Abstract
Cortical function and related cognitive, language, and communication skills are genetically influenced. The auditory brainstem response to speech is linked to language skill, reading ability, cognitive skills, and speech-in-noise perception; however, the impact of shared genetic and environmental factors on the response has not been assessed. We assessed auditory brainstem responses to speech presented in quiet and background noise from 1) 23 pairs of same sex, same learning diagnosis siblings (Siblings), 2) 23 unrelated children matched on age, sex, IQ, and reading ability to one of the siblings (Reading-Matched), and 3) 22 pairs of unrelated children matched on age and sex but not on reading ability to the same sibling (Age/Sex-Matched). By quantifying response similarity as the intersubject response-to-response correlation for sibling pairs, reading-matched pairs, and age- and sex-matched pairs, we found that siblings had more similar responses than age- and sex-matched pairs and reading-matched pairs. Similarity of responses between siblings was as high as the similarity of responses collected from an individual over the course of the recording session. Responses from unrelated children matched on reading were more similar than responses from unrelated children matched only on age and sex, supporting previous data linking variations in auditory brainstem activity with variations in reading ability. These results suggest that auditory brainstem function can be influenced by siblingship and auditory-based communication skills such as reading, motivating the use of speech-evoked auditory brainstem responses for assessing risk of reading and communication impairments in family members.
Keywords: auditory brainstem, speech, genetics, environment, siblings, reading
Introduction
Shared genetic and environmental influences can impact cognitive and neural function. Siblings are highly similar in their intellectual ability and other cognitive skills such as working memory, problem solving, reading, and language function (Barry, Yasin, & Bishop, 2007; Blokland et al., 2011; Deary, Spinath, & Bates, 2006; Hart, Petrill, Thompson, & Plomin, 2009; Paracchini, 2011; Petrill, Deater-Deckard, Thompson, DeThorne, & Schatschneider, 2006; Tomblin & Buckwalkter, 1998). These similarities in cognitive function are mirrored by similarities in cortical electrophysiology among siblings, true for oscillatory activity, obligatory sensory responses, and task-dependent evoked responses (Anokhin, Vedeniapin, Heath, Korzyukov, & Boutros, 2007; Boomsma, Anokhin, & de Geus, 1997; Katsanis, LIacono, McGue, & Carlson, 1997; van Baal, Boomsma, & De Geus, 2001; van Beijsterveldt, Molenaar, De Geus, & Boomsma, 1996; van Beijsterveldt & van Baal, 2002; Wright et al., 2001; Young, Lader, & Fenton, 1972). However, debate exists as to whether behavioral and neural similarities among siblings are a result of shared genetic material or shared home environment. Twins have been used as the primary model for assessing genetic versus environmental contribution to cognitive skills and electrophysiological activity and results suggest that genetic contributions play an important role in intersubject variations in cortical activity.
Oscillatory cortical activity at rest, which has been linked to cognitive abilities, language function, and risk for developmental disorders (Gou, Choudhury, & Benasich, 2011; Grice et al., 2001; Rojas, Maharajh, Teale, & Rogers, 2008), is highly correlated among monozygotic twins (Boomsma et al., 1997; van Baal et al., 2001; van Beijsterveldt et al., 1996; van Beijsterveldt & van Baal, 2002; Young et al., 1972). Correlations among dizygotic twins are lower than those for monozygotic twins, suggesting that shared genetics contribute a greater proportion of the shared variance among twins than a shared family environment (van Baal et al., 2001; van Beijsterveldt et al., 1996; van Beijsterveldt & van Baal, 2002; Young et al., 1972). Response similarity is also higher among dizygotic twins than unrelated participants matched on age and sex, further supporting the impact of genetics on oscillatory cortical activity (Young et al., 1972). While sex can affect cortical activity, studies generally find that there is no interaction between sex and heritability, suggesting the similarity among responses of female twin pairs and male twin pairs is equivalent (van Baal et al., 2001; van Beijsterveldt et al., 1996). Estimates of heritability, the amount of variance accounted for by genetic relative to environmental factors, can be as high as 79% for oscillatory cortical activity, and range from 33% to 73% for evoked cortical activity (Katsanis et al., 1997; van Beijsterveldt & van Baal, 2002).
The amplitudes of obligatory auditory cortical responses such as N1, P1, and N2 are similarly more highly correlated in monozygotic twins than dizygotic twins (Anokhin et al., 2007; Katsanis et al., 1997; Martin, Tremblay, & Stapells, 2007). Although these correlations are lower than those of oscillatory activity, the correlations between monozygotic twins can be as high as the correlation of activity across electrodes within an individual subject (Katsanis et al., 1997). As evoked cortical responses are known to be somewhat variable across subjects and even within subjects at different test times (Kileny & Kripal, 1987; Walhovd & Fjell, 2002), the strength of the relationship seen for monozygotic twins further supports that cortical activity has hereditary influences.
Task-dependent cortical activity also appears to be genetically influenced. The evoked P50 and P300 responses reflect attentional processing and change detection when stimuli are presented in an oddball paradigm (Anokhin et al., 2007; Katsanis et al., 1997; Wright et al., 2001). These task-dependent responses can be linked to cognitive skills and also predict risk for developmental disorders and addiction (Gaspar et al., 2011; Hill & Steinhauer, 1993; Orekhova et al., 2008). For both types of responses, activity of monozygotic twins is more similar than the activity of dizygotic twins, with heritability estimates of 48% to 61% for the P300 response and approximately 30% for the P50 response (Anokhin et al., 2007; Wright et al., 2001). These heritability estimates may be lower than for other cortical activity because these task-dependent responses, particularly the P50, are only weakly reliable within an individual (Anokhin et al., 2007). Evidence of moderate to strong heritability for cognitive skills among siblings supports that low heritability of the P50 is likely due to low reliability of the response within a subject (Blokland et al., 2011; Hart et al., 2009; Petrill et al., 2006; Wright et al., 2001).
