Abstract
Objective
Disability or death occurs more frequently in patients with hemorrhagic transformation (HT) after ischemic stroke. In rat models of stroke, sulfonylurea (SU) drugs such as glibenclamide (adopted US name, glyburide) confer protection against swelling and HT through actions on the novel SUR1-regulated NCCa-ATP channel. Here, we sought to determine whether the use of SU drugs in patients with diabetes mellitus (DM) presenting with acute ischemic stroke might influence the incidence of HT.
Methods
We retrospectively analyzed data on 220 patients with DM who presented with acute ischemic stroke, 43 of whom were managed with and continued to receive SU drugs, and 177 of whom were managed without (controls).
Results
During a median length of stay in hospital of 11 days, 20 control patients (11%) experienced symptomatic HT (sHT), while no patient in the SU group experienced sHT (P=0.016). No patient in the SU group died, compared to 18 (10%) in the control group (P=0.027). Similarly favorable outcomes were observed after matching for baseline imbalances and excluding outliers. In support of the proposed mechanism, we present a case of sHT in which an analysis of brain tissues obtained intraoperatively showed prominent upregulation of SUR1, the target of SU drugs, in microvessels and neurons.
Interpretation
We conclude that, in diabetic patients with acute ischemic stroke, prior and continued use of SU drugs is associated with reduced sHT compared to those whose treatment regimen does not include SU drugs.
Keywords: stroke, hemorrhagic transformation, diabetes mellitus, sulfonylurea receptor 1, glibenclamide
INTRODUCTION
Hemorrhagic transformation (HT) – the secondary occurrence of petechial or confluent hemorrhage in the area of stroke – is an important complication of ischemic stroke, particularly in patients treated with recombinant tissue plasminogen activator (rtPA). Depending on the cohort and the timing of imaging, rates of HT vary widely.1 In a systematic review of prospective studies since 1996, the overall frequency of any HT, asymptomatic or symptomatic, in patients without antithrombotic drugs was 8.5%, and ranged from 8% to 22% when patients were treated with aspirin and heparin.2 In a study of 245 consecutive patients with acute ischemic stroke, autopsy revealed HT in 29% of patients.3 Previous investigations identified several risk factors for HT, with the strongest factors (odds ratio ≥2) including: thrombolytic treatment, baseline stroke severity, infarct size, early hypodensity on CT, age ≥70 years, hyperglycemia or history of diabetes, cardioembolism, and antiplatelet therapy.1–12 Disability or death occurs more frequently in patients with HT after an ischemic stroke, especially in patients with parenchymal hematoma.4
It is important to distinguish between clinically asymptomatic and symptomatic HT, because they have different prognostic importance.13 Data from two studies of thrombolytic therapy in acute stroke, the National Institute of Neurological Disorders and Stroke (NINDS) study5 and PROACT-II,6 revealed dramatic consequences of symptomatic HT (sHT) after ischemic stroke, with mortality rates of 45% and 83%, respectively. Moreover, it is likely that sHT prolongs hospital stay and delays initiation of therapy with antiplatelet agents or oral anticoagulants.14 Finding ways to protect the ischemic brain from sHT likely will improve the outcome of patients with ischemic stroke, and may expand the use of thrombolytic treatment.
In stroke, edema formation and HT have been postulated to comprise a continuum of pathological microvascular dysfunction initiated by ischemia/hypoxia.15 Microvascular dysfunction has been linked to de novo expression of sulfonylurea receptor 1 (SUR1), and the sulfonylurea (SU) inhibitor, glibenclamide, has been found to confer significant protection from microvascular dysfunction. In non-lethal preclinical models of thromboembolic, permanent, and temporary occlusion, as well as models of malignant cerebral edema, protection is manifested as significant improvements in edema and brain swelling.16–18 In models that incorporate rtPA administration, protection is manifested as significant improvements in swelling and HT.19,20 Reductions in edema, swelling, and HT are accompanied by commensurate improvements in lesion size, white matter preservation, neurological function, and mortality.
In patients with type 2 diabetes mellitus (DM) presenting with acute non-lacunar ischemic stroke, the use of SU drugs as part of the treatment regimen for diabetes has been associated with better outcomes at discharge compared to patients whose diabetes is managed without the use of SU drugs.21 However, previous studies have not examined the association between HT and the use of SU drugs. The aim of the present study was to determine whether, in diabetic patients, the use of SU drugs prior to and during hospitalization for acute ischemic stroke might influence the incidence of HT.
