Abstract
Objective
Developing central white matter is subject to ischemic-type injury during the period that precedes myelination. At this stage in maturation, central axons initiate a programme of radial expansion and ion channel re-distribution. Here we test the hypothesis that during radial expansion axons display heightened ischemic sensitivity, when clusters of Ca2+ channels decorate future node of Ranvier sites.
Methods
Functionality and morphology of central axons and glia were examined during and after a period of modeled ischemia. Pathological changes in axons undergoing radial expansion were probed using electrophysiological, quantitative ultrastructural and morphometric analysis in neonatal rodent optic nerve and peri-ventricular white matter axons studied under modeled ischemia in vitro or after hypoxia-ischemia in vivo.
Results
Acute ischemic injury of central axons undergoing initial radial expansion was mediated by Ca2+ influx through Ca2+ channels expressed in axolemma clusters. This form of injury operated only in this axon population, which was more sensitive to injury than neighboring myelinated axons, smaller axons yet to initiate radial expansion, astrocytes or oligodendroglia. A pharmacological strategy designed to protect both small and large diameter pre-myelinated axons proved 100% protective against acute ischemia studied under modeled ischemia in vitro or after hypoxia-ischemia in vivo.
Interpretation
Recent clinical data highlight the importance of axon pathology in developing white matter injury. The elevated susceptibility of early maturing axons to ischemic injury described here may significantly contribute to selective white matter pathology and places these axons alongside pre-oligodendrocytes as a potential primary target of both injury and therapeutics.
Keywords: Axon, Ca2+ channel, Cerebral Palsy, Hypoxia-ischemia, Ischemia, Myelination, Periventricular Leukomalacia, White Matter Injury
Cerebral white matter injury (WMI) is increasingly recognized as a common form of perinatal brain injury that predisposes to cerebral palsy as well as cognitive and learning disabilities. WMI is now the leading cause of cerebral palsy (CP) in survivors of premature birth and peaks in incidence between ~23–32 post-conceptional weeks.1–4 In a large population-based study of children with CP, WMI was the most common finding.5 In a separate more mature cohort of term babies who developed CP, the most common antecedents were intra-cerebral hemorrhage and/or hypoxia-ischemia.6 Emerging neuroimaging data supports that WMI has been under-recognized in these infants.7 WMI is also the major form of cerebral injury in infants with congenital heart disease and is frequently observed prior to heart lesion repair.8, 9,10
Despite the growing impact of WMI, it is unclear whether common cellular and molecular mechanisms define WMI pathogenesis in these distinct cohorts of preterm and term neonates. WMI in preterm neonates coincides with early degeneration of pre-oligodendrocytes (pre-OLs) that are selectively vulnerable to glutamate receptor (GluR) activation and oxidative damage related to hypoxia-ischemia.11, 12 PreOLs display arrested maturation in chronic diffuse WMI,13,14 which may extend the window of white matter vulnerability to recurrent hypoxia-ischemia.15 A declining but substantial pool of preOLs persists in some regions of cerebral white matter at least until term16 and preOLs appear to populate the human cerebral cortex for months after birth.17 Hence, preOLs may contribute to WMI well beyond the period of peak vulnerability in preterm infants.
Although numerous studies have defined a role for glial vulnerability in WMI, there has been limited study of the susceptibility of immature axons. In a preterm cohort with WMI, axons degenerated in focal or diffuse lesions associated with pan-cellular necrosis,18 but axonal degeneration was not detected in non-necrotic diffuse WMI in pre-term sheep.19 However, it is currently unclear if axonal vulnerability increases during the rapid maturation that precedes the onset of myelination. As white matter maturation progresses, axons undergo profound structural and molecular changes that include a rapid increase in size and a redistribution of membrane ion channels. We have recently shown that central white matter axons express clusters of functional voltage-gated Ca2+ channels (VGCCs) as they prepare for myelination,20 and suffer ischemic damage via mechanisms distinct from those operating in small pre-myelinated and in early myelinated axons.21 In this study, we tested the hypothesis that early diameter expansion in central axons is accompanied by a period of heightened sensitivity to ischemic injury due to the unique ion channel expression patterns present at this stage.
Methods
All animal procedures conformed to local ethical guidelines. UK home office regulations were followed for all work performed in the UK. For rat optic nerve experiment, nerves were dissected from Lister-hooded rats between P8–12 (referred to as “P10”) or from adult (P80–100); and for mouse optic nerve, from P10, P18–22 (“P20”), or from adult (P100–110) THY-1/GFP-M (Line GFP-M) mice.22,23 In keeping with prior studies24, the effect of ischemic conditions upon optic nerve axons was uniform between P8-P12 (Supplementary Fig 1) and these data were therefore collected into a single group to allow meaninful statistical comparison with other age points. Nerves were perfused with artificial cerebrospinal fluid (aCSF), composition (in mM): NaCl, 126; KCl, 3; NaH2PO4, 2; MgSO4, 2; CaCl2, 2; NaHCO3, 26; glucose, 10; pH, 7.45, bubbled with 5% CO2 / 95% O2 and maintained at 37°C. Zero-Ca2+ aCSF: CaCl2 was omitted and 50 μM EGTA was added. For oxygen – glucose deprivation (OGD), aCSF was replaced by glucose-free aCSF (containing 10 mM sucrose to keep the osmolarity constant) saturated with a 95% N2/5% CO2 mixture. The chamber atmosphere was switched to 5% CO2 / 95% N2 during OGD perfusion. Osmolarity of solutions was measured and adjusted as required. Data are mean ± SEM, significance determined by t-test or ANOVA as appropriate. Glutamate receptor antagonists were from Tocris (UK), all other reagents were from Sigma (UK) unless otherwise stated. Drug treatments were initiated 10 min prior to the onset of OGD, throughout the OGD period and the 60 min recovery period.
Rat optic nerve (RON)
The electrophysiological recording techniques have been described recently.20 In brief, compound action potentials (CAPs) were evoked and recorded with glass electrodes and peak-to-peak amplitude was used to assess changes in excitability (see24). CAPs were evoked via square-wave constant current pulses (Iso stim A320, WPI), amplified (Cyber Amp 320, Axon Instruments), subtracted from a parallel differential electrode, filtered (low pass: 800–10000 Hz), digitized (1401 mini, Cambridge Electronic Design) and displayed on a PC running Signal software (Cambridge Electronic Design). Non-recoverable CAP loss from the optic nerve indicates irreversible failure of axon function.21 For immunohistochemistry, P10 RONs were fixed in 4% paraformaldehyde/ 0.1M PBS for 30 min prior to incubation in 0.1M PBS plus 20% sucrose w/v for 5 min and freeze-sectioning. 20 μm sections were subsequently blocked for 60 min in 0.1M PBS, 10% normal goat serum plus 0.5% Triton-X 100. Sections were then incubated in this solution plus primary antibody overnight at 4° C. Antibodies raised against neurofilament-200 (NF-H) and neurofilament-70 (NF-L) (Chemicon; 1:200 and 1:100 respectively) were detected using appropriate Alexa-conjugated secondary antibodies (1:1000, Cambridge Bioscience, see25 for further details). Primary antibody omission controls were blank. Images were collected using an Olympus confocal microscope. Intensity levels of neurofilament staining were assessed by mean pixel intensity levels within standard regions of interest, performed using Metamorph software (Universal Imaging). Three regions of interest from three nerves sections in at least four different optic nerves were averaged using identical image acquisition setting.
For electron microscopy, P10 RONs were washed in Sorenson's buffer and post-fixed in 3% gluteraldehyde/Sorenson's. Nerves were fixed (2% osmium tetroxide) and dehydrated prior to epoxy infiltration. Ultrathin sections were counterstained with uranyl acetate and lead citrate and examined with a Jeol 100CX electron microscope. Since effectively all axons in the adult RON are myelinated, non-myelinated axons of the P10 nerve can be considered to be either in an early or late stage of preparation for myelination. For morphometric analysis and viability scoring, axons within a minimum of three grid sections were outlined by hand (Image-J software, NIH) and axon area and perimeter measured. Axon diameter was taken as the mean of the longest and shortest widths. Grids were randomly selected and all identifiable axons within the area were included. Axon viability scores were assigned blind using the following scoring system. Axons were given one point for each of three well-established indicators of viability (see20); i) the presence of an intact axolemma, ii) the presence of microtubules, iii) the presence of a debris-free axoplasm. Axons that showed all three attributes were therefore given a viability score of "3", and axons with none were given a score of "0".