Although not previously assessed, auditory brainstem function may similarly show evidence of heritability. The auditory brainstem response to complex sounds, including speech, is a reliable electrophysiologic response that reflects environmental influences on auditory processing (Hornickel, Knowles, & Kraus, 2012b; Skoe & Kraus, 2010; Song, Nicol, & Kraus, 2011b). Unlike the click-evoked auditory brainstem response, the speech-evoked response mimics the acoustics of the stimulus with considerable fidelity, to the degree that the stimulus can be identified from an acoustic playback of the brainstem response (Chandrasekaran & Kraus, 2010; Galbraith, Arbagey, Branski, Comerci, & Rector, 1995; Skoe & Kraus, 2010); please see (Skoe & Kraus, 2010) for greater detail about the response characteristics and recording techniques). The speech-evoked auditory brainstem response reflects the simultaneous and synchronous activity of multiple neural generators, that can be influenced by lifelong experience, short-term auditory training, and even directed attention (Hornickel, Zecker, & Kraus, 2012; Krishnan, Gandour, & Bidelman, 2010; Parbery-Clark, Skoe, & Kraus, 2009; Parbery-Clark, Strait, & Kraus, 2011; Rinne, Balk, Autti, Alho, & Sams, 2008; Russo, Hornickel, Nicol, Zecker, & Kraus, 2010; Russo, Nicol, Zecker, Hayes, & Kraus, 2005; Song, Skoe, Banai, & Kraus, 2011; Song, Skoe, Wong, & Kraus, 2008). The auditory brainstem response can also reflect communication disorders such as poor reading ability and speech-in-noise perception (Anderson, Skoe, Chandrasekaran, & Kraus, 2010; Anderson, Skoe, Chandrasekaran, Zecker, & Kraus, 2010; Banai et al., 2009; Chandrasekaran, Hornickel, Skoe, Nicol, & Kraus, 2009; Chandrasekaran & Kraus, 2012; Hornickel, Anderson, Skoe, Yi, & Kraus, 2012; Hornickel, Chandrasekaran, Zecker, & Kraus, 2011; Hornickel, Skoe, Nicol, Zecker, & Kraus, 2009). Children with reading impairments have deficient auditory brainstem function relative to their typically-developing peers, particularly in response to the spectrotemporally dynamic portion of the speech syllable important for linguistic meaning (i.e., the formant transition; Banai et al., 2009; Chandrasekaran et al., 2009; Chandrasekaran & Kraus, 2012; Hornickel, Anderson, et al., 2012; Hornickel et al., 2011; Hornickel et al., 2009). Because these deficits are specific to acoustic elements important for distinguishing speech sounds, deficient auditory brainstem function may reflect and/or contribute to the poor phonological processing and phonological memory skills seen for children with reading impairments (Banai et al., 2009; Boets et al., 2011; Chandrasekaran et al., 2009; Chandrasekaran & Kraus, 2012; Dufva, Niemi, & Voeten, 2001; Gathercole, Alloway, Willis, & Adams, 2006; Gibbs, 2004; Goswami, Gerson, & Astruc, 2009; Hornickel, Anderson, et al., 2012; Hornickel et al., 2011; Hornickel et al., 2009; Ramus & Szenkovits, 2008; Richardson, 2004).
Reading ability and language function are known to be hereditary (Barry et al., 2007; Friend, DeFries, & Olson, 2008; Lind et al., 2010; Petrill et al., 2006; Tomblin & Buckwalkter, 1998), with heritability estimates of 45% to 77%; however, familial influence on the speech-evoked auditory brainstem response has not previously been explored. If siblingship contributes to impaired auditory brainstem function in poor readers, it may become possible to determine the risk of younger siblings of children with reading impairments for developing reading deficits. The present study was conducted to assess the influence of siblingship and similarity of literacy skills on the auditory brainstem response to speech. We collected auditory brainstem responses to speech from 1) same sex, same learning diagnosis (reading impaired or typically-developing) sibling pairs (Siblings), 2) controls matched to one of the siblings on reading ability, IQ, age, and sex (Reading-Matched), and 3) pairs of controls matched to the same sibling only on age and sex (Age/Sex-Matched). We calculated the similarity among responses from siblings and compared that to the similarity among responses from reading- and IQ- matched controls as well as to the similarity among responses from age- and sex-matched controls using intersubject-response correlations. We predicted that response similarity among siblings would be highest, followed by response similarity for children matched on reading ability, as reading ability is known to relate to specific characteristics of the auditory brainstem response. We also expected that the response similarity in both groups would be significantly greater than response similarity for unrelated children matched only on age and sex. Although shared family environment and genetic influences cannot be disentangled, these results would suggest that auditory brainstem responses to speech reflect effects of relatedness, with implications for their use as a biological marker of reading impairment.
Methods
Participants
Participants included 113 children (83 males, 30 females) between 6 years-5 months and 14 years -10 months of age (mean= 11 years-3 months). All participants had normal hearing defined as air conduction thresholds <20 dB HL for octaves from 250–8000 Hz with air-bone threshold gaps < 10 dB for octaves 500–4000 Hz, click-evoked brainstem responses within laboratory based norms (100 μs stimulus presented at 31.3 Hz and 80 dB SPL), and 95% confidence intervals for an estimate of IQ including scores > 85 (Wechsler Abbreviated Scale of Intelligence; Wagner, Torgesen, & Rashotte, 1999). None had any current or prior neurological disorders, but 35 children with diagnosed learning impairments were included to assess relationships across a continuum of reading ability.
Children were divided into four groups based on their shared siblingship, reading abilities, age, and sex, resulting in three comparisons: Siblings, Reading-Matched, and Age/Sex-Matched (see Table 1). Participants in the current study were tested as part of three larger studies with slightly differing stimulus presentation methods. Thus, participant pairs described below were matched on study so that intersubject-response correlations were only generated for pairs of participants in the same study.
Table 1.
Group information and mean intersubject-response correlations for the Siblings, Reading-Matched, and Age/Sex-Matched comparisons.
| Comparisons | Number of pairs | Number of participants with LD diagnoses | Intersubject-response correlation (r) | |
|---|---|---|---|---|
| Quiet M (SD) | Noise M (SD) | |||
| Siblings | 23 | 10 | 0.697 (0.10) | 0.535 (0.18) |
| Reading-Matched | 23 | 11 | 0.625 (0.12) | 0.490 (0.23) |
| Age/Sex-Matched | 22 | 19 | 0.470 (0.21) | 0.339 (0.22) |
Siblings
46 of the participants were same sex siblings with identical diagnoses of learning impairments (both learning impaired or both typically-developing), and were randomly divided into two groups (Sibling 1 and Sibling 2). There were four twin pairs in the siblings groups, but no data on zygosity were available. Responses from each pair of siblings were compared to each other, resulting in 23 pairs.
Reading-Matched
For subjects in the Sibling 1 group (randomly selected members of sibling pairs), unrelated children matched for sex, age (maximum deviation = 6 months), reading abilities as assessed by the Test of Oral Word Reading Efficiency (Torgesen, Wagner, & Rashotte, 1999) and Test of Silent Word Reading Fluency (Mather, Hammill, Allen, & Roberts, 2004; maximum deviation = 5 points), and IQ scores (maximum deviation = 5 points) were identified. Responses from members of the Sibling 1 group and their reading-matched control were compared to each other, resulting in 23 pairs.