METHODS
Patients and Data Collection
We performed a blinded, retrospective chart review of all patients with type 2 diabetes mellitus who were admitted with an acute ischemic stroke to the Department of Neurology of Charité Hospital Campus Mitte, Berlin, Germany, from January 2005 to December 2006. Patients were included in the study if they: (i) had been on an antidiabetic sulfonylurea (SU) drug (glibenclamide, glimepiride, or gliquidon) prior to stroke and continued on the same medication after hospitalization for stroke (SU group); or (ii) had not been on a SU drug for 1 month or more prior to stroke, and were not started on a SU drug after hospitalization for stroke (controls). (See online supplement and Fig S1.)
Demographic, clinical and radiological data were collected from charts and from electronic medical records by a research associate (K.T.), a neurologist (H.K.) and a radiologist (B.V.), all of whom were blinded to treatment group. (See online supplement.)
The primary outcome measure was symptomatic hemorrhagic transformation (sHT) prior to discharge or within 21 days of the ischemic stroke, as defined by the criteria of the European Cooperative Acute Stroke Study II (ECASS II).22 Secondary outcomes were any HT (symptomatic or asymptomatic), death in hospital, functional independence at discharge (mRS ≤2 points), and neurological improvement at discharge (an improvement in NIHSS score ≥4 points or NIHSS=0, as previously21). HT were categorized in accordance with the ECASS II criteria.11 The size of the qualifying stroke was categorized in accordance with the criteria described by Paciaroni et al.4 (See online supplement.)
Immunohistochemistry
Human brain tissues were obtained at operation from the ipsilateral cerebral cortex of a 71 year old male who underwent decompressive craniectomy for malignant cerebral edema complicated by sHT, or at autopsy from the ipsilateral and contralateral cerebral cortex of a 65 year old male who had suffered a recent non-fatal embolic stroke and died following cardiopulmonary arrest (control). Tissues were obtained under a protocol approved by the Institutional Review Board of the University of Maryland. Paraffin sections were processed and immunolabeled for SUR1. (See online supplement.)
Statistical Analyses
Associations between treatment group and outcomes were examined using cross tabulations and Fisher’s exact test. The time from the ischemic stroke to death in hospital was analyzed using Kaplan-Meier failure estimates and the log-rank test. Patients who survived were right-censored at their discharge date.
Mantel-Haenszel methods were used to assess whether the association between treatment group and outcomes was confounded by known predictors of sHT.
To compare outcomes for patients with similar sHT risk factors, we applied a weighted Euclidean matching methodology (pPAIRS©)23 to match SU and control patients using 6-dimensions (NIHSS score, age, glucose, treatment with rtPA, treatment with anti-platelet agent prior to or on day 1, and stroke sub-type), in order to identify a subpopulation of control patients whose baseline characteristics were matched to those of the SU group. When there was an excess of subjects in the control group, 1:2 (SU:control) matching was adopted. Matches with extreme values of distances between them were eliminated using a threshold (75th percentile + 1.5* IQR).23
RESULTS
Baseline characteristics
Among all 220 patients, the mean time from stroke onset to hospital admission was 1.5 days [standard deviation (SD), 3.3]. Baseline demographic and clinical characteristics are summarized in Table 1. Patients treated with SU drugs were more likely to be male, had lower stroke severity on admission (NIHSS median, 5; range, 1–16 versus median, 7; range, 0–34; P=0.023; online supplement, Fig S2, left panel), and were less likely to be treated with antiplatelet agents prior to admission or on the first day in hospital, compared to patients without SU treatment (controls). Patients in the two groups did not differ with regard to glucose level on admission, thrombolytic or heparin treatment, INR and aPTT on admission, antiplatelet therapy from admission to day 3, etiology of stroke, or per os intake at 24 hours. Although the proportion of patient with NIHSS ≤9 on admission was lower in SU-treated patients, the prevalence of “large” and “large or medium” stroke sizes was similar in both groups.
Table 1.
