Abstract
HIV infection is associated with metabolic bone disease resulting in bone demineralization and reduced bone mass. The molecular mechanisms driving this disease process have yet to be elucidated. Wnt/β-catenin signaling plays a key role in bone development and remodeling. We attempted to determine the effects of the HIV-1 protein, gp120, on Wnt/β-catenin signaling at an intracellular and transcriptional level in primary human osteoblasts (HOBs). This work, inclusive of experimental controls, was part of a greater project assessing the effects of a variety of different agents on Wnt/β-catenin signaling (BMC Musculoskelet Disord 2010 Sep 15;11:210). We examined the phenotypic effects of silencing and overexpressing the Wnt antagonist, Dickkopf-1 (Dkk1) in HOBs treated with gp120. HOBs exposed to gp120 displayed a significant reduction in alkaline phosphatase activity (ALP) activity and cell proliferation and increased cellular apoptosis over a 48h time course. Immunocytochemistry demonstrated a significant reduction in intracytosolic and intranuclear β-catenin in response to HIV-1 protein exposure. These changes were associated with a reduction of TCF/LEF-mediated transcription, the transcriptional outcome of canonical Wnt β-catenin signaling. Silencing Dkk1 expression in HOBs exposed to gp120 resulted in increased ALP activity and cell proliferation, and decreased cellular apoptosis relative to scrambled control. Dkk1 overexpression exacerbated the inhibitory effect of gp120 on HOB function, with decreases in ALP activity and cell proliferation and increased cellular apoptosis relative to vector control. Wnt/β-catenin signaling plays a key regulatory role in HIV-associated bone loss, with Dkk1, a putative central mediator in this degenerative process.
Keywords: HIV-1, Wnt, Dkk1, Osteoblast, Osteoporosis
Introduction
HIV infection is associated with a metabolic bone disease resulting in bone demineralization and reduced bone mass1. As a consequence, HIV infected patients have a higher incidence of osteopenia and osteoporosis2, leading to an increased risk of fragility fracture. Both HIV infection and treatment with highly active antiretroviral therapy (HAART) are believed to contribute to the pathogenesis of this HIV associated bone loss3,4. However, the molecular mechanisms driving this degenerative process remain fundamentally unclear and are as yet considerably under investigated. HIV infection has a chronic clinical evolution, characterized by a progressive depletion of CD4+ T lymphocytes and a global impairment of immune function5. This profound depression in cellular immunity resulting from chronic HIV infection is due to the effects of continued viral replication and immune activation in association with the putative biological and biochemical processes of apoptosis and oxidative stress6. Although significant bone loss has been demonstrated in treatment-naïve HIV patients7,8, initiation of HAART does appear to exacerbate this reduction in bone mass and is dependent on the treatment regimen9–12.
Normal bone remodeling involves coupling of the tightly regulated processes of bone formation by osteoblasts and bone resorption by osteoclasts. The impact of HIV infection on the cellular and molecular mechanisms underpinning bone homeostasis has yet to be fully elucidated. The Wnt/β-catenin signaling cascade is a key regulator of bone development and remodeling13, first identified, when the loss-of-function mutation of the LDL receptor-related protein (LRP5) was associated with osteoporosis psuedoglioma syndrome, a recessive disorder characterized by osteogenesis imperfecta with low bone mass and a predisposition to fractures as well as ocular defects14,15. Subsequently, gain-of-function mutations of the LRP5 were associated with disorders of increased bone mass, such as Van Buchem's disease, osteopetrosis, and endosteal hyperostosis16–18.
Prior work from our group has assessed the effects of a variety of different agents (including dexamethasone) on Wnt/β-catenin signaling in primary human osteoblasts; and this study has utilized the same experimental controls19,20. The objectives of this current study were firstly, to determine the effects of the HIV-1 protein, gp120, on intracellular and transcriptional Wnt/β-catenin signaling in HOBs, and secondly, to assess the phenotypic effects of transient silencing and overexpression of the Wnt antagonist, Dickkopf-1 (Dkk1) in the setting of gp120 exposure.
Materials and Methods
Cell Culture
Primary human osteoblasts (HOBs) obtained from normal human hip samples were purchased commercially (Promocell, Heidelberg, Germany). HOBs were cultured in Osteoblast Growth Medium (Promocell, Heidelberg, Germany) containing 10% FCS and antibiotics (100 IU/ml penicillin and 100µg/ml streptomycin) at 37°C, 5% CO2, as previously reported20. All experiments involved the use of HOBs between 2th and 5th passage. HOBs were treated with 100ng/mL of HIV-1 gp120 (NIH AIDS Research and Reference Reagent Program) over a 48h time course. The HIV-1 gp120 used in this line of experiments was one of a number of agents being assessed simultaneously for osteogenic effects in HOBs and the same experimental controls were used for all agents analyzed20.
Alkaline Phosphatase Activity
Alkaline phosphatase (ALP) activity was analyzed by the measurement of released p-nitrophenol from p-nitrophenolphosphate (pNPP), as previously reported20. Cell lysates after experimental treatment were collected by Cell Lytic MT (Sigma-Aldrich, Poole, UK) treatment and the extracted protein was frozen at −80°C for at least 2h prior to ALP activity assay in order to disrupt cellular membranes. 10µl of thawed cell lysates were incubated with 200µl pNPP reagent (Sigma-Aldrich, Poole, UK) for 30 min at room temperature. ALP activity was analyzed at 405nm and normalized to total protein extracted, which was measured using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rochford, Illinois) as per manufacturer’s protocols. One unit of ALP activity was defined as the amount required for the liberation of 1.0 µmol p-nitrophenol/ min/mg of cellular protein.
Cell Proliferation
HOBs were plated at a density of 2.5 × 104cells/100µl/well in 96-well plates. Mitogenic activity after experimental treatment was assayed using the colorimetric 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenol)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (CellTiter 96 AQ; Promega, Madison, WI, USA). At experiment termination, 20µl MTS/phenazinemethosulfate (PMS) solution was added to each well for 3h, at which time the light absorbance at 490 nm was measured using a spectrophotometric microplate reader. Cell proliferation and cell viability, represented as percent survival, was calculated as [(experimental absorbance – blank absorbance) / control absorbance − blank absorbance)] / 100, where the control absorbance is the optical density obtained for 2.5 × 104cells/well (number of cells plated at the start of the experiment), and blank absorbance is the optical density determined in wells containing medium and MTS alone. HOBstreated with 2nM docitaxol for 48h were used as a negative control.