Age/Sex-Matched
For this group, unique pairs of participants were formed (not including members of the Sibling 1 group), who were matched in age (maximum deviation = 6 months) and sex to a specific member of the Sibling 1 group and to each other. These participants were not matched on IQ or reading skill with the subjects in the Sibling 1 group or with each other. Due to the large number of children of similar age and sex who were already part of the Siblings and Reading-Matched comparisons, two independent participants were chosen for this group to eliminate overlap. For the same reason, a pair of age- and sex-matched participants could not be found for one member of the Sibling 1 group and one member of the Age/Sex-Matched group differed in age from their matched pair mate by eight months. Responses from age and sex-matched children were compared to each other, resulting in 22 pairs.
The groups did not differ in age (F4, 84 = 0.208, p > 0.9), IQ (F4, 84 = 0.257, p > 0.9), reading ability (TOWRE: F4, 84 = 1.046, p > 0.3, TOSWRF: F4, 84 = 0.894, p > 0.4), or maternal education as reported in a parental questionnaire (F4, 64 = 0.187, p = 0.671).
Electrophysiological Stimulus and Recording Parameters
The six-formant speech stimulus/da/, synthesized using KLATT (Klatt, 1980), was 170 ms in length (50 ms formant transition and 120 ms steady-state vowel) with a stable fundamental frequency (100 Hz) and fourth (3300 Hz), fifth (3750 Hz), and sixth (4900 Hz) formants. The first three formants were dynamic during the formant transition period, rising from 400 to 720 Hz, falling from 1700 Hz to 1240 Hz, and falling from 2580 to 2500 Hz, respectively. The stimulus was presented in quiet and in the presence of six-talker babble background noise made up of four female and two male voices. The voices spoke grammatically correct but nonsensical sentences and were mixed in Cool Edit Pro, Version 2.1 (Syntrillium Software, 2003). The babble track for 67 of the participants was 4.7 s long; for the remaining 46 participants it was 45 s long. The signal-to-noise ratio of the babble track was set at +10 dB based on the root mean square amplitude of the entire track.
Stimuli of alternating polarity were presented at 80 dB SPL through an insert earphone (ER-3, Etymotic Research) to the right ear using the stimulus presentation software Neuroscan Stim 2 (Compumedics) with an interstimulus interval of 60 ms (n = 67) or 81 ms (n = 46). A vertical electrode montage (active Cz, forehead ground, and ipsilateral earlobe reference) was used to record responses at a sampling rate of 20 kHz using Neuroscan Acquire 4.3 (Compumedics). During electrophysiological recording, participants were seated in a comfortable chair and allowed to watch a movie of their choice. The soundtrack of the movie was played at <40 dB SPL in the testing booth, audible through the participant’s unoccluded left ear. Movie viewing encouraged participants to sit quietly and relaxed for the testing session.
Data Processing
Data processing replicated previously published studies (Hornickel, Knowles, et al., 2012b). Responses were bandpass filtered from 70–2000 Hz (12 dB/octave roll-off) and broken into 230 ms analysis windows (n = 92) or 250 ms analysis windows (n = 21; 40 ms of pre-stimulus activity in both cases). Trials with amplitude greater than ±35 μV were excluded as artifact. Responses to individual polarities were averaged and then added to create a final average of 6000 sweeps (3000 of each polarity). The addition of responses to alternating polarities eliminates the cochlear microphonic and reduces the impact of stimulus artifact, along with our use of common mode referencing and insert earphones (Aiken & Picton, 2008; Campbell, Kerlin, Bishop, & Miller, 2012; Gorga, Abbas, & Worthington, 1985).
Data Analysis
To assess the similarity of responses between matched subjects, we calculated intersubject-response correlations over 0–180 ms of the response using Matlab 7.3 (Mathworks, Natick, MA). Responses were allowed to shift up to 1.5 ms in time to yield the largest correlation coefficient between responses. These correlation coefficients represented the similarity of responses for siblings (Siblings), age-, sex-, IQ-, and reading-matched children (Reading-Matched), and age- and sex-matched children (Age/Sex-Matched). While response-response correlations are often conducted within an individual to assess the impact of background noise on the response (Parbery-Clark et al., 2009; Russo et al., 2005), this type of intersubject correlation of responses has also been utilized to assess the genetic influence on evoked cortical responses by Young and colleagues (Young et al., 1972). As would be expected, variability is higher between individuals than within an individual; however, intersubject variability in auditory brainstem responses is often quite low (Edwards, Buchwald, Tanguay, & Schwafel, 1982; Lauter & Oyler, 1992; Tusa, Stewart, Shechter, Simon, & Liberman, 1994), sizably smaller than for cortical responses (Kileny & Kripal, 1987; Walhovd & Fjell, 2002). Our observed correlations between evoked brainstem responses were similar and in some cases larger than observed correlations among dizygotic twins for both resting EEG and cortical evoked responses (Katsanis et al., 1997; Young et al., 1972), suggesting our methodology is valid.
Additionally, the within-subject variance (Within-Subjects) for participants in the Sibling 1 group was assessed by calculating the straight correlation between responses collected during the first half of the recording session (3000 presentations) and those from the second half of the recording session (3000 presentations) over the same time range (0–180 ms). Again, the responses were allowed to shift up to 1.5ms in time to find the largest correlation coefficient. This type of measure of within-subject variance has been shown to be reliable (r = 0.741) over the course of one year of growth in children with a wide range of reading ability (Hornickel, Knowles, & Kraus, 2012a; Hornickel, Knowles, et al., 2012b).
All correlation coefficients were Fisher-transformed to z-scores for statistical analyses, but were converted back to correlation coefficients for visual clarity in the figures.
Statistical Analysis
All statistical analyses were run using SPSS (IBM Corporation, Armonk, NY). The similarity of responses (intersubject-response correlations) were compared for each pairing of participants (Siblings, Reading-Matched, Age/Sex-Matched) and the within-subject response variance (Within-Subject) using paired t-tests. We chose to employ paired t-tests because the subjects were matched on a number of behavioral (reading, IQ) and/or physical characteristics (age, sex). Analyses were conducted independently for responses in quiet and those in noise. Due to data collection malfunctions, responses in noise were not available for two participants. This resulted in one missing data point each for the Siblings, Reading-Matched, and Age/Sex-Matched comparisons.
