Abstract
Concentration–response curves (CRCs) of adenosine receptor (AR) agonists, NECA (nonspecific), CCPA (A1 specific), CGS-216870 (A2A specific), BAY 60-6583 (A2B specific), and Cl-IB-MECA (A3 specific) for mesenteric arteries (MAs) from 4 AR knockout (KO) mice (A1, A2A, A2B, and A3) and their wild type (WT) were constructed. The messenger RNA expression of MAs from KO mice and WT were also studied. Adenosine (10−5 to 10−4 M) and NECA (10−6 to 10−5 M) induced relaxation in all mice except A2B KO mice, which only showed constriction by adenosine at 10−6 to 10−4 and NECA at 10−8 to 10−5 M. The CCPA induced a significant constriction at 10−8 and 10−7 M in all mice, except A1KO. BAY 60-6583 induced relaxation (10−7 to 10−5 M) in WT and no response in A2BKO except at 10−5 M. The CRCs for BAY 60-6583 in A1, A2A, and A3 KO mice shifted to the left when compared with WT mice, suggesting an upregulation of A2B AR. No responses were noted to CGS-21680 in all mice. Cl-IB-MECA only induced relaxation at concentration greater than 10−7 M, and no differences were found between different KO mice. The CRC for Bay 60-6583 was not significantly changed in the presence of 10−5 M of L-NAME, 10−6 M of indomethacin, or both. Our data suggest that A2B AR is the predominant AR subtype and the effect may be endothelial independent, whereas A1 AR plays a significant modulatory role in mouse MAs.
Keywords: adenosine receptors, adenosine receptor knockout mice, 5′-(N-Ethyl-carboxamido) adenosine (NECA), CGS-21680, BAY 60-6583, Cl-IB-MECA
INTRODUCTION
Mesenteric arteries are part of a network of vascular beds that regulates vascular resistance. Mesenteric artery ischemia is often seen in people who have hardening of the arteries in other parts of the body (for example, those with coronary artery disease or peripheral vascular disease).1,2 The condition is more common in smokers and in patients with high blood pressure or plasma cholesterol.3 An imbalance between oxygen supply and demand (ischemia) leads to alterations in cellular release of adenosine.4 Once adenosine is produced by the action of ecto-5′-nucleotidase, it is released from the parenchymal tissue (including endothelium) that interacts with specific extracellular receptors located on smooth muscle and endothelial cells of the vessels to produce relaxation.5 Currently 4 adenosine receptor (AR) subtypes, A1, A2A, A2B, and A3, are known to mediate adenosine-induced vasodilation or vasoconstriction. Their role in coronary circulation has been extensively studied.5–9 A2A AR is the major contributor in regulating adenosine-induced coronary vasodilation, whereas A2B plays a minor role.5,6,8 A1 AR and A3 AR, on the other hand, negatively modulate the A2 AR–induced vasodilation.5,7,9 However, there are very few studies that investigated the effect of adenosine in mesenteric circulation in regard to the AR subtype that is involved in vascular reactivity. Based upon the vessel size (about 100–200 µm outside diameter, 50–100 µm inside diameter) and relevance in blood flow regulation, the first branches of superior mesenteric arteries in the mouse, which are considered as both conduit (feeder) and resistance arteries in small animals,10,11 serve as a good starting vascular segment in our study for the involvement of ARs in mesenteric artery flow regulation.
Using all 4 AR genetic knockout (KO) mice (A1, A2A, A2B, and A3) and their wild-type (WT) controls (C57BL/6), we constructed concentration–response curves (CRCs) for various AR agonists in submaximal preconstricted isolated mesenteric arterial rings and profiled the messenger RNA (mRNA) expression of ARs.
MATERIALS AND METHODS
Animals
All animals were cared for in accordance with protocol approved by the Animal Care and Use Committee of the Health Science Center at West Virginia University. A1, A2B, and A3 KO mice were obtained from Dr Stephen Tilley, University of North Carolina Chapel Hill, Chapel Hill, NC. A2AKO mice were also obtained from Dr Stephen Tilley, but they were originally from Dr Catherine Ledent of Universite Libre de Bruxelles, Brussels, Belgium.12 All KO mice were on C57BL/6J background. Standard laboratory food and water were available ad libitum. Temperature was held constant at 23 ± 2°C and humidity was 60% ± 10%. An inverted light–dark cycle of 12:12 hours was used (lights off at 1700 hrs). Experiments were conducted in accordance with national legislation and with the Declaration of Helsinki regarding the use of experimental animals.
