] |
| Global cognition standardized score | 0.52 [−2.89, −0.11, 1.08, 2.84] | 0.15 [−3.92, −0.61, 0.81, 2.63] | 0.23 [−3.92, −0.51, 0.87, 2.84] |
| Cognitive/social activity score | 22.5 [0, 16, 29.5, 60] | 21 [0, 15, 28, 63] | 21.5 [0, 15, 28, 63] |
| Physical/exercise score | 7 [0, 4.5, 9.8, 21] | 5.8 [0, 3.5, 8.8, 21] | 6 [0, 3.5, 9, 21] |
| Job level score | 4 [1, 3, 6, 6] | 4 [2, 3, 6, 6] | 4 [1, 3, 6, 6] |
| Amyloid burden | 1.40 [1.18, 1.32, 1.78, 3.03] | --- | --- |
| Glucose metabolism | 1.39 [0.83, 1.28, 1.48, 1.79] | --- | --- |
| Hippocampal volume | 7.1 [3.9, 6.4, 7.7, 9.7] | --- | --- |
| Lifetime intellectual activity “Principal Component 1” | −0.35 [−3.29, −1.06, 1.19, 3.61] | --- | --- |
| Current intellectual activity “Principal Component 2” | −0.07 [−2.01, −0.75, 0.52, 3.26] | --- | --- |
All study participants have APOE information while 2% of the APOE information is missing from the overall MCSA study participants.
PET imaging of subjects began in early 2006, so the table represents all study participants and non-participants between 1/1/2006 – 1/31/2011.
Correlation of Biomarkers and Cognition with Lifestyle Variables
None of the biomarkers (amyloid burden, glucose metabolism or hippocampal volume) was strongly correlated with any of the lifestyle measures (p>0.05) but the correlation of hippocampal volume with current intellectual activity showed a trend towards significance (rs = 0.08, p=0.08) (Table 2). In contrast, global cognition was correlated with lifetime intellectual activity (rs = 0.39, p<0.01), current intellectual activity (rs = 0.10, p=0.02) and with physical activity (rs = 0.10, p=0.03) (Table 2).
Table 2.
Age and gender adjusted Pearson correlations (95% CI) for the lifestyle activity variables versus the imaging variables and global cognition.
| Lifetime intellectual activity | P | Current intellectual activity | P | Current physical activity | P | |
|---|---|---|---|---|---|---|
| Amyloid burden | −0.02 (−0.10, 0.07) | 0.69 | −0.02 (−0.11, 0.07) | 0.65 | −0.07 (−0.16, 0.02) | 0.11 |
| Glucose metabolism | 0.05 (−0.04, 0.13) | 0.27 | 0.03 (−0.06, 0.12) | 0.51 | −0.01 (−0.10, 0.08) | 0.84 |
| Hippocampal volume | −0.02 (−0.10, 0.07) | 0.72 | 0.08 (−0.01, 0.16) | 0.08 | 0.03 (−0.06, 0.11) | 0.54 |
| Global cognition | 0.39 (0.32, 0.46) | <.01 | 0.10 (0.02, 0.19) | 0.02 | 0.10 (0.01, 0.18) | 0.03 |
Structural Equation Models
Table 3 summarizes the results of the path analyses. The table consists of standardized betas (βs), their standard errors, and p-values of the estimates from the full model. Both reduced models fit the data well (chi-square p-values 0.61 and 0.50 respectively), and showed substantial reductions in the BIC (a total reduction of 48) consistent with the pattern of significant and non-significant paths. The standardized betas can be interpreted as partial correlations. The second column indicates if we found the relationship between the two variables in that row to be significant or not. Figure 1 presents the path diagrams from the full model. Gray lines indicate associations which were tested but found to be non-significant. Red lines indicate the significant direct effects observed in our data. Numbers beside the red lines are standardized betas (p-values).
Table 3.
