Summary
Secondary lymphoid organs (SLOs), including lymph nodes, Peyer's patches, and the spleen, have evolved to bring cells of the immune system together. In these collaborative environments, lymphocytes scan the surfaces of antigen-presenting cells for cognate antigens, while moving along stromal networks. The cell-cell interactions between stromal and hematopoietic cells in SLOs are therefore integral to the normal functioning of these tissues. Not only do stromal cells physically construct SLO architecture, but they are essential for regulating hematopoietic populations within these domains. Stromal cells interact closely with lymphocytes and dendritic cells, providing scaffolds on which these cells migrate, and recruiting them into niches by secreting chemokines. Within lymph nodes, stromal cell-ensheathed conduit networks transport small antigens deep into the SLO parenchyma. More recently, stromal cells have been found to induce peripheral CD8+ T-cell tolerance and control the extent to which newly activated T cells proliferate within lymph nodes. Thus, stromal-hematopoietic crosstalk has important consequences for regulating immune cell function within SLOs. In addition, stromal cell interactions with hematopoietic cells, other stroma, and the inflammatory milieu have profound effects on key stromal functions. Here, we examine ways in which these interactions within the lymph node environment influence the adaptive immune response.
Keywords: fibroblastic reticular cell, lymphatic endothelial cell, blood endothelial cell, integrin α7+ pericyte, T cell, dendritic cell, tolerance/suppression
Introduction
Hallmarks of adaptive immusnity include the capacity to generate potent antigen-specific responses to diverse collections of pathogens and to develop immunological memory, which confers continued protection against many of these invaders. T and B lymphocytes express highly specific antigen recognition receptors that allow these cells to respond to infected cells or invading pathogens without causing undue harm to healthy tissues. One of the challenges facing the initiation of productive primary immune responses is that extremely few naive T cells recognize a given peptide-major histocompatibility complex (pMHC) complex. In fact, studies have estimated that within the entire naive T-cell repertoire of a mouse, only 20–1,200 T cells may be specific for a particular pMHC complex (1–3). Therefore, a fundamental challenge that exists for generating primary immunity is how to bring together rare antigen-specific T cells and their cognate antigens.
A clever solution to this problem involves highly organized secondary lymphoid organs (SLOs), such as lymph nodes, Peyer's patches (PPs), and the spleen, which facilitate immunosurveillance of tissue-derived or blood-borne antigens. Accordingly, rather than patrolling the entire body in search of cognate antigens, lymphocytes may survey these SLOs while still receiving representative information about the status of neighboring tissues.
Once naive lymphocytes enter SLOs, they must still actively traverse these specialized structures in search of their cognate antigens. SLOs are complex, compartmentalized tissues in which hematopoietic populations may interact. Lymph nodes, for example, can be divided into cortical, paracortical, and medullary areas, each of which is characterized by the presence of specific non-hematopoietic stromal subsets of mesenchymal and endothelial origin (Fig. 1). Lymph node stromal cells (LNSCs) are a heterogeneous population that can be divided into subclasses based on surface expression of the glycoproteins CD31 and podoplanin (gp38) and their localization within lymph nodes: fibroblastic reticular cells (FRCs)(gp38+CD31−), follicular dendritic cells (FDCs)(gp38+/−CD31−), lymphatic endothelial cells (LECs)(gp38+CD31+), blood endothelial cells (BECs)(gp38−CD31+), integrin α7+ pericytes (IAPs)(gp38−CD31−ITGA7+), and a small proportion (<5%) of otherwise undefined stroma (gp38−CD31−ITGA7−) (4, 5). FRCs, FDCs, and BECs can also be found in other SLOs, where they are likely to exhibit characteristics similar to those of their lymph node counterparts. We have also detected the presence of IAPs in primary lymphoid organs such as the thymus, but it remains to be determined whether these cells also exist in PPs and the spleen (authors’ unpublished data). Stromal cells have long been recognized as key structural components of SLOs (6–8). In fact, for many years, stromal cells of SLOs were believed to play purely structural roles in constructing and maintaining SLO environments. More recently, however, we have come to understand many ways in which stromal cells of SLOs interact with hematopoietic populations, actively influencing the development of adaptive immune responses.
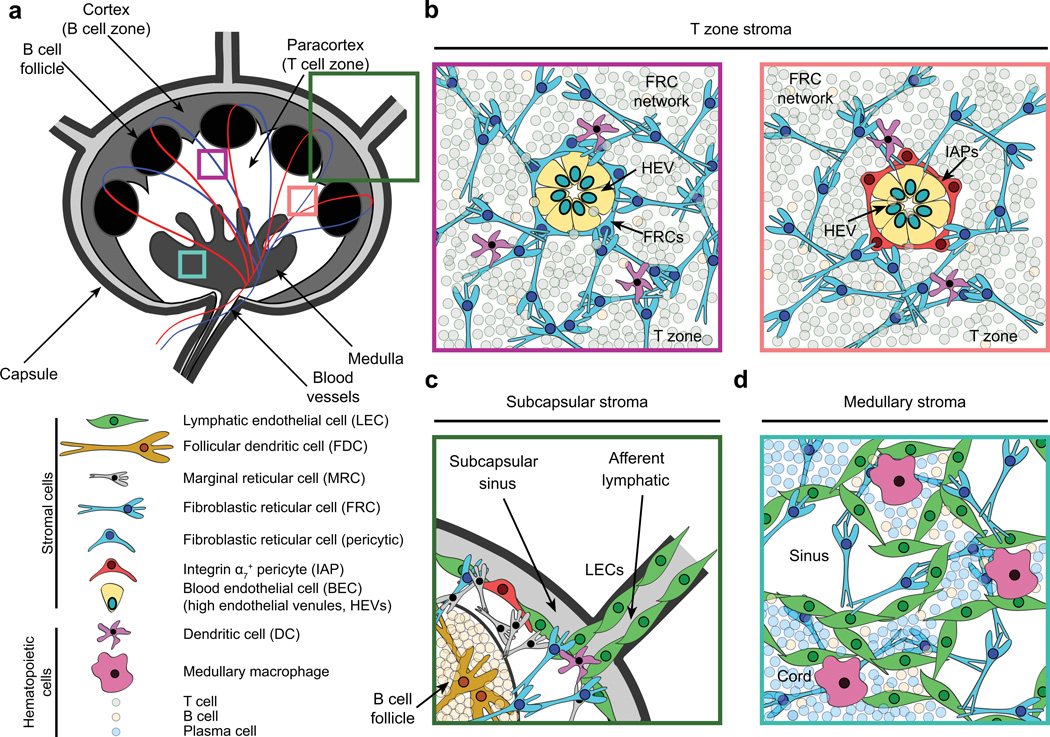
Fig. 1. Lymph node architecture and stromal cell localization.
(A) Cartoon depicting lymph node architecture and compartmentalization. (B) Naive lymphocytes gain access to lymph nodes through high endothelial venules (HEVs). HEVs are comprised of specialized blood endothelial cells (BECs) that regulate lymphocyte entry into lymph nodes via expression of molecules such as peripheral node addressins. These structures are routinely surrounded by a layer of fibroblastic reticular cells (FRCs, left). However, a small proportion (<15%) of these gateways are instead ensheathed by integrin α7+ pericytes (IAPs, right). Both types of HEVs appear similar histologically. Furthermore, they connect to the dense FRC network within the paracortex, providing a continuous scaffold on which naive lymphocytes and dendritic cells crawl and interact. FRCs also promote naive T-cell survival through the secretion of IL-7. (C) Interstitial fluid and migratory dendritic cells enter lymph nodes through afferent lymphatics, which are lined by lymphatic endothelial cells (LECs). Between the capsule and the lymph node parenchyma lies the subcapsular sinus (SCS), within which lymph percolates, allowing antigen uptake by macrophages. FRCs, CXCL13-secreting marginal reticular cells, and IAPs are also found proximal to the SCS. B-cell follicles contain a specialized stromal subset (follicular dendritic cells), which interacts with B lymphocytes and continually captures and displays antigens on its surface via complement receptors. (D) The lymph node medulla contains macrophages, B cells, plasma cells, and a dense LEC network that regulates lymphocyte egress through efferent lymphatics.
Indeed, we now know that hematopoietic cells do not exist in a void within SLOs, ignorant of the many stromal populations that surround them. Rather, leukocytes are in continual, close contact with different stromal subsets from the moment that they enter these tissues. Accordingly, SLO-resident stromal cells have been observed to play profound roles in promoting and regulating different aspects of adaptive immunity. Furthermore, this flow of information is not unidirectional, as studies have demonstrated that even fundamental functions of stromal cells are modulated by cues from hematopoietic cells, other stromal cells, and more broadly the SLO environment. Here we explore how these various interactions within lymph nodes affect adaptive immune responses, paying close attention to the role of FRCs.
Stromal cells facilitate antigen delivery to lymph nodes
Stromal-hematopoietic interactions are crucial for lymph node development and antigen-presentation in these organized tissues. In fact, crosstalk among lymphoid tissue inducer cells, mesenchymal stromal organizer cells, and LECs induces the generation of lymph nodes near branch points of blood vessels (reviewed in 9, 10). However, the mere existence of naive lymphocyte-filled SLOs is not sufficient for the initiation of adaptive immune responses to invading pathogens in the periphery, as tissue-derived antigens must still gain access to these specialized compartments.