Materials
Adenosine, 5′-(N-ethylcarboxamido)adenosine (NECA), 2-chloro-N6-cyclopentyladenosine (CCPA), 2-p-(2-Carboxyethyl) phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride hydrate (CGS-21680), 2-Chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (Cl-IB-MECA), phenylephrine (PE), indomethacin, L-NG-Nitroarginine methyl ester (L-NAME), and all the chemicals for the buffers were purchased from Sigma-Aldrich. BAY 60-6583 was a generous gift from Dr Thomas Krahn of Bayer Healthcare, Germany. All realtime reverse transcription
Mesenteric Artery Wire Myograph Experiments
The intestines from A1, A2A, A2B, and A3, KO mice and their WT mice were excised and placed in oxygenated (5% CO2 and 95% O2) modified Krebs–Henseleit buffer (in mM: NaCl120, NaHCO3 25, KCl 4.7, KH2PO4 1.2, CaCl2 1.8, MgSO4 1.2, glucose 15, and EDTA 0.05) at 37°C, pH 7.4. First-order branches of the superior mesentery arteries were isolated and cleaned of surrounding tissue. The arterial rings (3–5 mm long, 50–100 µm inside diameter) were mounted on an isometric myograph (Danish Myo Techology A/S, Aarhus, Denmark) based on the work of Mulvany and Nyborg.13 Each vascular ring was stretched to a resting passive tension (200 mg) and was allowed to equilibrate for at least 30 minutes. The optimal resting tension was determined by measuring the tension that produced the greatest contractile response after the addition of 50 mM KCl (determined separately). Viability of the vascular ring was verified by recording contraction after the addition of 50 mM KCl to the tissue bath. The integrity of endothelium was confirmed by the addition of the endothelium-dependent vasodilator acetylcholine (10−6 M) during the plateau phase of 10−6 M of PE-induced contraction, a submaximal contraction that was predetermined in separate experiments (data not shown). Vascular rings that did not contract after the addition of KCl or that did not relax after the addition of acetylcholine were eliminated from further study.
After equilibration and verification of arterial ring integrity, vascular rings were randomly assigned to 6 drug groups: adenosine, NECA (nonspecific agonist), CCPA (A1 AR agonist), CGS-21680 (A2A AR agonist), Bay 60-6583 (A2B agonist from Bayer, Germany), and Cl-IB-MECA (A3 AR agonist). The CRC for these adenosine analogues were obtained by cumulative addition of the agonists in the 10−6 M PE preconstricted vascular rings. One-way analysis of variance (ANOVA) was used to compare between mouse groups within the same drug groups.
In separate experiments, L-NG-Nitroarginine methyl ester [L-NAME (a nitric oxide synthase inhibitor)] and indo-methacin (a nonspecific cyclooxygenase inhibitor) were used to assess whether endothelium plays a role in the predominant A2B AR–mediated vasodilation in the mesenteric arteries. CRC for Bay 60-6583 was constructed, as described above, using the vascular rings from WT mice in the presence and absence of 10−5 M L-NAME, 10−6 of indomethacin, or both. Both of these drugs were incubated for half an hour before PE preconstruction and subsequent cumulative dosing of Bay 60-6583.
Real-Time Reverse Transcription–Polymerase Chain Reaction Experiments
Total RNA was isolated from the first branches of mice mesenteric arteries from all 4 KO mice and C57 using RNAEasy total RNA isolation kit from Qiagen. This was followed by conversion of 0.5 µg of total RNA into complementary DNA (cDNA) using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to the instructions of the manufacturer in a total volume of 100 µL. Because of the relatively low expression of ARs, polymerase chain reaction (PCR) PreAmplification Kit from ABI was used. Real-time PCR was performed using ABI PRISM 7300 Detection System (Applied Biosystems) using Taqman Universal Mastermix (Applied Biosystems, Branchburg, NJ) according to the instructions of the manufacturer. The reaction volume (25 µL) included 12.5 µL of 2X Taqman Universal Mastermix, 1 µL of cDNA, and 1.25 µL of 20X FAM-labeled Taqman gene expression assay master mix solution. For the real-time PCR for ARs genes, the Taqman inventoried gene expression product was purchased from Applied Biosystems. The 18S ribosomal RNA was used as an endogenous control. The fold difference in expression of target cDNA was determined using the comparative CT method. The ΔCT value was determined in each experiment by subtracting the average 18S CT value from the corresponding average CT for A1, A2A, A2B, and A3 AR in coronary arteries. The standard deviation was calculated using the formula . To set the relative unit to 1, ΔΔCT was calculated by subtraction of the ΔCT calibrator value (A1 AR ΔCT values in C57). The fold difference in gene expression of the target was calculated as the average value from 2−ΔΔCT + s and 2−ΔΔCT − s.14,15 One-way ANOVA was used for comparing between mouse groups in the same AR gene.
Statistical Analysis
All data were expressed as mean ± standard error of the mean. All CRCs were analyzed using nonlinear regression using GraphPad Prism3. When significant differences between curves were found, the data were further analyzed between groups at the same concentrations. One-way ANOVA followed by Bonferroni correction for multiple comparisons were used to analyze differences between groups. The value of P <0.05 was considered statistically significant.