Mouse optic nerve (MON)
Nerves were dissected as for rat and the protocol for perfusion and OGD were the same except a range of OGD periods (30, 60, 90, 120 min) and recovery times (60, 120, 180 min) were tested. MONs were subsequently fixed in 4% formaldehyde/PBS for 60 min and cryopreserved overnight in 30% sucrose/PBS pH 7.4. For immuno-staining, nerves were embedded in 1.5% low-melting point agar and sectioned (20μm) prior to blocking in 4% goat serum/0.5% Triton-X 100 and addition of primary antibody overnight at 4° C. Antibodies raised against GFAP (1:100, Immunostar, Inc), and APC (1:100, Sigma-Aldrich) were used and detected via Cy-3 conjugated secondary antibodies (1:200, Jackson Immunoresearch). Nuclei were stained with 10 μg/ml of Hoechst for 3 min and sections examined via an Nikon Eclipse TE300 epiflourescent microscope (see Supplementary Fig 2). For axon imaging, GFP-M expression in fixed nerve sections was examined in image stacks (0.25 μm thick) collected via a Biorad MRC-1024 confocal microscope. To obtain a representative picture of whole nerve, image stacks from three adjacent sections were collected and divided into 5x5 grids. Axons in each grid were given a score from 0 (no injury) to 3 (axon fragmentation) and each experiment was repeated 3 times (see Supplementary Fig 3). Morphometric electron microscopy was performed as for the RON.
In vivo hypoxia-ischemia
The model has been described in detail previously26 and is designed to generate a moderate form of injury while allowing good fixation for ultrastructural analysis. In brief, for the examination of early hypoxic-ischemic changes to axons the left common carotid artery was reversibly ligated in Sprague-Dawley rat pups at P7 under isoflurane anaesthesia. At this stage, following 2 hour post-operative recovery, pups were placed in a humidified 8% O2/ 92% N2 atmosphere and maintained at 37°C. Reversible ligation was achieved by arterial occlusion via a silastic ligature subsequently released under deep anesthesia immediately prior to fixation. Restoration of flow was verified by visual inspection and was consistently achieved up to 6 hours post-ligation. At the conclusion of a 1.5 hour hypoxic period, animals were deeply anesthetized for transcardiac perfusion, 0.05 cc of heparin (10,000 units/ml; Elkin-Sinn, Inc., NDC 0641-2470-41) was injected into the left ventricle and was followed by 50 ml of freshly made 3% glutaraldehyde (Electron Microscopy Sciences, #16320, Glutaraldehyde 50% Solution, EM Grade) in 0.01 M phosphate buffer, pH 7.4. Brains were cut into blocks running from the cortical surface to the ventricular surface, ~2–3 mm lateral from the mid-line at the level of the infundibulum. Blocks were post-fixed (2% osmium tetroxide), dehydrated and infiltrated in epoxy resin. Ultra-thin sections running from the cortical surface to the ventricular surface were cut, counterstained with uranyl acetate and lead citrate. Sections were examined with a JEOL 100CX electron microscope.
Immuno-Electron Microcopy for O4 Antibody Localization to Degenerating Cells
A detailed protocol was previously described for tissue preservation for ultrastructural analysis of O4 antibody labeling.26 Briefly, at the conclusion of a more severe period of hypoxia-ischemia than used for examination of early axonal changes above (2.5 h exposure to hypoxia), animals survived for 5 h, after which the brain was perfused with 50 ml of freshly made 4% paraformaldehyde in 0.01 mol/L PB, pH 7.4, containing 1% glutaraldehyde, 3% sucrose and 0.02% calcium chloride. To optimize penetration of the O4 antibody, tissue sections were permeabilized by the freeze-thaw method as previously described.27 Tissue was processed for O4 immunohistochemistry,28 and the primary antibody visualized by immunoperoxidase staining with diaminobenzidine. The brain tissue was post-fixed with 2% osmium tetroxide and tissue blocks from the cerebral cortex were processed for EM as described above.
Results
Maturation-dependent axonal vulnerability in the optic nerve
To define the developmental window of peak vulnerability to axon injury in white matter, we compared the magnitude of axonal damage at P10, P20 and adult in the rodent optic nerve. The ultrastructural examination of longitudinal sections of damaged axons in the optic nerve is complicated by the sigmoid path axons take in this structure. Occasional long sections of myelinated axons in P10 rat optic nerve (RON) were visualized and revealed generalized areas of axonal swelling with dissolution of axoplasmic inclusions (Fig 1A). Less damaged axons showed localized regions of sub-myelin swelling where the sheath occasionally buckled from the axolemma and formed vesicular debris (Fig 1A, boxed areas shown at higher gain below). A cross section of this phenomenon is shown in Fig 1B. Smaller pre-myelinated axons running parallel to myelinated axons cannot be readily distinguished in post-OGD ultra-micrographs.
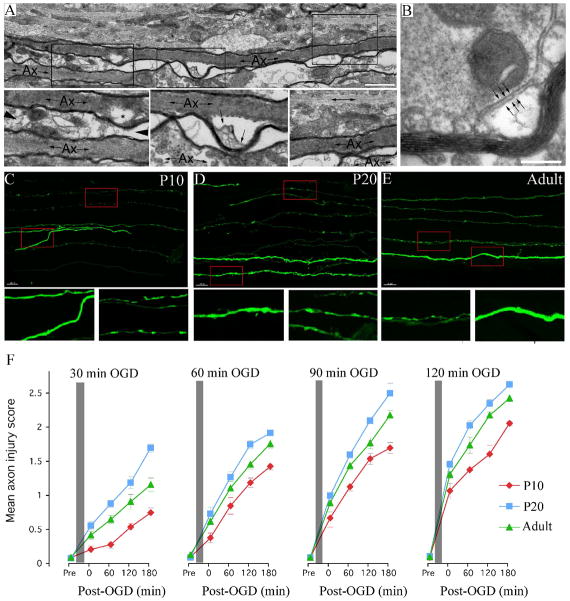
Figure 1.
OGD-induced injury in rodent axons. A: Long section ultra-micrograph from a post-OGD P10 RON, showing regional myelin detachment from the axoplasm, shown in cross section in “B”. “Ax” = myelinated axon. Note the bubbling of membrane within the expanded axo-glia space (arrows). Bar = 100 nm. C-E Pathological changes in GFP-M axons in THY-1/GFP-M mice at P10 (C), P20 (D) and adult (E) exposed to 60 min OGD + 60 min recovery. Boxed regions are expanded below. Note the differential injury in the smallest GFP-M(+) axons which show beading and localized swelling. Bar = 10 μm. F: The mean axon injury score of GFP-M(+) axons collected after 30, 60, 90 or 120 min of OGD (indicated by the grey bar). Data collected from P10 (red), P20 (blue) and adult (green) MONs is included. Note that the extent of injury increases in all age groups with the period of OGD and the length of recovery. Note also that axon injury in P20 MONs is higher under all conditions than at P10 or adult.
To examine injury in pre-myelinated axons, optic nerves of Thy-1/GFP-M mice were examined where a selective population of axons express fluorescent GFP, allowing the morphology of single axons to be mapped using confocal microscopy. Periods of OGD ranging between 30–120 min were tested with recovery periods of between 0–180 min. P10, P20 and adult MONs were included in the study to assess developmental changes in axonal injury. Examples of GFP-M expression in P10, P20 and adult mice 60 min after a 60 min period of OGD are shown in Fig 1C-E respectively, with the red boxed areas shown in greater detail below. Pathological changes included local swelling, axonal beading and fragmentation with smaller axons differentially effected (corresponding to the larger pre-myelinated axons and ensheathed axons; see Fig 2). Quantitative scoring of axon injury revealed a relationship between the period of OGD and the extent of axonal injury at all ages (Fig 1F), while statistically significant injury was found at all time points after all OGD periods. It is apparent that axons continue to deteriorate over the 180 min recovery period. Consistent with earlier studies28, there is heightened sensitivity to ischemic conditions in axons of the P20 optic nerve (P<0.001 vs., mean injury score in the other two age groups after all OGD durations).