Baseline variables for SU group versus all controls
| Control (N=177) n (% or IQR) |
SU Treatment (N=43) n (% or IQR) |
P-Value | |
|---|---|---|---|
| Age ≥ 70 years | 85 (48%) | 19 (44%) | 0.73 |
| Male sex | 92 (52%) | 31 (72%) | 0.025 |
| Admission NIHSS ≤ 9 | 116 (66%) | 37 (86%) | 0.0093 |
| Admission NIHSS, median (IQR) | 7 (4–14) | 5 (3–8) | 0.023* |
| Glucose ≥ 140 mg/dL | 115 (65%) | 24 (56%) | 0.29 |
| Received rtPA | 9 (5%) | 2 (5%) | 1.0 |
| INR on admission, median (IQR) | 1.05 (1.0–1.13) | 1.05 (0.98–1.12) | 0.55* |
| PTT, median (IQR) | 33.1 (30.6–36.2) | 33.4 (30.9–35.9) | 0.78* |
| Heparin treatment prior to stroke | 8 (5%) | 1 (2%) | 1.0 |
| Platelet count per nL, median (IQR) | 237 (196–287) | 228 (183–252) | 0.052* |
| Antiplatelet therapy prior to admission or on day 1 | 117 (66%) | 20 (47%) | 0.022 |
| Antiplatelet therapy on admission or day 1–3 | 153 (86%) | 33 (77%) | 0.15 |
| Stroke subtype (TOAST): large-vessel disease | 79 (45%) | 20 (47%) | 0.87 |
| Stroke subtype (TOAST): cardioembolic | 54 (31%) | 16 (37%) | 0.47 |
| Stroke subtype (TOAST): lacunar | 22 (12%) | 7 (16%) | 0.46 |
| Large stroke (IS score>=3) | 21 (12%) | 4 (9%) | 0.79 |
| Large or medium stroke (IS score >=2) | 72 (41%) | 13 (30%) | 0.23 |
| Prior stroke | 45 (25%) | 14 (33%) | 0.45 |
| Prior TIA | 14 (8%) | 7 (16%) | 0.14 |
| Per os intake at 24 hours# | 127 (72%) | 35 (81%) | 0.25 |
Abbreviations: NIHSS, National Institute of Health Stroke Scale; IQR, Interquartile range
Mann-Whitney U test; other P-values from Fisher’s exact test (two-tailed)
patients did not have relevant swallowing restrictions and received their food and medication per os; this excludes patients with a nasogastric tube and a single control patient with orders for intravenous infusion and nil per os
There were no important differences in the mean number of radiological examinations (2.3 versus 2.2; P=0.59), the median time between stroke onset and last imaging for stroke (74.4 versus 77.3 hours; P=0.97), and the proportion of magnetic resonance imaging for stroke evaluation (48% versus 54%; P=0.61) in the control versus treatment groups, respectively. The median length of stay in hospital was 11 and 10 days for control and treatment groups, respectively (P=0.77).
The analysis following Euclidean matching23 identified 70 out of the original 177 control patients whose baseline characteristics were matched to those of the SU group. The resulting baseline characteristics of the matched control subgroup and the SU group were similar, including per os intake at 24 hours (Table 2 and online supplement, Fig S2, right panel).
Table 2.
Baseline variables after matching using pPAIRS©
| SU Treatment (N=43) n (% or IQR) |
Matched Controls (N=70) n (% or IQR) |
Excluded Controls (N=107) n (% or IQR) |
P-Value Matched vs. Excluded |
P-Value Matched vs. SU Treatment |
|
|---|---|---|---|---|---|
| Age ≥ 70 years | 19 (44%) | 29 (41%) | 56 (52%) | 0.17 | 0.85 |
| Male sex | 31 (72%) | 39 (56%) | 53 (50%) | 0.45 | 0.11 |