RESULTS
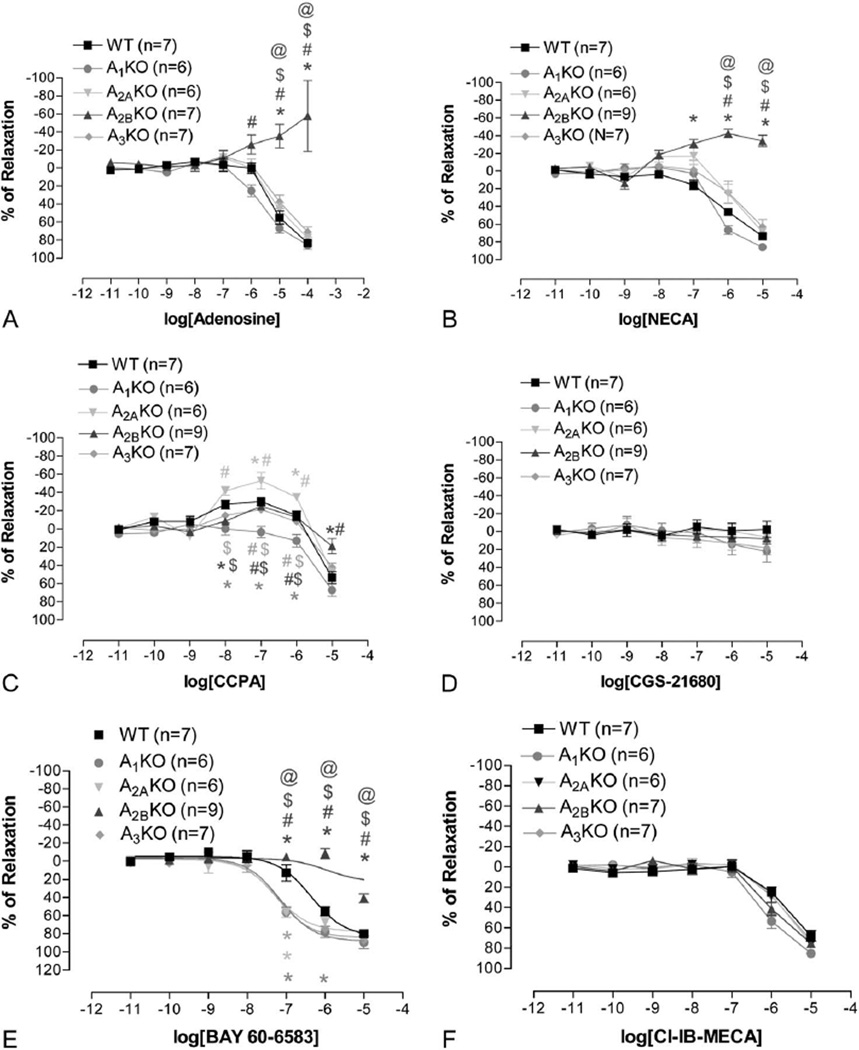
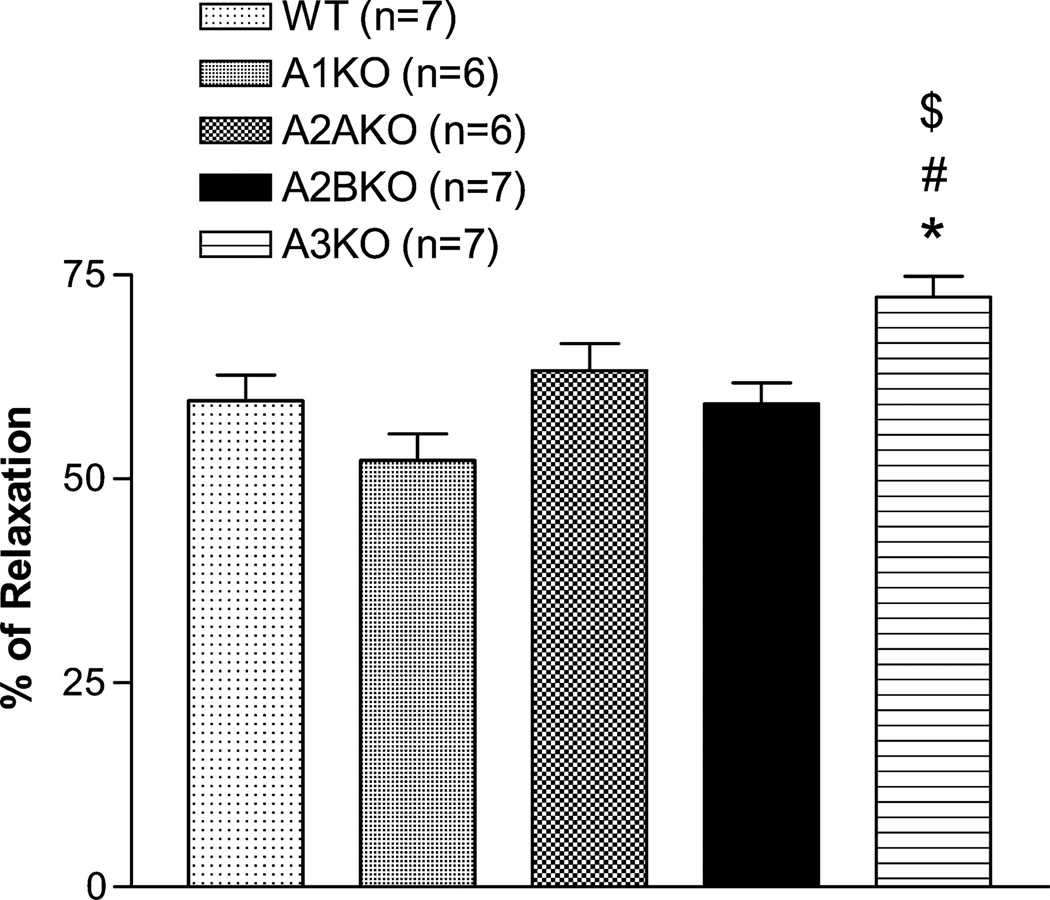
The CRCs for all agonists in 4 AR KO mice are shown in Figure 1. Only vasodilation was observed using no-selective agonists (adenosine and NECA) in WT, A1, A2A, and A3 KO mice, whereas vasoconstriction was observed only in A2B KO. CCPA, the A1 AR agonist, induced vasoconstriction from 10−8 to 10−7 M in all KO mice except A1 KO. However, at 10−5 M, CCPA induced vasodilation in all mice possibly due to nonselective effect of CCPA at a higher concentration. CGS-21680 had no significant vascular effect in all mice. A2B agonist, BAY 60-6583, induced only vasodilation that is similar to adenosine and NECA in WT mice and no effect in A2B KO mice except at 10−5 M. Interestingly, the BAY 60-6583 CRC shifted to the left in other 3 AR KO mice (A1, A2A, and A3). A3 agonist, Cl-IB-MECA, induced vasodilation only at concentrations greater than 10−7 M, and the responses were similar in all mice. Also, responses to acetylcholine between all mice were similar except A3 KO that showed a significant greater response to acetylcholine (Fig. 2).