Figure 2.
Central axons preparing for myelination have a heightened sensitivity to OGD. A: Electron micrograph of wild-type P20 MON showing pre-myelinated axons (arrows), ensheathed axons (arrow heads) and early myelinated axons (“Ax”) in cross section (bar = 1 μm). B–D: Diameter spectra for pre-myelinated axons (B), ensheathed axons (C) and myelinated axons (D). E: GFP-M fluorescent axons in transgenic P20 MON (bar = 5 μm). F: Diameter spectrum of fluorescent axons. G: Injury scores for fluorescent axons following 60 min OGD + 60 min recovery, plotted against axon diameter. Note that the smallest fluorescent axons, corresponding to large pre- myelinated and enstheathed axons, are the most damaged.
In keeping with a heightened sensitivity to energy deprivation for large pre-myelinated axons, quantitative immunofluorescence labeling of glia revealed comparable injury between this axon population and oligodendrocytes following 60 min of OGD followed by 60 min recovery (Supplementary Fig 4 C, arrow). Immuno-staining with APC and nuclear counter-staining was converted to a % injury score and plotted alongside axon injury to allow comparison. As expected, the degree of oligodendrocyte death at all periods of OGD and recovery times was lower in older mice (e.g., 83.1 ±1.3% of oligodendrocytes were dead after 60 min of OGD and 60 min recovery at P10 and 74.8 ±2.2% of oligodendrocytes were dead after the same protocol in the adult, P<0.05; Supplementary Fig 4 D-F). Axon (grouping all diameters together) and astrocyte injury (as assessed by GFAP immunofluorescence and analyzed as for APC) following 60 min OGD progressed more slowly with little difference between the two (Supplementary Fig 3 A, C). Despite this, even a short period of OGD (30 min) significantly reduced GFAP staining (Supplementary Fig 4 D-F: by 12.7 ±2,1% of control at P10, P<0.05; 26.4 ±3.5% at P20, P<0;05; and 37.7 ± 2.5% in adult, P<0.01). The loss of GFAP staining tended to progress during 120 min of recovery and was significantly greater in older animals, a pattern that held true for all periods of OGD tested.
Central axons preparing for myelination have a heightened sensitivity to OGD
Ultra-micrograph morphometric analysis of wild-type P20 MON revealed populations of premyelinated (0.10–0.56 μm), ensheathed (0.35–0.73 μm) and myelinated (0.54–2.20 μm) axons (Fig 2A–D). A range of GFP-M(+) axon diameters were observed in Thy-1/GFP-M mice of the same age over the range 0.43–1.90 μm (Fig 2E, F), and this model therefore allows imaging of large pre-myelinating, ensheathed and myelinated axons but not small pre-myelinating axons. This absence of fluorescence in small axons allows high-resolution imaging of the larger axon populations (Fig 2E). Following a standard 60 min OGD + 60 min recovery protocol applied to the transgenic P20 MON, injury scoring revealed an inverse relationship between axon pathology and diameter, with the large pre-myelinated axons damaged most and injury tailing off as axons progressed through ensheathment and myelination (Fig 2G). For example, 63% of axons in the 0.45–0.60 μm bin range had an injury score of 3 (fragmentation), 22% had a score of 2 (beading), 12% had a score of 1 (local swelling), and only 2% had a score of 1 (no pathology) (n=41 axons).
Hypoxia-ischemia in near term-equivalent cerebral white matter triggers selective degeneration of larger caliber pre-myelinated axons
We examined axon injury in cerebral white matter after a relatively short period of reversible hypoxia-ischemia (1.5 hr) using a P7 in vivo rat model.26 The white matter affected contains no compact myelin or ensheathed axons but a proportion of axons have increased in diameter from ~0.2 μm to 0.5–1.0 μm. The proportion of O4(+) preOLs in cerebral white matter is low at this age (~90% of O4(+) cells are immature oligodendrocytes).29 In terms of human development, this model most closely resembles term, although the absence of ensheathed axons is more typical of late mid-gestation (exact correlations between white matter development in man and rodents are not feasible).29 Signs of injury in smaller axons in this relatively mild model of injury were rare but most of the larger axons exhibited signs of pathology (Fig 3A-F). Injury ranged from local axon swelling where axoplasm contained numerous large convoluted profiles (Fig 3A, two axons indicated by large arrows). More severe regions of local axonal swelling were also seen, which often contained remnants of convoluted profiles and areas of flocculent debris (Fig 3B, one such axon is indicated by the series of small arrows). The diameter of the axon often narrowed adjacent to the swollen area forming a “neck” region. In these apparently graded forms of damage, axolemma integrity was maintained and the injury was often boarded on either side by a largely normal axon structure. Microtubules extended from a non-swollen region of axon into the swollen region in Fig 3B, where they appeared to contribute to the convoluted profiles (arrow heads). Areas of severe axon swelling displaced neighboring small diameter axons and on occasion were associated with transection of the axon (Fig 3C, large arrows).
Figure 3.
Large pre-myelinated axon damage in a model of limited hypoxic-ischemic periventricular white matter injury. A: Two large diameter pre-myelinated axons show focal pathology (large arrows) including axolemma swelling and numerous convoluted profiles within the axoplasm. Note neighboring smaller diameter axons appear normal. B: A large diameter pre-myelinated axon (arrows) exhibits severe local swelling (boxed area shown below). Note the disorganization of microtubules as they enter the swollen region (arrow heads) and the debris within the swollen axoplasm. C: A localized region of swelling (large arrows) appears to be associated with axon transection. Note the displacement of neighboring smaller diameter axons which otherwise have retained a normal appearance. D: Cross-section micrographs showing selective damage of large pre-myelinated axons (arrows), which can often be positively identified by the presence of remaining microtubule profiles (“*”). Neighboring small diameter axons retain a typical structure and include mitochondria with a normal appearance (arrow heads, top left example shown at higher gain in the insert). E: An oligodendrocyte (“Oli”) retains almost normal appearance with an intact cell membrane (arrow heads) and cellular inclusions such as mitochondria and Golgi apparatus. Near-by small diameter axon profiles are normal but several large diameter pre-myelinated axons exhibit pathology (arrows). F: Blind injury scoring of pre-myelinated axons that are either <0.4 μm (black bars) or >0.4 μm (open bars) in diameter. Note that after H-I there is a significant injury of the larger diameter axons (***= P<0.001).
Where axons were visualized in cross-section, the selective nature of axonal injury was again apparent. At higher magnification, damaged axons identifiable in many cases by the presence of some remaining microtubules, were surrounded by large fields of small diameter axons of normal appearance (Fig 3D, E). Flocculent debris and convoluted profiles were apparent in almost all large diameter axon cross sections while small axons retained membrane integrity, microtubules and mitochondria of normal appearance. Blinded injury scoring confirmed the selective nature of the axon damage, with significant injury found only in axons >0.4 μm in diameter (Fig 3F, P<0.001). Damaged large axon profiles were found neighboring oligodendroglial cells that showed only limited signs of pathology (Fig 3E). Consistent with prior studies,11 degenerating oligodendroglial cells in the white matter, identified on the basis of loss of membrane integrity, were rare, although sign of stress were apparent, which included cytoplasmic vacuoles and occasional swollen mitochondria.
In response to hypoxia-ischemia in term-equivalent rat cerebral cortex, preOLs degenerate with a spectrum of necrotic and apoptotic changes
To further define the mechanisms of death of oligodendrocyte lineage cells triggered by hypoxia-ischemia in the term-equivalent rat, we performed the first analysis by immuno-electron microscopy of the ultrastructural features of degenerating O4-labeled preOLs. Degenerating pre-Ols were not widely apparent following 1.5 hr of hypoxia-ischemia but were frequently detected throughout the overlying cerebral cortex following a 2.5 hour exposure (the minimum period required for reliable injury in cerebral cortex).26 Figure 4 demonstrates that the degenerating preOLs displayed a spectrum of ultrastructural changes consistent with features of both apoptotic (Fig 4A, C) and necrotic (Fig 4B) death. Apoptotic-like preOLs displayed features such as swollen mitochondria, clumping of nuclear chromatin and cytoplasmic membranous vesicles, all features of CNS apoptosis (e.g., 30). Vesicles were associated with apparent fragmentation of O4-labeled plasma membrane, which is more typical of necrotic cell death. By contrast, apparent apoptotic neurons (Fig 4C) contained plasma membrane that did not display fragmentation and had less disrupted intracellular organelles, features more typical of true apoptosis (Fig 4C). Necrotic cells had lost cell membrane integrity and had dispersed or fragmented nuclear chromatin. To estimate the percentage of cells with each type of cell death features, we analyzed a survey of 40 O4(+) cells, which revealed equal percentages of cells that displayed ultrastructural changes consistent with apoptotic-like or necrotic-like degeneration.