Cellular Apoptosis
HOBs were plated at a density of 2.5 × 104cells/100µl/well in 96-well plates. Apoptotic activity after experimental treatment was assessed by analyzing caspase-3 and caspase-7 activation using the substrate DEVD-aminoluciferin from Caspase-Glo 3/7 assay kit (Promega, Madison, WI, USA), as per manufacturer’s protocols. At experiment termination, 100µl of Caspase-Glo 3/7 Reagent was added to each well and incubated at room temperature for 3h, at which time the luminescence of each sample was measured. HOBs treated with 2nM docitaxol for 48h were used as a positive control.
Immunoflourescence Staining
Indirect immunocytochemistry was employed to visualize beta-catenin, utilizing a mouse anti β-catenin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as the primary antibody, as previously reported20. HOBs treated with HIV-1 gp120-containing media were fixed in ice-cold methanol, permeabilized for 10 min and subsequently blocked with 10% goat serum for 10 min at room temperature. Samples were incubated for 1h with primary antibody, followed by 30 min incubation with a goat anti-mouse TRITC-conjugate. HOBs were examined with a Zeiss Axioskop 40 fluorescence microscope using 20× objectives. Digital images captured with a ProgRes C10plus research digital camera (Jenoptik Laser OptikSysteme GmbH, Jena, Germany) were processed using the ProgResCapturePro 2.1 image analysis softwear package (Jenoptik Laser Optik Systeme GmbH, Jena, Germany).
Generation of Conditioned Media
Mouse Wnt3a overexpressing cells (L-Wnt3a) and control non-transfected L-cells were purchased commercially (ATCC, Manassas, USA) and cultured in DMEM with glutamax, 10% FBS, 100 IU/ml penicillin and 100 µg/ml streptomycin. L-Wnt3a culture medium was supplemented with 400 µg/ml geneticin to maintain selective pressure. Conditioned medium from L-Wnt3a and control L-cells was collected according as per manufacturer’s protocols and as previously reported20. Cells were passaged 1:10 in 8 ml medium without geneticin and left to grow for 10 days. Medium collected from each cell line was replaced with 8 ml fresh medium for a further 3 days. The second batch of medium was then collected and the cells discarded. First and second batches were combined, sterile filtered (0.2 µm) and stored at −20°C until required.
β-Catenin/TCF Transcription Reporter Assay
HOBs plated in 24-well plates at 2 × 104/cm2 were transiently transfected with the Wnt-luciferase reporter construct pBAR (1µg total; Dr. RT. Moon, University of Washington, Seattle, WA, USA) and the control reporter pfuBAR (1µg total; Dr. RT. Moon, University of Washington, Seattle, WA, USA) using GeneJuice (Novagen, Madison, WI, USA), as previously reported20. The β-catenin activated reporter (pBAR) used to transfect HOBs contains 12 TCF binding sites (5’-AGATCAAAGG-3’) separated by distinct 5 base linkers. These elements lie directly upstream of a minimal thymidine kinase promoter driving firefly luciferase expression. The control reporter pfuBAR is identical to its sister reporter, except for a two base substitution in each TCF binding site. Both reporters contain a separate PGK promoter constitutively driving the expression of a puromycin resistance gene, and both are in a lentiviral platform. Subsequent co-transfection with 0.5µg of pSL9EF1a(P)RLUC (Dr. RT. Moon, University of Washington, Seattle, WA, USA), the internal control reporter driving constitutive expression of Renilla luciferase, was performed, normalizing for transfection efficiency. Sixteen hours after transfection, HOBs were washed and cultured for 48h in media containing gp120 with 2% FCS, in the presence or absence Wnt3a-conditioned media. HOBs were lysated and luciferase assays performed with the Dual Luciferase Assay Kit (Promega, Madison, WI, USA) as per manufacturer’s protocols and as previously reported20. 10µl of cell lysates were first assayed for firefly luciferase and then Renilla luciferase activity. Firefly luciferase activity was subsequently normalized to Renilla luciferase activity.
siRNA-mediated Dkk1 Gene Silencing
Predesigned short interfering RNA (siRNA) targeting human Dkk1 (Hs_DKK1_1) and a control scrambled RNA targeting a sequence not sharing homology with the human genome (AllStars Negative Control) were purchased commercially (Qiagen, Crawley, UK). HOBs were transfected with siRNAs and control scrambled RNA using the RNAiFect transfection reagent (Qiagen, Crawley, UK) as per manufacturer’s protocols and as previously reported20. siRNA or scrambled RNA solutions were prepared 15–25 min before cell transfection, with a ratio of siRNA to the RNAiFect reagent of 1 µg siRNA to 3 µl transfection reagent. siRNA-RNAiFect transfection complexes were incubated for 15 min at room temperature (15–25°C). Osteoblast growth medium was exchanged for fresh medium and the siRNA-RNAiFect suspension was added drop-wise onto the HOBs. HOBs with adherent complexes were subsequently incubated for 24h at 37°C, 5% CO2, followed by a change of medium and commencement of experimentation. Transfection efficiency was established in three preliminary experiments in which a fluorescent control RNA-RNAiFect complex (Qiagen, Crawley, UK) was transfected into the HOBs instead of the siRNA-RNAiFect complex. The uptake of the fluorescent RNA assessed by fluorescence microscopy was in the range of 75–85%. The ratio of siRNA to the RNAiFect reagent was determined three preliminary experiments with a ratio of 1 µg siRNA:3µlRNAiFect providing a maximal gene silencing of 75% knockdown as determined by qRT-PCR. Knockdown was further confirmed on ELISA.
Dkk1 Gene Overexpression
Dkk1 cDNA (pDkk1) and control ‘empty vector’ DNA (pControl) plasmids were kindly provided as gifts (Dr. RT. Moon, University of Washington, Seattle, WA, USA). HOBs were transfected with pDkk1 and pControl using the GeneJuice transfection reagent (Novagen, Madison, WI, USA) as per manufacturer’s protocols. Briefly, pDkk1 or pControl transfection solutions were prepared 15–25 min before the cell transfection. The ratio of DNA to GeneJuice reagent was 1 µg DNA to 3 µl transfection reagent, as determined in preliminary optimization experiments. DNA-GeneJuice transfection suspensions were incubated for 15 min at room temperature (15–25°C). Osteoblast growth medium was replaced with fresh medium and the DNA-GeneJuice suspension was added drop-wise onto the HOBs. HOBs with added transfection suspension were incubated for 24h at 37°C, 5% CO2, at which point the medium was changed and experimentation was commenced. Expression of Dkk1 was determined by qRT-PCR and ELISA.