FIGURE 1.
The CRCs for the following: AR agonists—adenosine (A), NECA (B); A1 AR agonist—CCPA (C); A2A agonist—CGS-21680 (CGS, D); A2B agonist—BAY-60-6583 (E); and A3 agonist—Cl-IB-MECA (F) in mesenteric arteries from all 4 AR KO mice and WT mice. *Indicates significantly different from WT at the same concentration. #Indicates significantly different from A1KO at the same concentration. $Indicates significantly different from A2AKO. @Indicates significantly different from A3KO. P < 0.05 was considered significant.
FIGURE 2.
Relaxation responses to 10−6 M acetylcholine in phenylnephrine preconstricted mesenteric arteries from all 4 AR KO mice and WT mice. *Indicates significantly different from WT at the same concentration. #Indicates significantly different from A1KO at the same concentration. $Indicates significantly different from A2AKO.
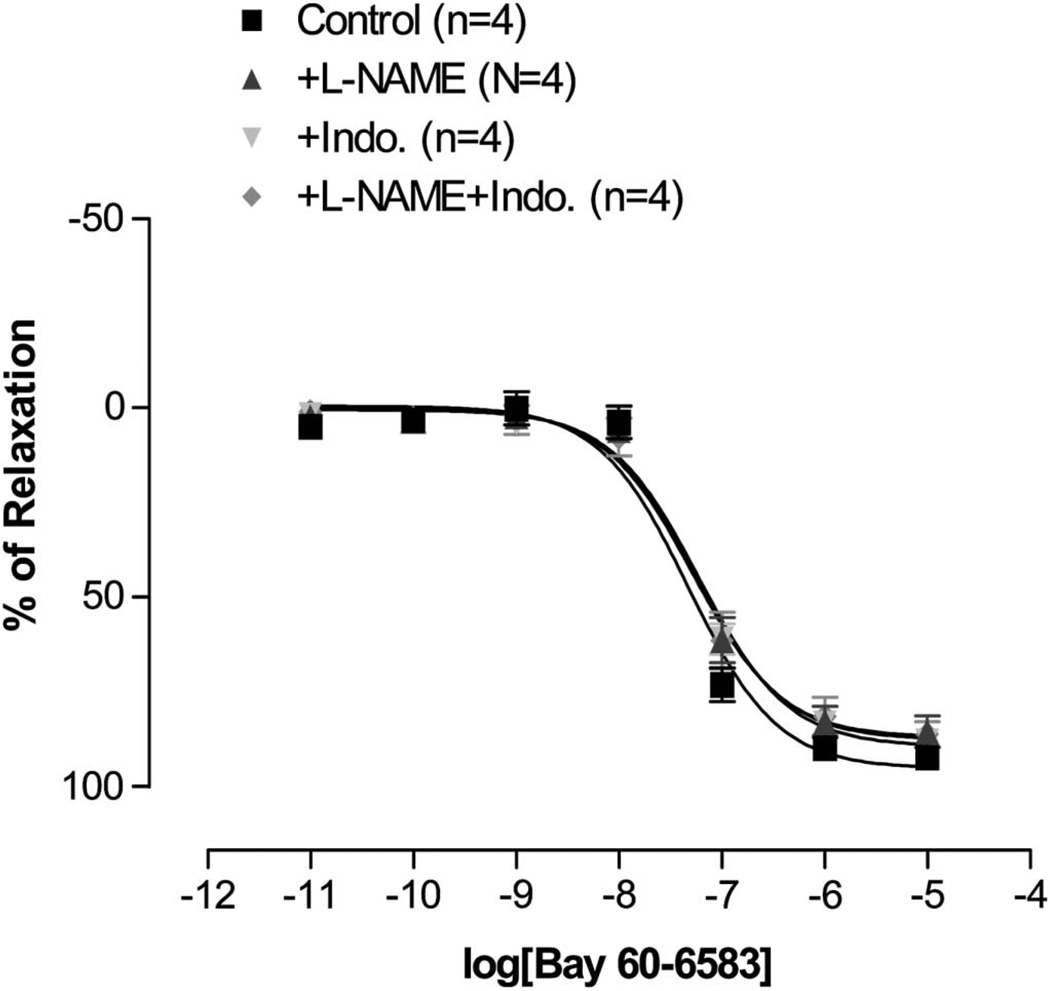
The CRC for Bay 60-6583 in WT mice was not significantly changed by the addition of 10−5 M of L-NAME, 10−6 M of indomethacin, or both (Fig. 3).
FIGURE 3.
The effects of 10−5 M of L-NAME and 10−6 M of indomethacin on Bay 606583-induced vascular relaxation in mesenteric artery rings from the wild-type mice. There is no significant difference between groups. L-NAME: L-NG-nitroarginine methyl ester. Indo.: indomethacin.