Figure 4.
Hypoxia-ischemia at P7 triggers preOL degeneration in rat cerebral cortex via a spectrum of apoptotic-necrotic injury. PreOLs were visualized by immuno-EM localization of the O4 antibody (see 13.29). In the intact cell, O4-labeling normally localizes to the plasma membrane, but in degenerating cells, cytoplasmic labeling was seen (see 54). A: Typical features of an O4 antibody-labeled cell showing injury towards the apoptotic end of the cell death spectrum, visualized by immuno-EM. Large arrowheads indicate immuno-label localized to the plasma membrane. Some labeling (short arrows) was associated with more superficial or internalized apparently membranous vesicles of varying size that appear to derive from the plasma membrane. Note the apoptotic-appearing nucleus (n) with condensed chromatin. Large intracellular vesicles (v) and apparent swollen mitochondria (long arrows) are typical features of apoptotic-like preOLs that have also been observed in vitro. The swollen mitochondrion (lower long arrow) is shown in detail in the inset at lower right in panel “C”. B: Typical features of an O4 antibody-labeled preOL showing features of necrotic-like degeneration. Note the necrotic-appearing nucleus (n) with glassy appearance and the extensive cytoplasmic and peri-nuclear O4-labeling (arrowheads) consistent with breakdown of the plasma membrane. C: The typical features of an apoptotic-like O4-labeled cell preOL (OL; centre) are distinct from the adjacent apoptotic cell at left, which is an apparent neuron with more normal-appearing mitochondria (m), perinuclear Golgi apparatus and evidence of the nuclear membrane, none of which is observed in the apoptotic preOL. The preOL displays swollen mitochondria (m), whereas the mitochondria in adjacent cells appear normal. The intact-appearing mitochondrion at upper right is shown in the inset at lower left. Note the cytoplasmic O4-labeling (arrows), which is associated with membranous vesicles that may be derived from internalized plasma membrane. Bars = 1 μm.
Block of intracellular Ca2+ release does not protect P10 RON axons
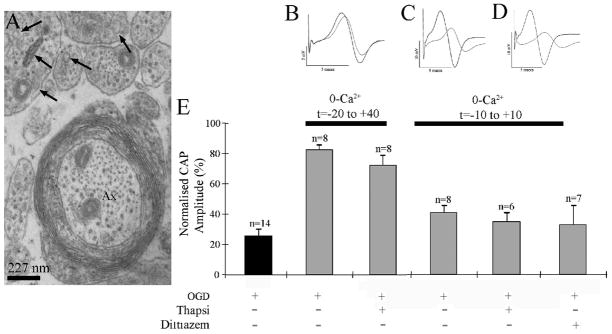
We next sought to identify the mechanism of injury operating in the highly vulnerable large premyelinated axon population during OGD. In the dorsal column of the adult rat spinal cord, L-type VGCC block can protect axons from ischemic-type injury by limiting Ca2+ release from intracellular stores.31 Developing RON axons contain numerous inclusions that are potential intracellular Ca2+ stores (Fig 5A, arrows), which may be endoplasmic reticulum or “tubular vesicular apparatus” 32 (membrane targeted to the axolemma during radial expansion). To investigate the potential significance of intracellular Ca2+ release during OGD, nerves were exposed to the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump inhibitor thapsigargin (100 μM) in the absence of extracellular Ca2+. Two zero-Ca2+ paradigms were used but in neither case did the presence of thapsigargin increase the protection against OGD-induced injury from that seen in zero Ca2+ alone (Fig 5B, C, E). Diltiazem, which blocks the voltage sensor of L-type VGCCs and would prevent ryanodine receptor-mediated release of endoplasmic Ca2+, also failed to increase recovery in the absence of extracellular Ca2+ (Fig 5D, E). These findings are not consistent with damaging intracellular Ca2+ release in P10 RON axons under ischemic conditions.
Figure 5.
Block of intracellular Ca2+ release in not protective of P10 RON axons. A: High-power electron micrograph showing the presence of intracellular inclusions with the features of endoplasmic reticulum in premyelinated and actively myelinating axons (e.g., arrows). B: CAPs recorded from a RON that was exposed to zero Ca2+/50 μM EGTA, plus thapsigargin from 20 min prior to the onset of OGD, to 40 min post OGD (t=-20 to + 40). C: CAPs from an experiment in which the RON was exposed to zero Ca2+/50 μM EGTA plus thapsigargin from t=-10 min to + 10 min. D: CAPs from an experiment in which the RON was exposed to zero Ca2+/50 μM EGTA plus diltiazem from t=-10 min to + 10 min. E: Data summary showing that neither thapsigargin protocol nor diltiazem significantly increased the degree of recovery compared to the relevant control.
Combined GluR and VGCC block is highly protective against degeneration of premyelinated axons
As reported in a prior study,21 compound action potentials (CAPs) recorded from the P10 RON recovered to 25.6 ±4.2% of control following 60 min OGD + 60 min of recovery (n=14), and combined block of NMDA and non-NMDA GluRs partially protected the CAP from 60 min of OGD. The presence of the L-type VGCC blocker diltiazem (50 μM; 41.2 ±7.7% CAP recovery, n=7; p>0.05 vs., OGD alone) or the P/Q-type blocker ω-agatoxin IVA (500 nM; 40.1±3.4% CAP recovery, n=7; p>0.05 vs., OGD alone) improved recovery from 60 min OGD, but not to a statistically significant degree (Supplemental Fig 6). A similar protocol performed in the presence of the VGCC blockers diltiazem + ω-agatoxin IVA + the NMDA GluR blocker MK-801 (10 μM) + and the non-NMDA glutamate receptor blocker NBQX (50 μM) resulted in complete recovery from 60 min of OGD (Fig 6A; “all treatment” 97.8 ±7.4% CAP recovery, n=7; p<0.001 vs., OGD alone). Immuno-staining of axons for either neurofilament light (NF-L) or heavy (NF-H) corroborated this finding, showing no significant staining loss following OGD during the “all treatment” protocol (Fig 6B ; P > 0.05 vs., control in both cases).
Figure 6.
Combined GluR and VGCC block is highly protective of all premyelinated axons. A: In the presence of ω-agatoxin VIA+diltiazem+MK-801+NBQX (“All treatments”) CAP area recovered fully from a 60 min period of OGD. The inserts at the top show representative CAPs recorded at time zero (black) and at the end of the recovery period (red); from left to right these traces represent control (where traces largely overlap due to excellent recording stability), OGD and “all treatments” paradigms respectively. Bars = 2 mV/ 1 msec. The mean recovery data is shown lower right. B: Electron-micrograph showing typical ultrastructural changes following the OGD protocol in the absence of drugs. Some premyelinated axons can be distinguished by the presence of microtubules, while a larger diameter ensheathed axons (arrow head) has lost all microtubules. Bar = 0.5 μm. C: NF-L and NF-H staining was also protected during OGD by the “all treatments” protocol. NF stained axons have a normal appearance following 60 min of OGD and recovery and mean staining intensity is not significantly different from control staining. D, E: The ultrastructural features of axons in nerves exposed to 60 min OGD and recovery in the presence of ω-agatoxin VIA+diltiazem+MK-801+NBQX appear largely normal. Even large pre- myelinated axons retain membrane integrity and contain microtubules (arrows), while several axonal mitochondria can be seen to have a normal structure. Myelinated axons (“Ax”) retain membrane integrity but appear to have few microtubules and exhibit myelin pathology. Bar = 1 μm. F: Axon health was assessed using an established scoring protocol (axon viability score), where a score of three represents a normal appearance and zero represents total axon breakdown. Axon viability scores generally fall between two and three following OGD in the presence of ω-agatoxin VIA+diltiazem+MK-801+NBQX when examined against axonal diameter. G: Mean viability score in treated RONs for small (<0.4μm diameter, black bars) and large (>0.4μm diameter, white bars) pre-myelinated axon does not differ significantly from control.
