Abstract
The dorsal premotor cortex (PMd) and the supplementary motor area (SMA) are critical for the acquisition and expression of sequential behavior, but little is known regarding how these regions are recruited when we must simultaneously acquire multiple sequences under different amounts of training. We hypothesized that these regions contribute to the retrieval of sequences at different familiarity levels, with the left PMd supporting sequences of moderate familiarity, and the SMA for sequences of greater familiarity. Double-pulse transcranial magnetic stimulation (TMS) was applied during the retrieval of six sequences previously learned under three different amounts of exposure during 30 days of training using a discrete sequence production (DSP) task. TMS led to a significant interaction of sequence error between depth of training and stimulation location. Stimulation of the left PMd increased error during moderate sequence retrieval, whereas stimulation of the SMA increased error during the retrieval of both moderately and extensively trained sequences. The lack of a double-dissociation fails to support a direct correspondence between brain region and putative behavioral learning stage. Instead the interaction suggests that SMA and PMd support the expression of sequences over different, albeit overlapping, time scales. Separate analysis of sequence initiation time did not demonstrate any significant difference between moderately and extensively trained sequences. Instead, stimulation to either region quickened sequence initiation for these sequences, but not for those sequences with poor retrieval performance. This supports the general role of these premotor regions in the maintenance of specific sequence knowledge prior to movement onset.
Keywords: Motor control, sequence learning, time scales, premotor cortex, TMS
Introduction
The dorsal premotor (PMd) cortex and the supplementary motor area (SMA) are intimately tied to the retrieval, selection and execution of sequential motor behavior (Wise 1985; Tanji 2001). Two lines of evidence suggest that PMd and SMA differ in terms of functional properties that are relevant to learning. One line, observed in human imaging experiments of initial sequence learning, is that PMd supports sequences that are explicitly defined and SMA supports implicit sequential movements. Increased PMd activation is observed when subjects become aware of repeating sequence patterns (Deiber et al. 1997), and decreases when performance becomes automatic (Jueptner et al. 1997). In contrast, activation of the SMA is observed during the retrieval of implicitly acquired sequences (Grafton et al. 1995; Bischoff-Grethe et al. 2004). The second line concerns the observation that PMd relies more on external cues whereas SMA is more strongly associated with internally generated sequential behavior (Mushiake et al. 1991; Jahanshahi et al. 1995; Jenkins et al. 2000).
These functional differences suggest that the PMd is more involved in retrieving a sequence when there are few training exposures (explicit, stimulus driven) whereas the SMA is more involved in the expression of sequences with many training exposures (implicit, internally driven). In terms of time scales, this can simply be restated so that the PMd contributes to learning over a relatively faster time scale than the SMA. This translates into a prediction whereby the two areas differ in the degree to which they support retrieval of a sequence based on the depth of training (i.e., a significant Region X Training interaction). The relative shift from externally to internally driven behavior has been used to argue that learning occurs in distinct stages. This motivates a stronger prediction: a double dissociation between region and training depth in which each stage of learning is supported by a distinct premotor substrate. The goal of this study was to test these two hypotheses using double-pulse transcranial magnetic stimulation (TMS) to create transient virtual lesions in PMd and SMA during the retrieval of sequences learned at different depths of training.
One approach would be to test performance of a single sequence during sham (no TMS), PMd and SMA stimulation at different time points of learning (early, middle, late training). However, this approach is problematic, because TMS in one session could alter the future trajectory of learning. Instead, we had subjects learn a set of six visually-cued, 10-element motor sequences that were practiced at three different training depths over a 30-day training regimen using a discrete sequence production (DSP) task (Rhodes et al. 2004). Trials were distributed evenly over a random training schedule, so that 2 sequences were minimally trained (MIN, 1 trial/session), 2 moderately trained (MOD, 10 trials/session), and 2 extensively trained (EXT, 64 trials/session). This was followed by a TMS experiment that tested how the left PMd and SMA support the retrieval of sequences learned under the three training depths. A concern with our approach is that most investigations describe the learning effects for a single motor sequence. However, in real life, we often simultaneously learn multiple skills at various depths of training. By manipulating the depth of training, we could test if the left PMd is particularly important for the expression of sequences with fewer prior training exposures (i.e., earlier stage of learning) compared to the SMA (i.e. later stage of learning). To do this, TMS was applied to either region as subjects retrieved sequences trained under different levels of practice. In this respect, we could then test the contributions of PMd and SMA at relatively fast, moderate, and slow stages of learning all during a single TMS session.
A double-dissociation of sequence error rate between region and depth of training would thus provide strong support for the prediction that the PMd and SMA operate under completely separable behavioral stages of sequence learning. On the other hand, a significant interaction effect on error rate between region and depth of training (without a complete double dissociation) would provide evidence that the two regions differentially support sequence learning in time, but not distinct behavioral stages of sequence learning.
We also tested the effect of TMS on the time needed to initiate a prepared sequence, or response time (RT). Given the experimental design, where subjects were given a symbolic cue to retrieve a sequence from memory, which was then followed by a go cue, we predicted that any effect of RT should only be observed if there was sufficient sequence information to retrieve from memory. Memory retrieval for the three levels of training depth was therefore tested. Given the few sequence exposures for the MIN sequences, we predicted that reliable retrieval would only be observed for MOD and EXT sequences, and that any RT effects should be particularly prominent for these sequences.