Fortuitously, lymph nodes are integrated into the lymphatic system, which drains interstitial fluid, or lymph, from tissues, ultimately returning it to the circulatory system. LECs form the branched lymphatic vessels, with specialized tips, that connect tissues to draining lymph nodes (11). Tissue-resident dendritic cells (DCs) have been found to migrate to draining lymph nodes via these lymphatic vessels, actively carrying antigens from upstream tissues (12–16). As DCs migrate to draining lymph nodes, they are in close contact with the LECs that form the lymphatic vessels within which they travel (11, 17). These lymphatic vessels have been found to increase expression of numerous cell adhesion molecules under inflammatory conditions, promoting DC entry into these structures (18). However, DC entry into afferent lymphatic vessels does not absolutely require integrin-mediated cell adhesion, and imaging studies have revealed that these cells can enter initial lymphatic vessels through gaps in the perilymphatic basement membrane (19, 20). Additionally, DCs have been shown to adhere to LECs in vitro, and interestingly direct contact between DCs and inflamed LECs leads to decreased levels of costimulatory molecule expression by DCs, highlighting functional implications of these interactions (21). We have recently demonstrated that DC migration to lymph nodes via lymphatic vessels is facilitated by interactions between the C-type lectin receptor, CLEC2 (expressed by DCs), and its ligand gp38 (expressed by LECs and FRCs) (4, 22, 23). In the absence of CLEC2-gp38 interactions, DC migration to lymph nodes was impaired as was the developing T-cell response (22). Afferent lymphatic vessels also facilitate the passive entry of tissue-derived antigens to lymph nodes by allowing lymph to drain into these structures where antigens can subsequently be accessed and processed by lymph node-resident antigen-presenting cells (24–28). In contrast, efferent lymphatics regulate lymphocyte egress from lymph nodes (reviewed in 29).
Within the T-cell zones of lymph nodes and the spleen, FRCs secrete and ensheath an extracellular matrix (ECM) based reticular or conduit network (5, 7, 24, 26, 30) (Figs 1 and 2). This conduit network allows molecules such as chemokines and small soluble antigens to rapidly flow from upstream tissues deep into the parenchyma of draining lymph nodes (26, 31). Chemokines that are produced within inflamed upstream tissues may thereby quickly be displayed by BECs that form the high endothelial venules (HEVs) through which leukocytes enter these sites (27, 28). In addition, lymph-borne antigens may be accessed by DCs that are in close contact with FRCs along the reticular network of the T-cell zone (26, 32). While future studies may identify the specific nature of the contacts between these cell types, it is likely that FRC conduit networks contribute to adaptive immunity by enhancing leukocyte recruitment to inflamed lymph nodes and by creating a concentrated pool of soluble antigens that can rapidly be sampled and displayed by resident antigen-presenting cells.
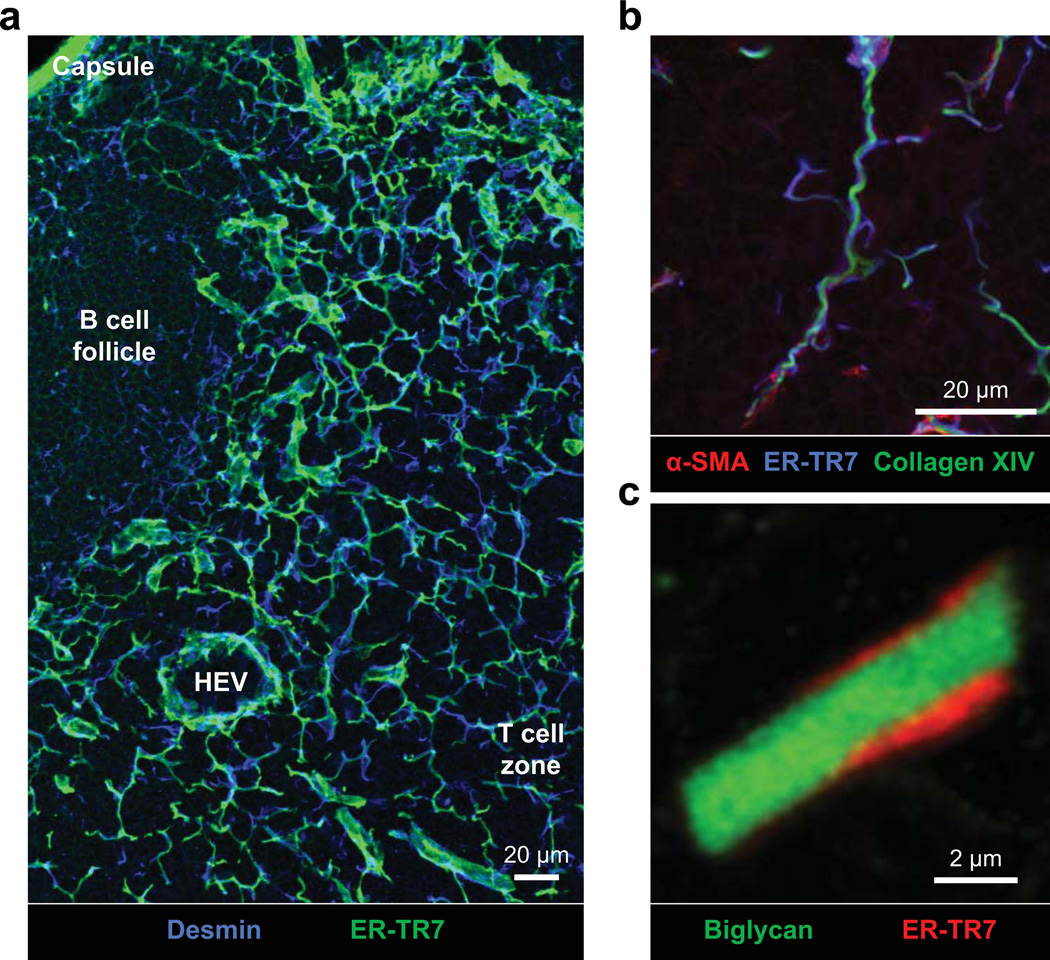
Fig. 2. Fibroblastic reticular cell network.
Fibroblastic reticular cells (FRCs) secrete, ensheath, and maintain an extracellular matrix (ECM)-based conduit or reticular network. The conduit network links the SCS to high endothelial venules (HEVs) within the lymph node paracortex, allowing small molecules such as chemokines to traffic from inflamed tissues to HEVs. Dendritic cells in close contact with the conduit network also gain rapid access to tissuederived antigens within the paracortex. In contrast to the paracortex, within B-cell follicles the FRC network is quite sparse, with little branching. (A) Confocal immunofluorescence analysis of 15 μm thick frozen skin-draining lymph node sections stained for desmin (blue, FRCs) and the ER-TR7 antigen (green, conduit network). ER-TR7 binds to an unidentified ECM component within the microfibrillar zone of the reticular network. (B) Confocal immunofluorescence analysis of 7.5 μm thick frozen skin-draining lymph node sections stained for α-smooth muscle actin (red, FRCs), the ER-TR7 antigen (blue, conduit microfibrillar zone), and collagen XIV (green, conduit core). (C) High magnification view of the conduit network. Sections were prepared as in (B), and stained for biglycan (green, collagen core) and the ER-TR7 antigen (red, conduit microfibrillar zone).
The conduit network consists of many different ECM components that together act as a molecular sieve, allowing small molecules to gain access to the lymph node paracortex (24, 26). We have recently found that the structure of the conduit network is more complicated than had previously been perceived, as FRCs express a plethora of ECM components and ECM regulatory molecules in resting and inflamed lymph nodes (5). Interestingly, the transcriptome of IAPs is similarly ECM-rich, although these pericytes have not yet been linked to conduit formation or maintenance.
Our findings have suggested novel mechanisms by which conduit pore size might be regulated. The conduit core contains long fibrils of collagen I (7, 24, 26), which provide tensile strength to these structures; however, we also observed expression of collagen XIV within this layer of the conduit network (5). In contrast to collagen I, which forms fibrillar chains, collagen XIV is a fibril-associated collagen with interrupted triple helices that has been reported to crosslink collagen I fibrils, limiting their diameter by preventing lateral fusion with adjacent fibrils (33, 34). We also found that the small leucine rich proteoglycan decorin localized to the conduit core, where it too can regulate collagen fibril diameters (35). FRCs and IAPs express the enzyme lysyl oxidase, which covalently crosslinks collagens I and XIV, preserving pore sizes (36). It seems likely that regulation of collagen fibril diameter and packing within the conduit network determine the effective pore size of this structure. Alterations in collagen fibril diameter or packing could affect the elasticity of lymph node architecture or the normal flow of lymph-borne antigens to the lymph node paracortex. However, the specific contributions of these ECM regulatory molecules remain to be assessed within the lymph node microenvironment.
Thus, stromal-hematopoietic interactions are crucial for promoting adaptive immunity, first by stimulating lymph node genesis and later by enhancing leukocyte entry into inflamed draining lymph nodes and rapid antigen-capture and display by antigen-presenting cells.
Stromal cells organize and support hematopoietic populations within secondary lymphoid organs
LNSCs continually provide important physical and chemical signals to hematopoietic cells, organizing these populations within SLO environments. These LNSC-derived cues are essential to the productive initiation of an immune response, as even after a naive T cell is in the same SLO as its cognate antigen (presented by a DC), it must still come in close contact with the antigen-presenting cell and exchange numerous signals before it can become activated (37, 38). As such, lymphocytes and DCs must be able to actively travel within SLOs. In vivo live-imaging studies have identified important roles for the T-cell zone FRC network in facilitating lymphocyte migration within SLOs. Rather than pushing off each other within maze-like structures as had previously been hypothesized (7), naive lymphocytes have been found to make intimate contacts with and actively migrate along FRC networks (39, 40). In lymph nodes, naive T and B cells were observed to exit HEVs, access FRC networks at specific sites, and actively crawl along the surfaces of these cells, though the molecular basis of these interactions has remained unclear (39). In contrast to interstitial fibroblasts, FRCs ensheath the majority of ECM that they secrete (7, 41). However, ultrastructural and light microscopic studies of lymph nodes from cynomolgus monkeys suggest that certain basement membrane components of the FRC conduit network, including fibronectin and collagen IV, are also exposed to hematopoietic cells (42). Accordingly, these ECM components may contribute to the migration of hematopoietic populations along the FRC network via interaction with cell surface integrins.