We have previously shown that GluR block at this point in white matter development protects oligodendroglial somata and processes from OGD (e.g.,25), and that limiting oligodendroglial process injury in this fashion greatly reduces injury in small pre-myelinated axons.21 Ultrastructural analysis supported the electrophysiological evidence that addition of VGCC block to GluR block augmented protection to include the larger pre-myelinated axons (Fig 6C-F). This population of axons has initiated radial expansion in preparation for myelination. At this stage, VGCC clusters on the axolemma contribute to the recorded CAP waveform, are important for the proper formation of the node of Ranvier20, and may act as a significant source of Ca2+ influx under ischemic conditions. Following OGD during “all treatments”, numerous larger diameter (>0.4 μm) pre-myelinating axons of normal appearance were found that contained typical axoplasmic inclusions such as microtubules and mitochondria (Fig 6C, D arrows). Myelinated axons lacked many of these features (Fig 6C, D “Ax”), although the myelin itself appeared compact. Note, myelinated axons represent only ~2% of axon cross-sections at this age.32 Injury levels in pre-myelinated axons of all diameters were low under these conditions (Fig 6E), and mean damage scores were not significantly different for larger (>0.4 μm) or small (<0.4 μm) pre-myelinated axons (Fig 6F). This contrasts with prior findings showing no protection of the larger pre-myelinated axons (>0.4 μm) from OGD in the presence of glutamate receptor blockers alone.21
Combined VGCC and GluR blockade protected larger pre-myelinated axons
Elevated [Ca2+]i is known to promote microtubule disassembly,33, 34 a key marker of axonal pathology during ischemia.35 We therefore examined microtubule number in cross section electron micrographs. Small pre-myelinated axons (<0.4 μm) contained ~8 microtubules on average in control nerves, a number that was significantly reduced following OGD (Fig 7A). In keeping with the electrophysiological and immuno-staining data, microtubules in small diameter axons were protected from OGD by GluR block and by “all treatments” (Fig 7A). Larger diameter pre-myelinated axons contained more microtubules (~16) which were not protected from OGD by GluR block but were by “all treatments” (Fig 7A), consistent with a role for VGCC in damage to this axon group. When microtubule number was plotted against axon diameter in control nerves, there was a strong correlation that included myelinated axons (Fig 7B). This relationship broke down in larger axons following OGD, an effect that was not prevented by GluR block but was limited by “all treatments” (Fig 7B). These data are pooled into 0.05 μm axon diameter bins to show mean tubule number (Fig 7C), revealing that OGD evoked a significant reduction in the number of tubules in axons of all diameters while GluR block prevented tubule loss following OGD in axons <=0.4 μm. The relationship between these parameters following OGD in “all treatments” is not significantly different from control across the diameter range.
Figure 7.
Addition of VGCC block to GluR block protected larger pre-myelinated axons. Morphometric analysis of OGD induced axon injury. A: The number of microtubules present in axons is plotted for small-diameter (<0.4μm) and large diameter (>0.4 μm) premyelinated axons in RONs collected after various protocols. Microtubule number in small axons falls significantly following OGD, an effect that is countered by GluR block; this effect is not augmented by combined GluR + VGCC block (“All treatments”). Microtubule number in larger axons falls significantly following OGD, an effect that is not reduced by GluR block but is prevented by combined GluR and VGCC block. B: Microtubule number plotted against axon diameter for individual axons. Data for premyelinated (filled symbols), ensheathed (open circles) and myelinated (open squares) are included. The data show that larger axons contain more microtubules, a relationship that breaks down following OGD (the largest diameter axons are include in the insert). During combined GluR and VGCC block, the relationship between microtubule number and diameter is largely preserved, while GluR block alone has no such effect. C: Mean microtubule number plotted against axon diameter (0.05 μm bins) for pre-myelinated axons. Note that OGD produces a significant reduction in the number of microtubules at any given axon diameter while GluR block protects the smaller axons and “all treatments” prevents the loss of microtubules across the whole diameter spectrum (“*” = P<0.05 vs., control).
The data for microtubule number under various conditions for ensheathed axons is included in Fig 7B (open circles), revealing that this group of larger axons, which have a very close morphological relationship with oligodendroglial processes, behave in a similar manner to the larger pre-myelinated axons yet to be ensheathed. This is confirmed when mean viability scores are examined, showing a significant fall from 3.0 ±0.0 (n=8) in control RON to 1.86 ±0.35 (n=7; P<0.001) following OGD; an effect that was complete blocked by ‘all treatments’ (3.0 ±0.0, n=8) but not by GluR block alone (1.80 ±.22, n=5; P<0.001 vs., control).
Astrocyte injury is distinct from axon injury
Acute astrocyte injury in actively myelinating white matter such as the P10 RON involves ischemic cell swelling mediated by the Na-K-Cl cotransport,36 although VGCC activation is linked to astrocyte injury at earlier stages in development.37 Astrocyte pathology was widespread in nerves exposed to the current “all treatments” paradigm during OGD. In particular, astrocyte processes often appeared to be disintegrating (supplemental Figure 6), while large flocculent zones bordered areas where astrocyte processes had lost membrane integrity (supplemental Figure 6 B arrows). These features were often in close proximity to axons and oligodendroglia of normal healthy appearance. Hence, astrocyte degeneration was not prevented by conditions that markedly protected against degeneration of large caliber axons.
Discussion
The current in vitro and in vivo findings have identified maturation-dependent susceptibility of developing axons in cerebral white matter to hypoxia-ischemia. Peak vulnerability occurs as axons undergo radial expansion during the initiation of early myelination, making this axon population exquisitely sensitive to injury. The findings suggest that damage to developing axons is a major, but previously under-appreciated, component of perinatal white matter injury. In addition, the data place late premyelinated axons alongside pre-OLs as principal targets for injury in the early evolution of hypoxic-ischemic lesions such as PVL and HIE. The heightened sensitivity to hypoxia-ischemia of these larger caliber axons is mediated by VGCC expression in the axolemma. Given the prolonged period over which early myelination occurs in the developing human brain, our data suggest that hypoxia-ischemia has the potential to trigger axonal degeneration in cerebral white matter over an extended time window of weeks-to-months before and after birth.
Spectrum of axon damage associated with preterm cerebral WMI
Preterm cerebral WMI frequently involves severe forms of pan-axonal injury that involve smaller caliber pre-myelinating axons.34 Recent fetal ovine studies (0.6 of gestation) suggest that the axon diameter in such early premyelinating white matter does not exceed 0.4 μm.19 Thus, injury during this developmental window appears to be distinct from the focus of the current study where more moderate injury to late pre-myelinating white matter selectively targeted larger caliber axons and spared the majority of small caliber axons. Prior to the onset of myelination, pan-axonal degeneration is triggered in preterm cerebral white matter under conditions of severe ischemia. The resultant WMI includes periventricular leukomalacia (PVL), which displays early coagulative necrosis and a variable progression to cavitary lesions with pan-cellular degeneration of both the glial and neuro-axonal elements. Such axonal degeneration is typified by retraction balls and clubs.2, 4, 38,19 Markers of early axonal pathology such as β-amyloid precursor protein (βAPP) and fractin visualize the trajectory of axons that degenerate within foci of necrosis in addition to sites distant to such foci, although reports vary as to the extent of this phenomenon. 34,18,39,14, 40 While the extent of axonal loss in PVL is difficult to quantify, there is an ~30% loss of cortical neurons which has been attributed to axotomy in necrotic lesions.41,42 Moreover, MRI studies have defined reduced white matter volumes in PVL that may involve axonal degeneration (reviewed in42, 43). Macroscopic focal necrotic lesions were previously common but have declined in recent years to comprise ~5% of the spectrum of WMI described in autopsy and neuro-imaging studies.