Methods
Subjects
Fifteen right-handed subjects (8 female, average age 24) volunteered with informed consent in accordance with the Institutional Review Board/Human Subjects Committee, University of California, Santa Barbara. All subjects had normal/corrected vision and no history of neurological disease or psychiatric disorders.
Procedure
Prior to TMS, subjects completed a training regimen involving the simultaneous acquisition of 6 different 10-element motor sequences using a DSP task. Subjects trained at home using their laptop computer and inside an MRI scanner during the collection of blood oxygenation level-dependant (BOLD) data. Training began with a session inside the scanner. Subjects then performed a minimum of 10 sessions (1 session/day) at home during a 14-day interval, and then returned to the scanner. This pattern repeated 3 times, completing at least 30 home training sessions and 4 scan sessions.
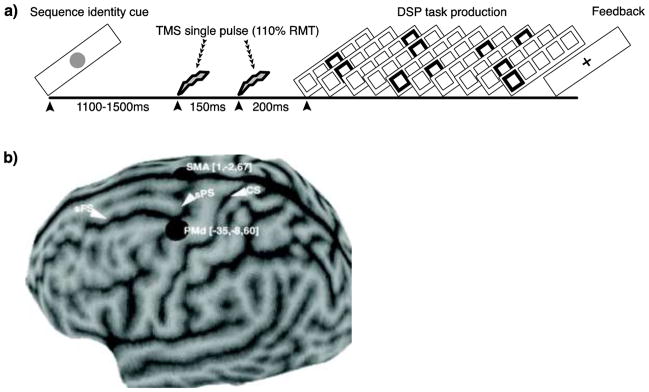
Subjects practiced visually cued DSP sequences using either a laptop keyboard (home training) or a button box (scanner training) with their right hand. A depiction of the DSP task is shown in Figure 1a. Each trial began with the presentation of a sequence identity cue, which preceded the initial DSP stimulus ‘go cue’, and allowed for the advance retrieval of sequence knowledge. Sequences were presented using a horizontal array of 5 square stimuli, with responses mapped from left to right, such that the thumb corresponded to the leftmost stimulus and the smallest finger to the rightmost stimulus. Red highlighted squares cued each response, with the next highlighted immediately after each correct response (no inter-stimulus interval). The sequence was paused at the point of an error and resumed after the correct key was pressed. Subjects had unlimited time to respond and a fixation ‘+’ signified trial completion. The structure of the sequences was organized so that each DSP stimulus location was presented twice, but without stimulus repetition (‘11’) and free of regularities such as trills (‘121’) and runs (‘123’).
Figure 1.
Trial structure and TMS sites. A trial (a) began with the presentation of a unique sequence identity cue that remained on screen for 1450–1850 ms. The initial TMS pulse was delivered 1100–1500 ms after identity cue onset, and followed by a second pulse after 150 ms. There was a 200 ms delay between the second pulse and the first discrete sequence production (DSP) stimulus, or go cue. Each correct key press led to the immediate presentation of the next DSP stimulus and so forth until all 10-elements were correctly executed. Feedback ‘+’ signaled sequence completion. If an incorrect key was hit, subjects received an error signal (not shown) and the DSP sequence paused until the correct response was made. Subject-specific functional localizers (b) for PMd and SMA were identified using BOLD data acquired during scanner practice sessions. The averaged group premotor foci are shown. Coordinates are in MNI-152 space. Abbreviations are as follows: sFS (superior frontal sulcus), sPS (superior precentral sulcus), CS (central sulcus).
Sequence familiarity was manipulated during home training at three exposure levels. Two sequences (rather than just one) were presented at each level for an additional analysis of the BOLD data collected during the scanner sessions. Each home training session consisted of 150 trials presented using a random schedule, so that two sequences trained extensively (EXT, 64 trials/sequence), two sequences trained moderately (MOD, 10 trials/sequence), and two sequences trained minimally (MIN, 1 trial/sequence). All subjects trained on the same sequence set and each at the same exposure level, which were maintained over the course of training. During each scan session, subjects received 50 practice trials for each sequence for the purpose of an additional analysis of the BOLD data. By the end of training subjects completed 34 practice (home and scanner) sessions (M ≈ 34.47, +/− 3.3 SD), and performed on average 2150 trials/EXT (+/− 212 SD), 505 trials/MOD (+/− 33 SD), and 230 trials/MIN (+/− 3 SD) sequence. Participation in the TMS study began 2 days (M ≈ 1.67, +/− 0.73 SD) after the completion of training, which was divided into two identical sessions and completed on consecutive days.
Prior to the start of the initial TMS session, subjects were given a brief sequence recollection memory test, which tested subjects’ ability to retrieve the sequences without the use of the DSP stimuli. They were instructed to report with accuracy and to not be concerned with speed. Each sequence was presented in blocks of 10 trials, with the sequence identity cue serving as the imperative for which sequence to produce. The first 5 trials of the block were presented with the DSP stimuli. The next 5 trials were presented without the DSP stimuli but the sequence could be retrieved based on the identity cue that introduced each trial. Subjects received error feedback and shown the correct response to make. Following correction, the trial would continue until the entire sequence was reported correctly.