| Admission NIHSS ≤ 9 | 37 (86%) | 55 (79%) | 61 (57%) | 0.0036 | 0.46 |
| Admission NIHSS, median (IQR) | 5 (3–8) | 5 (3–8) | 8 (4–14) | 0.0008* | 0.71* |
| Glucose ≥ 140 mg/dL | 24 (56%) | 45 (64%) | 70 (65%) | 0.87 | 0.43 |
| Received rtPA | 2 (5%) | 2 (3%) | 7 (7%) | 0.49 | 0.63 |
| INR on admission, median (IQR) | 1.05 (0.98–1.12) | 1.04 (1.01–1.1) | 1.07 (1.0–1.14) | 0.32* | 0.91* |
| PTT, median (IQR) | 33.4 (30.9–35.9) | 33.2 (30.1–36.8) | 33.0 (30.7–36.1) | 0.82* | 0.73* |
| Heparin treatment prior to stroke | 1 (2%) | 3 (4%) | 5 (5%) | 1.0 | 1.0 |
| Platelet count per nL, median (IQR) | 228 (183–252) | 234 (196–287) | 242 (195–289) | 0.38* | 0.22* |
| Antiplatelet therapy prior to admission or on day 1 | 20 (47%) | 38 (54%) | 79 (74%) | 0.0093 | 0.42 |
| Antiplatelet therapy on admission or day 1–3 | 33 (77%) | 59 (84%) | 94 (88%) | 0.51 | 0.33 |
| Stroke subtype (TOAST): large-vessel disease | 20 (47%) | 35 (50%) | 44 (41%) | 0.28 | 0.85 |
| Stroke subtype (TOAST): cardioembolic | 16 (37%) | 22 (31%) | 32 (30%) | 0.87 | 0.55 |
| Stroke subtype (TOAST): lacunar | 7 (16%) | 13 (19%) | 9 (8%) | 0.062 | 0.81 |
| Large stroke (IS score>=3) | 4 (9%) | 10 (14%) | 11 (10%) | 0.48 | 0.56 |
| Large or medium stroke (IS score >=2) | 13 (30%) | 31 (44%) | 41 (38%) | 0.44 | 0.17 |
| Prior stroke | 14 (33%) | 18 (26%) | 27 (25%) | 1.0 | 0.52 |
| Prior TIA | 7 (16%) | 5 (7%) | 9 (8%) | 1.0 | 0.21 |
| Per os intake at 24 hours# | 35 (81%) | 56 (80%) | 71 (66%) | 0.060 | 1.0 |
NIHSS, National Institute of Health Stroke Scale; IQR, Interquartile range
Mann-Whitney U test; other P-values from Fisher’s exact test (two-tailed)
patients did not have relevant swallowing restrictions and received their food and medication per os; this excludes patients either with a nasogastric tube or with orders for intravenous infusion and nil per os
The 107 patients omitted by the matching algorithm were more severely injured, with a higher median NIHSS of 8 (range 1–34 and IQR 4–14), compared to the matched controls, with a median NIHSS of 5 (range 0–18 and IQR 3–8). The omitted controls had a higher incidence of prior antiplatelet therapy (Table 2) and a higher incidence of sHT (12%) compared to the matched subgroup (10%).
HT and death
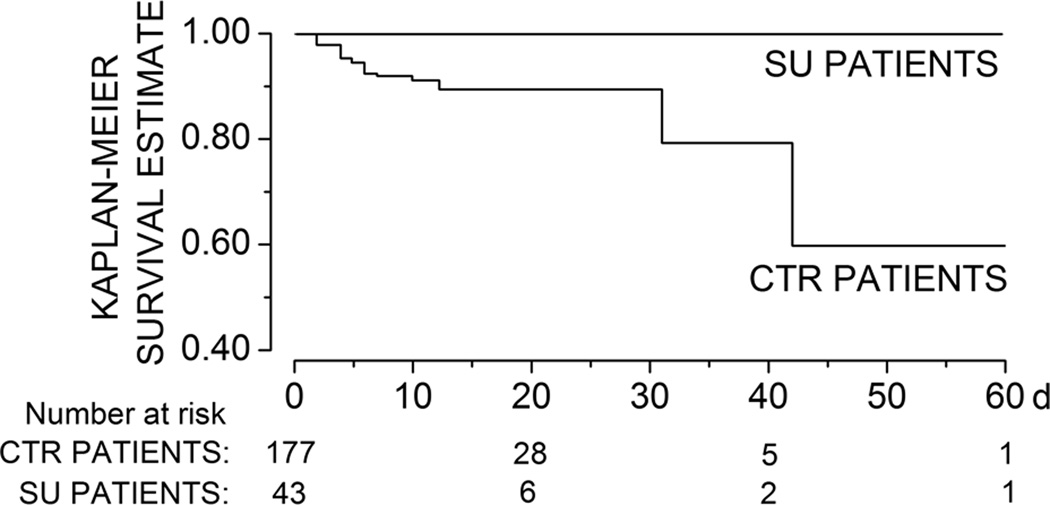
sHT occurred in 20 patients and 18 patients died. All patients with sHT or death in hospital were in the control group (Table 3A). Patients who experienced sHT were more likely to be female, to have higher NIHSS scores, and to have been treated with rtPA (online supplement, Table S1). The median time from stroke to diagnosis of sHT was 2 days (range, 0.5–19 days) and the median time from stroke to death was 5 days (range, 1–42 days). Patients taking SU drugs were significantly more likely to survive during hospitalization (Fig 1), and were significantly less likely to experience any HT (asymptomatic or symptomatic). Overall, 11 of 20 sHTs, and 15 of 50 HTs were parenchymal hematomas.
Table 3.