Under conditions of more moderate hypoxia-ischemia, the preterm white matter is susceptible to milder forms of microscopic necrosis (“non-cystic PVL”) that also damage smaller caliber axons. Although the incidence of these lesions is high,14 a recent human autopsy study found that the overall burden of small necrotic foci of axonal injury is low14. In milder forms of human and experimental preterm WMI, axonal degeneration appears to be restricted to foci of necrosis and axons are spared in regions of diffuse WMI characterized by early preOL degeneration, later preOL maturation arrest and reactive astrogliosis.14,34,42 Interestingly, the uninjured axons that localized to diffuse WMI in a preterm ovine model19 displayed a size range that is smaller than the axons shown to be susceptible here in early myelinating lesions.
Spectrum of axon damage associated with term neonatal leucoencephalopathy
Recent imaging studies have identified that cerebral WMI occurs in association with HIE,44 while the protective effects of head cooling may be related to reduced WMI.7, 8, 45 Axonal injury assessed by βAPP staining is present in white matter of neonates who suffered birth asphyxia,46 and WMI was observed in a neonatal pig model of HIE.47 MRI studies suggest that changes in axonal microstructure accompany early myelination of cerebral white matter. In human preterm cerebral white matter, anisotropic changes measured with diffusion tensor MRI may be consistent with the initiation of radial expansion in some pre-myelinated axons,48 and similar findings have been reported in rat cerebral white matter.49 Combined electrophysiological, immuno-staining and MRI studies of developing rabbit white matter showed action potential changes consistent with axon maturation prior to the onset of myelination,50 and similar results were found in rodent optic nerve.51 Since human white matter begins active myelinating in the third trimester,1, 16,52 the VGCC-mediated axonal injury identified here may contribute to WMI from late mid-term into the post-natal period and following milder insults not characterized by necrosis.
Mechanisms of axonal injury relevant to myelination failure
As preterm cerebral white matter initiates myelination, mechanisms related to both glial and axonal pathology may contribute to myelination failure in WMI. The onset of axon diameter expansion in the RON approximately coincides with maturation of the oligodendrocyte lineage as some preOLs transition to the immature OL stage.53–55 At birth, all RON axons are small (~0.2 μm: 32, 51, 56). By P6, the first ensheathed axons are closely associated within an oligodendroglial process but most have not received their first wrap of compact myelin and have a diameter range up to 0.6 μm. Ensheathed axons co-exist alongside naked axons cylinders of a similar diameter (32 and see Fig 2), showing that this first phase of diameter expansion occurs in early pre-myelinating axons prior to ensheathment. RON preOLs are highly sensitive to acute ischemic injury due to the presence of non-NMDA receptors on their somata and NMDA receptors on their processes, and display reduced ischemia-sensitivity as they mature.11, 12, 57 We previously demonstrated a loss of glutamate reactivity from axons and oligodendroglia in a P7 rat model of reversible hypoxia-ischemia.26 There appeared to be a net translocation of the excitotoxin to the extracellular space consistent with receptor-mediated preOL degeneration. Our present results with this model support that the majority of preOLs acutely degenerate from a combination of necrosis and apoptosis in cortical regions. The previously unrecognized early preOL degeneration in grey matter may also have implications for neuronal injury and cortical volume reduction associated with prematurity (e.g.,58). The redistribution of glutamate associated with preOL injury may drive neuronal damage; alternatively an as yet un-identified intermediate may contribute, as previously proposed for premyelinated axons.21
Both human and experimental studies support that preOLs that regenerate after acute WMI enter a state of maturation arrest that contributes to myelination failure.15 Myelination also requires a synergy between axons and their surrounding glia,59 and axons expressing VGCC clusters will be those engaged in synaptic interactions with developing oligodendrocytes (reviewed in 60). Interestingly, we have previously reported that subtle disruption to this form of communication impairs node of Ranvier formation.20 It is, thus, possible that myelination failure involves selective injury to a subpopulation of larger caliber axons engaged in highly specialized axo-glial interactions during the onset of myelination.
Maturation-dependent mechanisms of axonal vulnerability
As white matter maturation progresses, axons undergo important developmental changes that underlie their variable sensitivity to hypoxia-ischemia. In P10 RON, small diameter (~0.2 μm) pre-myelinated axons are damaged following NMDA receptor-mediated injury of neighboring oligodendroglial processes,21 presumably due to the release of an unidentified factor into the peri-axonal space. By contrast, myelinated RON axons sustain acute toxic Ca2+ overload during ischemia due to voltage-gated Ca2+ channel activation61 and reversal of Na-Ca exchange.62 Prior to the onset of myelination, axons initiate radial expansion and undergo ion channel re-organization in preparation for the formation of nodes of Ranvier and myelination. The appearance of P/Q- and L-type voltage-gated VGCC clusters at putative node sites is an early event in this process,20 and our results indicate that these VGCCs act as a significant route of toxic Ca2+ influx during hypoxia-ischemia, making axons more vulnerable to ischemic injury than at any other point in their maturation.
We found no evidence for a contribution from intracellular Ca2+ release to ischemic axon injury at this developmental stage and the additional protective effect of VGCC block is consistent with our prior observation of functional VGCCs on axons undergoing radial expansion prior to myelination.20 By imaging GFP-M expression that was restricted to pre-myelinated, ensheathed and early myelinated axons, we found highly selective injury of the smallest fluorescent axons. These corresponded to the larger pre-myelinated axons, which had greater ischemia-sensitivity than neighboring oligodendroglia. Comparison between all GFP-M(+) axons and other cell types at different ages revealed higher levels of pathology in oligodendrocytes than in axons and astrocytes and a higher sensitivity to ischemic injury in axons at P20 compared to P10 and adult. While caution must be used when interpreting injury data based upon immuno-staining (see 63), this latter observation corresponds with earlier reports that actively myelinating white matter has an elevated metabolic rate and correspondingly lower tolerance to energy deprivation. 24 The influence of maturation upon axon injury will vary between individual white matter structures, where even closely apposed tracts can differ significantly in their rate of development, axon diameter spectra and degree of myelination (e.g. 52,64).
Clinical Relevance
The current findings demonstrate that early maturing axons, having initiated diameter expansion and expressing clusters of functional VGCC, are exquisitely sensitive to ischemic injury. This is a property that this axon population shares with preOLs, previously regarded as the most ischemia-sensitive element with immature white matter. By contrast, axons yet to enter this developmental window, or which have passed through it to initiate myelination, have a much higher ischemic tolerance. The burden of WMI in a given region is thus likely to be influenced by the complement of pre-myelinated axons and preOLs in the affected white matter structures. In the RON, the rate of myelination reaches a peak between P15-P30 and in this 100% myelinated tract the proportion of larger pre-myelinated axons reaches a maximum several days before this.32, 51 This period corresponds to a developmental window when the nerve is particularly vulnerable to energy deprivation (24, Fig 1 and Supplementary Fig 3). In preterm human parietal white matter, the onset of myelination occurs during the third trimester and the degree of myelination varies between tracts.16,52 The period before the onset of myelination may define a period when preOLs and axons that have initiated diameter expansion co-exist and both contribute to WMI. Susceptibility of particular white matter regions to ischemic injury will therefore correlate with both axonal and oligodendrocyte maturation at that site.
The high degree of protection afforded to larger pre-myelinated axons by the multiple agent pharmacological strategy tested here suggests a strategy to reduce preterm WMI during the transition to active myelination, although detailed testing with in vivo models is first required. The principal finding of the current study is the previously unrecognized high ischemia-sensitivity of large pre-myelinating central axons, subject to toxic Ca2+ influx mediated by VGCCs. Several lines of evidence indicate that this axon population is at least as ischemia-sensitive as pre-OLs, which have to date been the focus of investigation in developing white matter injury.
Supplementary Material
Supplemental Figure 1. The mean CAP recovery recorded following 60 min of OGD + 60 min recovery at various ages. Note that the degree of CAP recovery is not significantly different between P8-P12, justifying the grouping of these nerves together.
Supplemental Figure 2. Hoechst staining used as cell death marker in APC(+) oligodendroglia. Examples of pyknotic cells show either clumping of heterochromatin (A) in the nucleus, or have nuclei which fluoresce brightly (B). On the other hand, viable cells have nuclei that do not stain brightly (C).
Supplemental Figure 3. Examples of injury scoring in THY-1/GFP-M mice.