Stromal subsets, and in particular IAPs, show high expression of many integrins. IAPs express high levels of transcript for integrin chains α1, α5, α7, α8, α9, αV, β1, and β5 (5). They also have low levels of expression of the integrin α2, α3, α4, α6, α10, α11, β3, and β4 chains (5). Together, these data suggest that IAPs may express numerous integrin heterodimers including: α1β1, α2β1, α3β1, α4β1, α5β1, α6β1, α6β4, α7β1, α9β1, α10β1, α11β1 and αVβ3. As with most functions of IAPs, the significance of this expression is unknown; however, the pericytic localization of these cells at the endothelial-parenchymal interface certainly lends itself to support of cell migration.
Following immunization, migratory DCs have been found to localize near HEVs within draining lymph nodes, increasing the likelihood of cognate antigen recognition by newly entering naive cells (43). In addition to providing a cellular scaffold on which hematopoietic cells may crawl, FRCs also secrete the homeostatic chemokines CCL19, CCL21a, and CXCL12 that help to recruit, organize, and promote interactions between CCR7- and CXCR4-expressing naive T cells and DCs (4, 5, 44, 45). Interestingly, in contrast to CCL19, CCL21a can bind to heparin sulfate residues, allowing it to be immobilized within the lymph node environment (46–48). CCL21a bound to the surface of FRCs induces integrin-dependent adhesion and motility of DCs in contrast to CCL19 and soluble CCL21a, which appear to confer directional cues (49). However, we have found that DC migration along the FRC network in a three-dimensional (3–D) deformable matrix can occur independently of chemotactic cues and integrin-engagement (22). Furthermore, DC migration along FRC networks was found to depend on CLEC2-gp38 signaling, which induces dramatic protrusive activity of these leukocytes (22). Disruption of this signaling pathway leads to diminished DC migration along FRC networks in vitro and in vivo, highlighting the importance of these DC-FRC interactions (22). It therefore appears that multiple molecular interactions between FRCs and DCs regulate the motility of this hematopoietic subset within lymph nodes. Additionally, FRCs enhance naive T-cell survival in the steady-state by secreting interleukin-7 (IL-7) (4). More recently, our microarray analyses of freshly isolated LNSCs have revealed that FRCs and IAPs express transcripts for numerous other cytokines and chemokines that may influence immune cell homeostasis and the initiation of immune responses (5). It seems likely that stroma support DC differentiation, maintenance, or expansion within SLOs given the close contacts between these populations and our observations that FRCs, LECs, and IAPs express high levels of transcript for the cytokine Flt3L (5, 50). In addition, we found that FRCs and IAPs express transcripts for a variety of chemokines such as CCL2, CCL7, and CXCL14, which may influence myeloid cell recruitment to or organization within lymph nodes (5). FRCs and IAPs also expressed transcripts for cytokines and chemokines that regulate B lymphocytes; though it is plausible that these transcripts reflect signatures of stroma that sparsely populate B-cell follicles (25). While FRCs clearly regulate naive T-cell homeostasis and promote lymphocyte-DC interactions, the ways in which FRCs affect the survival and function of non-lymphoid hematopoietic populations remain to be investigated. Moreover, any crosstalk between IAPs and the hematopoietic system is currently undefined. Thus, FRCs interact closely with naive lymphocytes and DCs in SLOs, promoting the survival and interaction of these hematopoietic populations while simultaneously regulating their recruitment to and localization within these tissues.
Lymph node stromal cells induce peripheral tolerance
During development, T cells are endowed with antigen-specific receptors (TCRs) with potential for self-reactivity. To prevent auto-inflammation and tissue destruction, there are a number of mechanisms in place to promote self-tolerance. Within the thymus, developing thymocytes undergo deletional tolerance or negative selection, a process by which self-reactive T cells can be eliminated from the T-cell repertoire (51–54). Thymic stromal cell-mediated display of peripheral tissue-restricted antigens (PTAs) is crucial for inducing tolerance to molecules that are largely expressed in other tissues (55, 56). For many years, these cells were thought to be the only stroma involved in inducing T-cell tolerance. In the recent past, however, LNSCs have been found to play important roles in enforcing CD8+ T-cell tolerance to self-antigens in the periphery via direct presentation of self-antigens to these lymphocytes (57–61).
A small proportion of T cells bearing self-reactive TCRs escape thymic deletion (62). Other tolerance-inducing mechanisms decrease the likelihood that those autoreactive T cells escaping negative selection will wreak havoc in most individuals. Some self-reactive T cells have been observed to undergo receptor editing, which presumably replaces the autoreactive TCR with a new TCRα-TCRβ pairing that has a lower affinity for self-pMHC complexes (63–66). Alternatively, self-reactive CD4+ T cells may develop into regulatory T cells (Tregs) in the thymus (natural Tregs) or even in the periphery (induced Tregs) via interaction with medullary thymic epithelial cells (mTECs) and/or DCs (67–69). Autoreactive T cells may also become anergic or may be deleted in the periphery (70).
In 2007, we and others identified a crucial role for LNSCs in inducing peripheral CD8+ T-cell tolerance (57–61). In contrast to DCs, which acquire antigens from tissues and subsequently cross-present processed peptides to CD8+ T cells in draining lymph nodes, LNSCs ectopically express and present PTAs directly to CD8+ T cells in a manner reminiscent of mTECs (59–61, 71, 72). An initial report from our laboratory demonstrated the ability of radio-resistant LNSCs to enforce CD8+ T-cell tolerance to truncated ovalbumin (tOVA), which was expressed as a model self-antigen specifically in the small intestine under control of the intestinal fatty-acid binding protein (iFABP) promoter (iFABP-tOVA; iFABP-tOVA mice) (59, 73). We found that when naive CD8+ T cells expressing a transgenic TCR specific for a tOVA-peptide (OT-I T cells) were transferred into iFABP-tOVA mice, these cells proliferated normally in mesenteric lymph nodes (MLNs) and PPs, where antigens from the small intestine would be expected to be presented by DCs (59). Intriguingly, OT-I T cells were also observed to proliferate in all non-draining lymph nodes examined, as well as to a lesser degree within the spleen. Using a number of approaches, Lee et al. (59) confirmed that OT-I T cells were indeed proliferating in response to signals that they received within these peripheral lymph nodes rather than being activated by DCs in MLNs or PPs and subsequently recirculating. In fact, CD45− radioresistant stromal cells were found to be responsible for the direct presentation of tOVA and the induction of limited activation and eventual deletion of these self-reactive lymphocytes within peripheral lymph nodes. Recent advances in stromal cell isolation techniques and high purity sorting of LNSC subsets have facilitated a more thorough analysis of PTA expression among these populations (57, 58, 74), identifying FRCs as the stromal source of tOVA expression in iFABP-tOVA mice (58). Furthermore, in vitro experiments confirmed the ability of FRCs to directly present this model PTA to OT-I T cells, stimulating proliferation of these lymphocytes. More broadly, these studies revealed that promiscuous expression of PTAs was a characteristic that was shared among all LNSC subsets that were examined, suggesting that LNSCs may also promote peripheral CD8+ T-cell tolerance to other self-antigens.
Nichols et al. (61) reported LNSC-mediated peripheral CD8+ T-cell tolerance to the endogenously expressed melanocyte antigen tyrosinase. FH T cells are CD8+ T cells that are transgenic for a tyrosinase-specific receptor that recognizes its cognate antigen in the context of the MHC class I molecule AAD. Earlier work had demonstrated that FH+AAD+ tyrosinase-expressing (FH+AAD+tyrosinase+) mice had fewer peripheral FH T cells than FH+AAD+tyrosinase− albino mice, suggesting that these lymphocytes were being deleted via an antigen-specific mechanism. Though PTA expression and display by mTECs has been found to be central to the deletion of many self-reactive developing thymocytes, a role for the thymic deletion of tyrosinase-specific CD8+ T cells was ruled out in this model, as transfer of tyrosinase+ thymi into FH+AAD+tyrosinase− mice did not lead to a decrease in peripheral FH+ T-cell numbers (56, 61, 75). After FH T cells were adoptively transferred to AAD+tyrosinase+ mice, these self-reactive lymphocytes were found to undergo activation, proliferation, and deletion in all lymph nodes, as was observed for OT-I T cells in the iFABP-tOVA model (59). While Nichols et al. (61) were able to localize this mechanism of deletional tolerance to the LNSC compartment by using a combination of bone-marrow chimeras and selective depletion of Langerhans cells, more recent studies have demonstrated that tyrosinase expression is specifically restricted to LECs (57, 58). As was the case for tOVA-expressing FRCs, LECs isolated from AAD+tyrosinase+ mice were also able to directly stimulate the proliferation of antigen-specific CD8+ T cells in vitro (57). Together, these data provide further evidence for the ability of LNSCs to directly induce and enforce peripheral CD8+ T-cell tolerance to self-antigens.
LNSC-dependent peripheral deletion of self-reactive CD8+ T cells was also observed in a model system in which hemagglutinin (HA), an influenza virus protein, was expressed as a self-antigen in enteric glial cells under regulation of the glial fibrillary acidic protein (GFAP) promoter (GFAP-HA; GFAP-HA mice) (60). In this study, Magnusson et al. (60) determined that transfer of naive transgenic HA-specific CD8+ T cells to GFAP-HA mice did not lead to overt intestinal pathology in these animals. Rather, as had previously been observed in the iFABP-tOVA and AAD+tyrosinase+ model systems, self-reactive lymphocytes exhibited partial activation, proliferation, and subsequent deletion in all lymph nodes that were examined (59–61). The authors then transferred naive HA-specific T cells into GFAP-HA hosts deficient in recombination activating gene 2 (RAG 2), to determine whether homeostatic expansion of self-reactive CD8+ T cells might be sufficient to lead to a breach in this mechanism of peripheral tolerance. Interestingly, while HA-specific T cells did undergo homeostatic proliferation as expected, treated mice were still protected from intestinal damage (60). A combination of in vitro coculture experiments, in vivo bone marrow chimeric experiments, and mRNA analysis of isolated ulex europeus agglutinin-1 (UEA-1)-expressing LNSCs reinforced the idea that these cells routinely express and directly present PTAs, such as GFAP-HA, to self-reactive CD8+ T cells, maintaining tolerance to specific self-antigens in the periphery (60).