Results of the path analyses based on the Structural Equation Models – the table consist of the standardized betas, standard errors and p-values of the paths analyzed between pairs of variables.
| Path | Significant | Standardized Beta | Std. Error | P-value |
|---|---|---|---|---|
| Paths between the lifestyle variables and imaging biomarkers | ||||
| lifetime intellectual activity bing → amyloid burden* | 0.008 | 0.041 | 0.839 | |
| current intellectual activity → amyloid burden* | 0.007 | 0.042 | 0.866 | |
| current physical activity → amyloid burden* | 0.046 | 0.042 | 0.270 | |
| lifetime intellectual activity → glucose metabolism | 0.044 | 0.042 | 0.299 | |
| current intellectual activity → glucose metabolism | 0.045 | 0.044 | 0.300 | |
| current physical activity → glucose metabolism | −0.023 | 0.043 | 0.590 | |
| lifetime intellectual activity → hippocampal volume | −0.019 | 0.040 | 0.627 | |
| current intellectual activity → hippocampal volume | 0.074 | 0.041 | 0.072 | |
| current physical activity → hippocampal volume | 0.014 | 0.041 | 0.728 | |
| Paths to the Global Cognition Variable | ||||
| lifetime intellectual activity → z(global) | Yes | 0.360 | 0.037 | 1.44E-22 |
| current intellectual activity → z(global) | Yes | 0.092 | 0.038 | 0.015 |
| current physical activity → z(global) | 0.034 | 0.038 | 0.364 | |
| amyloid burden*→ z(global) | Yes | 0.086 | 0.040 | 0.032 |
| glucose metabolism → z(global) | Yes | 0.200 | 0.041 | 8.29E-07 |
| hippocampal volume → z(global) | Yes | 0.192 | 0.043 | 7.30E-06 |
Coded as -ln(Amyloid Burden).
Figure 1.
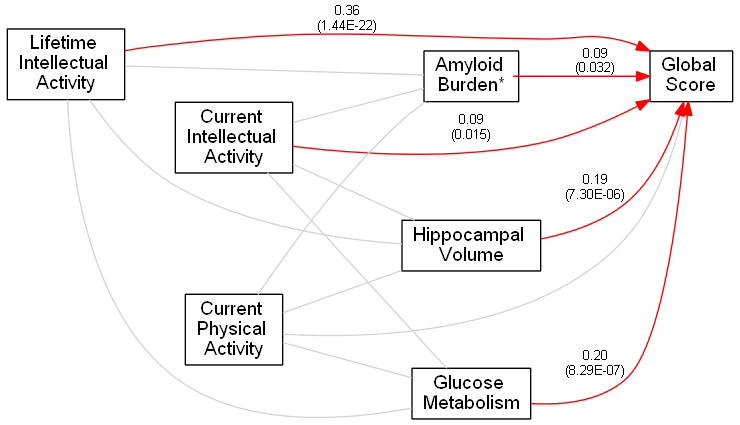
Results of the path analysis between the three sets of variables in the study – lifestyle activities, imaging biomarkers and global cognition. Gray lines indicate associations which were tested but found to be non-significant. Red lines indicate the significant direct effects observed in our data. Numbers beside the red lines are standardized betas (p-values). * Coded as -ln(Amyloid Burden).
Lifestyle activities and Cognition
In the path analysis with global Z-score as a function of lifestyle variables, both lifetime (βs=0.36 and p<0.001) and current (βs=0.09 and p=0.015) intellectual activities were significant predictors of global cognition. On the other hand, current physical activity was not related to global cognition in the presence of the intellectual activity variables.
Lifestyle activities and AD Biomarkers
None of the paths between lifestyle activities and the biomarker variables were significant (p>0.05). There was only a trend between current intellectual activity and hippocampal volume (p=0.072).
Biomarkers and Cognition
In the path analysis with global Z-score as a function of biomarkers, amyloid burden (βs=0.09 and p=0.032), glucose metabolism (βs=0.20 and p<0.001) and hippocampal volume (βs =0.19 and p<0.001) were significant predictors of global cognition.
COMMENT
The major conclusions of this study were 1) both lifetime and current intellectual activity but not physical activity explained variability in the cognitive performance of non-demented elderly subjects; 2) intellectual and physical activities were not associated with AD biomarkers and 3) all AD biomarkers (amyloid PET, glucose metabolism and hippocampal volume) explained variability in the cognitive performance. These results suggest that intellectual lifestyle/activities may delay the onset of dementia but do not significantly influence the expression of AD pathophysiology.