During the TMS experiment, subjects produced the sequences using the same button box and direct mapping as in scanner training. Each TMS session contained 4 blocks of 75 trials, with 2 blocks for each stimulation site. Each block of 75 trials was divided into 3 smaller, 25 trial exposure condition blocks that were grouped according to practice exposure (MIN, MOD, EXT). Within each exposure condition block, there was an approximately equal amount of trials for each of the two sequences, with half of the trials presented with TMS and the other half with no TMS. Feedback detailing the number correct and the average time needed to complete a trial was given after each block. The order of stimulation blocks was counterbalanced over the two sessions, such that if PMd was the initial stimulation site on Day 1, SMA was the initial stimulation site on Day 2.
The TMS task was presented using a laptop computer running MATLAB (Version 7.1, Mathworks, Natick, MA) and PsychtoolBox (Version 3, psychtoolbox.org). A NIDAQ PCIMIA interface handled communication to the stimulator. An external monitor displayed the task to subjects at their eye-level. A custom fiber-optic button box and transducer collected key-press responses and response times (button box: HHSC-1×4-L; transducer: fORP932; Current Designs, Philadelphia, PA).
Localization and TMS
High-resolution T1-weighted sagittal images of the whole brain were acquired for each subject (3.0 T Siemens Trio with a 12-channel phased-array head coil), and a cortical surface representation displayed using Brainsight software (Rogue-Research). Stimulation sites were based on subject-specific imaging results from performing the sequences during the collection of BOLD. An event-related design was used, which allowed for the collection of 50 trials/sequence for each scan session. Functional images were processed and analyzed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK). Images were first realigned, then coregistered to the native T1, normalized to the MNI-152 template with a re-sliced resolution of 3×3×3 mm, and smoothed with a kernel of 8 mm full-width at half-maximum. For each subject, all sequencing events were modeled using a single design matrix and estimated using the general linear model (GLM). First-level models were created with stimulus vectors for each exposure condition and scanning session. The main effect of sequence production was generated for each subject with a t-test collapsed across exposure condition and session. This isolated brain regions that were sensitive to the task, and local maxima at PMd and SMA were located in MNI space and mapped back into native space. The search for premotor local maxima was constrained using known landmarks (Picard and Strick 2001). The left PMd was constrained to vicinity of the intersection of the precentral and superior frontal sulci, and the SMA site was constrained rostrally from the cingulate sulcus, and between the anterior commissure and the paracentral lobule. Unlike the PMd, this site was not constrained to the left hemisphere. Each stimulation site was then marked on the surface representation (Figure 1b).
Coil position with respect to the cortical anatomy of a subject was continuously monitored in real time using a Polaris Optical Tracking System (Northern Digital, Inc.). This allowed for the center and orientation of the TMS coil with respect to each stimulation site to be tracked throughout the experiment.
A Neutonus PNS stimulator (model no. N-0233-A-110V) with an air-cooled iron-core butterfly-shaped coil was used to deliver magnetic stimulation, with two individual pulses separated by a duration of 150 ms. A dual-pulse approach was used to generate a wider temporal effect during preparation. Pulse duration for the stimulator to the coil is 180 μs (at 100% of the operating power). The TMS motor threshold was 110% of the stimulator intensity required to produce a visible contraction of the intrinsic hand muscles at least 50% time with the TMS coil positioned over the hand area of the left primary motor cortex (M1). The same motor threshold was used for both TMS sessions. Stimulation occurred during the ‘foreperiod’ between the onset of the sequence identity cue and the go cue. The initial pulse was delivered at a random interval between 1100–1500 ms after the identity cue and the second pulse followed 150 ms later. In order to minimize motor artifacts due to TMS, a 200 ms gap separated the second pulse from the go cue.
Data analysis: Effect of interference on training performance
The training regimen presented sequences in a random schedule, which introduced the possibility that retrograde interference (RI) could have disrupted, in particular, the performance of MIN and MOD sequences, because these were presented less frequently than EXT sequences. To test if interference was detrimental to MIN and MOD, comparisons of the time needed to complete each 10-element sequence, or movement time (MT), could be made across the three exposure conditions (MIN, MOD, EXT) by selecting trials with an equivalent number of prior exposures. For each subject and sequence, training trials were grouped into bins of 25 consecutive trials, and excluding error trials, averaged for MT. All subjects completed at least 200 MIN trials/sequence and 450 MOD trials/sequence. These limits formed the basis for MT performance comparison across the different exposure conditions.
Data analysis: Sequence knowledge
Tests of sequence knowledge were based on the recollection memory task and on predictive motor performance. These tests measured the transition from a state that relied on the DSP stimuli to another whereby the sequences were generated from memory. For the recollection memory test, error was the dependent measure, calculated both in terms of the number of key press errors a subject made when reporting a sequence from memory, as well as the number of correct key presses that could be reported before making an initial error.