In spite of the fact that all LNSC subsets have been found to express PTAs, individual PTAs such as tyrosinase and retinal S antigen appear to be differentially expressed by different stromal populations (57, 58). Furthermore, it remains to be investigated whether PTA expression within a given stromal cell type is homogeneous or whether individual cells of a particular stromal class may stochastically express a subset of the total pool of PTAs characteristic of that population, as occurs in mTECs. In addition, future work may determine whether corresponding stromal populations in tertiary lymphoid organs or other tissues express PTAs, and what the functional consequences of such expression might be.
Though mTECs and LNSCs exhibit a shared capacity to express PTAs, the mechanisms by which these populations regulate promiscuous gene expression appear to be different. The transcriptional modulator Aire is not responsible for PTA expression among FRCs, LECs, and BECs, in contrast to mTECs which rely heavily on Aire expression (55, 57, 58). Among LNSCs, extremely low levels of Aire transcript were only detectable in bulk gp38−CD31− stromal cells, though no protein was detected. Conversely, all LNSC subsets were found to express high levels of an Aire-like transcriptional regulator known as DF1 (58). DF1 was previously shown to regulate PTA expression by LNSCs in pancreatic lymph nodes of mice (76). DF1 was expressed normally in the pancreatic lymph nodes of young NOD mice; however, Yip et al. (76) found that over time, DF1 expression decreased while expression of the DF1 splice variant (DF1-VAR1) increased. In contrast to DF1, DF1-VAR1 does not contain a nuclear localization sequence and is therefore unable to enter the nucleus to regulate PTA expression. To make matters worse, DF1-VAR1 was found to interact with DF1, likely retaining DF1 in the cytoplasm and further limiting PTA expression within pancreatic lymph nodes. It seems plausible that diminished PTA expression within pancreatic lymph nodes of aging NOD mice may contribute to the development of disease in these animals. Future studies may elucidate the molecular mechanisms that control LNSC expression of the transcriptional regulators Aire and DF1. In addition, given that the expression of specific PTAs is not uniform among LNSC subsets, it is likely that other transcriptional regulators may contribute to the modulation of PTA expression by these cells. The identities of these other transcriptional regulators remain to be determined.
While numerous studies have demonstrated that promiscuous PTA expression and direct presentation by LNSCs is sufficient to promote peripheral CD8+ T-cell tolerance to specific self-antigens, it has been unclear whether LNSCs can similarly influence CD4+ T-cell tolerance. In contrast to professional antigen-presenting cells such as DCs, LNSCs express low levels of MHC class II molecules in the steady-state. However, we and others have recently shown that FRCs, LECs, and BECs can upregulate surface expression of MHC class II molecules under inflammatory or infectious conditions (5, 77). Various inflammatory stimuli have been found in vivo to promote a suppressive LNSC phenotype that is characterized by increased expression of the inhibitory molecule programmed cell death ligand 1 (58, 78). In addition, FRCs isolated from inflamed iFABP-tOVA mice were subsequently found to be less efficient than FRCs from untreated animals at stimulating proliferation of naive OT-I T cells in vitro. In light of these findings, it is unclear whether the observed increase in surface MHC class II expression by LNSCs will contribute to immunity, the induction of regulatory T cells, or CD4+ T-cell tolerance, and future work must investigate these possibilities.
Many open questions remain. However, these studies have convincingly demonstrated a role for LNSCs in maintaining peripheral CD8+ T-cell tolerance via direct stromal presentation of self-antigens to self-reactive CD8+ T cells.
Lymph node stroma as an active regulator of developing immune responses
LNSCs promote the initiation of adaptive immune responses through a number of mechanisms. Despite the role that FRCs play in facilitating this process, little was known about how these cells might directly influence the developing T-cell response due to difficulties in isolating and studying this rare stromal population.
Recent advances in stromal cell isolation and culturing techniques have allowed researchers to begin to assess the ways in which LNSCs may contribute directly to developing T-cell responses (74). Within the lymph node, naive T cells become activated in the FRC-rich paracortex. Accordingly, we and others were interested in understanding how the presence of FRCs might affect the progression of the T-cell response (79). Using a reductionist approach, naive lymphocytes were activated (using antigen-bearing bone marrow-derived DCs (BMDCs) or αCD3 + αCD28 stimulation) in the presence or absence of in vitro expanded primary FRCs or LECs, or cell lines derived from these populations (79–81). Interestingly, these studies demonstrated that FRCs and LECs possess a potent suppressive capability, as T cells activated in their presence exhibited a significant block in proliferation (79–81) (Fig. 3).
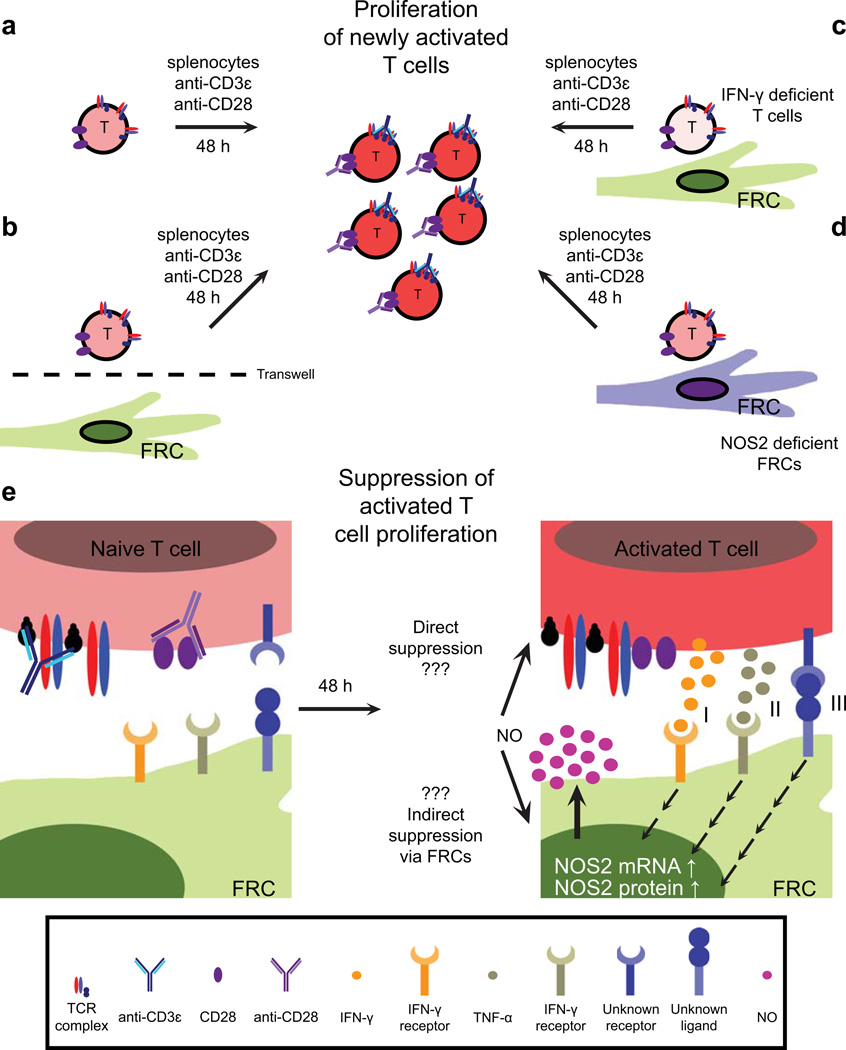
Fig. 3. Regulation of activated T-cell proliferation by fibroblastic reticular cells.
(A) Experimental setup: naive splenocytes were cultured in the presence of anti-CD3ε and anti-CD28 antibodies, leading to T-cell activation and proliferation. (B) When splenocytes were cultured in the presence of fibroblastic reticular cells (FRCs) with a transwell filter separating the two populations, T cells proliferated normally and little nitric oxide (NO) was detected. (C,D) Coculture and activation of T cells deficient in IFN-γ expression with wildtype (WT) FRCs, or WT T cells with FRCs deficient in NOS2 expression allows normal proliferation of T cells. (E) Activation and coculture of WT T cells with WT FRCs leads to high levels of NO in the culture supernatant, and a significant block in activated T-cell proliferation. Activated T-cell-derived IFN-γ (I), TNF-α (II), and an unknown membrane-bound signal (III) synergize to increase NOS2 mRNA and protein levels in FRCs. This in turn enhances FRC production and secretion of NO, which directly or indirectly curbs proliferation of these T cells.
While expansion of newly activated T cells was substantially reduced in the presence of FRCs, early stages of T-cell activation were not significantly impaired (79, 80). In the course of DC-mediated activation of naive T cells, FRCs may be exposed to a variety of different cytokines including interferon-γ (IFN-γ), IL-4, IL-12, and tumor necrosis factor-α (TNF-α). A combination of experiments using antibody blockade and genetic tools demonstrated that FRC-mediated suppression of activated T-cell proliferation was crucially dependent on intimate crosstalk between these two populations (79–81) (Fig. 3). Furthermore, activated T-cell-derived IFN-γ was found to be essential for FRC-mediated suppression (79, 80).
IFN-γ is a potent inflammatory stimulus, inducing expression of numerous molecules. Investigation of suppressive mechanisms that interfere with metabolic pathways on which activated T cells are crucially dependent revealed a role for inducible nitric oxide synthase (Nos2) in this process (79–81) (Fig. 3D). Nos2 expression has been reported to be induced by IFN-γ in both hematopoietic and stromal cell populations (82, 83). Indeed, we and others (79–81) observed that Nos2 transcripts were strongly upregulated by FRCs following treatment with recombinant IFN-γ. Expression of other inhibitory molecules including indoleamine 2,3-dioxygenase 1 (Ido1), arginase 1 (Arg1), and cyclooxygenases 1/2 (Cox1/2) was not found to change in response to recombinant IFN-γ stimulation or suppressive contributions of these molecules were negligible (79–81). In contrast, when proliferation assays were performed in the presence of Nos2−/− FRCs or inhibitors to nitric oxide synthases, there was a substantial reversal in the suppression of activated T cells, reinforcing the idea that FRC-derived nitric oxide is crucial for the observed block in T-cell proliferation (79–81). Interestingly, in one study, COX-1/2 were found to play a role in the suppression of T cells that were activated by antigen-loaded BMDCs (81). However, the mechanism of increased COX-1/2 activity in this setting remains to be investigated, as Cox2 mRNA levels in FRCs did not increase in response to IFN-γ stimulation (81). In addition, future studies will determine the specific contributions of FRCs and BMDCs to prostaglandin production in this coculture system.