Quantitative Real Time PCR
Dkk-1 mRNA regulation in HOBs treated with HIV-1 gp120 was measured by quantitative Real Time PCR using human Dkk-1 QuantiTect assay (Qiagen, Crawley, UK), as previously reported20. The QuantiTect probe sequence for Dkk-1 was 5'-CACACCAAAGGACAAGA-3'. The Dkk-1 forward primer sequence was 5'-GGGAATTACTGCAAAAATGGAATA-3', and the reverse primer sequence was 5'-ATGACCGGAGACAAACAGAAC-3'. Total RNA extracted by TRI-reagent/chlorophorm method was assayed in duplicate using a Rotorgene 3.0 Real Time PCR instrument (Corbett Research, Cambridge, UK) and the Real Time PCR amplification kit SYBR Green I (Qiagen, Crawley, UK). Gene specific primer pairs were used, with Dkk1 gene products reported as a function of crossing time (Ct), the cycle number at which PCR amplification becomes linear. mRNA expression was normalized to control and GAPDH expression resulting in Mean Fold Change values or ΔΔCt. Following cycling ensuring specificity, melt curve analysis verified the amplification of PCR products starting at 65°C and ramping to 95°C at 0.1°C/sec. One peak in the melt curve indicated no secondary, non-specific products were formed.
Dkk1 ELISA
Human Dkk1 ELISA kit (R&D Systems Europe Ltd, Abingdon, UK) was purchased commercially to analyse Dkk1 protein expression on cell supernatant. ELISA was performed as per manufacturer’s protocols and as previously reported20.
Statistical Analysis
All data presented were obtained from three independent experiments, each in triplicate. Data were expressed as mean ± SE. Statistical differences were calculated using Student’s t-test or ANOVA for multiple comparisons. p values <0.05 was considered statistically significant.
Results
Effect of gp120 on Primary Human Osteoblast Differentiation and Function
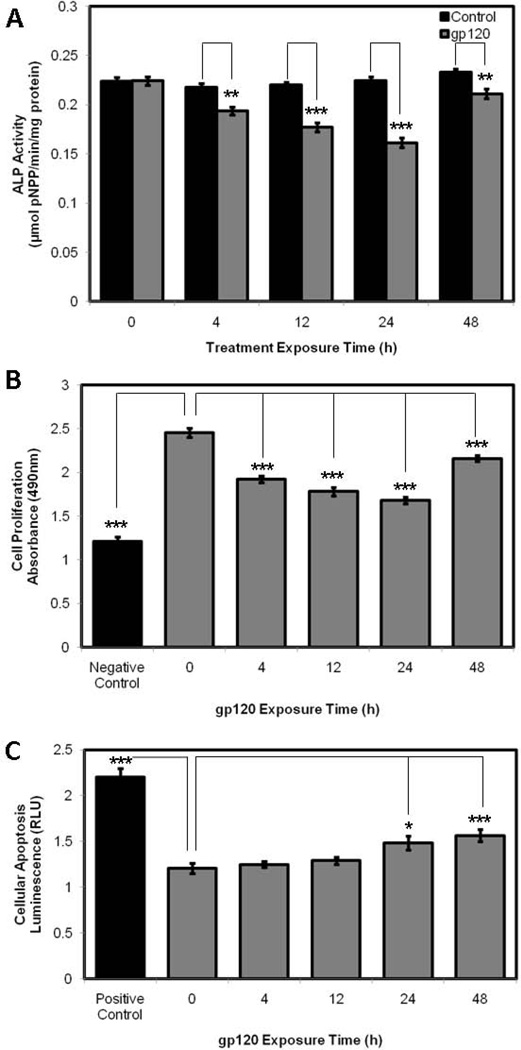
In order to study the effects of gp120 treatment on HOB differentiation and function, we examined ALP activity by pNPP assay, cell proliferation and cell viability by MTS assay and cellular apoptosis by measuring caspase-3/7 activation. HOBs exposed to gp120 displayed reductions in ALP activity over a 48h time course, with maximal reductions identified at 24h exposure. Significant reductions were demonstrated at 4h (p=0.0012), 12h (p<0.0001), 24h (p<0.0001) and 48h (p=0.0047) of gp120 exposure (Fig. 1A) when compared to control HOBs for each time point. HOBs treated with gp120 displayed reductions in cell proliferation and cell viability over 48h, with maximal reductions identified at 24h. Significant reductions were demonstrated at 4h (p<0.0001), 12h (p<0.0001), 24h (<0.0001) and 48h (p=0.0002) of gp120 exposure (Fig. 1B) when compared to untreated HOBs. As a negative control, HOBs were treated with 2nM docitaxol for 48h. HOBs exposed to gp120 displayed time-dependent increases in cellular apoptosis over 48h. Significant increases in caspase-3 and caspase-7 activity were noted after 24h (p=0.0103) and 48h (p=0.0008) of gp120 treatment (Fig. 1C) when compared to untreated HOBs. As a positive control, HOBs were treated with 2nM docitaxol for 48h.
Figure 1.
pNPP assay (A), MTS assay (B) and Caspase 3/7 assay (C) in HOBs exposed to 100ng/mL gp120 over 48h.