Predictive performance was based on the duration between any 2 key presses, or inter-key intervals (IKIs) made on correct trials during the TMS experiment. If a response is predictive, it should be faster than a reactive response to an unknown stimulus. We chose the threshold for predictive IKIs to be the 75th percentile of the no TMS MIN sequence trials. This suggests that IKIs faster than the 75% cutoff were produced without direct use of the DSP stimuli. This is a conservative estimate because subjects were not naïve to MIN, having practiced at least 200 trials/sequence during training. We tested a range of thresholds (98–50%), with little effect on the distribution of predictive movements. Those IKIs faster than the threshold were counted for each exposure and stimulation condition. Previous work on long-term sequence learning in monkeys used a similar approach (Matsuzaka et al. 2007).
Data analysis: TMS effects
There were three variables of interest: (1) the time elapsed between the go cue and first button press, or response time (RT); (2) the time needed to complete each 10-element sequence, or movement time (MT); (3) error trials that included any incorrect response. Error was expressed as the mean number of trials for each exposure condition that contained at least one incorrect button press. To measure behavioral effects due to TMS, difference scores were calculated by subtracting the dependent variable during TMS trials from the matching no TMS control trials. Distribution normality was tested and confirmed for each variable using the Lilliefors modification of the Kolmogorov-Smirnov test. Repeated-measures ANOVA and additional post-hoc T-tests were carried out for each dependent measure.
Results
Effects of interference on training performance
We first tested if interference during training could explain TMS session performance. By the end of training, all subjects performed 200 MIN trials, but only needed a third as many practice sessions to perform 200 MOD trials. If interference is detrimental, then MIN sequences should be slower than MOD sequences following 200 trials. Comparing trial bins revealed no significant difference in performance (T ≈ 0.64, P ≈ 0.53), indicating that increased interference did not disrupt performance. A similar comparison could be made between MOD and EXT following 450 trials, which again failed to show a difference in performance (T ≈ 2.01, P ≈ 0.064). These results suggest that performance is determined by physical practice and solidified during training that combines a random schedule across multiple sessions separated by considerable temporal delays (such as 30 nights of sleep).
Sequence knowledge
We next tested if there were differences of recollection memory between exposure conditions. There were different error rates [F(2,28) ≈ 9.95, P < 0.001, Figure 2a] and differences in the number of correct elements before an initial error [F(2,28) ≈ 13.19, P = 0.0001, Figure 2b]. Differences were significant for EXT and MOD with respect to MIN sequences for the number of errors made/trial (EXT vs MIN: T ≈ 3.62, P < 0.005; MOD vs MIN: T ≈ 3.13, P < 0.01) and the number of correct button presses made before an initial error (EXT vs MIN: T ≈ 4.00, P = 0.001; MOD vs MIN: T ≈ 3.76, P < 0.005). These effects suggest that motor representations were formed for both EXT and MOD, and less so for the MIN sequences, which relied more on the DSP stimuli. This is supported by differences in predictive sequence movements [F(2,28) ≈ 61.42, P < 0.00001, Figure 2c]. There was no effect of TMS on predictive movements, so comparisons between the exposure levels are collapsed across the no TMS and TMS trials. Both EXT and MOD had more predictive movements compared to MIN (EXT vs MIN: T ≈ 16.41, P < 10e-19; MOD vs MIN: T ≈ 7.50; P < 10e-8). Moreover, EXT demonstrated far more predictive movements than MOD (EXT vs MOD: T ≈ 14.51, P < 10e-17), suggesting that EXT production relied even less on DSP stimuli than MOD. The larger amount of predictive movements for EXT revealed that increased practice leads to deeper motor sequence knowledge. The recall and predictive sequence tests suggest that MIN sequences, with respect to MOD and EXT, are poorly learned because execution relies more on the presence of the DSP stimuli. Because stimulation occurred during the preparatory period when subjects could retrieve in advance each upcoming sequence, it is less certain how stimulation influenced the retrieval of the MIN sequences. On the other hand, the MOD and EXT sequences had similar performance on the recall test, indicating that both could be retrieved similarly from memory based on the presentation of the sequence identity cue. For these reasons, tests of regional sensitivity to TMS are evaluated using two separate models; one model that includes all exposure levels, and another that excludes MIN and focuses on differences between MOD and EXT.
Figure 2.
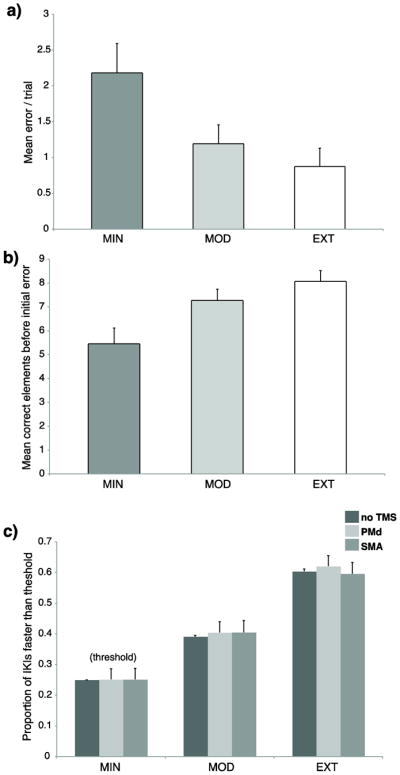
Sequence knowledge. Relative to MIN, subjects made fewer errors/trial (a), and produced more elements before creating an error (b) when they produced MOD and EXT sequences without DSP stimuli. Subjects generated significantly more predictive movements (c) for MOD and EXT sequences relative to the MIN threshold. More predictive movements were made for EXT relative to MOD sequences. The predictive threshold was based on the upper 25% of the IKI distribution from the no TMS MIN trials. Error bars show standard error of the mean (SEM).