In contrast to LNSC-mediated induction of CD8+ T-cell tolerance, suppression of activated T-cell proliferation by FRCs was not dependent on direct presentation of antigens, thereby allowing FRCs to limit the expansion of both CD8+ and CD4+ T cells (57–61, 79–81). However, intimate contacts between activated T cells and FRCs were important, as suppression was reversed when these cells were separated by semi-permeable membranes in transwell experiments, regardless of the addition of recombinant IFN-γ (79–81) (Fig. 3B). Nitric oxide is extremely reactive and unlikely to persist in tissue environments due to its ability to rapidly nitrosylate nearby proteins (83). Therefore, this suppressive mechanism is likely to operate only on those activated T cells that are in direct contact with stimulated FRCs. Secretion of nitric oxide must also be tightly regulated to avoid undesirable tissue destruction or off-target stimulatory effects. Accordingly, we and others (79, 80) found that while IFN-γ signaling in FRCs was sufficient to dramatically increase Nos2 transcript levels, nitrite (a stable end product of nitric oxide) remained undetectable in the culture supernatant. In Lukacs-Kornek et al. (79), it was found that recombinant IFN-γ and TNF-α synergize to dramatically increase FRC expression of Nos2, generating protein levels comparable to those induced by activated splenocytes. Recombinant IFN-γ and TNF-α also induced nitric oxide production by these stromal cells in the absence of T cells (79). However, suppression of activated T-cell proliferation was not completely reversed in the absence of TNF-α signaling in FRCs, and nitrite could still be detected in these cocultures, suggesting that another factor can at least partially substitute for this cue (79). The identity of this other, potentially membrane-bound factor remains to be elucidated.
While these studies have illuminated a direct role for FRCs in inhibiting the proliferation of newly activated T cells through a NOS2-dependent mechanism, this stromal subset also indirectly affects T-cell proliferation by altering the stimulatory capacity of BMDCs (81). Siegert and colleagues (81) found that antigen-pulsed BMDCs cultured with an FRC cell line prior to reisolation and coculture with OT-I T cells (in the absence of FRCs), induced less T-cell proliferation than BMDCs that had not been precultured with FRCs. However, the ways in which FRCs alter the stimulatory capacity of DCs are unclear. Preculture with FRCs may change the costimulatory potential of the BMDCs, though the specific mode of crosstalk between DCs and FRCs remains to be investigated. Earlier reports suggest that long-term cocultures of splenic stromal cells and DCs drives these antigen-presenting cells towards a suppressive phenotype (84, 85). Recent work from our lab has shown that FRCs express transcripts for numerous molecules that may influence DC biology, supporting possible crosstalk between these populations (5). In addition, Siegert et al. (81) found that when FRCs and BMDCs were cultured together in the presence of IFN-γ, a larger proportion of each population expressed NOS2 protein than when cultured only in the presence of IFN-γ, reinforcing the likelihood of ongoing crosstalk.
NOS2-mediated suppression of activated T-cell proliferation was found to operate in vivo in models of viral infection, tolerance induction, and BMDC-mediated priming (79–81). Though expression of Nos2 could not selectively be eliminated from the stromal compartment of the lymph node, due to a lack of genetic tools and technical issues concerning reconstitution of bone marrow chimeras, in each of these three models increased proliferation of antigen-specific T cells was observed in the absence of Nos2 expression (79–81). Differences in T-cell proliferation were evident at early time points when this suppressive mechanism is thought to be operating, and intracellular NOS2 could be detected in FRCs (and mesenteric lymph node LECs in the model of tolerance induction) (79–81, 86). While NOS2 was not detectable within the myeloid compartment in the non-inflammatory model of tolerance induction, this protein was observed in DCs isolated from the lymph nodes of mice 24 h after infection with OVA-expressing vesicular stomatitis virus, a time point that corresponds to NOS2 expression in FRCs (79, 81). Importantly in each case, NOS2 protein was found within a small proportion of FRCs, suggesting that in vivo FRCs, activated T cells and antigen-bearing DCs may form niches in which crosstalk occurs, allowing this suppressive mechanism to operate (79–81). We found that following IFN-γ stimulation, FRCs upregulate expression of chemokines such as CXCL9 and CXCL10 (authors’ unpublished data), which can be recognized by activated T cells, potentially retaining these cells within this niche and facilitating FRC regulation of activated T-cell expansion.
The suppressive capacity of stromal cells is not restricted to FRCs or the lymph node environment, as we observed similar suppressive behavior from LECs, and it has previously been shown that fibroblasts (79, 87–89) from other sites can restrict the proliferation of activated T cells. Along these lines, Siegert et al. (81) found that fibroblasts and epithelial cells isolated from a variety of tissues exhibited this suppressive phenotype. However, it is not clear whether these cells act through a NOS2-dependent mechanism.
While FRC-mediated suppression of activated T-cell proliferation clearly acts during early phases of a primary immune response, it is unclear whether FRCs similarly constrain memory T-cell expansion upon restimulation. Indeed, memory T cell pools in Nos2−/− (90, 91) and Ifngr1−/− (92) mice are known to be larger following antigenic stimulation, though this may involve contributions from other stromal cell types in the body, in addition to FRCs. We too found that memory T cells proliferated to a larger extent in Nos2−/− mice, suggesting that FRCs may continue to regulate these populations (79). In addition, the underlying mechanism by which FRC-derived nitric oxide suppresses the proliferation of activated T cells also remains to be illuminated.
Activated CD4+ T cells have been found to differentiate into Foxp3+ Tregs in the presence of chemical donors of nitric oxide (93). While we found that neither CD4+ T cells nor IL-10 were required for FRC-mediated suppression of activated CD8+ T-cell proliferation in vitro, it is plausible that FRCs may generate Tregs under other conditions in vivo (79). In support of this possibility, we have found that under inflammatory conditions FRCs, LECs, and BECs upregulate surface expression of MHC II molecules and increase transcription of many components of the MHC II antigen-processing and presentation pathway (5). FRCs in particular also alter their cytokine and chemokine profiles, suggesting these cells may be able to influence Treg development (5). IAPs also respond to inflammation, changing their expression of PTAs in response to TLR3 stimulation (58). However, IAPs were the only LNSC subset not to upregulate surface MHC class II levels on exposure to LPS, OVA, and OT-I T cells, suggesting they do not engage CD4+ T cells under inflammatory conditions (5).
FRCs promote the initiation of the adaptive immune response through a number of different mechanisms. However, the work described in this review has shown that these cells also strongly curb the expansion of activated T cells in vitro and in vivo (79–81). Though perhaps counter-intuitive, FRC-mediated regulation of T-cell proliferation may be crucial for a number of reasons. Unrestrained expansion of activated T cells could have detrimental effects on the integrity of the FRC network and more generally the lymph node environment. In addition, it may be advantageous to allow for the expansion of multiple T-cell clones early in the adaptive immune response, and local FRC-mediated regulation of T-cell proliferation may increase access to antigen-bearing DCs and the availability of limited survival factors.
Contact-dependent regulation of stromal cell function
SLO stromal cells are in continual contact with each other and with assorted hematopoietic cell populations. Earlier in this review, we have detailed some of the ways in which stromal cells influence hematopoietic cell recruitment to and function within SLOs. Here we examine how the lymph node environment and cell-cell contacts among SLO stromal and hematopoietic subsets regulate key aspects of stromal cell function within these sites.
FRCs form complex networks on which T and B cells migrate in search of cognate antigens in SLOs (39, 40) (Fig. 2). Given the intimate contacts between these populations, FRCs are routinely exposed to lymphocyte-derived factors within these tissues. Lymphocyte-FRC interactions may therefore influence FRC function. Indeed, we have already discussed one example in which FRC exposure to activated T-cell-derived IFN-γ and TNF-α induces notable NOS2-dependent suppression of activated T-cell proliferation by FRCs (79–81). More broadly, crosstalk between FRCs and lymphocytes has also been found to regulate fundamental aspects of FRC biology. In particular, ECM production is a characteristic duty of FRCs, as these cells continually generate and remodel the ECM-based conduit network that they ensheath (Fig. 2). Interestingly, in vitro studies have demonstrated that FRC secretion of ECM is dramatically enhanced by the presence of T cells, suggesting a role for lymphocyte-derived factors in influencing this process (30). In this study, Katakai et al. (30) found that signaling through tumor necrosis factor receptors 1 and 2 (TNFR) and lymphotoxin β receptor (LTβR) synergized to recapitulate levels of ECM production by FRCs that were comparable or higher to those of FRCs cultured in the presence of lymphocytes. Additionally, TNFR and LTβR stimulation of FRCs promoted the proliferation of these cells, suggesting one mechanism by which the FRC compartment of SLOs might be able to expand in concert with an ongoing T-cell response (30). Notably, while three dimensional (3D) co-culture of FRCs and lymphocytes enhanced the generation of a reticular network that was reminiscent of SLO architecture, Katakai et al. (30) found that this structure was still immature. Therefore, in vivo, additional environmental or cell-derived cues are likely to be integrated, collectively regulating FRC deposition and organization of ECM and the reticular network.