Outcomes
| A: SU group versus all controls | |||
|---|---|---|---|
| Control (n=177) n (%) |
SU Treatment (n=43) n (%) |
P-value* | |
| sHT | 20 (11%) | 0 (0%) | 0.016 |
| death | 18 (10%) | 0 (0%) | 0.027 |
| any HT | 50 (28%) | 4 (9%) | 0.0096 |
| Change in NIHSS ≥4 or NIHSS=0 | 24 (14%) | 12 (28%) | 0.036 |
| Discharge mRS ≤ 2 | 59 (33%) | 25 (58%) | 0.0047 |
| B: SU group versus controls matched using pPAIRS© | |||
|---|---|---|---|
| Control (N=70) n (%) |
SU Treatment (N=43) n (%) |
P-value* | |
| sHT | 7 (10%) | 0 (0%) | 0.043 |
| death | 6 (9%) | 0 (0%) | 0.081 |
| any HT | 20 (29%) | 4 (9%) | 0.018 |
| Change in NIHSS ≥4 or NIHSS=0 | 4 (6%) | 12 (28%) | 0.0017 |
| Discharge mRS ≤ 2 | 29 (41%) | 25 (58%) | 0.12 |
P-values from Fisher’s exact test (two-tailed)
Figure 1. Diabetic patients taking sulfonylurea drugs are more likely to survive during hospitalization for stroke.
Risk of in-hospital death in diabetic patients treated with sulfonylurea (SU) drugs versus those managed without SU drugs; Kaplan-Meier survival estimates; log rank test, χ2= 4.89; P=0.03.
Several factors have been found to be important predictors of HT after ischemic stroke (see Introduction). Separate Mantel-Haenszel analyses were carried out to assess potential effects of age ≥70 years, NIHSS>9 on admission, large size of ischemic stroke, antiplatelet therapy prior to admission or on day 1 and use of rtPA. In these analyses, SU treatment remained significantly associated with the risk of sHT. Similarly, there was no evidence that the association between SU treatment and in-hospital death was confounded by any of the aforementioned variables.
Comparison of the Euclidean matched control subgroup with the SU cohort showed that, despite removing the most severely injured patients, the difference in sHT remained significantly lower in the SU group, and there was a strong but non-significant trend toward a reduction in mortality (Table 3B). Patients in the SU group were significantly less likely to experience any HT.
Outcomes at discharge
Patients treated with a SU drug were significantly more likely to have a better neurological outcome (improvement in NIHSS score ≥4 points or NIHSS score=0) and a better functional outcome (mRS≤2) at the time of discharge (Table 3A). Based on Mantel-Haenszel analyses controlling for age, gender or NIHSS on admission, these covariables did not influence the association between SU treatment and functional outcome. Baseline matching also showed significantly better neurological outcomes and a strong trend toward better functional outcomes (Table 3B).
Case report
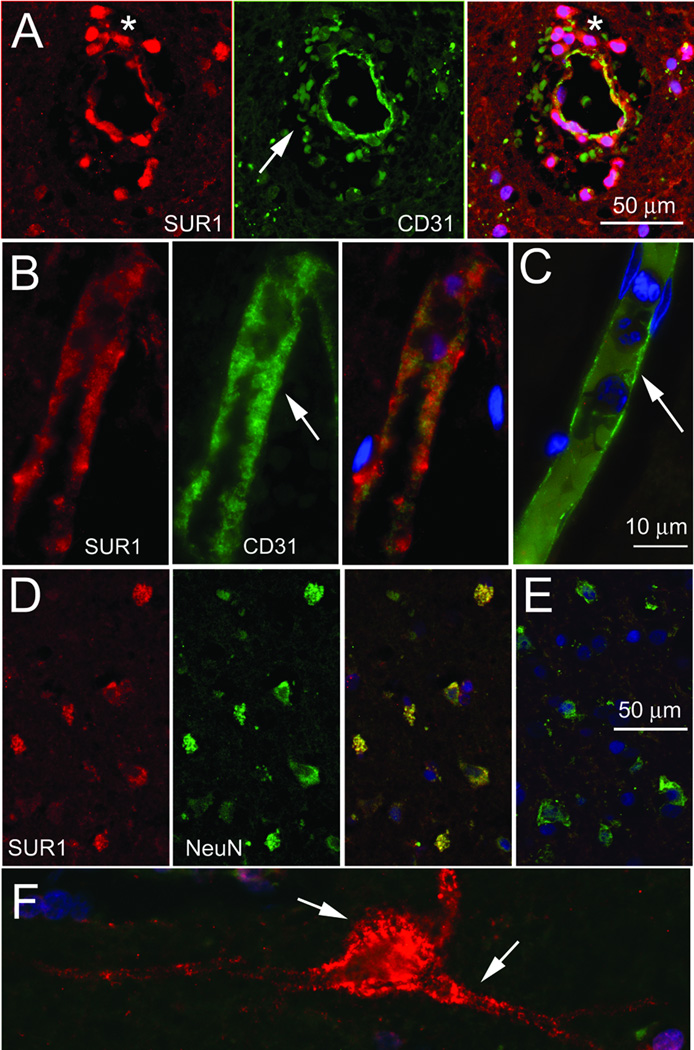
A 71 year old male presented with a right middle cerebral artery (MCA) syndrome. Imaging showed early changes of a large MCA stroke secondary to occlusion of the M1 trunk (online supplement, Fig S3A–C). He received conventional treatment with rtPA within 3 hours of symptom onset. Neurological and radiological deterioration at ~36 hours confirmed sHT (Fig S3D), prompting decompressive craniectomy (Fig S3E). He initially improved, but within 24 hours, further neurological and radiological deterioration (Fig S3F) prompted return to the operating room for debridement of infarcted, hemorrhagic tissues, at which time biopsies of adjacent viable tissues also was performed. Analysis of biopsy specimens showed widespread upregulation of SUR1 in microvessels and in neurons (Fig 2). The finding of SUR1 upregulation in stroke was corroborated using tissues obtained at autopsy from a patient with a recent non-fatal stroke, with contralateral tissues serving as negative control.
Figure 2. Sulfonylurea receptor 1 (SUR1) is upregulated in a patient with symptomatic hemorrhagic transformation (sHT).