TMS effects
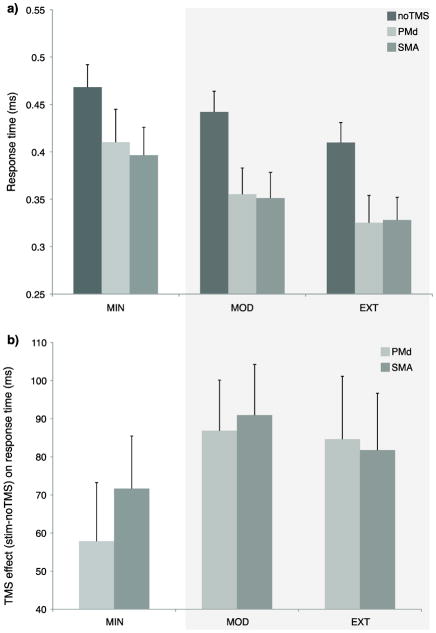
In order to measure how the two premotor regions supported learning at different training depths, effects of TMS were evaluated according to changes in RT, MT and error. There was an effect of RT across the exposure conditions [F(2,28) ≈ 18.30, P < 0.0001, Figure 3a], stimulation conditions [F(2,28) ≈ 30.72, P < 0.0001], and an interaction [F(4,28) ≈ 2.52, P ≈ 0.05], which was driven by a greater TMS effect for MOD and EXT relative to MIN. Stimulation to either region during MOD and EXT led to an overall decrease in RT compared to MIN (EXT vs MIN: T ≈ 2.49, P ≈ 0.02; MOD vs MIN: T ≈ 3.53, P ≈ 0.001, Figure 3b), revealing that MOD and EXT were more sensitive to TMS than MIN. Note, Figure 3b shows the RT difference from no TMS with the sign inverted, so that the more positive the value, the faster the RT is with TMS. This effect was similar for both regions, suggesting that both left PMd and SMA are involved in the implementation of known (MOD, EXT) motor representations. Because MIN trials were poorly learned, a separate model was used to evaluate RT for only MOD and EXT sequences. This analysis identified RT effects across exposure conditions [F(1,14) ≈ 4.64, P ≈ 0.05], stimulation conditions [F(1,14) ≈ 35.26, P < 0.0001], but no interaction [F(1,14) ≈ 0.44, P ≈ 0.65]. This is consistent with an interpretation that TMS to either region leads to faster RT so long as there has been sufficient learning for the sequences to be prepared in advance.
Figure 3.
RT performance. RT was faster as a function of practice exposure (a) and stimulation led to faster RT for the MOD and EXT sequences (b) relative to the MIN effect of stimulation. Note that RT stimulation effects are plotted so that larger positive values reflect faster RTs for the stimulation condition (PMd, SMA) relative to the no TMS control. Shading reflects the model that included only the MOD and EXT sequences. Error bars show SEM.
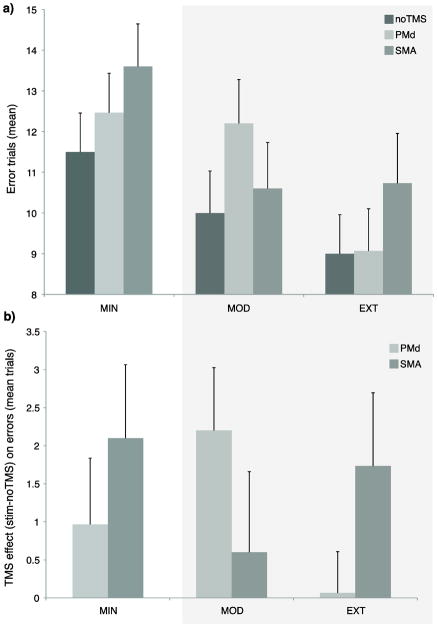
There was an effect of error across exposure conditions [F(2,28) ≈ 5.82, P ≈ 0.008, Figure 4a], and a modest effect of stimulation condition [F(2,28) ≈ 3.21, P ≈ 0.056, Figure 4a]. Planned comparisons revealed that error was higher for MIN relative to both MOD (T ≈ 2.15, P ≈ 0.05) and EXT (T ≈ 2.81, P ≈ 0.01) sequences, suggesting that MIN sequence knowledge was less developed. To evaluate stimulation effects, the difference in error between no TMS and stimulation trials were entered into an ANOVA. No differences were found using a model that included the three exposure conditions. Given the evidence for weak MIN learning, a separate model was used to compare error rates between just the MOD and EXT sequences. It revealed a significant interaction in error frequency between region and exposure condition [F(1,14) ≈ 4.20, P < 0.01, Figure 4b, shaded region]. Comparisons between EXT and MOD confirmed that the interaction was driven by a larger TMS effect for left PMd (T ≈ 2.14, P ≈ 0.05), than SMA (T ≈ 0.82, P ≈ 0.4). The interaction is not due to a shift in a simple speed-accuracy tradeoff, because RTs sped up similarly during both MOD and EXT TMS trials. The difference in error shows that each region is sensitive to TMS for sequences that have been practiced for different amounts. The errors are the consequence of TMS during the ongoing preparation of sequences prior to the go cue. Interestingly, pair-wise comparisons between no TMS and SMA stimulation trials revealed a modest effect on error rates of for EXT (one-tailed T ≈ 1.74, P ≈ 0.05), but not for MOD (one tailed, T ≈ 0.55, P ≈ 0.3). This suggests that the contribution of SMA for sequence retrieval increases as a function of the amount of practice. To determine the effect of TMS on error rates during MIN sequences, comparisons were performed between the stimulation conditions. Stimulation of the SMA increased error relative to the no TMS condition (T ≈ 2.11, P ≈ 0.05), but not for the PMd (T ≈ 1.08, P ≈ 0.3).