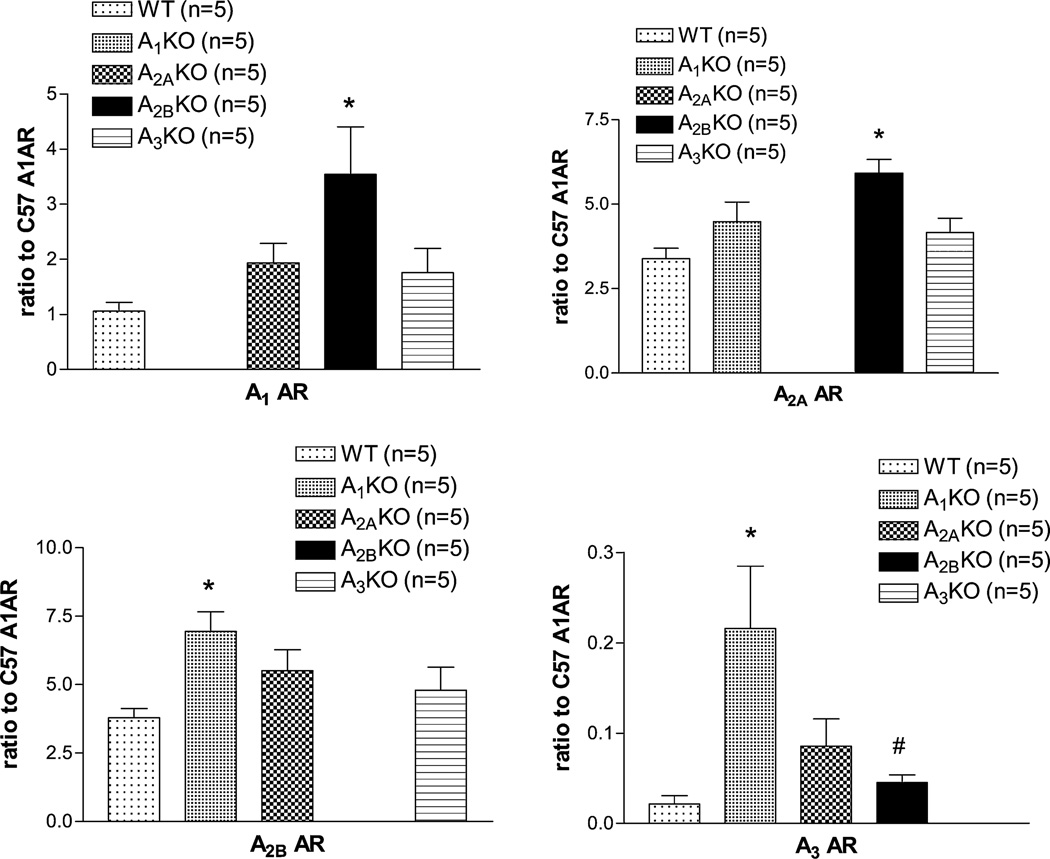
Real-time reverse transcription–polymerase chain reaction results confirm all individual KO mice which have no expression of respective ARs. In WT mice, A2A and A2B had the highest level of expression (3.38 ± 0.69 and 3.80 ± 075 fold of A1 AR expression). A3 AR had the lowest expression level (0.02-fold ± 0.02-fold of A1 AR expression). We also found that if one of the AR was deleted, the other ARs have tendency to compensate with an increase in expression, but only 1 or 2 AR expression showed significant difference from WT. Comparing among mice groups, only A2BKO showed a significant upregulation of A1 AR expression (Fig. 4A, about 3.5-fold more than WT) and only A1KO showed significant A2B AR upregulation (Fig. 4C, about 2-fold more than WT). A2A AR was upregulated in A2BKO (Fig. 4B). A3 AR expression were upregulated in A1 and A2B KO mice (Fig. 4D).
FIGURE 4.
AR mRNA expression profile of the mesenteric arteries from all 4 AR KO mice and WT mice. *Indicates significantly difference from WT.
DISCUSSION
It is well established that A2A and A2B AR mediate vasodilation, whereas A1 and A3 AR mediate vasoconstriction in adenosine-induced vascular reactivity in most vascular tissues.5,16,17 Using genetic KO mice and specific agonists, our study provides direct evidence that A2B AR predominantly mediates adenosine-induced vasodilation and A1 AR–mediated vasoconstriction modulates the effect of A2B AR in mesenteric arteries, which was also suggested in other studies.18,19
It is striking to find a complete lack of vasodilation in response to the nonspecific AR agonists stimulation (adenosine and NECA in Figs. 1A, B) in A2BKO, which suggests an A2B predominant effect, and a significant vasoconstriction at a higher concentration, which implies a vasoconstriction role for other ARs. Indeed, CCPA, the A1 AR agonist, is the only specific agonist that demonstrated a significant dose-dependent vasoconstriction (up to 10−7 M) in all KO mice and WT mice except A1KO (Fig. 1C). Previous studies from other laboratories and ours also confirm the role of A1 AR in regulating blood pressure and vasoconstriction in mouse mesenteric arteries19,20 (Dovenia article). Furthermore, the lack of vasoconstriction to adenosine and NECA in WT and the “unmasking” of the adenosine and NECA-induced vasoconstricting effect in A2BKO strongly support the predominant role of A2B AR and major modulatory role for A1 AR in mesenteric arteries.