Lifestyle activities and Cognition
Each of the lifestyle activities was correlated with global cognition after adjusting for age and gender (p<=0.03). Using the path analyses both current and lifetime intellectual activities explained variability in global cognition (p<0.02) but physical activity did not. Since intellectual activities are correlated with physical activity in this study, the combined path analyses helped identify the independent contributions of each of the lifestyle activities when all the variables were considered together. The overall finding that higher level of lifestyle activities is related to better global cognition is consistent with the literature. Better lifestyle choices may lower the risk or delay the onset of symptoms because it takes longer for global cognition to progress to clinically detectable cognitive impairment that impacts daily functioning.
Higher levels of education, occupation and cognitive activity have all been independently shown to be associated with a lower risk of dementia 3–5. Since each of these variables is highly correlated with each other, we found that the application of PCA helped us segregate the effects of lifetime learning and current cognitive activity. Even though both lifetime and current intellectual activities were significantly associated with cognition, we found that lifetime intellectual activity had a bigger contribution (standardized beta of 0.360) compared to current intellectual activity (standardized beta of 0.09). This is logical because lifelong intellectual conditioning due to education and occupation have a major impact on the intellectual capacity of an individual that in turn greatly influences the current general cognitive performance.
Physical activity has been shown to be related to better cognitive performance 10, 24. In contrast we did not find strong evidence that current physical activity was related to better cognitive performance. A possible explanation for the discrepancy might be that we adjusted for the concurrent influence of intellectual lifestyle activities on the relationship between cognition and physical activity. Also, we only studied the effect of current physical activity and perhaps lifetime physical activity would have been more predictive of cognitive performance.
Lifestyle Activities and AD Biomarkers
We were surprised to find that in both the correlation and path analyses none of the lifestyle activities correlated or explained variability in the AD biomarkers (p>0.05). While lifestyle activities may lead to better cognitive performance due to more efficient cognitive networks and cognitive conditioning, lifestyle activities appear to have minimal effect on the accumulation of pathophysiology. We also repeated the correlation analyses in cognitively normal subjects and came to the same conclusion. The inclusion of MCIs in this paper only strengthens our findings since MCIs might have increased the correlations between lifestyle activities and cognitive function but had no impact on the correlation between lifestyle activities and biomarkers.
The influence of lifestyle activities on the expression of AD-P biomarkers has not been studied extensively in humans. A recent study found that there is an association between exercise and AD biomarkers but they found that several of these associations especially with cerebrospinal fluid based AD biomarkers reduced to non significant trends after controlling for covariates 25. We found that controlling for covariates was important for detecting the true influence of lifestyle activities on AD biomarkers. Additionally in the path analyses we were able to account for correlations between the three groups of variables and separate the effects between each of them. Another recent study found that there was an association between lifetime cognitive activity and PIB uptake but did not find an association between exercise and PIB uptake 26. While Landau et al. used a self report questionnaire for reporting of the lifetime cognitive activity based on common cognitively demanding activities, we extracted lifetime cognitive activity based on education, occupation and current cognitive activity which would explain the key differences between the two studies.
While the number of human studies in this area has been very limited, there have been several studies conducted in transgenic AD mice. Results have been inconsistent. Environmental stimulation and exercise have been shown to reduce 27–28, worsen 29 as well as not effect amyloid deposition 30 in these animals. Studies on the effect of these variables on cortical plasticity have consistently found that exercise in particular is related to larger hippocampal volumes 31, 32. We however did not find strong support for this effect.
AD biomarkers and Cognition
The paths between AD biomarkers (amyloid burden, glucose metabolism and hippocampal volume) and cognitive performance were significant (p<0.05). However the relationship between neuronal biomarkers and cognitive performance (p<0.001) was much stronger than amyloid burden and cognitive performance (p=0.03). These results are consistent with the notion that amyloid deposition occurs early in the disease 33, prior to overt cognitive symptoms, and does not directly cause clinical symptoms 34, 35 and has a weaker association with cognition than neuronal biomarkers 36. On the other hand abnormal neuronal biomarkers are believed to be downstream pathological events that progressively worsen in the presence of a relatively static total load of amyloid and which lead directly to cognitive impairment 37, 38.