Figure 4.
Mean number of error trials. Error decreased as a function of practice exposure (a) and stimulation during the retrieval of these sequences led to an interaction between stimulation site and sequence type (b), such that the MOD sequences were more sensitive to stimulation of the PMd and both the MOD and the EXT sequences were sensitive to the stimulation of the SMA. Shading reflects the model that included only the MOD and EXT. Error bars show SEM.
There was an effect of MT across the exposure conditions [F(2,28) ≈ 51.06, P < 0.0001], but no effect of TMS on MT, which commenced at least 500 ms after TMS. By this point, any residual TMS effects were likely to be minimal. Depth of training, however, did have a substantial influence on MT performance. EXT sequences were produced much faster than MOD (T ≈ 6.91, P < 0.00001) and both EXT (T ≈ 8.29, P < 0.000001) and MOD (T ≈ 4.96, P < 0.001) were faster than MIN. These results confirm that sequence acquisition is strongly influenced by the amount of physical practice, which is dramatically illustrated by the continuation of practice from MOD (~500 trials) to EXT (~2000 trials).
Discussion
The motor system supports our ability to learn a multitude of skills simultaneously throughout daily life. Not all movements that we simultaneously practice are learned at the same rate. Consequently, one must be able to select, retrieve and produce motor programs that have a wide range of familiarity in order to achieve a behavioral state. For example, when learning to drive a car with a manual transmission, we might spend more time practicing shifting to first than reverse, but both sequences are needed in order to pass our driver’s exam. The objective of the current study was to investigate how the left PMd and the SMA support the simultaneous acquisition and expression of multiple sequences that are learned under different rates of exposure. We found that TMS disruption of the left PMd led to increased error during the retrieval of sequences that received moderate practice (MOD) but not for sequences that were extensively practiced (EXT). A different pattern was observed for the SMA, with TMS leading to similar increases in error during the retrieval of sequences practiced for both MOD and EXT intensities. These results suggest that PMd and SMA support the expression of sequences learned over different depths of training. An alternative interpretation is that these two regions contribute to learning on different time scales, with the SMA having a more extended influence over learning relative to the left PMd.
Because we failed to find evidence of a double-dissociation between region and depth of training, our results are inconsistent with a hypothesis that PMd and SMA support entirely distinct behavioral learning stages. Instead, our data show that these regions support the expression of sequences learned under functions with different albeit overlapping time scales. Of note, Figure 4b shows that the double-dissociation between region and exposure condition on error rate is approaching significance, and might reach significance with a larger sample size. It is also possible that a longer training period is needed to fully distinguish the slower stage of learning influence of the SMA. While the amount of training for EXT (~2100 trials/sequence) is consistent with previous long-term sequence learning studies (Lehéricy et al. 2005), it is far shorter than the intense levels of training (10,000+ trials) in non-human primate studies (Matsuzaka et al. 2007).
The differential involvement of the two premotor areas is consistent with some of their functional attributes. The left PMd is involved in visually cued sequencing (Wise 1985) is independent of the effector used (Grafton et al. 1998; Haaland et al. 2004) and is modulated by sequence complexity (Haaland et al. 2004; Verstynen et al. 2005). This is suggestive of an executive sequencing role that would be particularly important during an earlier stage of sequence learning. Our results support this interpretation, showing that left PMd supports the retrieval of sequences learned under a relatively fast time scale, as characterized by the MOD sequences. Although MOD and EXT sequences were performed with similar error rates and RTs, compared to heavily trained EXT, the MOD sequences contained fewer predictive movements and substantially slower MTs. Despite having similar explicit representations as the EXT sequences, MOD sequence production required more of the DSP spatial cues to guide the movements, consistent with the role of the PMd in the production of externally generated (EG) movements (Deiber et al. 1997; Doyon et al. 2002).
In contrast with PMd, the SMA is known to be preferentially involved in the storage and retrieval of sequential motor information that is internally generated (Mushiake et al. 1991; Jahanshahi et al. 1995; Jenkins et al. 2000) and is also involved in planning and execution of movements based on external cues (Doyon et al. 2002). We found SMA to be sensitive to TMS during the preparation of both MOD and EXT sequences. Consistent with a role of the SMA in sequence retrieval over an extended period of training, both the PMd and SMA are critical for MOD, whereas EXT requires only the SMA. Further, EXT sequences were produced with more predictive sequence movements, suggesting that sequence retrieval relies more on internally generated information with increased practice.