The complete lack of vasodilation to CGS-21680 (Fig. 1D), an A2A specific agonist, in all KO mice and WT mice is surprising because previous study from rabbits and some studies in rat suggested an A2A-predominant responses in mesenteric arteries,21–23 although one study in rat isolated and perfused mesenteric arteries did suggest a A2B AR–mediated vasodilation.18 The differences may be due to species differences and is supported by another study that also found little responses to CGS-21680 in isolated mouse mesenteric arteries.19 The differences in experimental preparation (in vivo vs. in vitro and methoxamine vs. PE preconstriction) may also explain varying results. In our experiments, only the first branch of supramesenteric arteries was used. The further downstream branches may have different functional profiles. Further studies are needed to investigate this observation.
The predominant role of A2B AR in this vascular preparation is obvious based upon the lack of vasodilatory responses to BAY 60-6583, adenosine, and NECA in A2B KO. A previous study did suggest a predominant role for A2B AR in mouse mesenteric arteries due to observed different responses to CGS-21680 and NECA in WT mice.19 Furthermore, the predominant role of A2B AR in this vascular tissue could also be demonstrated by the enhanced effect of BAY 60-6583 in all KO mice except A2B KO (Fig. 1E), when compared with WT mice.
Activation of A2B AR has been shown to have both endothelial-dependent and endothelial-independent effects in various vascular tissues.18,24–26 Previous study in rat mesenteric arteries demonstrated that the L-NAME had no effect on adenosine-induced vasodilation.18 To rule out the involvement of factors released from endothelium due A2B AR activation (Bay 60-6583), in this study we used L-NAME and indomethacin. Both L-NAME and indomethacin alone or in combination were without an effect (Fig. 3). However, it is possible that the concentrations of these agents were not sufficient to block the release of mediators from endothelium. Also, our data do not address the involvement of EDHF. Therefore, further studies are needed to investigate these mechanisms.
Because A2B AR is a low-affinity AR, it has limited role under normal physiological conditions but could play an important role under ischemic condition, where local adenosine concentration increase significantly. Indeed, A2B AR has been shown to play a significant role in postischemic protection.27,28 A recent study from our group found that postischemic infarct size increases in A2B KO mice (unpublished data). However, there is limited information on the effect of A2B AR in mesenteric ischemia. With these newly developed A2B AR agonists,29 such as Bay 60-6583, there is renewed interest in investigating the role of A2B AR in ischemia in this tissue.
The role of A3 AR in vascular reactivity is unclear due to its low expression (<10% of A1 AR expression in WT mice, Fig. 4D). Using Cl-IB-MECA, the A3 AR–specific agonist, we still cannot confirm the role of A3 AR in this tissue (Fig. 1F). There is no difference between different KO mice in response to Cl-IB-MECA including A3KO. The vasodilatory effect at high concentration presumably indicates the nonspecific effects of Cl-IB-MECA, which is consistent with the study showing that Cl-IB-MECA–induced vasodilation in rat mesenteric artery cannot be blocked by A3 AR–specific antagonist, BWA-1433.30 However, we observed a higher vasodilatory response to acetylcholine in A3KO, suggesting a possible A3 AR–mediated endothelial-dependent vasoconstricting effect. Previous study from our laboratory has shown that the vasoconstricting effect of Cl-IB-MECA was blocked by COX-1 inhibitor in mouse aorta, and the expression of A3 AR and COX-1 were mostly in endothelium.31 Although we did not observe Cl-IB-MECA–induced vasoconstriction, the higher acetylcholine-induced vasodilatory effect in A3KO suggests A3 AR may at least provide modulating effect to adenosine responses.
Compensatory upregulation of ARs in KO mice has been previously reported.32,33 For instance, A2B AR has been shown to be upregulated to compensate for the removal of predominant A2A AR in coronary arteries in A2A KO mice and vice versa.32,33 The contractile function and the protein expression of A1 AR was found to be upregulated in aorta from A2AKO.34 Small increases in A1, A2B, and A3 AR were found in the spleen of A2A KO.35 In our study, we also observed an mRNA upregulation of A2A AR in A2B KO (Fig. 4B). However, it did not translate to functional response. There is no response to CGS-21680 in all 4 KO mice, which indicates that A2A AR may not play a significant role in vascular reactivity in this branch of mesenteric arteries. However, it is possible that the upregulation of A2A AR in mesenteric arteries may affect other A2A AR function, such as reducing inflammation and KATP channel regulation.33,36,37 Alternatively, because A1 AR was also upregulated in A2BKO (Fig. 4A), the modulating effect of A1 AR may cancel out any effect that was induced by the A2A AR upregulation. Similarly, we observed significant mRNA upregulation of A3 AR in A1KO (Fig. 4D), which suggested a compensatory upregulation. However, the mRNA upregulation also did not translate to vascular functional responses. Further investigations are needed to address these issues.
The predominant role of A2B AR and A1 AR in mesenteric arteries may have some clinical implication. For the treatment of mesenteric ischemia, surgical revascularization remains the treatment of choice, but thrombolytic medical treatment and vascular interventional radiological techniques have a growing role.38 In nonocclusive mesenteric ischemia, intra-arterial injection of papaverine to superior mesenteric artery has been shown to be effective in preventing bowel infarct.39 At 10−6 M, A2B agonist Bay 60-6583 induces close to 90% endothelial-independent relaxation in PE preconstricted vascular rings. A2B AR agonist may be a viable alternative in relieving nonocclusive mesenteric ischemia.