Effect of Intellectual and Physical Activity on Alzheimer’s disease Biomarkers and Cognition
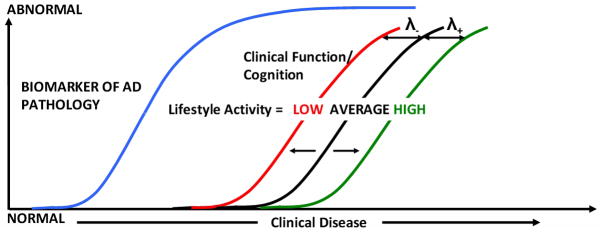
The key observations in this study are illustrated in Figure 2. In an elderly individual with average lifestyle activities, the cognitive decline or clinical function in AD represented by the black curve can be viewed as a downstream process caused by an increasing pathological burden as measured by the imaging biomarkers (the blue curve). In this paper we found that the lifestyle activities do not influence the biomarkers of AD pathology i.e. pathology accumulation curves but only influences the cognitive performance curve. In an individual with high lifestyle activities (green curve), the global cognition of the person is higher than the individual with average lifestyle activities. This effectively translates into moving the clinical function or cognition curve further right by λ+ thus delaying the onset of the disease. Similarly for low lifestyle activities the curve is moved to the left by λ− i.e. shortening the time between accumulation of pathology and onset of disease. The model illustrated in Figure 2 is also consistent with one of our earlier papers where we found that cognitive reserve as measured by verbal IQ may delay the onset of cognitive decline 39.
Figure 2.
Model illustrating the effect of lifestyle activities on AD biomarkers and cognition or clinical function in subjects. Clinical disease stage is indicated on the horizontal axis and the magnitude of biomarker abnormalities and cognition (from normal to maximally abnormal) on the vertical axis. The cognition or clinical function curve is moved left-right based on the individual’s lifestyle activity. The variable λ for lifetime intellectual activity is greater than that for current intellectual activity and the variable λ for physical activity is close to zero.
Our results suggest that lifetime intellectual activity appears to have the strongest association with cognitive performance in non-demented elderly subjects – with an influence that exceeds that of AD-P biomarkers. This implies that the λ parameters for lifetime intellectual activity are larger than the λ parameters for current intellectual activity. On the other hand λ parameters for physical activity were close to zero because there did not seem to be an association between cognition and physical activity when adjusting for intellectual activities.
Strengths of this Study
One of the key strengths of this study is the availability of a large population based sample of non-demented elderly with lifestyle activity measures as well as imaging measures. A second strength is the statistical approaches that we employed in the paper a) principal component analysis to clearly demarcate the contribution of the lifetime and current intellectual activities; b) path analysis or structural equation analysis to discern the independent effect of lifestyle variables in the presence of each other. Also unlike studies that use informant based assessment for cognitive performance, we combined domain specific measures based on a neuropsychological battery to obtain the global cognitive performance measures.
Limitations of this Study
We recognize that, as with all cross-sectional association studies, we cannot prove cause and effect. Therefore, the results do not preclude the possibility that active intervention in the form of exercise or cognitive activity may reduce amyloid deposition or increase cortical plasticity. However our results suggest that if there is a causal effect of lifestyle activities on AD biomarkers (i.e. reduced amyloid deposition, increased hippocampal volume and glucose metabolism), it is fairly weak. Another limitation of the study pertains to the measurement of current cognitive and physical activities. We used simple self-report questionnaires that may not have captured some relevant dimensions of current activities that influence cognitive functioning.
Supplementary Material
Acknowledgments
This work was supported by the NIH grants K99 AG37573, R01 AG11378, P50 AG16574, U01 AG06786; the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation, U.S.A and Opus building NIH grant C06 RR018898.
Appendix: Lifestyle Variables
Current Cognitive and Physical Activity Questionnaires
The cognitive activity questionnaire has eleven components that ask the subject to indicate how often a certain cognitive task is performed on average over the last 12 months. The answer to each component gets a weighted average based on a weekly level. The cognitive activity score is summed over the first ten components. Television is the eleventh component and is not included in the cognitive activity score.
The physical activity questionnaire has six components that ask the subject to indicate how often a certain physical task is performed on average over the last 12 months. The answer to each component gets a weighted average based on a weekly level. The physical/exercise score is averaged over the six components. However, there is no additional weighting based on physical exertion level similar to the sensitivity analysis in 40.