We also found SMA to support the production of MIN sequences, which suggests that it is critical for the accurate production of all exposure levels. However, as evidenced by recollection and predictive movements, as well as effects of error and MT across exposure conditions, it is clear that MIN sequences have a different representation than MOD and EXT sequences. The effect for MIN is consistent with a rapid increase of SMA activation observed in single-session learning studies (Jenkins et al. 1994; Jueptner et al. 1997). We suggest that the SMA supports multiple stages of sequence learning, which is consistent with the involvement of SMA during both fast and slow stages of learning (Dayan and Cohen 2011). Similarly, we found that TMS of the PMd led to increased error for MOD but not MIN sequences. It is perhaps counterintuitive that we did not find an effect of error for MIN given the functional involvement of the PMd during initial sequence learning (Jenkins et al. 1994; Jueptner et al. 1997; Doyon et al. 2002). There is no consensus for the direction of PMd functional change as some have reported increases (Doyon et al., 2002) and other decreases (Jenkins et al. 1994; Jueptner et al. 1997) in activation during initial learning. Previous neuroimaging studies of PMd activation conflate preparation and execution processes during sequencing tasks. In our study, we applied TMS at a specific point during the preparatory period, which in turn, influenced retrieval and immediate expression of the learned sequences. For MIN sequences, there appears to be less sequence information to retrieve from memory in this period.
During home training, subjects practiced all 6 sequences according to a random practice schedule. Learning in this scenario might be problematic due to RI, whereby a competing representation interferes with an initially learned motor memory that has not been stabilized through the process of memory consolidation (Goedert and Willingham 2002; Walker et al. 2003). Further, RI is practice-dependent, such that less frequently trained sequences experience even greater effects of interference (Krakauer et al. 2005; Ghilardi et al. 2009). Because three exposure levels were used during each home training session (MIN, MOD, EXT), it is possible that each condition was disrupted by a different amount of RI. Several lines of evidence suggest, however, that this does not create a significant problem for the interpretation of our results. Previous studies of RI involve 1–2 training sessions (Walker et al. 2003; Krakauer et al. 2005). In contrast, our subjects completed a minimum of 30 training sessions, and therefore it was possible that the repeating pattern of a training session followed by sleep led to deeper learning and memory stabilization for all sequences. Furthermore, we show that the subjects’ performance was determined by prior physical practice. For instance, performance was no different for MIN and MOD sequences following the completion of 200 training trials or between MOD and EXT sequences following 450 trials of training. Performance was, therefore, contingent entirely upon physical practice for a given sequence despite the fact MIN and MOD trials were more infrequently distributed across the entire experiment relative to EXT, and thus potentially exposed to greater interference. In addition, the use of preparatory cues to indicate which sequence should be retrieved provides a powerful contextual cue to reduce the likelihood of interference. It is well known that retrieval processes, when coupled with a contextual cue (Osu et al. 2004) help to solidify sequence representations from competing sources of interference. Finally, practicing different sequences in a random order, as structured in our experiment, leads to superior retention compared to practicing the same sequences in a blocked order (Shea and Morgan 1979). This has been explained in part as a result of increased recruitment of brain regions including SMA and PMd during the preparation of motor sequences (Cross et al. 2007).
It is also important to consider that we applied bilateral stimulation of the SMA, and that this might have clouded the time scale specificity or stages of learning influence of the SMA during sequence retrieval. Because localization of SMA was based on the effect of sequencing derived from acquired BOLD data, local maxima could be isolated to either hemisphere. Given its location, stimulation to either left or right SMA most likely spanned both hemispheres, which is of similar effect to previous TMS studies of SMA during sequencing that stimulated at the midline vertex (Gerloff et al. 1997; Verwey et al. 2002).
Our second main finding was that TMS to either premotor region led to a non-selective decrease in RT for sequences that could be planned in advance (MOD, EXT) relative to MIN sequences. This effect on RT is due to a specific, rather than a general TMS effect on sequence preparation. Similar to a choice reaction time task, both action selection and response preparation take place during the temporal interval between the identity cue and the go cue. Subjects could equally retrieve MOD and EXT sequences from memory during the recollection test, suggesting that the temporal delay prior to the onset of stimulation (1100–1500ms) was sufficient for selecting the appropriate sequence and unlikely that TMS overlapped with action selection. In contrast, the MIN sequences were poorly retrieved, and had less sequence information available during the same foreperiod. It is logical then, that RT would be altered for MOD and EXT compared to MIN sequences.
