Abstract
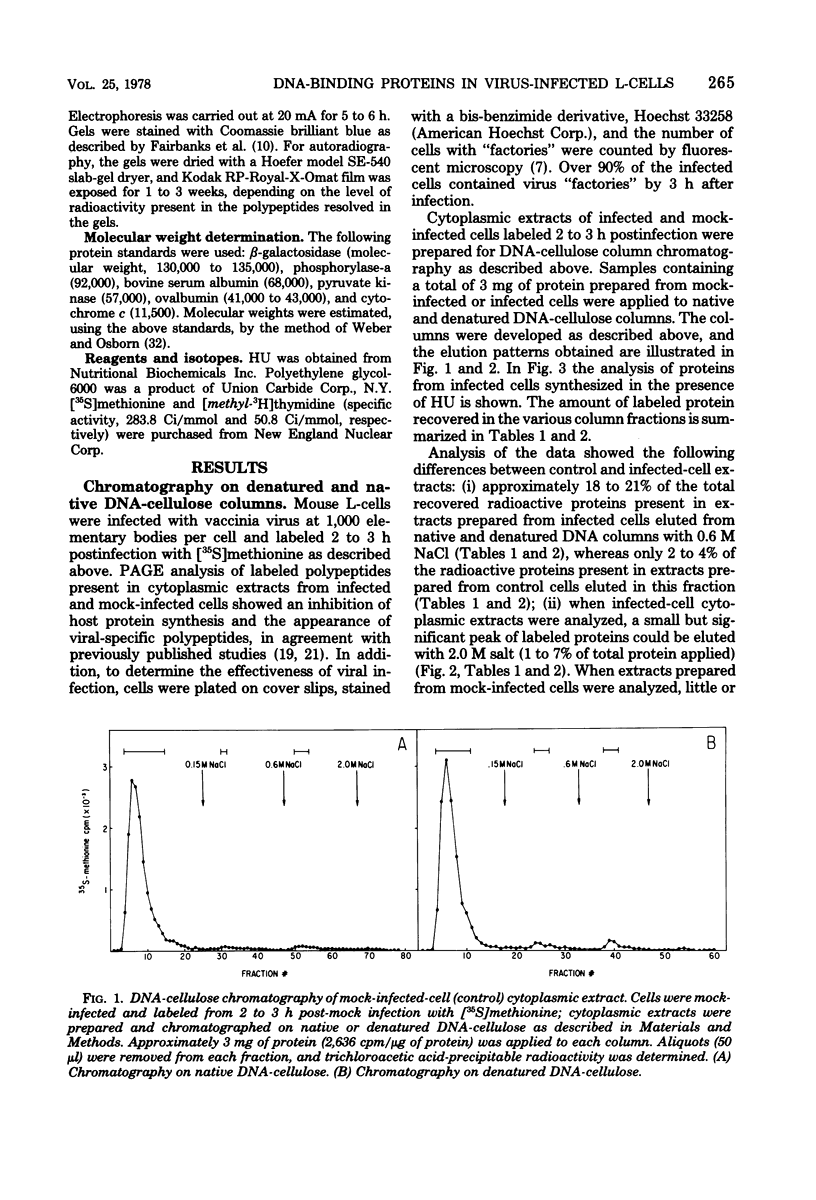
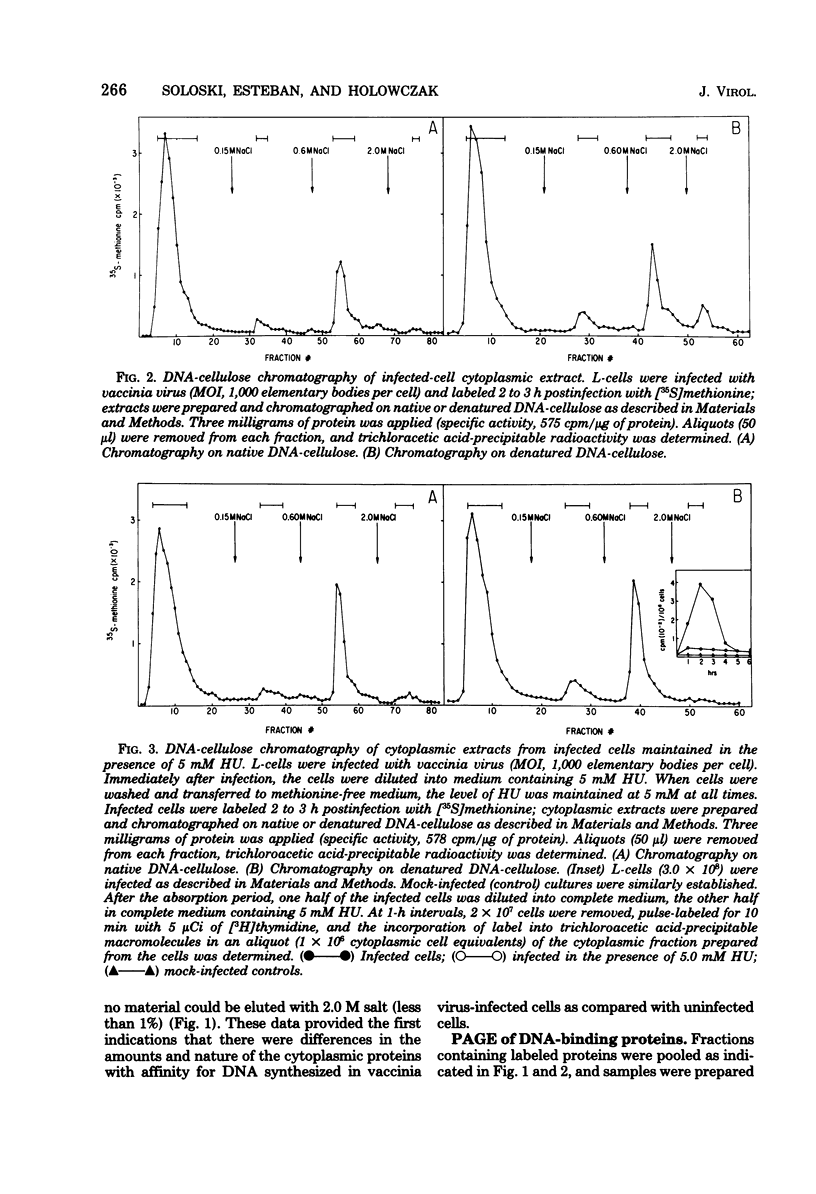
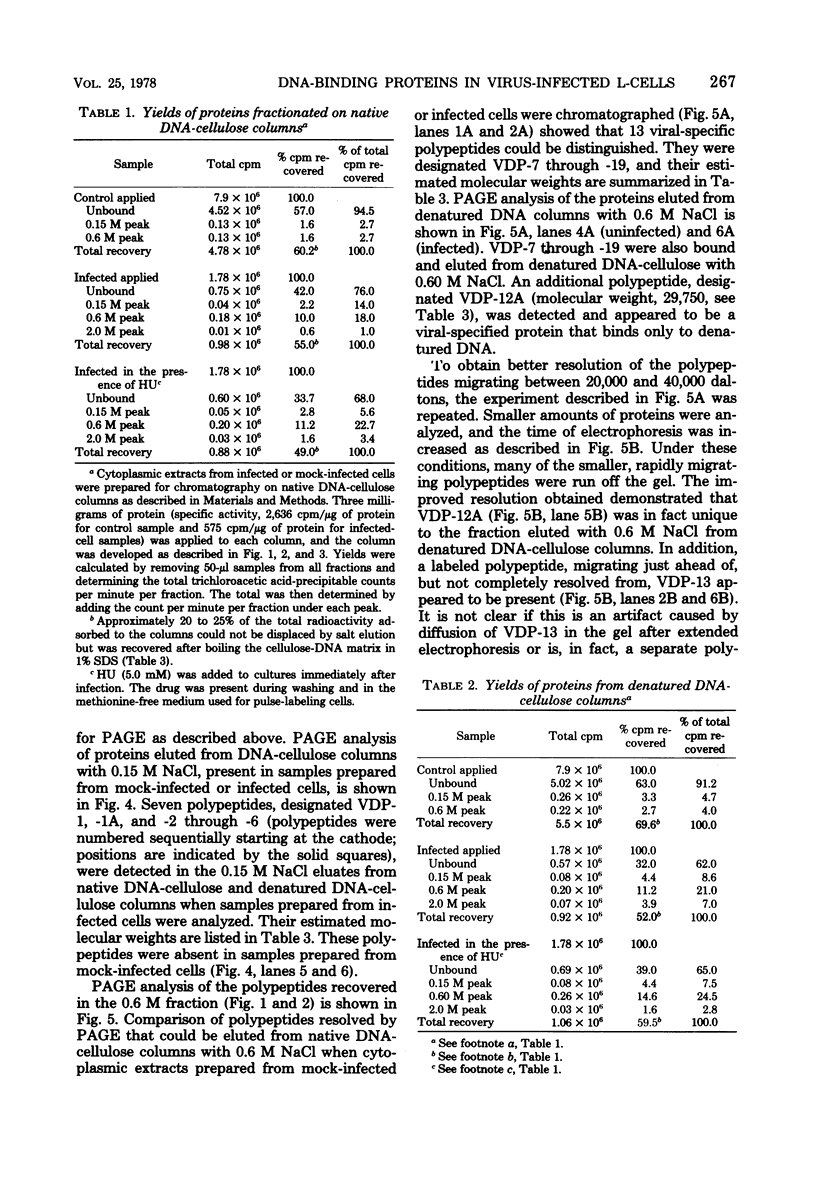
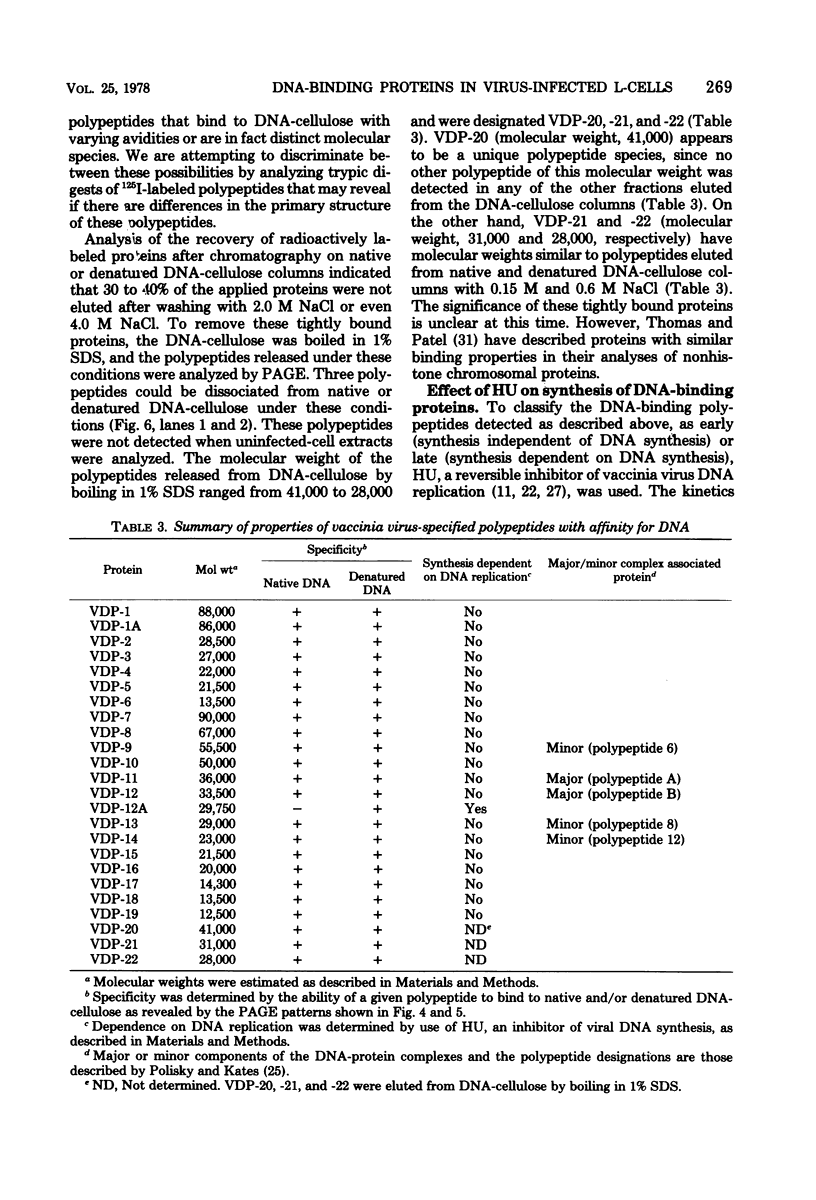
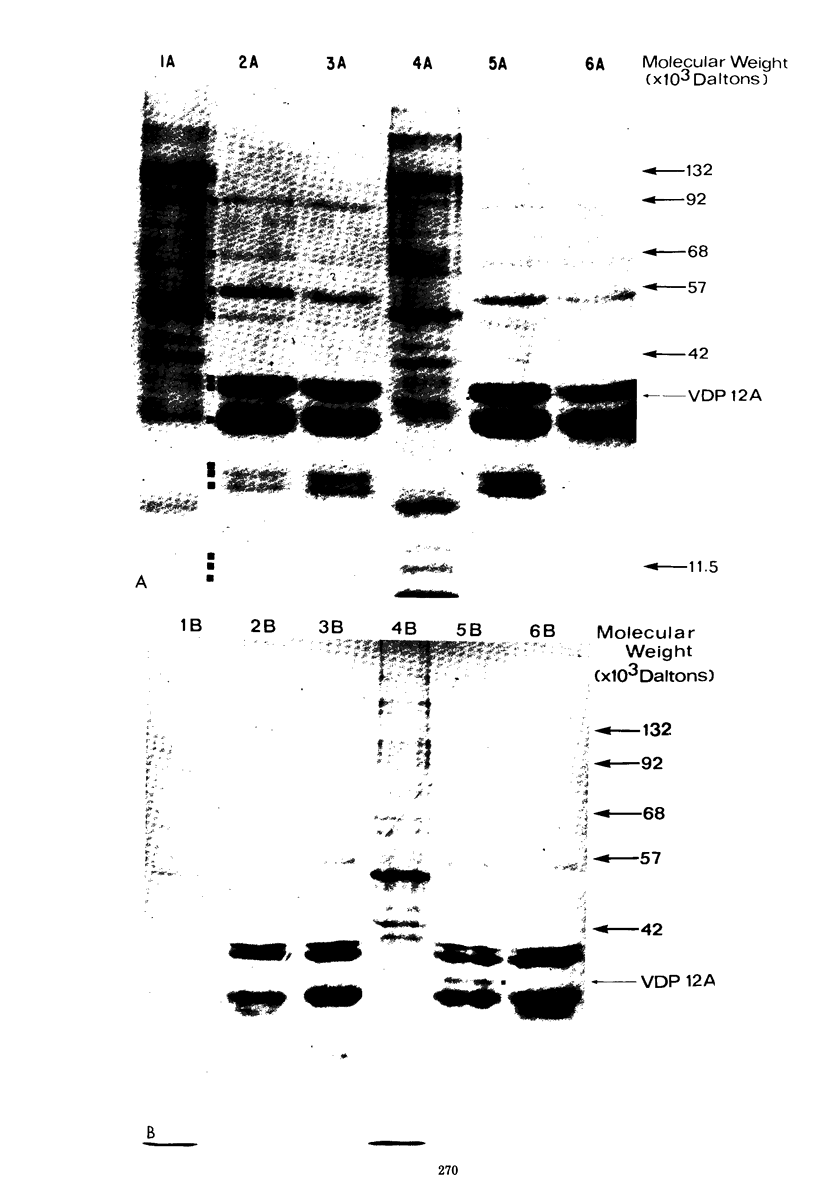
Mouse L cell fibroblasts were infected with vaccinia virus and labeled 2 to 3 h postinfection with [35S]methionine. Labeled proteins were fractionated on native and denatured DNA-cellulose columns and then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Twenty-four 90,000 to 12,500, were detected. VDP-12A (molecular weight, 29,750) had affinity for denatured but not native DNA, and its synthesis was dependent on viral DNA replication. VDP-20 (molecular weight, 41,000) bound very tightly to native and denatured DNA and was displaced only after boiling the protein-DNA-cellulose matrix in 1% sodium dodecyl sulfate. VDP-8,-11,-12,-13, -and-14 behaved electrophoretically like the polypeptide species previously shown to be present in DNA-protein complexes prepared from infected cells. The molecular weights of VDP-10 (50,000), VDP-11 (36,000), and VDP-8 (67,000) were similar to the polypeptide subunits of polyadenylate polymerase