A–C: Sections of cortex obtained at operation from the ipsilateral cerebral cortex of a patient who underwent decompressive craniectomy for malignant cerebral edema complicated by sHT (A), or at autopsy from the ipsilateral (B) and contralateral (C) cerebral cortex of a patient who had suffered a recent non-fatal embolic stroke (control), double immunolabeled for SUR1 (red) and for the endothelial cell marker, PECAM-1 (CD31; green); merged images of the double labelling are shown in (C), and in the panels on the right in (A) and (B), with co-localization indicated by the yellow colour. In (A), note the extravasated erythrocytes that autofluoresce in the green channel (arrow), and the extravasated neutrophils (asterisk) which are believed to express SUR1-KATP channels31 surrounding the SUR1-positive vessel (yellow). In (B) and (C), note the swollen SUR1-positive endothelium (B, arrow), compared to the thin, SUR1-negative endothelium in the control tissues (C, arrow). D–F: Sections of cortex from the patient with sHT (D,F) and from the control tissues (E), double immunolabeled for SUR1 (red) and for the neuronal marker, NeuN (green); merged images of the double labelling are shown in (E), and in the panel on the right in (D), with co-localization indicated by the yellow colour. Note the SUR1-positive cortical neurons with blebbing in (D,F), compared to the SUR1-negative cortical neurons in the control tissues (E); in (F), blebs are observed in the perikaryon and in large processes (arrows).
DISCUSSION
The present study is the first to show an association between the occurrence of sHT, upregulation of SUR1 in brain microvessels, and treatment with SU drugs in humans with acute ischemic stroke. The association with SU treatment was supported by a post-hoc Mantel-Haenszel procedure to test for baseline influences, as well as by explicit matching with outlier elimination based on factors known to be associated with an increase in likelihood of sHT.
Given that sHT after ischemic stroke results in significant increases in disability and death,4 there is an urgent need to identify treatments to protect the ischemic brain from sHT. Our findings here are limited to patients with DM. However, diabetic patients comprise one third of stroke patients, diabetics are among those who suffer the worst outcomes from stroke, and stroke in diabetics has a high rate of recurrence,24,25 making it difficult to overestimate the importance of improving outcomes in this subgroup of stroke patients. A convergence of data suggests that an increased risk of sHT is a major factor in the poorer outcomes observed in diabetic patients,26,27 making the principal finding of the present study highly pertinent. Given that the principal adverse effect of glibenclamide is hypoglycemia, and that a high proportion of diabetic and non-diabetic patients presenting with stroke are hyperglycemic,26 it is anticipated that diabetic patients presenting with stroke would be relatively unlikely to experience an adverse event from administration of glibenclamide, especially at the low dose shown to be beneficial in preclinical models.16,17 In addition, it is possible that SU drugs could ameliorate sHT more broadly, i.e., in non-diabetic patients, including those treated with rtPA, as suggested by the preclinical data.19,20
The favorable outcome in the SU group could have been due to one or more causes. While it is possible that SU protection shifts the etiology of stroke toward less severe causes (a trend toward higher lacunar stroke in the SU arm was observed), it also is possible that prior use of SU drugs reduces the initial stroke severity for a given stroke etiology. The latter possibility is consistent with the strong imbalance in baseline NIHSS scores of the control versus the SU groups, a finding also identified in a previous study,21 and is consistent with the observation of an apparent dissociation between the size of stroke and the admission NIHSS score in the SU group. Whereas both the control and the SU groups had the same proportions of “large” and “large or medium” strokes, the SU group presented with significantly better NIHSS scores than the control group.