Effect of gp120 on Wnt/β-CateninSignaling in Primary Human Osteoblasts
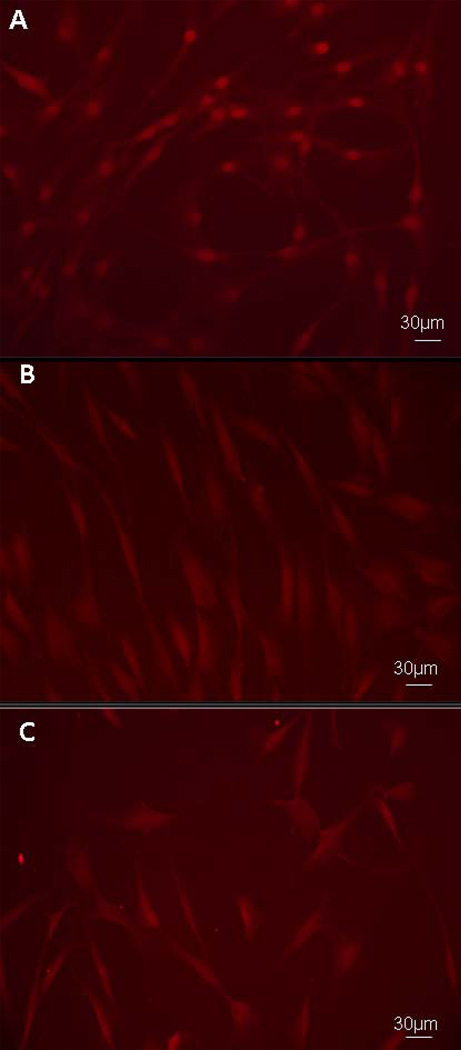
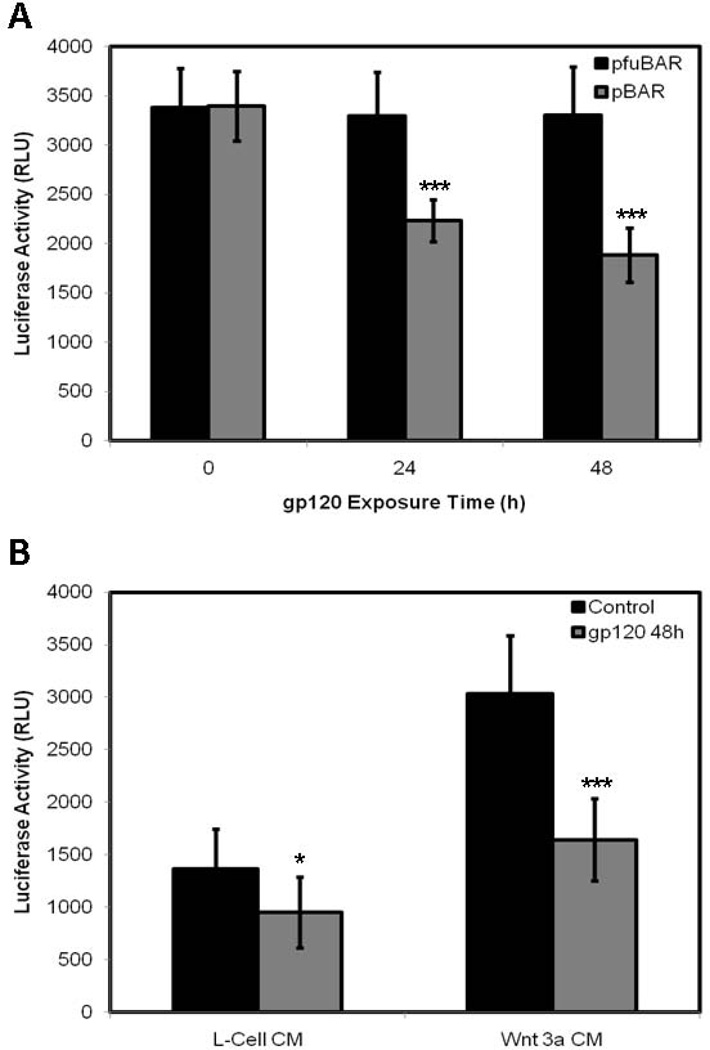
In order to study the effects of gp120 treatment on Wnt/β-catenin signaling in HOBs, we firstly examined β-catenin trafficking using immunoflourescence analysis, and secondly, examined Wnt/β-catenin-mediated transcription using a Wnt-luciferase reporter assay. HOBs grown to confluency were exposed to gp120 over a 48h time course. Control HOBs, not exposed to gp120, cultured in osteoblast growth medium alone, demonstrated a strong perinuclear and intranuclear staining of β-catenin, representing activated Wnt/β-catenin signaling (Fig. 2A). HOBs exposed to gp120 demonstrated a reduction in intracellular staining for β-catenin at 24h (Fig. 2B) and this reduction in β-catenin staining persisted at 48h (Fig. 2C), representing a significant inhibition of Wnt/β-catenin signaling. HOBs transiently transfected with the highly sensitive Wnt-luciferase reporter construct pBAR and the control reporter pfuBAR were exposed to gp120 over a 48h time course. Significant reductions in luciferase activity were identified at 24h (p<0.0001) and 48h (p<0.0001) of gp120 treatment in the pBAR reporter cells, with luciferase activity in the pfuBAR reporter cells remained unchanged during treatment (Fig. 3A).
Figure 2.
Immunoflourescence analysis of HOBs exposed to 100ng/mL gp120 over a 48h time course, demonstrating control HOBs (A) and HOBs at 24h (B) and 48h (C) of treatment. Experimental controls reproduced with permission from BMC Musculoskelet Disord 2010 Sep 15;11:210.
Figure 3.
HOBs transfected with pBAR and pfuBAR, exposed to 100ng/mL gp120 over 48h (A). HOBs transfected with pBAR treated with Wnt 3a conditioned media and L-cell conditioned media (B). Experimental controls reproduced with permission from BMC Musculoskelet Disord 2010 Sep 15;11:210.
In order to validate these findings HOBs transfected with pBAR were treated with Wnt 3a conditioned media (Wnt agonist) and L-cell conditioned media (control) over a 48h time course. Significant reductions in luciferase activity were identified when 100ng/mL of gp120 was added to both Wnt 3a conditioned media (p<0.0001) and control L-cell conditioned media (p=0.0262) over the 48h time course (Fig. 3B). These reductions in luciferase activity represent a reduction in Wnt/β-catenin signaling through the inhibition of TCF/LEF mediated transcription.