Results
As was predicted, responses from two siblings were more similar than responses from either Reading-Matched or Age/Sex-Matched pairs; however, responses from Reading-Matched pairs were also more similar than responses from Age/Sex-Matched pairs. Similarity in response characteristics due to shared familial factors or literacy skills were seen for responses to speech both in quiet and in noise. These results suggest that the auditory brainstem response to speech reflects both siblingship and similarities in reading proficiency.
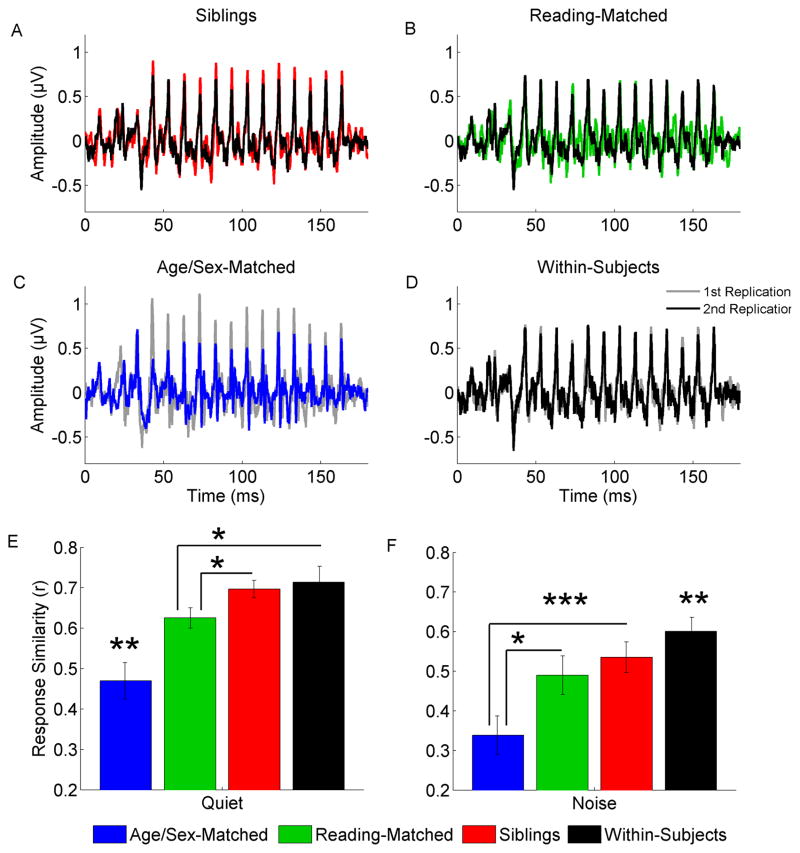
The intersubject-response correlations among pairs of siblings with the same sex and learning diagnosis (Siblings) were significantly greater than correlations among responses for two unrelated children matched on age and sex only (Age/Sex-Matched) for responses in quiet (t21 = 5.282, p < 0.001; see Figure 1. A, C, E and Table 1) and in noise (t19 = 3.799, p = 0.001; see Figure 1. F). Given that auditory brainstem response characteristics are known to pattern with reading ability, we expected that responses from Reading-Matched children would be more similar than responses from Age/Sex-Matched children. The intersubject-response correlations for Reading-Matched pairs were significantly greater than intersubject-response correlations for Age/Sex-Matched pairs for responses in quiet (t21 = 4.298, p < 0.001; see Figure 1. B, C, E and Table 1) and in noise (t19 = 2.865, p = 0.010; see Figure 1. F).
Figure 1. Auditory brainstem responses to speech in quiet and background noise reflect influences of both siblingship and similar reading ability.
A–D. A set of representative response waveforms for responses to/da/in quiet. In panels A, B, and D, the response from an individual in the Sibling 1 group is represented in black along with (A) the response of his sibling in red, (B) the response of his reading-matched control in green, and (D) the replication of his own response in gray. In panel C, the two independent children of the same age and sex are represented in gray and blue. E. Intersubject-response correlations among age-and sex-matched children were significantly weaker than for all other comparisons for responses in quiet (p ≤ 0.01). The Sibling group had stronger response similarity than children matched on reading ability, IQ, age and sex (Reading-Matched; p < 0.05), and were not significantly different from the similarity of responses within an individual, reinforcing the influence of siblingship on auditory brainstem responses to speech. Because responses from the Reading-Matched group were more similar than responses from age-and sex-matched children, there is support that auditory brainstem response morphology varies with reading skill independent of shared genes and family environment. F. As noise is known to degrade the response, the overall strength of correlations dropped when compared to speech in quiet, but Reading-Matched and Siblings groups still had more similar responses than age-and sex-matched controls (p < 0.05 and p = 0.001, respectively). The Reading-Matched and Siblings groups no longer differed in response similarity. Within-Subjects response correlations were significantly stronger than all other comparisons (p ≤ 0.01) except for the Siblings comparison where the difference was trending (p < 0.08).
When comparing the correlations among responses for two siblings (Siblings) and two unrelated but reading-matched children (Reading-Matched), the correlations between siblings were significantly greater in quiet (t22 = 2.661, p = 0.014; see Figure 1. A, B, E and Table 1), but not in noise (t21 =0.845, p = 0.407; see Figure 1. F). Background noise is known to degrade the response (Burkard & Don, 2007) and perhaps increases intersubject variability resulting in weaker similarities among responses from siblings.
Correlations among responses from siblings (Siblings) were also compared to the response variance of an individual across the recording (Within-Subject). The intersubject-response correlations from siblings were not significantly different from the within-subject correlations for responses in quiet (Within-Subjects: M = 0.709, SD = 0.19; t22 = 0.422, p = 0.677; see Figure 1. A, D, E). In noise, however, within-subject correlations tended to be larger than the inter-response correlations of siblings (Within-Subjects: M = 0.601, SD = 0.17; t21 = 1.875, p = 0.075; see Figure 1. F). The lack of difference between within-subjects correlations and inter-subject correlations from siblings is similar to observations that cortical response similarity between monozygotic twins was as great as response similarity across different scalp electrodes within an individual or responses within an individual across time (Katsanis et al., 1997; van Beijsterveldt et al., 1996). On the other hand, intersubject-response correlations for Reading-Matched children were significantly smaller than within-subject correlations for both quiet (t22 = 2.343, p = 0.015) and noise (t21 = 3.465, p = 0.002; see Figure 1. E and F, respectively).