Supplemental Figure 4. Comparison of injury in different cellular compartments of MON. A: Representative images showing GFAP reactivity at P10, P20 and adult in control and 60 min OGD + 60 min recovery. Note that injury, indicated as loss of GFAP reactivity, is greater in older animals. B: Representative images showing APC reactivity (green) and nuclear morphology (blue) at P10, P20 and adult in control and 60 min OGD + 60 min recovery. C: The degree of astrocyte (plotted red), oligodendrocyte (plotted blue) and axon (plotted green) injury in P10 MON following 60 min of OGD (indicated by the gray bar). Note the similar degree of injury in axons and astrocytes, and the higher level of injury in oligodendrocytes. Also note, that when only the smallest GFP(+) axons are counted, the degree of injury exceeds that of oligodendrocytes (arrow and green triangle). D-F: Similar analysis for four periods of ischemic injury (30, 60, 90 and 120 min) at P10, P20 and adult MON.
Supplemental Figure 5. L- or P/Q-type VGCC block is not significantly protective against ischemic-type injury in the P10 RON. A, B: CAPs recorded before the initiation of a 60 min period of OGD and after 60 min recovery in aCSF, in the presence of either diltiazem (A) or ω-agatoxin VI-A (B). C, D: Mean CAP area plotted against time for OGD experiments performed in either diltiazem (C) or ω-agatoxin VI-A (D). E: Data summary showing increased mean CAP recovery compared to OGD in the absence of both drugs which does not reach statistical significance.
Supplemental Figure 6. Astrocyte pathology persists following OGD in the presence all treatments. A: Low-power electron micrograph of P10 RON subjected to 60 min OGD and 60 min recovery in the presence of all treatments. Note the presence of two oligodendrocyte somata of normal appearance containing healthy organelles such as mitochondria and Golgi apparatus (“Oli”). An astrocyte process shows signs of disintegration (arrow). Bar = 2 μm. B: A large astrocyte process contains multiple vacuoles and has lost membrane integrity directly adjacent to a large area of debris (arrows). Bar = 1 μm.
Acknowledgments
We wish to thank Miss Natalie Allcock for technical assistance with electron microscopy. The GFP-M mice were a kind gift from Dr. Leonardo Sacconi, European Laboratory for Non-linear Spectroscopy, University of Florence, and Department of Physics, University of Florence, Italy. This work was supported by the National Institutes of Neurological Disorders and Stroke (NS44875) to RF and 1RO1NS054044, R37NS045737 to SAB and 1F30NS066704 to AR and the Department of Veterans Affairs Merit Review Program to CKM. Supported by the American Heart Association (11GRANT7510072 to SAB) and the March of Dimes Birth Defects Foundation (SAB).
References
- 1.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 2.Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28:405–414. doi: 10.1053/j.semperi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpe JJ. Neurology of the newborn. Philadelphia: W.B. Saunders Company; 1995. [Google Scholar]
- 5.Bax M, Tydeman C, Flodmark O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. Jama. 2006;296:1602–1608. doi: 10.1001/jama.296.13.1602. [DOI] [PubMed] [Google Scholar]
- 6.Himmelmann K, Hagberg G, Uvebrant P. The changing panorama of cerebral palsy in Sweden. X. Prevalence and origin in the birth-year period 1999–2002. Acta Paediatr. 99:1337–1343. doi: 10.1111/j.1651-2227.2010.01819.x. [DOI] [PubMed] [Google Scholar]
- 7.Li AM, Chau V, Poskitt KJ, et al. White matter injury in term newborns with neonatal encephalopathy. Pediatr Res. 2009;65:85–89. doi: 10.1203/PDR.0b013e31818912d2. [DOI] [PubMed] [Google Scholar]
- 8.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 9.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. discussion 536–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McQuillen PS, Miller SP. Congenital heart disease and brain development. Ann N Y Acad Sci. 1184:68–86. doi: 10.1111/j.1749-6632.2009.05116.x. [DOI] [PubMed] [Google Scholar]
- 11.Fern R, Moller T. Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. J Neurosci. 2000;20:34–42. doi: 10.1523/JNEUROSCI.20-01-00034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riddle A, Dean J, Buser JR, et al. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann Neurol. 70:493–507. doi: 10.1002/ana.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buser JR, Maire J, Nelson K, et al. Myelination failure in human peinatal white matter injury: a disrupted repair mechanisms linked to pre-oligodendrocyte maturation arrest. Annals of Neurology. 2012 In Press. [Google Scholar]
- 15.Segovia KN, McClure M, Moravec M, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Back SA, Luo NL, Borenstein NS, et al. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean JM, Moravec MD, Grafe M, et al. Strain-specific differences in perinatal rodent oligodendrocyte lineage progression and its correlation with human. Dev Neurosci. 33:251–260. doi: 10.1159/000327242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes RL, Billiards SS, Borenstein NS, et al. Diffuse Axonal Injury in Periventricular Leukomalacia as Determined by Apoptotic Marker Fractin. Pediatr Res. 2008 doi: 10.1203/PDR.0b013e31816c825c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddle A, Maire J, Gong X, et al. Differential Susceptibility to Axonopathy in Necrotic and Non-Necrotic Perinatal White Matter Injury. Stroke. doi: 10.1161/STROKEAHA.111.632265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alix JJ, Dolphin AC, Fern R. Vesicular apparatus, including functional calcium channels, are present in developing rodent optic nerve axons and are required for normal node of Ranvier formation. J Physiol. 2008;586:4069–4089. doi: 10.1113/jphysiol.2008.155077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alix JJ, Fern R. Glutamate receptor-mediated ischemic injury of premyelinated central axons. Ann Neurol. 2009;66:682–693. doi: 10.1002/ana.21767. [DOI] [PubMed] [Google Scholar]
- 22.Feng G, Mellor RH, Bernstein M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 23.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 24.Fern R, Davis P, Waxman SG, Ransom BR. Axon conduction and survival in CNS white matter during energy deprivation: a developmental study. J Neurophysiol. 1998;79:95–105. doi: 10.1152/jn.1998.79.1.95. [DOI] [PubMed] [Google Scholar]
- 25.Salter MG, Fern R. NMDA Receptors are Expressed in Developing Oligodendrocyte Processes and Mediate Injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 26.Back SA, Craig A, Kayton RJ, et al. Hypoxia-ischemia preferentially triggers glutamate depletion from oligodendroglia and axons in perinatal cerebral white matter. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600344. [DOI] [PubMed] [Google Scholar]
- 27.Meshul CK, McGinty JF. Kappa opioid receptor immunoreactivity in the nucleus accumbens and caudate-putamen is primarily associated with synaptic vesicles in axons. Neuroscience. 2000;96:91–99. doi: 10.1016/s0306-4522(99)90481-5. [DOI] [PubMed] [Google Scholar]
- 28.Back SA, Luo NL, Borenstein NS, et al. Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol. 2002;61:197–211. doi: 10.1093/jnen/61.2.197. [DOI] [PubMed] [Google Scholar]
- 29.Craig A, Ling Luo N, Beardsley DJ, et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol. 2003;181:231–240. doi: 10.1016/s0014-4886(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 30.Martin LJ, Sieber FE, Traystman RJ. Apoptosis and necrosis occur in separate neuronal populations in hippocampus and cerebellum after ischemia and are associated with differential alterations in metabotropic glutamate receptor signaling pathways. J Cereb Blood Flow Metab. 2000;20:153–167. doi: 10.1097/00004647-200001000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Ouardouz M, Nikolaeva MA, Coderre E, et al. Depolarization-induced Ca2+ release in ischemic spinal cord white matter involves L-type Ca2+ channel activation of ryanodine receptors. Neuron. 2003;40:53–63. doi: 10.1016/j.neuron.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Hildebrand C, Waxman SG. Postnatal differentiation of rat optic nerve fibers: electron microscopic observations on the development of nodes of Ranvier and axoglial relations. J Comp Neurol. 1984;224:25–37. doi: 10.1002/cne.902240103. [DOI] [PubMed] [Google Scholar]
- 33.Schlaepfer WW. Structural alterations of peripheral nerve induced by the calcium ionophore A23187. Brain Res. 1977;136:1–9. doi: 10.1016/0006-8993(77)90126-3. [DOI] [PubMed] [Google Scholar]