Effect of gp120 on Dickkopf-1 Expression in Primary Human Osteoblasts
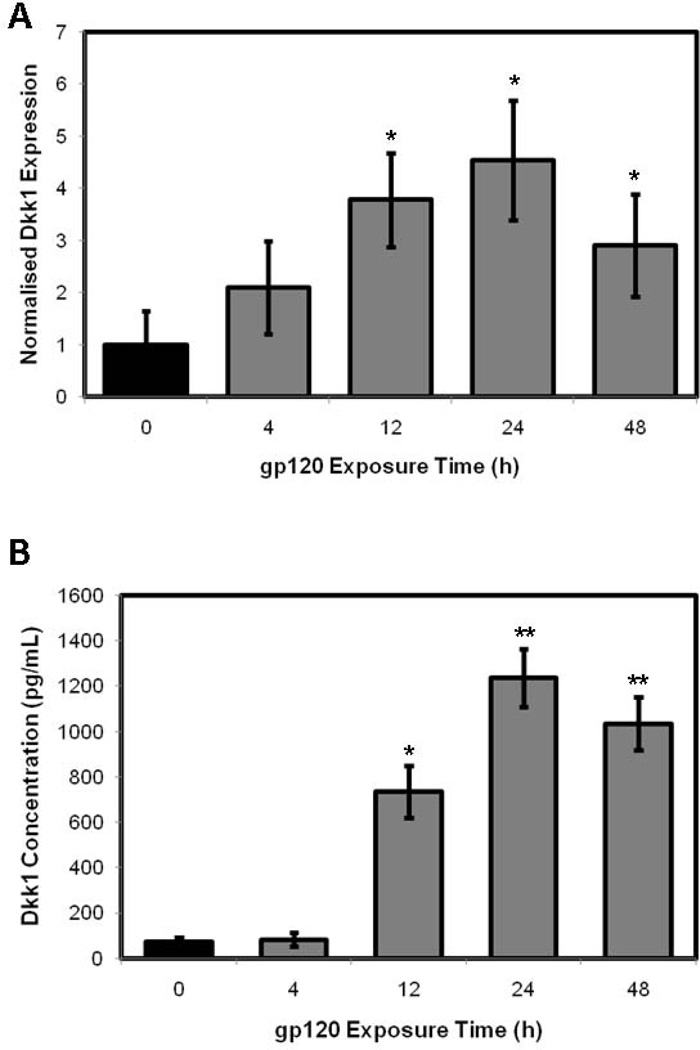
To further explore the effect of gp120 exposure on Wnt/β-catenin signaling in HOBs, we analysed the gene and protein expression of the potent Wnt antagonist, Dickkopf-1, in the setting of gp120 exposure. Dkk1 gene expression was significantly altered in response to gp120 exposure over a 48h time course. Dkk1 mRNA expression increased dramatically in response to gp120 exposure, with relative Dkk1 mRNA concentration increasing over the 48h time courses, with maximal increases in expression occurring at 24h exposure. Using quantitative RT-PCR, a 2.1-fold increase in Dkk1 mRNA expression was observed at 4h (p=0.1416), followed by a 3.8-fold increase at 12h (p=0.0183), 4.5-fold increase at 24h (p=0.0119) and 2.9-fold increase at 48h (p=0.0483) of gp120 treatment when compared to control HOBs (Fig. 4A). The impact on gene expression analysis was mirrored by similar changes in protein expression, with Dkk1 protein expression significantly altered in response to HIV-1 protein exposure over 48h, with maximal increases in expression occurring at 24h exposure. ELISA assessed changes in Dkk1 concentration in cell supernatant. A 10.1-fold increase in Dkk1 protein expression was observed at 12h (p=0.0102), followed by a 17.1-fold increase at 24h (p=0.0026) and 14.3-fold increase at 48h (p=0.0022) of gp120 exposure compared to control HOBs (Fig. 4B).
Figure 4.
RT-PCR assessing Dkk1 gene expression (A) and ELISA assessing Dkk1 protein expression (B) in HOBs exposed to 100ng/mL gp120 over 48h.
Effect of Silencing Dickkopf-1 Expression on Primary Human Osteoblasts exposed to gp120
To examine the phenotypic effects that aberrations in Wnt/β-catenin signaling have on HOBs exposed to HIV-1 proteins, we previously silenced Dkk1 expression using siRNA20. HOBs transfected with siRNA targeting Dkk1 expression demonstrated 75.38% reduction in Dkk1 gene expression using quantitative RT-PCR and 84.94% reduction in Dkk1 protein expression using ELISA20. Furthermore, when HOBs were transfected with the Wnt-luciferase reporter construct pBAR and the control reporter pfuBAR, and subsequently treated with siRNA targeting Dkk1 expression or scrambled control RNA; a significant increase in luciferase activity was observed in the siRNA transfected cells when compared to scrambled control cells (p<0.0001) in the pBAR reporter cells, whilst the luciferase activity in the pfuBAR reporter cells remained unchanged during treatment20.
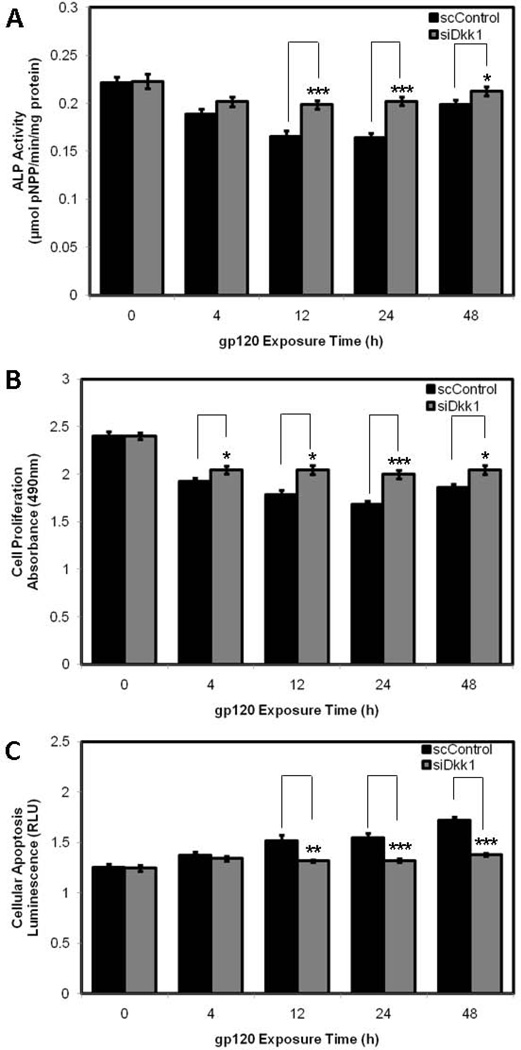
When HOBs were transfected with siRNA attenuating Dkk1 expression, there was an increase in ALP activity at each time point when compared to scrambled control, with significant increases identified at 12h (0.199 ± 0.004 V 0.166 ± 0.006 µmolpNPP/min/mg protein; p=0.0006), 24h (0.202 ± 0.004 V 0.164 ± 0.004 µmolpNPP/min/mg protein; p=0.001) and 48h (0.213 ± 0.004 V 0.199 ± 0.005 µmolpNPP/min/mg protein; p=0.0448) of gp120 exposure (Fig. 5A). HOBs transfected with siRNA demonstrated increases in cell proliferation at each time point when compared to scrambled control, with significant increases identified at at 4h (2.045 ± 0.042 V 1.923 ± 0.038 OD; p=0.0474), 12h (2.043 ± 0.047 V 1.781 ± 0.051 OD; p=0.0017), 24h (1.998 ± 0.044 V 1.680 ± 0.035 OD; p<0.0001) and 48h (2.043 ± 0.047 V 1.858 ± 0.037 OD; p=0.0068) of gp120 exposure (Fig. 5B). HOBs transfected with siRNA demonstrated a reduction in cellular apoptosis at each time point when compared to scrambled control, with significant reductions in caspase-3 and caspase-7 activity identified at 12h (1.317 ± 0.012 V 1.514 ± 0.058 RLU; p=0.0045), 24h (1.321 ± 0.016 V1.546 ± 0.045 RLU; p=0.0002) and 48h (1.377 ± 0.016 V 1.719 ± 0.037 RLU; p<0.0001) of gp120 exposure (Fig. 5C).