Discussion
The aim of the present study was to shed light on the influence of shared genetic and environmental factors on the auditory brainstem response to speech. Surprisingly few studies exist investigating on the heritability of auditory brainstem function, even though responses to simple stimuli are widely used to assess hearing ability and auditory nervous system health (Hall, 2006; Hood, 1998; Sininger, 2007). In the present study we found that auditory brainstem responses from two siblings of the same sex and learning diagnosis were more similar than responses from pairs of children matched on age and sex alone, or pairs of children additionally matched on IQ and reading ability. This suggests that siblingship has a greater impact on auditory brainstem response morphology than does reading ability. In fact, responses from siblings were as similar as two responses of an individual collected within the same recording session.
A number of studies have shown that cortical activity including oscillatory activity, obligatory evoked responses, and task-dependent evoked responses are genetically influenced. In these studies, monozygotic twins had more similar responses than did dizygotic twins, indicating that much of the variance in cortical activity can be accounted for by shared genetic material beyond the impact of shared home, familial, and even uterine environment (Anokhin et al., 2007; Boomsma et al., 1997; Katsanis et al., 1997; van Baal et al., 2001; van Beijsterveldt et al., 1996; van Beijsterveldt & van Baal, 2002; Wright et al., 2001; Young et al., 1972). These studies give heritability estimates of 33%–79% for cortical oscillatory and evoked responses, which are similar to heritability estimates for risk factors of Type II diabetes (Jermendy et al., 2011; Poulsen, Dyvik, Vaag, & Beck-Neilsen, 1999). As with the risk for developing diabetes, there are environmental factors that can influence cortical activity, leading to heritability estimates of less than 100%. The strength of intersubject-response correlations we saw for our sibling pairs is similar to the strength of correlations seen for dizygotic twins in a number of studies because, presumably, our sibling pairs share the same amount of genetic material on average as dizygotic twins and similarly have a shared home environment (Katsanis et al., 1997; Young et al., 1972). There is evidence that the auditory brainstem response to speech is relatively stable during the elementary and junior high school years (Hornickel, Knowles, et al., 2012a, 2012b; Johnson, Nicol, & Kraus, 2008), but the strength of correlations seen for our sibling pairs even though they were up to 74 months apart in age (average 26 months) further supports the strong influence of relatedness on auditory brainstem function.
Similarity in auditory brainstem function also appears to be linked to common reading ability. Numerous studies have shown that poor readers have characteristic deficits in the representation of timing and harmonic elements of speech relative to their typically-developing peers (Anderson, Skoe, Chandrasekaran, & Kraus, 2010; Banai et al., 2009; Chandrasekaran et al., 2009; Chandrasekaran & Kraus, 2012; Hornickel, Anderson, et al., 2012; Hornickel et al., 2011; Hornickel et al., 2009). We expected and found that children who were matched on reading ability would show more similar auditory brainstem response morphology than children who were matched on age and sex alone, reinforcing previous results that specific auditory brainstem response characteristics are linked with reading ability. This reinforces the notion that auditory brainstem responses to speech and other complex sounds could provide a metric for assessing risk for communication disorders such as reading impairments, auditory processing disorders, and poor speech-in-noise perception. The results of the present study further support that there are characteristic differences between poor readers and their typically-reading peers in auditory brainstem responses to speech. These similarities are beyond those due to age and sex alone, both factors known to impact auditory evoked responses (Burkard & Don, 2007; Hood, 1998; Ponton & Eggermont, 2007).
Because the auditory brainstem response is reliable from test to retest in the absence of focused intervention or training (Hornickel, Knowles, et al., 2012b; Song, Nicol, & Kraus, 2011a; Song, Nicol, et al., 2011b), it may contribute to the assessment of genetic influence on auditory function. Previous studies of cortical activity report differing heritability estimates, largely because of increased within-subject variability that can occur in cortical activity (Anokhin et al., 2007; Kileny & Kripal, 1987; Walhovd & Fjell, 2002; Wright et al., 2001). If responses are only weakly reliable within a subject, strong correlations of responses between twins may not reflect heritability of the response characteristics but random variations in the individual responses. The reliability of the auditory brainstem response to speech also recommends it for clinical use, similar to the well-established use of auditory brainstem responses to simple stimuli for peripheral hearing and neural assessments (Hall, 2006; Hood, 1998; Sininger, 2007).
A number of studies have shown that task-dependent cortical activity, such as the P50 and P300 responses, can be affected by addiction, psychiatric disorders, and developmental disorders (Gaspar et al., 2011; Hill & Steinhauer, 1993; Orekhova et al., 2008; Turetsky, Cannon, & Gur, 2000). Importantly, unaffected family members of patients with these different conditions often have similar response properties as their affected family members (Benegal, Jain, Subbsukrishna, & Channabasavanna, 1995; Hill & Steinhauer, 1993; Maziade et al., 2000; Steinhauer, Hill, & Zubin, 1987; Turetsky et al., 2000). Although the family members are currently unaffected, this could suggest a higher risk for development of addiction, etc., and clearly supports that these risk factors are mediated by shared genetic and environmental factors. The genetic contribution to reading impairments and dyslexia has been supported by recent genome sequencing studies and from evidence that learning disabilities and language disorders often co-occur in family members (Barry et al., 2007; Lind et al., 2010; Paracchini, 2011; Petrill et al., 2006; Tomblin & Buckwalkter, 1998). Our results support that the auditory brainstem response to speech, known to be linked to reading ability (Anderson, Skoe, Chandrasekaran, & Kraus, 2010; Banai et al., 2009; Chandrasekaran et al., 2009; Chandrasekaran & Kraus, 2012; Hornickel, Anderson, et al., 2012; Hornickel et al., 2011; Hornickel et al., 2009), is influenced by shared genes and home environment above and beyond the similarities in reading ability.
A limitation of the current study is that only four of the sibling pairs involved were twin pairs, with unknown zygosity. Previous analyses of the genetic influence on cortical activity compared monozygotic and dizygotic twins to assess heritability. We are unable to determine heritability in the present study and are also unable to disentangle the impact of shared genes and shared home environment. As socio-economic status and parent education level impact reading ability and cognitive skills through a host of factors, and even modulate the heritability of reading skills (Friend et al., 2008; Hanscombe et al., 2012), it is possible that home environment is driving the sibling similarities in auditory brainstem responses. When performing a non-parametric analysis comparing intersubject-response correlations from our four twin pairs and the remaining nineteen sibling pairs we see no significant difference in response similarity between twins and non-twin siblings (quiet: Mann-Whitney U = 30, p = 0.557; noise: U = 24, p = 0.342). Again, we did not have any information about the zygosity of our twins and, as dizygotic twins share the same amount of genetic material on average as non-twin siblings, we would not anticipate a difference unless we could confirm monozygosity in our twin pairs. As these data also cannot disentangle shared genetic influence from shared environment, we conducted an additional analysis of maternal education, as provided by self-report from the parents. Recall that the groups did not differ on maternal education, and we additionally found that maternal education did not predict response similarity for siblings (quiet: r = −0.028, p = 0.901; noise: r = 0.016, p = 0.946) or for reading-matched children (quiet: r = 0.019, p = 0.930; noise: r = −0.143, p = 0.526). This gives some support for genetic influences on response similarity among siblings; however, future work should include a controlled study of twin pairs to assess the heritability of auditory brainstem function.