Secondly, it is possible that continued use of SU drugs after stroke exerts a direct protective effect attributable to inhibition of ischemia-induced upregulation of SUR1. This possibility is supported by the strong confirmatory evidence that emerged from our analysis, performed after matching the control and SU groups for baseline variables, which demonstrated a significant benefit regarding sHT and neurological improvement. This interpretation is consistent with preclinical data in models of stroke, both without16–18 and with rtPA,19,20 in which glibenclamide was shown to confer significant protection in terms of edema, brain swelling, HT, lesion size, white matter preservation, neurological function, and mortality. Moreover, this interpretation is consistent with the finding of marked upregulation of SUR1 in microvessels and neurons of a patient who suffered from sHT and who required decompressive craniectomy, similar to findings of SUR1 upregulation in rat models of malignant cerebral edema.16,18,20
A single report suggested that the use of SU drugs before stroke may not influence outcome favorably.28 As discussed elsewhere,29 shortcomings with methodology and with the dataset used for this report limit the validity of its conclusion. Overall, preclinical and retrospective clinical studies strongly support the use of SU drugs in the context of cerebral ischemia. The data presented here, as well as previously,21 suggest that patients with DM who are managed with SU drugs and who present with an acute ischemic stroke should not have their SU drug discontinued, unless a contraindication is present.
The present study has several limitations, in part due to its retrospective design. The specific data that were collected were not decided a priori, and continuing versus discontinuing the use of a SU drug after hospitalization for stroke was not determined by study design. Although the latter could have biased the study toward excluding patients from the SU group who were too ill to take an oral medication, per os intake at 24 hours was not different in the two groups, and was essentially identical after matching. Finally, we could not calculate the odds ratio for sHT, since sHT was not identified in any patient in the SU group.
We conclude that, in diabetic patients with acute ischemic stroke, prior and continued use of SU drugs is associated with a more favorable outcome than in diabetic patients whose treatment regimen does not include SU drugs. This study, in agreement with a prior study,21 provides evidence that, absent contra-indications, diabetic patients taking SU drugs and presenting with acute ischemic stroke should have these drugs continued, not stopped. The findings of the present study re-affirm the need for prospective trials to test the protective effects of sulfonylureas in stroke.30
Supplementary Material
Acknowledgement
Funding was provided by grants to JMS from the National Heart, Lung and Blood Institute (HL082517) and the National Institute of Neurological Disorders and Stroke (NS061808), and to PM and TAK from the Institute of Clinical and Translational Research at the Baylor College of Medicine and the Department of Veterans Affairs (VISN 16 PGP).
Footnotes
CONFLICT OF INTEREST. JMS holds a US patent (#7,285,574), "A novel non-selective cation channel in neural cells and methods for treating brain swelling", and is a member of the scientific advisory board and holds shares in Remedy Pharmaceuticals. No support, direct or indirect, was provided to JMS, or for this project, by Remedy Pharmaceuticals. PM and TAK hold the copyright for pPAIRS©. They have no commercial interest in its use. All other authors declare no COI.