and phosphohydrolase I, enzymes purified from virions which have also been shown to have affinity for DNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- BECKER Y., JOKLIK W. K. MESSENGER RNA IN CELLS INFECTED WITH VACCINIA VIRUS. Proc Natl Acad Sci U S A. 1964 Apr;51:577–585. doi: 10.1073/pnas.51.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss G. J., Marsden H. S., Hay J. Herpes simplex virus proteins: DNA-binding proteins in infected cells and in the virus structure. Virology. 1975 Nov;68(1):124–134. doi: 10.1016/0042-6822(75)90154-3. [DOI] [PubMed] [Google Scholar]
- Dahl R., Kates J. R. Synthesis of vaccinia virus "early" and "late" messenger RNA in vitro with nucleoprotein structures isolated from infected cells. Virology. 1970 Oct;42(2):463–472. doi: 10.1016/0042-6822(70)90289-8. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- Esteban M., Holowczak J. A. Replication of vaccinia DNA in mouse L cells. I. In vivo DNA synthesis. Virology. 1977 May 1;78(1):57–75. doi: 10.1016/0042-6822(77)90078-2. [DOI] [PubMed] [Google Scholar]
- Esteban M., Holowczak J. A. Replication of vaccinia DNA in mouse L cells. II. In vitro DNA synthesis in cytoplasmic extracts. Virology. 1977 May 1;78(1):76–86. doi: 10.1016/0042-6822(77)90079-4. [DOI] [PubMed] [Google Scholar]
- Esteban M., Holowczak J. A. Replication of vaccinia DNA in mouse L cells. III. Intracellular forms of viral DNA. Virology. 1977 Oct 15;82(2):308–322. doi: 10.1016/0042-6822(77)90006-x. [DOI] [PubMed] [Google Scholar]
- Esteban M. Rifampin and vaccinia DNA. J Virol. 1977 Feb;21(2):796–801. doi: 10.1128/jvi.21.2.796-801.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fil W., Holowczak J. A., Flores L., Thomas V. Biochemical and electron microscopic observations of vaccinia virus morphogenesis in HeLa cells after hydroxyurea reversal. Virology. 1974 Oct;61(2):376–396. doi: 10.1016/0042-6822(74)90275-x. [DOI] [PubMed] [Google Scholar]
- Holowczak J. A., Diamond L. Poxvirus DNA. II. Replication of vaccinia virus DNA in the cytoplasm of HeLa cells. Virology. 1976 Jul 1;72(1):134–146. doi: 10.1016/0042-6822(76)90318-4. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Kornberg A., Alberts B. M. Stimulation of T4 bacteriophage DNA polymerase by the protein product of T4 gene 32. J Mol Biol. 1971 Nov 28;62(1):39–52. doi: 10.1016/0022-2836(71)90129-x. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., van der Vliet P. C., Rosenwirth B., Anderson C., Rabek J., Levinson A., Anderson S. Characterization of an adenovirus early protein required for viral DNA replication: a single strand specific DNA binding proteins. Mol Cell Biochem. 1976 Apr 28;11(2):79–95. doi: 10.1007/BF01792789. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Paoletti E. Polyadenylate polymerase from vaccinia virions. Nat New Biol. 1973 Sep 12;245(141):59–63. doi: 10.1038/newbio245059a0. [DOI] [PubMed] [Google Scholar]
- Moss B., Salzman N. P. Sequential protein synthesis following vaccinia virus infection. J Virol. 1968 Oct;2(10):1016–1027. doi: 10.1128/jvi.2.10.1016-1027.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolette E., Rosemond-Hornbeak H., Moss B. Two nucleid acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3273–3280. [PubMed] [Google Scholar]
- Pennington T. H. Vaccinia virus polypeptide synthesis: sequential appearance and stability of pre- and post-replicative polypeptides. J Gen Virol. 1974 Dec;25(3):433–444. doi: 10.1099/0022-1317-25-3-433. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Biogenesis of vaccinia: separation of early stages from maturation by means of hydroxyurea. Virology. 1971 Jan;43(1):144–151. doi: 10.1016/0042-6822(71)90232-7. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Katz J. R., Dales S. Biogenesis of poxviruses: synthesis and phosphorylation of a basic protein associated with the DNA. Virology. 1975 Apr;64(2):531–543. doi: 10.1016/0042-6822(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Polisky B., Kates J. Vaccinia virus intracellular DNA-protein complex: biochemical characteristics of associated protein. Virology. 1972 Jul;49(1):168–179. doi: 10.1016/s0042-6822(72)80018-7. [DOI] [PubMed] [Google Scholar]
- Polisky B., Kates J. Viral-specific polypeptides associated with newly replicated vaccinia DNA. Virology. 1975 Jul;66(1):128–139. doi: 10.1016/0042-6822(75)90184-1. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. DNA-binding proteins induced by herpes simplex virus type 2 in HEp-2 cells. J Virol. 1976 Aug;19(2):717–731. doi: 10.1128/jvi.19.2.717-731.