The present results suggest that the auditory brainstem response could be a particularly useful metric for assessing risk of reading impairment in children who have family members with reading disorders. Further expansion of this work could include an evaluation of the similarity among responses from parents and their children and an investigation of response similarities for siblings who differ in learning diagnosis. Both of these results would lend further support of the auditory brainstem response to speech as an appropriate marker of risk for reading impairment among family members.
Acknowledgments
The authors would like to thank the children and their families for participating and the members of the Auditory Neuroscience Lab for their assistance with data collection. We would also like to thank Trent Nicol, Samira Anderson, and Karen Chan for their review of the manuscript. This work was supported by The National Institutes of Health (R01DC01510), The National Science Foundation (SMA-1015614 and BCS-0921275\001), a Cognitive Science Interdisciplinary Research Fellowship from Northwestern University (awarded to JH), and the Hugh Knowles Center at Northwestern University.
References
- Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hearing Research. 2008;245:35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Anderson S, Skoe E, Chandrasekaran B, Kraus N. Neural timing is linked to speech perception in noise. Journal of Neuroscience. 2010;30(14):4922–4926. doi: 10.1523/JNEUROSCI.0107-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Skoe E, Chandrasekaran B, Zecker SG, Kraus N. Brainstem correlates of speech-in-noise perception in children. Hearing Research. 2010;270(1–2):151–157. doi: 10.1016/j.heares.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Vedeniapin AB, Heath AC, Korzyukov O, Boutros NN. Genetic and environmental influences on sensory gating of mid-latency auditory evoked responses: A twin study. Schizophrenia Research. 2007;89:312–319. doi: 10.1016/j.schres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Banai K, Hornickel J, Skoe E, Nicol T, Zecker SG, Kraus N. Reading and subcortical auditory function. Cerebral Cortex. 2009;19(11):2699–2707. doi: 10.1093/cercor/bhp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry J, Yasin I, Bishop D. Heritable risk factors associated with language impairments. Genes, Brain, and Behavior. 2007;6:66–76. doi: 10.1111/j.1601-183X.2006.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benegal V, Jain S, Subbsukrishna D, Channabasavanna S. P300 amplitudes vary inversely with continuum of risk in first degree male relatives of alcoholics. Psychiatric Genetics. 1995;5:149–156. doi: 10.1097/00041444-199524000-00001. [DOI] [PubMed] [Google Scholar]
- Blokland G, McMahon K, Thompson P, Martin NG, Zubicaray G, Wright MJ. Heritability of working memory brain activation. Journal of Neuroscience Methods. 2011;31:10882–10890. doi: 10.1523/JNEUROSCI.5334-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets B, Vandermosten M, Poelmans H, Luts H, Wouters J, Ghesquiere P. Preschool impairments in auditory processing and speech perception uniquely predict future reading problems. Research in Developmental Disabilities. 2011;32(2):560–570. doi: 10.1016/j.ridd.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Anokhin AP, de Geus EJC. Genetics of Electrophysiology: Linking Genes, Brain, and Behavior. Current Directions in Psychological Science. 1997;6(4):106–110. [Google Scholar]
- Burkard RF, Don M. The auditory brainstem response. In: Burkard RF, Don M, Eggermont JJ, editors. Auditory Evoked Potentials: Basic Principles and Clinical Applications. Baltimore, MD: Lippincott Williams & Wilkins; 2007. pp. 229–253. [Google Scholar]
- Campbell T, Kerlin JR, Bishop CW, Miller LM. Methods to eliminate stimulus transduction artifact from insert earphones during electroencephalography. Ear and Hearing. 2012;33(1):144–150. doi: 10.1097/AUD.0b013e3182280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Hornickel J, Skoe E, Nicol T, Kraus N. Context-dependent encoding in the human auditory brainstem relates to hearing speech in noise: Implications for developmental dyslexia. Neuron. 2009;64:311–319. doi: 10.1016/j.neuron.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N. The scalp-recorded brainstem response to speech: Neural origins. Psychophysiology. 2010;47:236–246. doi: 10.1111/j.1469-8986.2009.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N. Biological factors contributing to reading ability: Subcortical auditory function. In: Benasich AA, Fitch RH, editors. Developmental dyslexia: Early precursors, neurobehavioral markers and biological substrates. Baltimore, MD: Paul H. Brookes Publishing Co; 2012. [Google Scholar]
- Deary I, Spinath F, Bates T. Genetics of intelligence. European Journal of Human Genetics. 2006;14:690–700. doi: 10.1038/sj.ejhg.5201588. [DOI] [PubMed] [Google Scholar]
- Dufva M, Niemi P, Voeten MJM. The role of phonological memory, word recognition, and comprehension skills in reading development: From preschool to grade 2. Reading and Writing. 2001;14:91–117. [Google Scholar]
- Edwards RM, Buchwald JS, Tanguay PE, Schwafel JA. Sources of variability in auditory brain stem evoked potential measures over time. Electroencephalography and Clinical Neurophysiology. 1982;53:125–132. doi: 10.1016/0013-4694(82)90018-9. [DOI] [PubMed] [Google Scholar]
- Friend A, DeFries J, Olson R. Parental Education Moderates Genetic Influences on Reading Disability. Psychological Science. 2008;19:1124–1130. doi: 10.1111/j.1467-9280.2008.02213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith GC, Arbagey PW, Branski R, Comerci N, Rector PM. Intelligible speech encoded in the human brain stem frequency-following response. Neuroreport. 1995;6:2363–2367. doi: 10.1097/00001756-199511270-00021. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Ruiz S, Zamorano F, Altayo M, Perez C, Bosman C, et al. P300 amplitude is insensitive to working memory load in schizophrenia. BMC Psychiatry. 2011;11(29) doi: 10.1186/1471-244X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Alloway TP, Willis C, Adams AM. Working memory in children with reading disabilities. Journal of Experimental Child Psychology. 2006;93:265–281. doi: 10.1016/j.jecp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Gibbs S. Phonological awareness: An investigation into the developmental role of vocabulary and short-term memory. Educational Psychology. 2004;24(1):13–25. [Google Scholar]