We suggest that during preparation, TMS interacts with the effective connectivity between the premotor regions and M1 and then modulates cortico-spinal (CS) excitability. Stimulation of M1 prior to a go cue can lead to either excitation or inhibition (Stinear et al. 2009) of motor evoked potentials (MEPs). Because PMd and SMA both have inhibitory and excitatory influence on M1 (Koch et al. 2007; Hamada et al. 2009), it is unclear how TMS influenced CS excitability. Faster RTs could be the product of reduced inhibition (Duque and Ivry 2009) or increased excitation related to the selected action (Leocani et al. 2000). There is little direct evidence of how premotor structures modulate these processes, however a recent study has shown that left PMd modulates inhibition during preparation (Duque et al. 2012). Using an innovative paired-pulse rTMS procedure, Duque et al. (2012) found that 1Hz stimulation of the left PMd reduced MEP inhibition prior to the onset of a go cue. This is consistent with an interpretation of our results that TMS reduces the hold on prepared movements, which allows for the quicker release following the go cue.
Acknowledgments
Supported by Public Health Service grant NS44393 and the Institute for Collaborative Biotechnologies through Contract W911NF-09-D-0001 from the US Army Research Office, and the National Science Foundation (DMS-0645369).
References
- Bischoff-Grethe A, Goedert KM, Willingham DT, Grafton ST. Neural substrates of response-based sequence learning using fMRI. J Cogn Neurosci. 2004;16:127–138. doi: 10.1162/089892904322755610. [DOI] [PubMed] [Google Scholar]
- Cross ES, Schmitt PJ, Grafton ST. Neural substrates of contextual interference during motor learning support a model of active preparation. J Cogn Neurosci. 2007;19:1854–1871. doi: 10.1162/jocn.2007.19.11.1854. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber MP, Wise SP, Honda M, Catalan MJ, Grafman J, Hallett M. Frontal and parietal networks for conditional motor learning: a positron emission tomography study. J Neurophysiol. 1997;78:977–991. doi: 10.1152/jn.1997.78.2.977. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA. 2002;99:1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci. 2012;32:806–816. doi: 10.1523/JNEUROSCI.4299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120:1587–1602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Moisello C, Silvestri G, Ghez C, Krakauer JW. Learning of a sequential motor skill comprises explicit and implicit components that consolidate differently. J Neurophysiol. 2009;101:2218–2229. doi: 10.1152/jn.01138.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert KM, Willingham DB. Patterns of interference in sequence learning and prism adaptation inconsistent with the consolidation hypothesis. Learn Mem. 2002;9:279–292. doi: 10.1101/lm.50102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. J Cogn Neurosci. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB. Abstract and effector-specific representations of motor sequences identified with PET. J Neurosci. 1998;18:9420–9428. doi: 10.1523/JNEUROSCI.18-22-09420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland KY, Elsinger CL, Mayer AR, Durgerian S, Rao SM. Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. J Cogn Neurosci. 2004;16:621–636. doi: 10.1162/089892904323057344. [DOI] [PubMed] [Google Scholar]
- Hamada M, Hanajima R, Terao Y, Okabe S, Nakatani-Enomoto S, Furubayashi T, Matsumoto H, Shirota Y, Ohminami S, Ugawa Y. Primary motor cortical metaplasticity induced by priming over the supplementary motor area. J Physiol. 2009;587:4845–4862. doi: 10.1113/jphysiol.2009.179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RSJ, Passingham RS. Motor sequence learning: A study with positron emission tomography. J Neurosci. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol. 1997;77:1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Mochizuki H, Marconi B, Caltagirone C, Rothwell JC. Interactions between pairs of transcranial magnetic stimuli over the human left dorsal premotor cortex differ from those seen in primary motor cortex. J Physiol. 2007;578:551–562. doi: 10.1113/jphysiol.2006.123562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci. 2005;25:473–478. doi: 10.1523/JNEUROSCI.4218-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele P-F, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123:1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Picard N, Strick PL. Skill representation in the primary motor cortex after long-term practice. J Neurophysiol. 2007;97:1819–183. doi: 10.1152/jn.00784.2006. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- Osu R, Hirai S, Yoshioka T, Kawato M. Random presentation enables subjects to adapt to two opposing forces on the hand. Nat Neurosci. 2004;7:111–112. doi: 10.1038/nn1184. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Rhodes BJ, Bullock D, Verwey WB, Averbeck BB, Page MPA. Learning and production of movement sequences: Behavioral, neurophysiological, and modeling perspectives. Hum Mov Sci. 2004;23:699–746. doi: 10.1016/j.humov.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Shea JB, Morgan RL. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J Exp Psychol Hum Learn Mem. 1979;5:179–187. [Google Scholar]
- Stinear CM, Barber PA, Coxon JP, Verryt TS, Acharya PP, Byblow WD. Repetitive stimulation of premotor cortex affects primary motor cortex excitability and movement preparation. Brain Stimul. 2009;2:152–162. doi: 10.1016/j.brs.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Tanji J. Sequential organization of multiple movements: involvement of cortical motor areas. Annu Rev Neurosci. 2001;24:631–651. doi: 10.1146/annurev.neuro.24.1.631. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol. 2005;93:1209–1222. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- Verwey WB, Lammens R, van Honk J. On the role of the SMA in the discrete sequence production task: a TMS study. Neuropsychologia. 2002;40:1268–1276. doi: 10.1016/s0028-3932(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Wise SP. The primate premotor cortex: past, present, and preparatory. Annu Rev Neurosci. 1985;8:1–19. doi: 10.1146/annurev.ne.08.030185.000245. [DOI] [PubMed] [Google Scholar]