- 34.Schlaepfer WW. Vesicular disruption of myelin simulated by exposure of nerve to calcium ionophore. Nature. 1977;265:734–736. doi: 10.1038/265734a0. [DOI] [PubMed] [Google Scholar]
- 35.Waxman SG, Black JA, Ransom BR, Stys PK. Protection of the axonal cytoskeleton in anoxic optic nerve by decreased extracellular calcium. Brain Res. 1993;614:137–145. doi: 10.1016/0006-8993(93)91027-p. [DOI] [PubMed] [Google Scholar]
- 36.Thomas R, Salter MG, Wilke S, et al. Acute ischemic injury of astrocytes is mediated by Na-K-Cl cotransport and not Ca2+ influx at a key point in white matter development. J Neuropathol Exp Neurol. 2004;63:856–871. doi: 10.1093/jnen/63.8.856. [DOI] [PubMed] [Google Scholar]
- 37.Fern R. Intracellular calcium and cell death during ischemia in neonatal rat white matter astrocytes in situ. J Neurosci. 1998;18:7232–7243. doi: 10.1523/JNEUROSCI.18-18-07232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banker B, Larrocher J-C. Periventricular leukomalacia of infancy: a form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- 39.Arai Y, Deguchi K, Mizuguchi M, Takashima S. Expression of beta-amyloid precursor protein in axons of periventricular leukomalacia brains. Pediatr Neurol. 1995;13:161–163. doi: 10.1016/0887-8994(95)00149-a. [DOI] [PubMed] [Google Scholar]
- 40.Meng SZ, Arai Y, Deguchi K, Takashima S. Early detection of axonal and neuronal lesions in prenatal-onset periventricular leukomalacia. Brain Dev. 1997;19:480–484. doi: 10.1016/s0387-7604(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 41.Andiman SE, Haynes RL, Trachtenberg FL, et al. The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons. Brain Pathol. 20:803–814. doi: 10.1111/j.1750-3639.2010.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ment LR, Hirtz D, Huppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8:1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- 43.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Vries LS, Groenendaal F. Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiology. 52:555–566. doi: 10.1007/s00234-010-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parikh NA, Lasky RE, Garza CN, et al. Volumetric and anatomical MRI for hypoxic-ischemic encephalopathy: relationship to hypothermia therapy and neurosensory impairments. J Perinatol. 2009;29:143–149. doi: 10.1038/jp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell JE, Becher JC, Wyatt B, et al. Brain damage and axonal injury in a Scottish cohort of neonatal deaths. Brain. 2005;128:1070–1081. doi: 10.1093/brain/awh436. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan SM, Bjorkman ST, Miller SM, et al. Morphological changes in white matter astrocytes in response to hypoxia/ischemia in the neonatal pig. Brain Res. 1319:164–174. doi: 10.1016/j.brainres.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Huppi PS, Maier SE, Peled S, et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 49.Wimberger DM, Roberts TP, Barkovich AJ, et al. Identification of "premyelination" by diffusion-weighted MRI. J Comput Assist Tomogr. 1995;19:28–33. doi: 10.1097/00004728-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Drobyshevsky A, Song SK, Gamkrelidze G, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foster RE, Connors BW, Waxman SG. Rat optic nerve: electrophysiological, pharmacological and anatomical studies during development. Brain Res. 1982;255:371–386. doi: 10.1016/0165-3806(82)90005-0. [DOI] [PubMed] [Google Scholar]
- 52.Jakovcevski I, Filipovic R, Mo Z, et al. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat. 2009;3:5. doi: 10.3389/neuro.05.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Small RK, Riddle P, Noble M. Evidence for migration of oligodendrocyte--type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987;328:155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- 54.Colello RJ, Devey LR, Imperato E, Pott U. The chronology of oligodendrocyte differentiation in the rat optic nerve: evidence for a signaling step initiating myelination in the CNS. J Neurosci. 1995;15:7665–7672. doi: 10.1523/JNEUROSCI.15-11-07665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ueda H, Levine JM, Miller RH, Trapp BD. Rat optic nerve oligodendrocytes develop in the absence of viable retinal ganglion cell axons. J Cell Biol. 1999;146:1365–1374. doi: 10.1083/jcb.146.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tennekoon GI, Cohen SR, Price DL, McKhann GM. Myelinogenesis in optic nerve. A morphological, autoradiographic, and biochemical analysis. J Cell Biol. 1977;72:604–616. doi: 10.1083/jcb.72.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Back SA, Gan X, Li Y, et al. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JD, Park HJ, Park ES, et al. Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain. 134:1199–1210. doi: 10.1093/brain/awr021. [DOI] [PubMed] [Google Scholar]
- 59.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- 60.Alix JJ, Domingues AM. White matter synapses: form, function, and dysfunction. Neurology. 76:397–404. doi: 10.1212/WNL.0b013e3182088273. [DOI] [PubMed] [Google Scholar]
- 61.Fern R, Ransom BR, Waxman SG. Voltage-gated calcium channels in CNS white matter: role in anoxic injury. J Neurophysiol. 1995;74:369–377. doi: 10.1152/jn.1995.74.1.369. [DOI] [PubMed] [Google Scholar]
- 62.Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J Neurosci. 1992;12:430–439. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shannon C, Salter M, Fern R. GFP imaging of live astrocytes: regional differences in the effects of ischaemia upon astrocytes. J Anat. 2007;210:684–692. doi: 10.1111/j.1469-7580.2007.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roessmann U, Gambetti P. Astrocytes in the developing human brain. An immunohistochemical study. Acta Neuropathol. 1986;70:308–313. doi: 10.1007/BF00686089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The mean CAP recovery recorded following 60 min of OGD + 60 min recovery at various ages. Note that the degree of CAP recovery is not significantly different between P8-P12, justifying the grouping of these nerves together.
Supplemental Figure 2. Hoechst staining used as cell death marker in APC(+) oligodendroglia. Examples of pyknotic cells show either clumping of heterochromatin (A) in the nucleus, or have nuclei which fluoresce brightly (B). On the other hand, viable cells have nuclei that do not stain brightly (C).
Supplemental Figure 3. Examples of injury scoring in THY-1/GFP-M mice.
Supplemental Figure 4. Comparison of injury in different cellular compartments of MON. A: Representative images showing GFAP reactivity at P10, P20 and adult in control and 60 min OGD + 60 min recovery. Note that injury, indicated as loss of GFAP reactivity, is greater in older animals. B: Representative images showing APC reactivity (green) and nuclear morphology (blue) at P10, P20 and adult in control and 60 min OGD + 60 min recovery. C: The degree of astrocyte (plotted red), oligodendrocyte (plotted blue) and axon (plotted green) injury in P10 MON following 60 min of OGD (indicated by the gray bar). Note the similar degree of injury in axons and astrocytes, and the higher level of injury in oligodendrocytes. Also note, that when only the smallest GFP(+) axons are counted, the degree of injury exceeds that of oligodendrocytes (arrow and green triangle). D-F: Similar analysis for four periods of ischemic injury (30, 60, 90 and 120 min) at P10, P20 and adult MON.
Supplemental Figure 5. L- or P/Q-type VGCC block is not significantly protective against ischemic-type injury in the P10 RON. A, B: CAPs recorded before the initiation of a 60 min period of OGD and after 60 min recovery in aCSF, in the presence of either diltiazem (A) or ω-agatoxin VI-A (B). C, D: Mean CAP area plotted against time for OGD experiments performed in either diltiazem (C) or ω-agatoxin VI-A (D). E: Data summary showing increased mean CAP recovery compared to OGD in the absence of both drugs which does not reach statistical significance.
Supplemental Figure 6. Astrocyte pathology persists following OGD in the presence all treatments. A: Low-power electron micrograph of P10 RON subjected to 60 min OGD and 60 min recovery in the presence of all treatments. Note the presence of two oligodendrocyte somata of normal appearance containing healthy organelles such as mitochondria and Golgi apparatus (“Oli”). An astrocyte process shows signs of disintegration (arrow). Bar = 2 μm. B: A large astrocyte process contains multiple vacuoles and has lost membrane integrity directly adjacent to a large area of debris (arrows). Bar = 1 μm.