- Gorga M, Abbas P, Worthington D. Stimulus calibration in ABR measurements. In: Jacobsen J, editor. The Auditory Brainstem Response. San Diego: College-Hill Press; 1985. pp. 49–62. [Google Scholar]
- Goswami U, Gerson D, Astruc L. Amplitude envelope perception, phonology and prosodic sensitivity in children with developmental dyslexia. Reading and Writing. 2009;23(8):995–1019. [Google Scholar]
- Gou Z, Choudhury N, Benasich AA. Resting frontal gamma power at 16, 24, and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behavioural Brain Research. 2011;220(2):236–270. doi: 10.1016/j.bbr.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice SJ, Spratling MW, Karmiloff-Smith A, Hailt H, Csibra G, de Haan M, et al. Disordered visual processing and oscillatory brain activity in autism and William’s syndrome. NeuroReport. 2001;12(12):2697–2700. doi: 10.1097/00001756-200108280-00021. [DOI] [PubMed] [Google Scholar]
- Hall JW. New Handbook of Auditory Evoked Responses. Boston, MA: Allyn & Bacon; 2006. [Google Scholar]
- Hanscombe K, Trzaskowski M, Haworth C, Davis O, Dale P, Plomin R. Socioeconomic Status (SES) and Children’s Intelligence (IQ): In a UK-Representative Sample SES Moderates the Environmental, Not Genetic, Effect on IQ. PLoS ONE. 2012;7(2) doi: 10.1371/journal.pone.0030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SA, Petrill SA, Thompson LA, Plomin R. The ABCs of Math: A Genetic Analysis of Mathematics and Its Links With Reading Ability and General Cognitive Ability. Journal of Educational Psychology. 2009;101(388) doi: 10.1037/a0015115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S, Steinhauer S. Assessment of Prepubertal and Postpubertal Boys and Girls at Risk for Developing Alcoholis, with P300 from a Visual Discrimination Task. Journal of Studies on Alcohol and Drugs. 1993;54:350–358. doi: 10.15288/jsa.1993.54.350. [DOI] [PubMed] [Google Scholar]
- Hood LJ. Clinical applications of the auditory brainstem response. Clifton Park, NY: Delmar Learning; 1998. [Google Scholar]
- Hornickel J, Anderson S, Skoe E, Yi H, Kraus N. Subcortical representation of speech fine structure relates to reading ability. Neuroreport. 2012;23(1):6–9. doi: 10.1097/WNR.0b013e32834d2ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J, Chandrasekaran B, Zecker SG, Kraus N. Auditory brainstem measures predict reading and speech-in-noise perception in school-aged children. Behavioural Brain Research. 2011;216(2):597–605. doi: 10.1016/j.bbr.2010.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J, Knowles E, Kraus N. Reliability of the auditory brainstem responses to speech over one year in school-age children: A reply to Drs. McFarland and Cacace. Hearing Research. 2012a doi: 10.1016/j.heares.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J, Knowles E, Kraus N. Test-retest consistency of speech-evoked auditory brainstem responses in typically-developing children. Hearing Research. 2012b;284(1–2):52–58. doi: 10.1016/j.heares.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J, Skoe E, Nicol T, Zecker SG, Kraus N. Subcortical differentiation of stop consonants relates to reading and speech-in-noise perception. Proceedings of the National Academy of Sciences. 2009;106(31):13022–13027. doi: 10.1073/pnas.0901123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J, Zecker S, Kraus N. Experience-dependent plasticity engendered by classroom FM system use. Paper presented at the Association for Research in Otolaryngology.2012. [Google Scholar]
- Jermendy G, Horvath T, Littvay L, Steinback R, Jermendy A, Tarnoki A, et al. Effect of genetic and environmental influences on cardiometabolic risk factors: a twin study. Cardiovascular Diabetology. 2011;10:96. doi: 10.1186/1475-2840-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Nicol T, Kraus N. Developmental plasticity in the human auditory brainstem. Journal of Neuroscience. 2008;28(15):4000–4007. doi: 10.1523/JNEUROSCI.0012-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanis J, Liacono WG, McGue MK, Carlson SR. P300 event-related potential heritability in monozygotic and dizygotic twins. Psychophysiology. 1997;34:47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Kileny P, Kripal J. Test-retest variability of auditory event-related potentials. Ear and Hearing. 1987;8(2):110–114. doi: 10.1097/00003446-198704000-00008. [DOI] [PubMed] [Google Scholar]
- Klatt DH. Software for a cascade/parallel formant synthesizer. Journal of the Acoustical Society of America. 1980;67(3):971–995. [Google Scholar]
- Krishnan A, Gandour J, Bidelman GM. The effects of tone langauge experience on pitch processing in the brainstem. Journal of Neurolinguistics. 2010;23(1):81–95. doi: 10.1016/j.jneuroling.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter JL, Oyler RF. Latency stability of auditory brainstem responses in children aged 10–12 compared with younger children and adults. British Journal of Audiology. 1992;26:245–253. doi: 10.3109/03005369209076643. [DOI] [PubMed] [Google Scholar]
- Lind P, Luciano M, Wright MJ, Montomery G, Martin NG, Bates T. Dyslexia and DCDC2: normal variation in reading and spelling is associated with DCDC2 polymorphisms in an Australian population sample. European Journal of Human Genetics. 2010;18:668–673. doi: 10.1038/ejhg.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Tremblay KL, Stapells DR. Principles and applications of cortical auditory potentials. In: Burkard RF, Eggermont JJ, Don M, editors. Auditory Evoked Potentials: Basic Principles and Clinical Application. Baltimore, MD: Lippincott Williams & Wilkins; 2007. pp. 482–507. [Google Scholar]
- Mather N, Hammill DD, Allen EA, Roberts R. Test of Silent Word Reading Fluency (TOSWRF) Austin, TX: Pro-Ed; 2004. [Google Scholar]
- Maziade M, Merette C, Cayer M, Roy M, Szatmari P, Cote R, et al. Prolongation of brainstem auditory-evoked responses in autistic probands and their unaffected relative. Archives of General Psychiatry. 2000;57:1077–1083. doi: 10.1001/archpsyc.57.11.1077. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, Elam M. Sensory gating in young children with autism: Relation to age, IQ, and EEG gamma oscillations. Neuroscience Letters. 2008;434:218–223. doi: 10.1016/j.neulet.2008.01.066. [DOI] [PubMed] [Google Scholar]