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz H. S., Rose H. M., Morgan C., Hsu K. C. The effect of hydroxyurea on virus development. II. Vaccinia virus. Virology. 1966 Apr;28(4):510–519. doi: 10.1016/0042-6822(66)90235-2. [DOI] [PubMed] [Google Scholar]
- Sarov I., Joklik W. K. Isolation and characterization of intermediates in vaccinia virus morphogenesis. Virology. 1973 Mar;52(1):223–233. doi: 10.1016/0042-6822(73)90411-x. [DOI] [PubMed] [Google Scholar]
- Scherzinger E., Klotz G. Studies on bacteriophage T7 DNA synthesis in vitro. II. Reconstitution of the T7 replication system using purified proteins. Mol Gen Genet. 1975 Dec 1;141(3):233–249. doi: 10.1007/BF00341802. [DOI] [PubMed] [Google Scholar]
- Scherzinger E., Seiffert D. Studies on bacteriophage T7 DNA synthesis in vitro. I. Resolution of the T7 replication system into its components. Mol Gen Genet. 1975 Dec 1;141(3):213–232. doi: 10.1007/BF00341801. [DOI] [PubMed] [Google Scholar]
- Thomas T. L., Patel G. L. DNA unwinding component of the nonhistone chromatin proteins. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4364–4368. doi: 10.1073/pnas.73.12.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]