As lymph flows through the FRC-ensheathed conduit network, FRCs are continually exposed to fluid flow. In vitro studies suggest that FRCs are extremely sensitive to this movement of interstitial fluid (94). When FRCs were cultured in a 3D matrix and exposed to low flow rates, they aligned and dramatically reorganized the surrounding ECM, forming channel-like structures (94). Higher flow rates, which might be consistent with edema in an inflamed upstream tissue (95–101), led to enhanced proliferation of FRCs in vitro, suggesting another mechanism by which these cells may expand in response to infection or ongoing inflammation (94).
FRC secretion of the homeostatic chemokines CCL19, CCL21a, and CXCL12 also appears to be regulated by a combination of fluid flow and cell-cell contacts. We and others (22, 30) have found that in vitro expansion of FRCs leads to a rapid decrease in the expression of CCL21a. While there are a variety of possible explanations for this observation, in vitro and in vivo studies have demonstrated that fluid flow directly influences FRC secretion of CCL21a (94). In vitro, fluid flow was sufficient to increase FRC expression of CCL21a transcript and protein, and correspondingly in vivo, the absence of fluid flow led to a significant decrease in FRC transcription of this chemokine (94). While lymph flow can induce FRC production of CCL21a, homeostatic chemokine secretion by these cells also appears to be modulated by cell-cell contacts. FRCs interact and form junctions with each other along the conduit network (5, 7). Junctional adhesion molecule C (JAM-C) is a type I transmembrane glycoprotein that has previously been reported to be expressed by SLO LECs and BECs (102). More recently, Frontera et al. (103) found that a subset of thrombomodulin-expressing FRCs coexpressed JAM-C in murine lymph nodes. The authors reported that this subpopulation of FRCs expressed the highest levels of mRNA for CCL19, CCL21a, and CXCL12. In addition, CCL21a and CXCL12 protein levels were significantly diminished in vivo following treatment with anti-JAM-C antibodies or in JAM-C deficient mice. Decreased homeostatic chemokine levels appear to be the result of post-translational regulation, as anti-JAM-C treatment did not lead to a drop in transcript levels for these molecules within the thrombomodulin-expressing subset of FRCs (94). While the authors found no differences in the ability of adoptively transferred naive lymphocytes to home to lymph nodes in anti-JAM-C treated mice, they did observe increased retention of naive T cells (94). Thus, fluid flow and cell adhesion molecules such as JAM-C appear to modulate FRC production of key homeostatic chemokines. Future studies may identify the specific mechanotransducers that allow FRCs to detect and respond to fluid flow. In addition, it may be determined whether signaling through other cell adhesion molecules, such as cadherin-11 which we recently showed to be expressed in junctions between FRCs, similarly controls stromal cell functions (5).
The newly described IAP subset also transcribes high levels of CCL19, CCL21a, and CXCL12 genes, though these cells have not yet been shown to secrete protein (5). Chemokines are expressed by IAPs at lower mean expression values than FRCs, suggesting FRCs may be more specialized for this purpose; however, it is also possible that the sorting strategy used to isolate IAPs (as bulk gp38−CD31− stroma) resulted in an underestimate of gene expression by this population, since IAPs comprise only 60–65% of DNCs. Addressing this would require microarray analysis of sorted IAPs. Nothing is yet known about IAP-lymphocyte interactions; however, the similarities between IAPs and FRCs and their shared expression of a multitude of chemokines and cytokines suggest immunologically relevant functions for this newly discovered cell type.
Though the specific mechanism is unclear, the presence of mature B cells in lymph nodes leads to a dramatic remodeling of the FRC network (104). Bajenoff et al. (104) found that in the absence of mature B cells, a dense, branching FRC network extended throughout the lymph node. However, within two weeks of adoptive transfer of mature B cells (or 21 days after birth of wildtype mice), normal follicle development was evident, and the conduit network within these structures became less branched and more sparse (104) (Fig. 2). Synchronous with observed conduit dispersion, follicle development was associated with the differentiation and/ or expansion of an FDC network (104). Recent work by Krautler et al. (105) has demonstrated that FDCs can arise from a ubiquitous perivascular precursor that expresses PDGFRβ. Given the perivascular localization of IAPs and certain FRCs, it remains to be investigated whether these stromal populations serve as FDC precursors within the lymph node environment. It is unknown whether B-cell-derived or FDC-derived signals specifically contribute to the significant reorganization of the FRC network within these regions. However, it is clear that even though these sites were previously occupied by T cells, the presence of mature B cells either directly or indirectly leads to a dramatic reorganization of these capsule-proximal lymph node regions.
More generally, lymph nodes are dynamic environments that undergo substantial expansion and reorganization during the course of an immune response. Following infection or immunization, draining lymph nodes swell, and stromal cell networks are altered in a variety of ways. To accommodate a larger influx of blood-borne leukocytes, the feeding arteriole is remodeled and there is an expansion of the BEC network (106–113). Additionally, HEVs undergo a number of phenotypic changes that promote leukocyte entry into lymph nodes (114–117). An increase in the number of LECs allows for enhanced entry of DCs into lymph nodes (118–120). This expansion in lymphatic endothelium has been associated with B-cell-derived vascular endothelial growth factor A (VEGF-A) in some settings (118, 119). The FRC network has also been observed to grow in certain inflammatory models, likely supporting expanding T-cell pools (30, 112).
Stromal cell proliferation and lymph node remodeling following infection or immunization appear to stem from crosstalk among a number of stromal and hematopoietic subsets. Continuous secretion of VEGF-A has been found to be required for the normal proliferation of LECs and non-HEV BECs in the steady-state (110). Using VEGF-A reporter mice in which lacZ expression was driven by the VEGF-A promoter (VEGF-A-lacZ mice), Chyou et al. (110) identified FRCs as the major source of VEGF-A in resting lymph nodes. FRC secretion of this factor was found to be positively regulated by LTβR signaling, highlighting a role for hematopoietic cell control of stromal cell function (110). In vivo administration of LTβR-Ig led to lower VEGF-A levels and diminished proliferation of LECs and non-HEV BECs in lymph nodes of naive mice (110). Within one day of immune stimulation, VEGF-A expression has been observed to increase twofold in lymph nodes in an L-selectin-dependent manner (109). In addition, a previous study had reported that VEGF-A colocalized with B cells in stimulated lymph nodes, suggesting a role for hematopoietic cell-derived VEGF-A in the regulation of endothelial cell proliferation (118). Therefore, Chyou et al. (110) sought to determine whether transcription of VEGF-A was increased within the hematopoietic compartment after immune stimulation. This did not appear to be the case as following footpad injection of mature DCs or immunization with OVA emulsified in complete Freund's adjuvant (OVA-CFA), FRCs continued to be the primary source of VEGF-A transcript in lymph nodes of VEGF-A-lacZ mice (110). In contrast to endothelial cell proliferation in resting lymph nodes, VEGF-A was required for proliferation of all endothelial subsets following DC transfer, highlighting possible changes to the regulatory programs of these cells (110). Treatment of mice with anti-VEGF-A antibodies or the VEGF-A-antagonist Aflibercept, led to a reduction in endothelial cell proliferation that was associated with a decrease in total lymph node cellularity eight days after footpad delivery of DCs (110). Similarly, intravenously injected, congenically marked splenocytes were found to traffic less efficiently to DC-stimulated lymph nodes of Aflibercept-treated mice at this time, indicating that blockade of VEGF-A signaling may lead to alterations in immune function (110).
FRC, LEC, and BEC expansion following immunization was found to operate in two distinct stages, each with requirements for specific hematopoietic cell types: initiation (increase in stromal cell proliferation) and expansion (increase in stromal cell numbers) (112). The initiation phase (days 1–2) was independent of lymphocytes, as it could be induced in RAG1−/− mice (109, 112). However, the initial increase in LNSC proliferation was dependent on the presence of CD11c+ cells and was diminished when these cells were locally depleted in lymph nodes of mice that expressed the diphtheria toxin receptor under the control of the CD11c promoter (CD11c-DTR mice) (109, 112, 121). While the identity of the CD11c-expressing cell that is required for the induction of normal initiation remains somewhat of a mystery, Chyou et al. (112) were able to exclude contributions from skin DCs and both radioresistant and radiosensitive migratory cells that require CCR7 to traffic to lymph nodes. However, CCR7-dependent radioresistant cells may influence the degree to which FRCs and endothelial cells proliferate (112).
Despite undergoing normal initiation, endothelial cells failed to increase in number in lymph nodes of RAG1−/− mice that were stimulated with DCs five days earlier, suggesting that successful expansion required the presence of B and T cells at this time (112). Furthermore, at day 5, endothelial cells in stimulated lymph nodes of RAG1−/− mice also exhibited lower rates of proliferation, highlighting a necessity for lymphocytes throughout the course of vascular expansion (112). Chyou et al. (112) obtained comparable results when immunizing mice with OVA-CFA, suggesting that these findings were not stimulus dependent, and T cells and B cells were found to play partially redundant roles during the expansion phase. FRCs also demonstrated similar cellular requirements in these two phases, raising the possibility of coordinate regulation of LNSC proliferation and expansion following immune activation (112). Future studies may identify the nature of the non-redundant signals that B lymphocytes provide to FRCs and endothelial cells in this process, as well as the specific cues that promote FRC survival during the expansion phase.
FRCs were found to increase expression of VEGF-A during the initiation phase (112). Given the requirement for CD11c+ cells in this process and the close contact between DCs and FRCs in lymph nodes, Chyou et al. (112) raised the possibility that DC-FRC interactions might foster an increase in FRC expression of VEGF-A, leading to enhanced endothelial cell proliferation. Indeed, coculture of BMDCs (that had been depleted of granulocytes) and NIH-3T3 fibroblasts supported this hypothesis (112). Therefore, it seems likely that DCs may induce FRC upregulation of VEGF-A expression. However, the specific molecular pathway or pathways that lead to this increase in VEGF-A expression remain to be identified.