- Paracchini S. Dissection of genetic associations with language-related traits in population-based cohorts. Journal of Neurodevelopmental Disorders. 2011;3:365–373. doi: 10.1007/s11689-011-9091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbery-Clark A, Skoe E, Kraus N. Musical experience limits the degradative effects of background noise on the neural processing of sound. Journal of Neuroscience. 2009;29:14100–14107. doi: 10.1523/JNEUROSCI.3256-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbery-Clark A, Strait DL, Kraus N. Context-dependent encoding in the auditory brainstem subserves enhanced speech-in-noise perception in musicians. Neuropsychologia. 2011;49:3338–3345. doi: 10.1016/j.neuropsychologia.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrill SA, Deater-Deckard K, Thompson LA, DeThorne L, Schatschneider C. Reading Skills in Early Readers: Genetic and Shared Environmental Influences. Journal of Learning Disabilities. 2006;39:48–55. doi: 10.1177/00222194060390010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ. Electrophysiological measures of human auditory system maturation. In: Burkard RF, Eggermont JJ, Don M, editors. Auditory Evoked Potentials: Basic Principles and Clinical Application. Baltimore, MD: Lippincott, Williams & Wilkins; 2007. pp. 385–402. [Google Scholar]
- Poulsen P, Dyvik K, Vaag A, Beck-Neilsen H. Heritability of Type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerace - a population-based twin study. Diabetologia. 1999;42:139–145. doi: 10.1007/s001250051131. [DOI] [PubMed] [Google Scholar]
- Ramus F, Szenkovits G. What phonological deficit? The Quarterly Journal of Experimental Psychology. 2008;61(1):129–141. doi: 10.1080/17470210701508822. [DOI] [PubMed] [Google Scholar]
- Richardson U, Thomson JM, Scott SK, Goswami U. Auditory processing skills and phonological representation in dyslexic children. Dyslexia. 2004;10:215–233. doi: 10.1002/dys.276. [DOI] [PubMed] [Google Scholar]
- Rinne T, Balk MH, Autti T, Alho K, Sams M. Auditory selective attention modulates activation of human inferior colliculus. Journal of Neurophysiology. 2008;100:3323–3327. doi: 10.1152/jn.90607.2008. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale PD, Rogers SJ. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry. 2008;8(66) doi: 10.1186/1471-244X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Hornickel J, Nicol T, Zecker S, Kraus N. Biological changes in auditory function following training in children with autism spectrum disorders. Behavioral and Brain Functions. 2010;6:60. doi: 10.1186/1744-9081-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Nicol T, Zecker S, Hayes E, Kraus N. Auditory training improves neural timing in the human brainstem. Behavioural Brain Research. 2005;156:95–103. doi: 10.1016/j.bbr.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Sininger YS. The use of auditory brainstem response in screening for hearing loss and audiometric threshold prediction. In: Burkard RF, Eggermont JJ, Don M, editors. Auditory Evoked Potentials: Basic Principles and Clinical Application. Baltimore, MD: Lippincott Williams & Wilkins; 2007. pp. 254–274. [Google Scholar]
- Skoe E, Kraus N. Auditory brainstem response to complex sounds: A tutorial. Ear and Hearing. 2010;31(3):302–324. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Nicol T, Kraus N. Reply to test-retest reliability of the speech-evoked ABR is supported by tests of covariance. Clinical Neurophysiology. 2011a;122:1890–1898. doi: 10.1016/j.clinph.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Nicol T, Kraus N. Test-retest reliability of the speech-evoked auditory brainstem response. Clinical Neurophysiology. 2011b;122(2):346–355. doi: 10.1016/j.clinph.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Skoe E, Banai K, Kraus N. Training to improve hearing speech in noise: Biological mechanisms. Cerebral Cortex. 2011;122:1890–1898. doi: 10.1093/cercor/bhr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Skoe E, Wong PCM, Kraus N. Plasticity in the adult human auditory brainstem following short-term linguistic training. Journal of Cognitive Neuroscience. 2008;20(10):1892–1902. doi: 10.1162/jocn.2008.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer S, Hill S, Zubin J. Event-Related Potentials in Alcoholics and Their First-Degree Relatives. Alcohol and Alcoholism. 1987;4:307–314. doi: 10.1016/0741-8329(87)90028-0. [DOI] [PubMed] [Google Scholar]
- Tomblin J, Buckwalkter P. Heritability of Poor Language Achievement Among Twins. Journal of Speech, Language, and Hearing Research. 1998;41:188–199. doi: 10.1044/jslhr.4101.188. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency (TOWRE) Austin, TX: Pro–Ed; 1999. [Google Scholar]
- Turetsky B, Cannon T, Gur R. P300 Subcomponent Abnormalities in Schizophrenia: III. Deficits in Unaffected Siblings of Schizophrenic Probands. Society of Biological Psychiatry. 2000;47:380–390. doi: 10.1016/s0006-3223(99)00290-5. [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Stewart WF, Shechter AL, Simon D, Liberman JN. Longitudinal study of brainstem auditory evoked responses in 87 normal human subjects. Neurology. 1994;44:528–532. doi: 10.1212/wnl.44.3_part_1.528. [DOI] [PubMed] [Google Scholar]
- van Baal GCM, Boomsma DI, De Geus EJC. Longitudinal Genetic Analysis of EEG Coherence in Young Twins. Behavior Genetics. 2001;31(6) doi: 10.1023/a:1013357714500. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CEM, Molenaar PCM, De Geus EJC, Boomsma DI. Heritability of Human Brain Function as Assessed by Electroencephalography. American Journal of Human Genetics. 1996;58:561–573. [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CEM, van Baal GCM. Twin and family studies of the human electroencephalogram: A review and a meta-analysis. Biological Psychology. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Comprehensive Test of Phonological Processing (CTOPP) Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Walhovd KB, Fjell AM. One-year test-retest reliability of auditory ERPs in young and old adults. International Journal of Psychophysiology. 2002;46(1):29–40. doi: 10.1016/s0167-8760(02)00039-9. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Hansell NK, Geffen GM, Geffen LB, Smith GA, Martin NG. Genetic Influence on the Variance in P3 Amplitude and Latency. Behavior Genetics. 2001;31(6) doi: 10.1023/a:1013393327704. [DOI] [PubMed] [Google Scholar]
- Young JPR, Lader MH, Fenton GW. A Twin Study of the Genetic Influences on the Electroencephalogram. Journal of Medical Genetics. 1972;9(13) doi: 10.1136/jmg.9.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]