Figure 5.
pNPP assay (A), MTS assay (B) and Caspase 3/7 assay (C) in HOBs transfected with siRNA or scrambled control RNA and exposed to 100ng/mL gp120 over 48h.
Effect of Overexpressing Dickkopf-1 on Primary Human Osteoblasts exposed to gp120
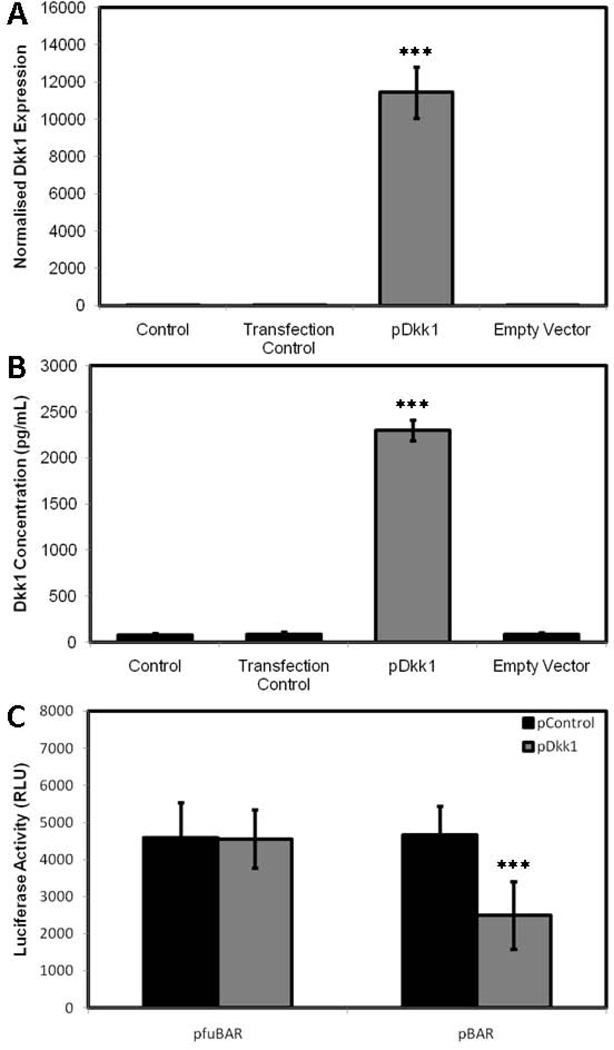
To further validate these phenotypic effects of silencing Dkk1 expression, we transciently overexpressed Dkk1, using a Dkk1 cDNA plasmid. HOBs were transfected with Dkk1 cDNA (pDkk1) and ‘empty vector’ DNA (pControl) and Dkk1 expression was assessed using both qRT-PCR and ELISA to confirm overexpression. Quantitative RT-PCR confirmed a significant increase in Dkk1 gene expression in the pDkk1 transfected cells relative to untransfected control cells (Fig. 6A). This represented a significant increase when compared to the empty vector control and transfection control cells (ie. cells treated with GeneJuice alone) (p<0.0001), which demonstrated no change in Dkk1 gene expression. This was further confirmed by ELISA, demonstrating a 29.21% increase in Dkk1 protein expression in the pDkk1 transfected cells relative to untransfected control cells (Fig. 6B), representing a significant increase when compared to both the empty vector control and transfection control cells (p=0.00052), which demonstrated no change in Dkk1 protein expression. To confirm that overexpression of Dkk1 inhibited Wnt/β-catenin signaling HOBs were transfected with pBAR and pfuBAR, and subsequently treated with pDkk1 and pControl. A significant decrease in luciferase activity was observed in the pDkk1 transfected cells when compared to empty vector control cells (p<0.0001) in the pBAR reporter cells, whilst the luciferase activity in the pfuBAR reporter cells remained unchanged during treatment (Fig. 6C).
Figure 6.
HOBs transfected with pDkk1 or empty vector exposed to 100ng/mL gp120 over 48h, assessing Dkk1 gene expression (A), Dkk1 protein expression (B) and Luciferase activity (C).
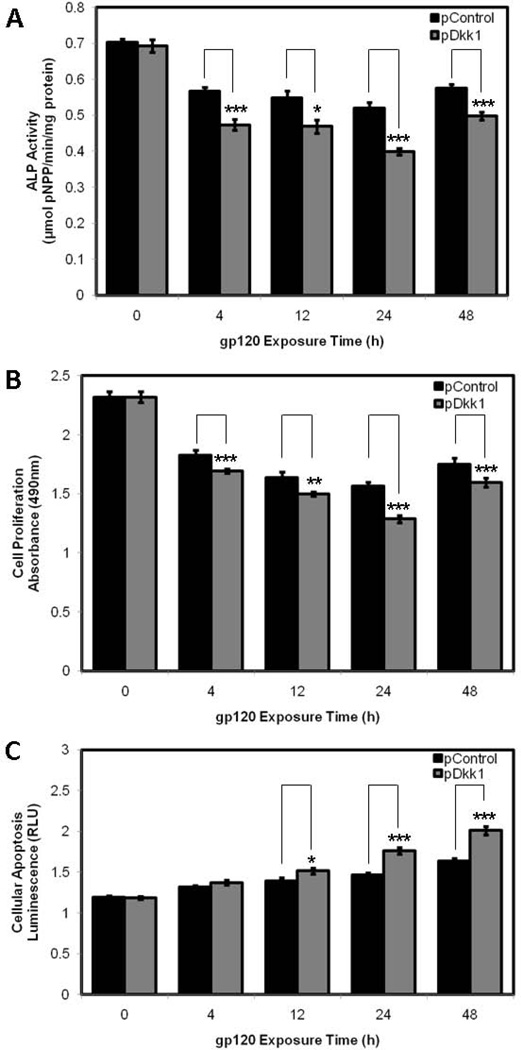
HOBs transfected with plasmid DNA overexpressing Dkk1 demonstrated a reduction in ALP activity at each time point when compared to vector control, with significant reductions identified at 4h (0.473 ± 0.015 V 0.567 ± 0.011 µmolpNPP/min/mg protein; p=0.0005), 12h (0.469 ± 0.019 V 0.548 ± 0.020 µmolpNPP/min/mg protein; p=0.0160), 24h (0.399 ± 0.009 V 0.520 ± 0.015 µmolpNPP/min/mg protein; p < 0.0001) and 48h (0.498 ± 0.011 V 0.575 ± 0.010 µmolpNPP/min/mg protein; p=0.0004) of gp120 exposure (Fig. 7A). HOBs transfected with plasmid DNA overexpressing Dkk1 displayed a reduction in cell proliferation at each time point when compared to vector control, with significant reductions identified at 4h (1.693 ± 0.018 V 1.827 ± 0.042 OD; p=0.0101), 12h (1.498 ± 0.016 V 1.637 ± 0.047 OD; p=0.0133), 24h (1.285 ± 0.032 V 1.563 ± 0.036 OD; p<0.0001) and 48h (1.595 ± 0.039 V 1.751 ± 0.051 OD; p=0.0272) of gp120 exposure (Fig. 7B). HOBs transfected with plasmid DNA overexpressing Dkk1 demonstrated an increase in cellular apoptosis at each individual time point when compared to vector control, with significant increases in caspase-3 and caspase-7 activity identified at 12h (1.515 ± 0.035 V 1.396 ± 0.037 RLU; p=0.0325), 24h (1.762 ± 0.043 V 1.468 ± 0.024 RLU; p<0.0001) and 48h (2.012 ± 0.052 V 1.639 ± 0.028 RLU; p<0.0001) of gp120 exposure (Fig. 7C).