Following LNSC expansion after immune stimulation, there is a period of vascular stabilization and quiescence that is characterized by a decrease in endothelial cell proliferation and maintenance of endothelial cell numbers (121). During the initiation and expansion phases, perivascular FRCs were observed to become more loosely organized around HEVs (121). However, by day 8 after DC transfer, FRCs were reassembling around these structures (121). CD11chiMHC IImed DCs gradually accumulated near HEVs, with numbers peaking five days after footpad injection of mature DCs (121). CD11chiMHC IImed cells were determined to be essential for maintaining endothelial cell quiescence and promoting vascular stabilization, as depletion of these cells six days after DC transfer led to elevated levels of endothelial cell proliferation and disruption of FRC reassembly around HEVs (121). Similar results were obtained when mice were immunized with OVA-CFA, suggesting that CD11chiMHC IImed cells may routinely regulate these processes in lymph nodes (121).
Given that elevated VEGF-A levels are associated with the initiation phase following immune activation, it was possible that vascular quiescence was linked to a decrease in VEGFA production. Indeed, Tzeng et al. (121) observed diminished VEGF-A levels during this phase. Depletion of CD11chiMHC IImed cells prevented this drop in VEGF-A levels, leading to elevated rates of endothelial cell proliferation, though these cells failed to accumulate further (121). Interestingly, prevention of vascular stabilization and quiescence was associated with damped T-dependent B-cell responses, reinforcing the importance of this phase of lymph node reorganization (121). It is unclear how CD11chiMHC IImed cells attenuate VEGF-A expression, whether this attenuation occurs via direct CD11chiMHC IImed cell-FRC interactions, what the nature of these interactions might be, and what other signaling pathways might contribute to this process. However, collectively these studies suggest significant roles for stromal-hematopoietic and stromal-stromal interactions in regulating LNSC maintenance and expansion following immunization or infection.
Our microarray analyses of freshly-isolated LNSCs have revealed FRC expression of numerous other molecules that might contribute to endothelial cell maintenance including VEGF-C, ANGPTL2, HGF, GREM1, and SERPINF1 (5). Similarly, IAPs express VEGF-C, ANGPTL2, HGF, and SERPINF1 (5). Future studies may investigate whether and in what ways FRC and IAP secretion of these molecules affect lymph node endothelial cell homeostasis or expansion. Given the proximity of IAPs to the lymph node vasculature and their transcription of pro-angiogenic factors, it seems likely that IAPs will contribute to the regulation of BECs, and this is one area of future research. Thus, not only are stromal-hematopoietic cell interactions important for supporting, organizing, and regulating hematopoietic populations within SLOs, but crosstalk among all of these cell types is also crucial for promoting key stromal cell functions and maintaining the SLO environment.
Conclusions
Adaptive immunity relies on the existence of SLOs, which bring together three prerequisites for this process: lymphocytes, cognate antigens, and antigen-presenting cells. Various studies have revealed the essential roles that SLO stromal cells play in orchestrating adaptive immune responses. In addition to recruiting, organizing, and promoting the survival of key hematopoietic populations, SLO stromal cells have been found to be active players in adaptive immunity. Advances in SLO stromal cell isolation techniques have allowed us to further dissect effects that stromal cells have on hematopoietic populations. Stromal cells directly present PTAs to CD8+ T cells, maintaining self-tolerance in the periphery. Furthermore, FRCs interact closely with DCs and naive T cells in these tissues. We now know that FRCs respond to cues from newly activated T cells, curbing the proliferation of these lymphocytes in lymph nodes. The interplay between FRCs, hematopoietic cells, other stromal cells, and the SLO environment also regulates many aspects of stromal cell function within these tissues. In addition to modulating chemokine production by stromal cells, these interactions affect SLO dynamics by influencing ECM production and stromal cell expansion. Interactions between stromal and hematopoietic cells continue to be a rich area of research. Future studies may illuminate the molecular mechanisms by which some of these phenomena are regulated, as well as other ways in which SLO stromal cells influence lymphocytes, DCs, and other hematopoietic populations, including macrophages and natural killer cells.
Acknowledgements
This work was supported by National Institutes of Health grants R01 DK074500 and P01 AI045757 (to Shannon J. Turley).
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Kotturi MF, et al. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol. 2008;181:2124–2133. doi: 10.4049/jimmunol.181.3.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra D, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012;13:499–510. doi: 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson AO, Anderson ND. Studies on the structure and permeability of the microvasculature in normal rat lymph nodes. Am J Pathol. 1975;80:387–418. [PMC free article] [PubMed] [Google Scholar]
- 7.Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaldjian EP, Gretz JE, Anderson AO, Shi Y, Shaw S. Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epitheliumlike monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Int Immunol. 2001;13:1243–1253. doi: 10.1093/intimm/13.10.1243. [DOI] [PubMed] [Google Scholar]
- 9.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 10.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 11.Baluk P, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 13.Cavanagh LL, Von Andrian UH. Travellers in many guises: the origins and destinations of dendritic cells. Immunol Cell Biol. 2002;80:448–462. doi: 10.1046/j.1440-1711.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- 14.Manickasingham SP, Reis e Sousa C. Mature T cell seeks antigen for meaningful relationship in lymph node. Immunology. 2001;102:381–386. doi: 10.1046/j.1365-2567.2001.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randolph GJ. Dendritic cell migration to lymph nodes: cytokines, chemokines, and lipid mediators. Semin Immunol. 2001;13:267–274. doi: 10.1006/smim.2001.0322. [DOI] [PubMed] [Google Scholar]
- 16.Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–135. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 17.Stoitzner P, Pfaller K, Stossel H, Romani N. A close-up view of migrating Langerhans cells in the skin. J Invest Dermatol. 2002;118:117–125. doi: 10.1046/j.0022-202x.2001.01631.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lammermann T, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 20.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009;206:2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podgrabinska S, et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol. 2009;183:1767–1779. doi: 10.4049/jimmunol.0802167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acton SE, et al. Podoplanin-rich stromal networks induce DC motility via activation of CLEC-2. Immunity. 2012;37:1–14. doi: 10.1016/j.immuni.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farr AG, Berry ML, Kim A, Nelson AJ, Welch MP, Aruffo A. Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J Exp Med. 1992;176:1477–1482. doi: 10.1084/jem.176.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roozendaal R, Mebius RE, Kraal G. The conduit system of the lymph node. Int Immunol. 2008;20:1483–1487. doi: 10.1093/intimm/dxn110. [DOI] [PubMed] [Google Scholar]
- 25.Roozendaal R, et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sixt M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Baekkevold ES, et al. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med. 2001;193:1105–1112. doi: 10.1084/jem.193.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palframan RT, et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 30.Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–795. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayakawa M, Kobayashi M, Hoshino T. Direct contact between reticular fibers and migratory cells in the paracortex of mouse lymph nodes: a morphological and quantitative study. Arch Histol Cytol. 1988;51:233–240. doi: 10.1679/aohc.51.233. [DOI] [PubMed] [Google Scholar]
- 33.van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–2823. [PubMed] [Google Scholar]
- 34.Young BB, Gordon MK, Birk DE. Expression of type XIV collagen in developing chicken tendons: association with assembly and growth of collagen fibrils. Dev Dyn. 2000;217:430–439. doi: 10.1002/(SICI)1097-0177(200004)217:4<430::AID-DVDY10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev. 2001;122:735–755. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 37.Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol. 2008;8:675–684. doi: 10.1038/nri2379. [DOI] [PubMed] [Google Scholar]
- 38.Pittet MJ, Mempel TR. Regulation of T-cell migration and effector functions: insights from in vivo imaging studies. Immunol Rev. 2008;221:107–129. doi: 10.1111/j.1600-065X.2008.00584.x. [DOI] [PubMed] [Google Scholar]
- 39.Bajenoff M, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol. 2008;181:3947–3954. doi: 10.4049/jimmunol.181.6.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lammermann T, Sixt M. The microanatomy of T-cell responses. Immunol Rev. 2008;221:26–43. doi: 10.1111/j.1600-065X.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- 42.Sobocinski GP, Toy K, Bobrowski WF, Shaw S, Anderson AO, Kaldjian EP. Ultrastructural localization of extracellular matrix proteins of the lymph node cortex: evidence supporting the reticular network as a pathway for lymphocyte migration. BMC Immunol. 2010;11:42. doi: 10.1186/1471-2172-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bajenoff M, Granjeaud S, Guerder S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J Exp Med. 2003;198:715–724. doi: 10.1084/jem.20030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peduto L, et al. Inflammation recapitulates the ontogeny of lymphoid stromal cells. J Immunol. 2009;182:5789–5799. doi: 10.4049/jimmunol.0803974. [DOI] [PubMed] [Google Scholar]
- 46.de Paz JL, Moseman EA, Noti C, Polito L, von Andrian UH, Seeberger PH. Profiling heparin-chemokine interactions using synthetic tools. ACS Chem Biol. 2007;2:735–744. doi: 10.1021/cb700159m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirose J, et al. Chondroitin sulfate B exerts its inhibitory effect on secondary lymphoid tissue chemokine (SLC) by binding to the C-terminus of SLC. Biochim Biophys Acta. 2002;1571:219–224. doi: 10.1016/s0304-4165(02)00232-5. [DOI] [PubMed] [Google Scholar]
- 48.Patel DD, Koopmann W, Imai T, Whichard LP, Yoshie O, Krangel MS. Chemokines have diverse abilities to form solid phase gradients. Clin Immunol. 2001;99:43–52. doi: 10.1006/clim.2000.4997. [DOI] [PubMed] [Google Scholar]
- 49.Schumann K, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Watowich SS, Liu YJ. Mechanisms regulating dendritic cell specification and development. Immunol Rev. 2010;238:76–92. doi: 10.1111/j.1600-065X.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 52.Swat W, Ignatowicz L, von Boehmer H, Kisielow P. Clonal deletion of immature CD4+8+ thymocytes in suspension culture by extrathymic antigen-presenting cells. Nature. 1991;351:150–153. doi: 10.1038/351150a0. [DOI] [PubMed] [Google Scholar]