References
- 1.Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke. 2007;38:431–440. doi: 10.1161/01.STR.0000254524.23708.c9. [DOI] [PubMed] [Google Scholar]
- 2.Lindley RI, Wardlaw JM, Sandercock PA, et al. Frequency and risk factors for spontaneous hemorrhagic transformation of cerebral infarction. J Stroke Cerebrovasc Dis. 2004;13:235–246. doi: 10.1016/j.jstrokecerebrovasdis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Kerenyi L, Kardos L, Szasz J, et al. Factors influencing hemorrhagic transformation in ischemic stroke: a clinicopathological comparison. Eur J Neurol. 2006;13:1251–1255. doi: 10.1111/j.1468-1331.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 4.Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke. 2008;39:2249–2256. doi: 10.1161/STROKEAHA.107.510321. [DOI] [PubMed] [Google Scholar]
- 5.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 6.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 7.Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 8.Bruno A, Levine SR, Frankel MR, et al. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59:669–674. doi: 10.1212/wnl.59.5.669. [DOI] [PubMed] [Google Scholar]
- 9.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 10.Larrue V, von KR, del Zoppo GJ, et al. Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke. 1997;28:957–960. doi: 10.1161/01.str.28.5.957. [DOI] [PubMed] [Google Scholar]
- 11.Larrue V, von Kummer RR, Muller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 12.Yong M, Kaste M. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke. 2008;39:2749–2755. doi: 10.1161/STROKEAHA.108.514307. [DOI] [PubMed] [Google Scholar]
- 13.Park JH, Ko Y, Kim WJ, et al. Is asymptomatic hemorrhagic transformation really innocuous? Neurology. 2012;78:421–426. doi: 10.1212/WNL.0b013e318245d22c. [DOI] [PubMed] [Google Scholar]
- 14.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 15.Simard JM, Kent TA, Chen M, et al. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(Ca- ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simard JM, Yurovsky V, Tsymbalyuk N, et al. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2009;40:604–609. doi: 10.1161/STROKEAHA.108.522409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simard JM, Tsymbalyuk N, Tsymbalyuk O, et al. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke. 2010;41:531–537. doi: 10.1161/STROKEAHA.109.572644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simard JM, Geng Z, Silver FL, et al. Does inhibiting SUR1 complement rtPA in cerebral ischemia? Ann N Y Acad Sci. 2012 doi: 10.1111/j.1749-6632.2012.06705.x. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simard JM, Woo SK, Tsymbalyuk N, et al. Glibenclamide – 10 hour treatment window in a clinically-relevant model of stroke. Transl Stroke Res. 2011 doi: 10.1007/s12975-012-0149-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunte H, Schmidt S, Eliasziw M, et al. Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke. 2007;38:2526–2530. doi: 10.1161/STROKEAHA.107.482216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 23.Mandava P, Kalkonde YV, Rochat RH, et al. A matching algorithm to address imbalances in study populations: application to the National Institute of Neurological Diseases and Stroke Recombinant Tissue Plasminogen Activator acute stroke trial. Stroke. 2010;41:765–770. doi: 10.1161/STROKEAHA.109.574103. [DOI] [PubMed] [Google Scholar]
- 24.Callahan A, Amarenco P, Goldstein LB, et al. Risk of stroke and cardiovascular events after ischemic stroke or transient ischemic attack in patients with type 2 diabetes or metabolic syndrome: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Arch Neurol. 2011;68:1245–1251. doi: 10.1001/archneurol.2011.146. [DOI] [PubMed] [Google Scholar]
- 25.Ergul A, Kelly-Cobbs A, Abdalla M, et al. Cerebrovascular Complications of Diabetes: Focus on Stroke. Endocr Metab Immune Disord Drug Targets. 2011 doi: 10.2174/187153012800493477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007;27:435–451. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- 27.Elgebaly MM, Ogbi S, Li W, et al. Neurovascular injury in acute hyperglycemia and diabetes: A comparative analysis in experimental stroke. Transl Stroke Res. 2011;2:391–398. doi: 10.1007/s12975-011-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favilla CG, Mullen MT, Ali M, et al. Sulfonylurea use before stroke does not influence outcome. Stroke. 2011;42:710–715. doi: 10.1161/STROKEAHA.110.599274. [DOI] [PubMed] [Google Scholar]
- 29.Simard JM, Kent TA, Kunte H. Letter by Simard et al regarding article, "Sulfonylurea use before stroke does not influence outcome". Stroke. 2011;42:e409. doi: 10.1161/STROKEAHA.111.620666. [DOI] [PubMed] [Google Scholar]
- 30.Arboix A. Potential impact of sulfonylureas in the outcome of type 2 diabetic patients with ischemic stroke. Stroke. 2007;38:2413–2414. doi: 10.1161/STROKEAHA.107.488361. [DOI] [PubMed] [Google Scholar]
- 31.Da Silva-Santos JE, Santos-Silva MC, Cunha FQ, et al. The role of ATP-sensitive potassium channels in neutrophil migration and plasma exudation. J Pharmacol Exp Ther. 2002;300:946–951. doi: 10.1124/jpet.300.3.946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.