Figure 7.
pNPP assay (A), MTS assay (B) and Caspase 3/7 assay (C) in HOBs transfected with pDkk1 and empty vector and exposed to 100ng/mL gp120 over 48h.
Discussion
The role of HIV infection on bone metabolism first came to light before the widespread use of HAART. Prior histomorphometry studies21, followed by more recent cross-sectional studies7 and randomized control trials9 have demonstrated a higher prevalence of osteopenia and osteoporosis in treatment-naïve HIV infected patients, emphasizing the deleterious effect of HIV infection on bone mass regulation. As normal bone remodeling requires the balanced activity of osteoblastic bone formation and osteoclastic bone resorption, HIV infection putatively disturbs the distinct molecular mechanisms and signal transduction pathways driving bone homeostasis.
The RANK/RANKL/OPG pathway, made up of three members of the TNF and TNF receptor super family, is the principal regulator of osteoclastic bone resorption22. In HIV infected patients, an inverse relationship exists between serum RANKL levels and BMD in HIV infected patients23, with treatment-naïve HIV infected patients with reduced BMD demonstrating a decreased plasma OPG/RANKL ratio24. Previous in vitro studies by our group demonstrated that HOBs treated with the HIV-1 protein gp120, display impaired osteoblastic activity in conjunction with reduced RANKL expression25. Data demonstrating that ritonovir and saquinavir, abrogate the primary physiological block to RANKL activity in primary human osteoclast precursor cells, may explain the apparent acceleration of BMD loss in patients on HAART11. Furthermore, HIV infection and antiretroviral therapy has been demonstrated to be associated with higher bone turnover markers and increased differentiation of osteoclast-like cells, especially in women on regimens containing ritonavir, placing HIV positive postmenopausal women receiving ritonavir at greater risk for bone loss26.
Wnt/β-catenin signaling plays an important role in the development and maintenance of many tissues and organs, with disturbances implicated in multiple disease processes27–29. The Wnt/β-catenin pathway plays a critical role in bone mass acquisition and maintenance with aberrations in canonical Wnt signaling resulting in disorders of bone mass30. Wnt signaling has now been implicated in HAART-induced bone loss in vitro, with ritonavir suppressing genes involved in Wnt/β-catenin signalling in osteoclast precursor cells, in addition to blocking the cytoplasmic to nuclear translocation of β-catenin, the key regulator of the Wnt signalling pathway, in association with enhanced β-cateninubiquitination31. Our data clearly delineates the role played by Wnt/β-catenin signaling in HIV associated bone loss in vitro. The HIV-1 proteingp120 has been demonstrated to block the cytoplasmic to nuclear translocation of β-catenin, in addition to inhibiting of TCF/LEF mediated transcription in HOBs.
Dickkopf proteins are a four-member family of extracellular proteins shown to dysregulate Wnt/β-catenin signaling in multiple different organisms and cell types. Both in vitro and in vivo data has demonstrated Dkk1 and Dkk2 to be associated with the osteopenic phenotype, with considerable evidence supporting Dkk1 as a powerful negative regulator of osteoblast function31–38. Our data implicates Dkk1 as a key mediator in HIV associated bone loss, with HOBs transfected with siRNA targeting Dkk1 expression exhibiting improved phenotypic markers of HOB differentiation and function when compared to scrambled control, in the setting of gp120 exposure. Furthermore, overexpression of Dkk1 has been shown to further exacerbate the inhibitory effects of gp120 on HOB differentiation and function.
The exact contribution of the virus and treatment in the pathogenesis of HIV associated bone loss remains unclear. Despite successful HAART making serum levels of virus almost undetectable, residual latent infection in various tissue types can still remain. Moreover, it is plausible that exposure to HIV-1 proteins early in infection or infection of osteoblasts may 'prime' these cells to the inhibitory effects of HAART. Furthermore, osteoblasts interact with numerous other cell types throughout their development, and the disruption of these interactions, rather than disruption of osteoblasts themselves, may be a contributory factor in the clinically observed losses in bone mass.
The investigations performed in this study and prior work from our group have assessed the effects of a variety of different agents (including dexamethasone) on Wnt/β-catenin signaling in primary human osteoblasts, providing significant evidence for a central role of Wnt/β signaling and Dkk1 expression in the maintenance of normal bone homeostasis and its dysregulation19,20. The use of such distinct in vitro models of reduced bone mass confirm that Dkk1 bioactivity in this setting may represent a final common pathway of effect on the primary human osteoblast. Current and prior data support the hypothesis that modulation of Dkk1 by osteoporotic stimuli initiates a series of signaling events, mediated, at least in part, by the Wnt/β-catenin pathway, which results in significant changes in osteoblast function, which may contribute to decreased BMD seen in patients.
In conclusion, we suggest that the Wnt/β-catenin pathway is a major signaling cascade in bone biology, playing a key role in bone development and remodeling. Canonical Wnt signaling, we suggest, plays a critical role in the bone loss exhibited in the HIV infected population. Our data demonstrates impaired β-catenin trafficking and inhibited TCF/LEF-mediated transcription in HOBs exposed to gp120, highlighting the key regulatory role played by Wnt/β-catenin signaling in HIV associated bone loss. Dkk1, a powerful antagonist of canonical Wnt/β-catenin signaling, is likely to be a central mediator is this degenerative process.
Acknowledgements
We wish to acknowledge the NIH AIDS Research and Reference Reagent Program for providing the HIV-1 proteins; and the European Union, Cappagh Hospital Trust, Mater College and the Irish Programme for Research in Third Level Institutions for funding support.