- 53.Buch T, Rieux-Laucat F, Forster I, Rajewsky K. Failure of HY-specific thymocytes to escape negative selection by receptor editing. Immunity. 2002;16:707–718. doi: 10.1016/s1074-7613(02)00312-6. [DOI] [PubMed] [Google Scholar]
- 54.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205:2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 56.Gardner JM, Fletcher AL, Anderson MS, Turley SJ. AIRE in the thymus and beyond. Curr Opin Immunol. 2009;21:582–589. doi: 10.1016/j.coi.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen JN, et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207:681–688. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fletcher AL, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689–697. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JW, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 60.Magnusson FC, et al. Direct presentation of antigen by lymph node stromal cells protects against CD8 T-cell-mediated intestinal autoimmunity. Gastroenterology. 2008;134:1028–1037. doi: 10.1053/j.gastro.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 61.Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 62.Lohse AW, Dinkelmann M, Kimmig M, Herkel J, Meyer zum Buschenfelde KH. Estimation of the frequency of self-reactive T cells in health and inflammatory diseases by limiting dilution analysis and single cell cloning. J Autoimmun. 1996;9:667–675. doi: 10.1006/jaut.1996.0087. [DOI] [PubMed] [Google Scholar]
- 63.McGargill MA, Derbinski JM, Hogquist KA. Receptor editing in developing T cells. Nat Immunol. 2000;1:336–341. doi: 10.1038/79790. [DOI] [PubMed] [Google Scholar]
- 64.Wang F, Huang CY, Kanagawa O. Rapid deletion of rearranged T cell antigen receptor (TCR) Valpha-Jalpha segment by secondary rearrangement in the thymus: role of continuous rearrangement of TCR alpha chain gene and positive selection in the T cell repertoire formation. Proc Natl Acad Sci USA. 1998;95:11834–11839. doi: 10.1073/pnas.95.20.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Girgis L, Davis MM, Fazekas de St Groth B. The avidity spectrum of T cell receptor interactions accounts for T cell anergy in a double transgenic model. J Exp Med. 1999;189:265–278. doi: 10.1084/jem.189.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oehen S, Feng L, Xia Y, Surh CD, Hedrick SM. Antigen compartmentation and T helper cell tolerance induction. J Exp Med. 1996;183:2617–2626. doi: 10.1084/jem.183.6.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Proietto AI, et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci USA. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 70.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 71.Belz GT, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–10104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vezys V, Olson S, Lefrancois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12:505–514. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 74.Fletcher AL, et al. Reproducible isolation of lymph node stromal cells reveals sitedependent differences in fibroblastic reticular cells. Front Immunol. 2011;2:35. doi: 10.3389/fimmu.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colella TA, et al. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J Exp Med. 2000;191:1221–1232. doi: 10.1084/jem.191.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yip L, et al. Deaf1 isoforms control the expression of genes encoding peripheral tissue antigens in the pancreatic lymph nodes during type 1 diabetes. Nat Immunol. 2009;10:1026–1033. doi: 10.1038/ni.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ng CT, Nayak BP, Schmedt C, Oldstone MB. Immortalized clones of fibroblastic reticular cells activate virus-specific T cells during virus infection. Proc Natl Acad Sci USA. 2012;109:7823–7828. doi: 10.1073/pnas.1205850109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mueller SN, et al. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci USA. 2007;104:15430–15435. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lukacs-Kornek V, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011;12:1096–1104. doi: 10.1038/ni.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khan O, Headley M, Gerard A, Wei W, Liu L, Krummel MF. Regulation of T cell priming by lymphoid stroma. PLoS One. 2011;6:e26138. doi: 10.1371/journal.pone.0026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siegert S, et al. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS One. 2011;6:e27618. doi: 10.1371/journal.pone.0027618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bogdan C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001;11:66–75. doi: 10.1016/s0962-8924(00)01900-0. [DOI] [PubMed] [Google Scholar]
- 83.Krumenacker JS, Hanafy KA, Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res Bull. 2004;62:505–515. doi: 10.1016/S0361-9230(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 84.Zhang M, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 85.Svensson M, Maroof A, Ato M, Kaye PM. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;21:805–816. doi: 10.1016/j.immuni.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 86.Beuneu H, et al. Visualizing the functional diversification of CD8+ T cell responses in lymph nodes. Immunity. 2010;33:412–423. doi: 10.1016/j.immuni.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 87.Haniffa MA, et al. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 88.Bouffi C, Bony C, Jorgensen C, Noel D. Skin fibroblasts are potent suppressors of inflammation in experimental arthritis. Ann Rheum Dis. 2011;70:1671–1676. doi: 10.1136/ard.2010.143297. [DOI] [PubMed] [Google Scholar]
- 89.Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179:2824–2831. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- 90.Vig M, et al. Inducible nitric oxide synthase in T cells regulates T cell death and immune memory. J Clin Invest. 2004;113:1734–1742. doi: 10.1172/JCI20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei XQ, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 92.Huang S, et al. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 93.Niedbala W, Cai B, Liu H, Pitman N, Chang L, Liew FY. Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40. Proc Natl Acad Sci USA. 2007;104:15478–15483. doi: 10.1073/pnas.0703725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomei AA, Siegert S, Britschgi MR, Luther SA, Swartz MA. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J Immunol. 2009;183:4273–4283. doi: 10.4049/jimmunol.0900835. [DOI] [PubMed] [Google Scholar]
- 95.Brand CU, Hunziker T, Braathen LR. Isolation of human skin-derived lymph: flow and output of cells following sodium lauryl sulphate-induced contact dermatitis. Arch Dermatol Res. 1992;284:123–126. doi: 10.1007/BF00372702. [DOI] [PubMed] [Google Scholar]
- 96.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He C, Young AJ, West CA, Su M, Konerding MA, Mentzer SJ. Stimulation of regional lymphatic and blood flow by epicutaneous oxazolone. J Appl Physiol. 2002;93:966–973. doi: 10.1152/japplphysiol.00212.2002. [DOI] [PubMed] [Google Scholar]
- 98.Matsumoto N, Koike K, Yamada S, Staub NC. Caudal mediastinal node lymph flow in sheep after histamine or endotoxin infusions. Am J Physiol. 1990;258:H24–H28. doi: 10.1152/ajpheart.1990.258.1.H24. [DOI] [PubMed] [Google Scholar]
- 99.Modi S, Stanton AW, Mortimer PS, Levick JR. Clinical assessment of human lymph flow using removal rate constants of interstitial macromolecules: a critical review of lymphoscintigraphy. Lymphat Res Biol. 2007;5:183–202. doi: 10.1089/lrb.2007.5306. [DOI] [PubMed] [Google Scholar]
- 100.Mullins RJ, Hudgens RW. Increased skin lymph protein clearance after a 6-h arterial bradykinin infusion. Am J Physiol. 1987;253:H1462–H1469. doi: 10.1152/ajpheart.1987.253.6.H1462. [DOI] [PubMed] [Google Scholar]
- 101.Staberg B, Klemp P, Aasted M, Worm AM, Lund P. Lymphatic albumin clearance from psoriatic skin. J Am Acad Dermatol. 1983;9:857–861. doi: 10.1016/s0190-9622(83)70198-2. [DOI] [PubMed] [Google Scholar]
- 102.Lamagna C, et al. Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion. Mol Biol Cell. 2005;16:4992–5003. doi: 10.1091/mbc.E05-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frontera V, et al. Cutting edge: JAM-C controls homeostatic chemokine secretion in lymph node fibroblastic reticular cells expressing thrombomodulin. J Immunol. 2011;187:603–607. doi: 10.4049/jimmunol.1003441. [DOI] [PubMed] [Google Scholar]
- 104.Bajenoff M, Germain RN. B-cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells. Blood. 2009;114:4989–4997. doi: 10.1182/blood-2009-06-229567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krautler NJ, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150:194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soderberg KA, Payne GW, Sato A, Medzhitov R, Segal SS, Iwasaki A. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc Natl Acad Sci USA. 2005;102:16315–16320. doi: 10.1073/pnas.0506190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Herman PG, Yamamoto I, Mellins HZ. Blood microcirculation in the lymph node during the primary immune response. J Exp Med. 1972;136:697–714. doi: 10.1084/jem.136.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anderson ND, Anderson AO, Wyllie RG. Microvascular changes in lymph nodes draining skin allografts. Am J Pathol. 1975;81:131–160. [PMC free article] [PubMed] [Google Scholar]
- 109.Webster B, Ekland EH, Agle LM, Chyou S, Ruggieri R, Lu TT. Regulation of lymph node vascular growth by dendritic cells. J Exp Med. 2006;203:1903–1913. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chyou S, et al. Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J Immunol. 2008;181:3887–3896. doi: 10.4049/jimmunol.181.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mebius RE, Breve J, Duijvestijn AM, Kraal G. The function of high endothelial venules in mouse lymph nodes stimulated by oxazolone. Immunology. 1990;71:423–427. [PMC free article] [PubMed] [Google Scholar]
- 112.Chyou S, et al. Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells. J Immunol. 2011;187:5558–5567. doi: 10.4049/jimmunol.1101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumamoto Y, Mattei LM, Sellers S, Payne GW, Iwasaki A. CD4+ T cells support cytotoxic T lymphocyte priming by controlling lymph node input. Proc Natl Acad Sci USA. 2011;108:8749–8754. doi: 10.1073/pnas.1100567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guarda G, et al. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. 2007;8:743–752. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- 115.Martin-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 116.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 117.Bajenoff M, Egen JG, Qi H, Huang AY, Castellino F, Germain RN. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends Immunol. 2007;28:346–352. doi: 10.1016/j.it.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 118.Angeli V, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 119.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 120.Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110:3158–3167. doi: 10.1182/blood-2007-01-066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tzeng TC, et al. CD11c(hi) dendritic cells regulate the re-establishment of vascular quiescence and stabilization after immune stimulation of lymph nodes. J Immunol. 2010;184:4247–4257. doi: 10.4049/jimmunol.0902914. [DOI] [PMC free article] [PubMed] [Google Scholar]