Job-level score
Based on the occupation that people held majority of their life, we categorized the occupations that were recorded for each subject based on the Department of Labor occupation list are into six different job level scores. Level 1 included people who had no occupation. Level 2 included private household occupations, service occupations, transportation and material moving. Level 3 included sales occupation, administrative support, protective services, farming and machine operators. Level 4 included technicians and precision production workers. Level 5 included executive, administrative and managerial services. Level 6 included professional specialty occupations.
Footnotes
Disclosure: The authors report no conflicts of interest
Statistical analysis was conducted by Timothy Lesnick and Scott Przybelski.
References
- 1.Stern Y, Gurland B, Tatemichi TK, et al. Influence of education and occupation on the incidence of Alzheimer’s disease. Jama. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 2.Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology. 2007;68:223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- 3.Lupton MK, Stahl D, Archer N, et al. Education, occupation and retirement age effects on the age of onset of Alzheimer’s disease. Int J Geriatr Psychiatry. 2010;25:30–36. doi: 10.1002/gps.2294. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 5.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 6.Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- 7.Newson RS, Kemps EB. General lifestyle activities as a predictor of current cognition and cognitive change in older adults: a cross-sectional and longitudinal examination. J Gerontol B Psychol Sci Soc Sci. 2005;60:P113–120. doi: 10.1093/geronb/60.3.p113. [DOI] [PubMed] [Google Scholar]
- 8.Bielak AA. How can we not ‘lose it’ if we still don’t understand how to ‘use it’? Unanswered questions about the influence of activity participation on cognitive performance in older age--a mini-review. Gerontology. 2010;56:507–519. doi: 10.1159/000264918. [DOI] [PubMed] [Google Scholar]
- 9.Jopp DS, Hertzog C. Assessing adult leisure activities: an extension of a self-report activity questionnaire. Psychol Assess. 2010;22:108–120. doi: 10.1037/a0017662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 16.Lowe VJ, Kemp BJ, Jack CR, Jr, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50:878–886. doi: 10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senjem ML, Lowe V, Kemp B, et al. Automated ROI analysis of 11C Pittsburgh compound B images using structural magnetic resonance imaging atlases, Alzheimer’s and Dementia. Alzheimer’s Association International Conference on Alzheimer’s Disease; Elsevier Inc; 2008. [Google Scholar]
- 19.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 20.Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–1972. [PubMed] [Google Scholar]
- 21.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox J. Structural Equation Modeling. Vol. 13. Lawrence Erlbaum Assoc, Inc; 2006. Structural Equation Modeling With the sem Package in R; pp. 465–486. [Google Scholar]
- 23.Gansner ER, North SC. An open graph visualization system and its applications to software enineering. Softw Pract Exper. 2000;30:1203–1233. [Google Scholar]
- 24.Taaffe DR, Irie F, Masaki KH, et al. Physical activity, physical function, and incident dementia in elderly men: the Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2008;63:529–535. doi: 10.1093/gerona/63.5.529. [DOI] [PubMed] [Google Scholar]
- 25.Liang KY, Mintun MA, Fagan AM, et al. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landau SM, Marks SM, Mormino EC, et al. Association of Lifetime Cognitive Engagement and Low beta-Amyloid Deposition. Arch Neurol. doi: 10.1001/archneurol.2011.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarov O, Robinson J, Tang YP, et al. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jankowsky JL, Xu G, Fromholt D, et al. Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:1220–1227. doi: 10.1093/jnen/62.12.1220. [DOI] [PubMed] [Google Scholar]
- 30.Arendash GW, Garcia MF, Costa DA, et al. Environmental enrichment improves cognition in aged Alzheimer’s transgenic mice despite stable beta-amyloid deposition. Neuroreport. 2004;15:1751–1754. doi: 10.1097/01.wnr.0000137183.68847.4e. [DOI] [PubMed] [Google Scholar]
- 31.Yuede CM, Zimmerman SD, Dong H, et al. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;35:426–432. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster PP, Rosenblatt KP, Kuljis RO. Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer’s disease. Front Neurol. 2011;2:28. doi: 10.3389/fneur.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fagan AM, Mintun MA, Shah AR, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: Diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingelsson M, Fukumoto H, Newell KL, et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62:925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 38.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vemuri P, Weigand SD, Przybelski SA, et al. Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain. 2011;134:1479–1492. doi: 10.1093/brain/awr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geda YE, Roberts RO, Knopman DS, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.