References
- 1.Borderi M, Gibellini D, Vescini F, et al. Metabolic bone disease in HIV infection. AIDS. 2009 Jul 17;23(11):1297–1310. doi: 10.1097/QAD.0b013e32832ce85a. [DOI] [PubMed] [Google Scholar]
- 2.Lin D, Rieder MJ. Interventions for the treatment of decreased bone mineral density associated with HIV infection. Cochrane Database Syst Rev. 2007 Apr 18;(2):CD005645. doi: 10.1002/14651858.CD005645.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Chew NS, Doran PP, Powderly WG. Osteopenia and osteoporosis in HIV: pathogenesis and treatment. Curr Opin HIV AIDS. 2007 Jul;2(4):318–323. doi: 10.1097/COH.0b013e3281a3c092. [DOI] [PubMed] [Google Scholar]
- 4.Mallon PW. HIV and bone mineral density. Curr Opin Infect Dis. 2010 Feb;23(1):1–8. doi: 10.1097/QCO.0b013e328334fe9a. [DOI] [PubMed] [Google Scholar]
- 5.Fauci AS. HIV and AIDS: 20 years of science. Nat Med. 2003 Jul;9(7):839–843. doi: 10.1038/nm0703-839. [DOI] [PubMed] [Google Scholar]
- 6.Montagnier L. 25 years after HIV discovery: prospects for cure and vaccine. Virology. 2010 Feb 20;397(2):248–254. doi: 10.1016/j.virol.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 7.Amiel C, Ostertag A, Slama L, et al. BMD is reduced in HIV-infected men irrespective of treatment. J Bone Miner Res. 2004;19:402–409. doi: 10.1359/JBMR.0301246. [DOI] [PubMed] [Google Scholar]
- 8.Bruera D, Luna N, David DO, et al. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS. 2003;17:1917–1923. doi: 10.1097/00002030-200309050-00010. [DOI] [PubMed] [Google Scholar]
- 9.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vsstavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004 Jul 14;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 10.Tebas P, Powderly WG, Claxton S, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000;14:F63–F67. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibellini D, Borderi M, De Crignis E, et al. RANKL/OPG/TRAIL plasma levels and bone mass loss evaluation in antiretroviral naive HIV-1-positive men. J Med Virol. 2007 Oct;79(10):1446–1454. doi: 10.1002/jmv.20938. [DOI] [PubMed] [Google Scholar]
- 12.Yin MT, McMahon DJ, Ferris DC, et al. Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2010 Feb;95(2):620–629. doi: 10.1210/jc.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009 Feb;24(2):171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong YQ, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 15.Gong YQ, Vikkula M, Boon L, et al. Osteoporosis-pseudoglioma syndrome, a disorder affecting skeletal strength and vision, is assigned to chromosome region 11q12-13. Am J Hum Genet. 1996;59:146–151. [PMC free article] [PubMed] [Google Scholar]
- 16.Boyden LM, Mao JH, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 17.Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Wesenbeeck L, Cleiren E, Gram J, et al. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet. 2003;72:763–771. doi: 10.1086/368277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurson CJ, Butler JS, Keating DT, et al. Gene expression analysis in human osteoblasts exposed to dexamethasone identifies altered developmental pathways as putative drivers of osteoporosis. BMC Musculoskelet Disord. 2007 Feb 12;8:12. doi: 10.1186/1471-2474-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler JS, Queally JM, Devitt BM, et al. Silencing Dkk1 expression rescues dexamethasone-induced suppression of primary human osteoblast differentiation. BMC Musculoskelet Disord. 2010 Sep 15;11:210. doi: 10.1186/1471-2474-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serrano S, Mariñoso ML,Soriano JC, et al. Bone remodelling in human immunodeficiency virus-1-infected patients. A histomorphometric study. Bone. 1995;16:185–191. doi: 10.1016/8756-3282(94)00028-x. [DOI] [PubMed] [Google Scholar]
- 22.Vega D, Maalouf NM, Sakhaee K. CLINICAL Review #: the role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: clinical implications. J Clin Endocrinol Metab. 2007 Dec;92(12):4514–4521. doi: 10.1210/jc.2007-0646. [DOI] [PubMed] [Google Scholar]
- 23.Konishi M, Takahashi K, Yoshimoto E, et al. Association between osteopenia/osteoporosis and the serum RANKLin HIV-infected patients. AIDS. 2005;19:1240–1241. doi: 10.1097/01.aids.0000176231.24652.02. [DOI] [PubMed] [Google Scholar]
- 24.Cotter EJ, Malizia AP, Chew N, et al. HIV proteins regulate bone marker secretion and transcription factor activity in cultured human osteoblasts with consequent potential implications for osteoblast function and development. AIDS Res Hum Retroviruses. 2007 Dec;23(12):1521–1530. doi: 10.1089/aid.2007.0112. [DOI] [PubMed] [Google Scholar]
- 25.Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem. 2003;278:48251–48258. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 26.Modarresi R, Xiang Z, Yin M, et al. WNT/beta-catenin signaling is involved in regulation of osteoclast differentiation by human immunodeficiency virus protease inhibitor ritonavir: relationship to human immunodeficiency virus-linked bone mineral loss. Am J Pathol. 2009 Jan;174(1):123–135. doi: 10.2353/ajpath.2009.080484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 28.Moon RT, Kohn AD, De Ferrari GV, et al. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004 Sep;5(9):691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 29.Johnson ML, Harnish K, Nusse R, et al. LRP5 and Wnt signaling: a union made for bone. J Bone Miner Res. 2004 Nov;19(11):1749–1757. doi: 10.1359/JBMR.040816. [DOI] [PubMed] [Google Scholar]
- 30.Bafico A, Liu GZ, Yaniv A, et al. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 31.Yin MT, Modarresi R, Shane E, et al. Effects of HIV infection and antiretroviral therapy with ritonavir on induction of osteoclast-like cells in postmenopausal women. Osteoporos Int. 2011 May;22(5):1459–1468. doi: 10.1007/s00198-010-1363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson G, Mao BY, Barrantes ID, et al. Kremen proteins interact with Dickkopf1 to regulate anteroposterior CNS patterning. Development. 2002;129:5587–5596. doi: 10.1242/dev.00154. [DOI] [PubMed] [Google Scholar]
- 33.Kuhnert F, Davis CR, Wang HT, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XF, Liu P, Liu WZ, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 35.Tian E, Zhan FH, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Sarosi I, Cattley RC, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006 Oct;39(4):754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald BT, Joiner DM, Oyserman SM, et al. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007 Sep;41(3):331–339. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morvan F, Boulukos K, Clément-Lacroix P, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006 Jun;21(6):934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]