Furthermore, mesenteric ischemia-reperfusion injury is common in surgical and trauma patients.40 One of the preventive treatments for the injury is ischemic preconditioning (IP), which is a phenomenon whereby exposure of a tissue to brief periods of ischemia protects them from the deleterious effects of prolonged ischemia reperfusion (IR) injury.41 The mechanisms of IR have been intensively studied in coronary circulation and all 4 ARs have been shown to play prominent roles in different stages of IR.42 However, few have been done in mesenteric circulation. Previous study in rat demonstrated that adenosine is one of the mediators of ischemia preconditioning that ameliorate ischemia and reperfusion–induced intestinal mucosal hyperpermeability.43 Further studies in this area are needed to clarify the possible role of ARs in the IP and as a possible therapeutic alternative.
In conclusion, it is clear from this study that A2B AR is the predominant AR in vascular reactivity in the first branch of superior mesenteric artery from mouse and A1 AR plays a significant modulatory role. Although A2A AR does not play a significant role, A3 AR may still play some role in modulating vascular reactivity. It is interesting to note that once one AR gene was deleted, the RNA expression of other ARs had the tendency to be upregulated, suggesting a complex compensatory relationship between ARs.
Acknowledgments
Supported by NIH HL 094447 and HL 027339.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Landis MS, Rajan DK, Simons ME, et al. Percutaneous management of chronic mesenteric ischemia: outcomes after intervention. J Vasc Interv Radiol. 2005;16:1319–1325. doi: 10.1097/01.RVI.0000171697.09811.0E. [DOI] [PubMed] [Google Scholar]
- 2.Schaefer PJ, Schaefer FK, Mueller-Huelsbeck S, et al. Chronic mesenteric ischemia: stenting of mesenteric arteries. Abdom Imaging. 2007;32:304–309. doi: 10.1007/s00261-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 3.Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954–968. doi: 10.1016/s0016-5085(00)70183-1. [DOI] [PubMed] [Google Scholar]
- 4.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- 5.Mustafa SJ, Morrison RR, Teng B, et al. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol. 2009;193:161–188. doi: 10.1007/978-3-540-89615-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison RR, Talukder MA, Ledent C, et al. Cardiac effects of adenosine in A(2A) receptor knockout hearts: uncovering A(2B) receptors. Am J Physiol Heart Circ Physiol. 2002;282:H437–H444. doi: 10.1152/ajpheart.00723.2001. [DOI] [PubMed] [Google Scholar]
- 7.Talukder MA, Morrison RR, Jacobson MA, et al. Targeted deletion of adenosine A(3) receptors augments adenosine-induced coronary flow in isolated mouse heart. Am J Physiol Heart Circ Physiol. 2002;282:H2183–H2189. doi: 10.1152/ajpheart.00964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talukder MA, Morrison RR, Ledent C, et al. Endogenous adenosine increases coronary flow by activation of both A2A and A2B receptors in mice. J Cardiovasc Pharmacol. 2003;41:562–570. doi: 10.1097/00005344-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Tawfik HE, Teng B, Morrison RR, et al. Role of A1 adenosine receptor in the regulation of coronary flow. Am J Physiol Heart Circ Physiol. 2006;291:H467–H472. doi: 10.1152/ajpheart.01319.2005. [DOI] [PubMed] [Google Scholar]
- 10.Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res. 2001;38:1–12. doi: 10.1159/000051024. [DOI] [PubMed] [Google Scholar]
- 11.Mulvany MJ. Small artery remodelling in hypertension. Basic Clin Pharmacol Toxicol. 2012;110:49–55. doi: 10.1111/j.1742-7843.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- 12.Ledent C, Vaugeois JM, Schiffmann SN, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 13.Mulvany MJ, Nyborg N. An increased calcium sensitivity of mesenteric resistance vessels in young and adult spontaneously hypertensive rats. Br J Pharmacol. 1980;71:585–596. doi: 10.1111/j.1476-5381.1980.tb10977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison RR, Teng B, Oldenburg PJ, et al. Effects of targeted deletion of A1 adenosine receptors on postischemic cardiac function and expression of adenosine receptor subtypes. Am J Physiol Heart Circ Physiol. 2006;291:H1875–H1882. doi: 10.1152/ajpheart.00158.2005. [DOI] [PubMed] [Google Scholar]
- 15.Nadeem A, Fan M, Ansari HR, et al. Enhanced airway reactivity and inflammation in A2A adenosine receptor-deficient allergic mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1335–L1344. doi: 10.1152/ajplung.00416.2006. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallon V, Osswald H. Adenosine receptors and the kidney. Handb Exp Pharmacol. 2009;193:443–470. doi: 10.1007/978-3-540-89615-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubino A, Ralevic V, Burnstock G. Contribution of P1-(A2b subtype) and P2-purinoceptors to the control of vascular tone in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1995;115:648–652. doi: 10.1111/j.1476-5381.1995.tb14981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Yang JN, Arner A, et al. Adenosine A(1) receptors and vascular reactivity. Acta Physiol (Oxf) 2010;199:211–220. doi: 10.1111/j.1748-1716.2010.02093.x. [DOI] [PubMed] [Google Scholar]
- 20.Ansari HR, Teng B, Nadeem A, et al. A(1) adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2009;297:H1032–H1039. doi: 10.1152/ajpheart.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Brito MT, Canto A, Correia JH, et al. Adenosine A(2A) receptors in portal hypertension: their role in the abnormal response to adenosine of the cranial mesenteric artery in rabbits. Br J Pharmacol. 2002;135:1324–1330. doi: 10.1038/sj.bjp.0704575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Paola R, Melani A, Esposito E, et al. Adenosine A2A receptorselective stimulation reduces signaling pathways involved in the development of intestine ischemia and reperfusion injury. Shock. 2010;33:541–551. doi: 10.1097/SHK.0b013e3181c997dd. [DOI] [PubMed] [Google Scholar]
- 23.Hiley CR, Bottrill FE, Warnock J, et al. Effects of pH on responses to adenosine, CGS 21680, carbachol and nitroprusside in the isolated perfused superior mesenteric arterial bed of the rat. Br J Pharmacol. 1995;116:2641–2646. doi: 10.1111/j.1476-5381.1995.tb17220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ansari HR, Nadeem A, Talukder MA, et al. Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am J Physiol Heart Circ Physiol. 2007;292:H719–H725. doi: 10.1152/ajpheart.00593.2006. [DOI] [PubMed] [Google Scholar]
- 25.Lee TK, Koh HC. Involvement of NO and KATP channel in adenosine A2B receptors induced cardiovascular regulation in the posterior hypothalamus of rats. J Cardiovasc Pharmacol. 2009;53:167–172. doi: 10.1097/FJC.0b013e318198ca6b. [DOI] [PubMed] [Google Scholar]
- 26.Olanrewaju HA, Mustafa SJ. Adenosine A(2A) and A(2B) receptors mediated nitric oxide production in coronary artery endothelial cells. Gen Pharmacol. 2000;35:171–177. doi: 10.1016/s0306-3623(01)00107-0. [DOI] [PubMed] [Google Scholar]
- 27.Przyklenk K. Role of adenosine A2B receptor stimulation in ischaemic postconditioning: dawn of a new paradigm in cardioprotection. Cardiovasc Res. 2012 doi: 10.1093/cvr/cvs181. [DOI] [PubMed] [Google Scholar]
- 28.Kuno A, Critz SD, Cui L, et al. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007;43:262–271. doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baraldi PG, Tabrizi MA, Fruttarolo F, et al. Recent improvements in the development of A(2B) adenosine receptor agonists. Purinergic Signal. 2008;4:287–303. doi: 10.1007/s11302-008-9097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prentice DJ, Payne SL, Hourani SM. Activation of two sites by adenosine receptor agonists to cause relaxation in rat isolated mesenteric artery. Br J Pharmacol. 1997;122:1509–1515. doi: 10.1038/sj.bjp.0701524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ansari HR, Nadeem A, Tilley SL, et al. Involvement of COX-1 in A3 adenosine receptor-mediated contraction through endothelium in mice aorta. Am J Physiol Heart Circ Physiol. 2007;293:H3448–H3455. doi: 10.1152/ajpheart.00764.2007. [DOI] [PubMed] [Google Scholar]
- 32.Teng B, Ledent C, Mustafa SJ. Up-regulation of A(2B) adenosine receptor in A(2A) adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol. 2008;44:905–914. doi: 10.1016/j.yjmcc.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharifi Sanjani M, Teng B, Krahn T, et al. Contributions of A2A and A2B adenosine receptors in coronary flow responses in relation to KATP channel using A2B and A2A/2B double knockout mice. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00052.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponnoth DS, Nayeem MA, Kunduri SS, et al. Role of omega-hydroxylase in adenosine-mediated aortic response through MAP kinase using A2Areceptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2012;302:R400–R408. doi: 10.1152/ajpregu.00481.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukashev DE, Smith PT, Caldwell CC, et al. Analysis of A2a receptordeficient mice reveals no significant compensatory increases in the expression of A2b, A1, and A3 adenosine receptors in lymphoid organs. Biochem Pharmacol. 2003;65:2081–2090. doi: 10.1016/s0006-2952(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 36.Olanrewaju HA, Gafurov BS, Lieberman EM. Involvement of K+ channels in adenosine A2A and A2B receptor-mediated hyperpolarization of porcine coronary artery endothelial cells. J Cardiovasc Pharmacol. 2002;40:43–49. doi: 10.1097/00005344-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Sitkovsky MV, Lukashev D, Apasov S, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 38.Sreenarasimhaiah J. Diagnosis and management of intestinal ischaemic disorders. BMJ. 2003;326:1372–1376. doi: 10.1136/bmj.326.7403.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivitz SM, Geller SC, Hahn C, et al. Treatment of acute mesenteric venous thrombosis with transjugular intramesenteric urokinase infusion. J Vasc Interv Radiol. 1995;6:219–223. doi: 10.1016/s1051-0443(95)71098-3. Discussion 224-218. [DOI] [PubMed] [Google Scholar]
- 40.Mallick IH, Yang W, Winslet MC, et al. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–1377. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- 41.Richard V, Kaeffer N, Tron C, et al. Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation. 1994;89:1254–1261. doi: 10.1161/01.cir.89.3.1254. [DOI] [PubMed] [Google Scholar]
- 42.Headrick JP, Lasley RD. Adenosine receptors and reperfusion injury of the heart. Handb Exp Pharmacol. 2009;193:189–214. doi: 10.1007/978-3-540-89615-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCallion K, Wattanasirichaigoon S, Gardiner KR, et al. Ischemic preconditioning ameliorates ischemia- and reperfusion-induced intestinal epithelial hyperpermeability in rats. Shock. 2000;14:429–434. doi: 10.1097/00024382-200014040-00002. [DOI] [PubMed] [Google Scholar]