Abstract
Background
Thyrotropin receptor (TSHR) antibodies that stimulate the thyroid (TSAb) cause Graves' hyperthyroidism and TSHR antibodies which block thyrotropin action (TBAb) are occasionally responsible for hypothyroidism. Unusual patients switch from TSAb to TBAb (or vice versa) with concomitant thyroid function changes. We have examined case reports to obtain insight into the basis for “switching.”
Summary
TBAb to TSAb switching occurs in patients treated with levothyroxine (LT4); the reverse switch (TBAb to TSAb) occurs after anti-thyroid drug therapy; TSAb/TBAb alterations may occur during pregnancy and are well recognized in transient neonatal thyroid dysfunction. Factors that may impact the shift include: (i) LT4 treatment, usually associated with decreased thyroid autoantibodies, in unusual patients induces or enhances thyroid autoantibody levels; (ii) antithyroid drug treatment decreases thyroid autoantibody levels; (iii) hyperthyroidism can polarize antigen-presenting cells, leading to impaired development of regulatory T cells, thereby compromising control of autoimmunity; (iv) immune-suppression/hemodilution reduces thyroid autoantibodies during pregnancy and rebounds postpartum; (v) maternally transferred IgG transiently impacts thyroid function in neonates until metabolized; (vi) a Graves' disease model involving immunizing TSHR-knockout mice with mouse TSHR-adenovirus and transfer of TSHR antibody-secreting splenocytes to athymic mice demonstrates the TSAb to TBAb shift, paralleling the outcome of maternally transferred “term limited” TSHR antibodies in neonates. Finally, perhaps most important, as illustrated by dilution analyses of patients' sera in vitro, TSHR antibody concentrations and affinities play a critical role in switching TSAb and TBAb functional activities in vivo.
Conclusions
Switching between TBAb and TSAb (or vice versa) occurs in unusual patients after LT4 therapy for hypothyroidism or anti-thyroid drug treatment for Graves' disease. These changes involve differences in TSAb versus TBAb concentrations, affinities and/or potencies in individual patients. Thus, anti-thyroid drugs or suppression/hemodilution in pregnancy reduce initially low TSAb levels even further, leading to TBAb dominance. In contrast, TSAb emergence after LT4 administration may be sufficient to counteract TBAb inhibition. The occurrence of “switching” emphasizes the need for careful patient monitoring and management. Finally, whole genome screening of relatively rare “switch” patients and appropriate Graves' and Hashimoto's controls could provide unexpected and valuable information regarding the basis for thyroid autoimmunity.
Introduction
Two types of thyrotropin receptor (TSHR) autoantibodies are responsible for two distinct clinical syndromes.
Thyroid-stimulating autoantibodies (TSAb)—by activating the TSHR—are the direct cause of Graves' disease, the most common form of hyperthyroidism in humans, with a prevalence of ∼1% in the population (reviewed in Ref. 1). Extremely rarely, TSHR autoantibodies that lack agonistic activity but are competitive inhibitors of TSH binding can cause hypothyroidism, as reported more than 30 years ago (2,3). Transplacental passage of TSH blocking autoantibodies (TBAb) causing transient neonatal hypothyroidism, described in 1980 by Matsuura et al. (4), validated the occurrence of this disease. Very recently, the physical distinction between TSAb and TBAb was elegantly confirmed by the cloning and molecular analysis of TSAb K1–18 and TBAb (K1–70) from the same patient (5).
A remarkable phenomenon, and the focus of the present review, is the instance of patients who evolve from TBAb-induced hypothyroidism to TSAb-induced hyperthyroidism, or vice versa. Not surprisingly, since TBAb-induced hypothyroidism is itself very rare, alternating between these two states is highly uncommon and is usually described in case reports. Zakarija et al. (6) described a delay in the onset of neonatal hyperthyroidism because of the coexistence of TSAb and TBAb in the same serum, with more potent TBAb initially suppressing the activity of TSAb (6). Such a change in TSHR autoantibody activities passively transmitted from the mother to an immunologically normal neonate can only occur consequent to a post-transfer difference in the affinities or clearance rates of the TSAb and TBAb. However, a true alteration over time in the generation of these two TSHR autoantibodies may take place, as will be described.
In considering the literature on TSHR autoantibody-related fluctuations in thyroid function, TBAb-induced hypothyroidism should be distinguished from Hashimoto's thyroiditis (HT; even more common than Graves' disease) in which massive lymphocytic infiltration and fibrosis overwhelms the TSH-driven regenerative capacity of the thyroid gland. In some instances, a distinction between hypothyroidism caused by HT and TBAb may be difficult, because the humoral markers of HT, autoantibodies to thyroid peroxidase (TPOAb) and thyroglobulin (TgAb), frequently coexist with TSHR antibodies. Conversely, the detection of TBAb in a patient with destructive HT may not indicate the cause of hypothyroidism. The most important clinical feature that distinguishes TBAb induced hypothyroidism from HT is thyroid atrophy that occurs in the former at the onset of clinical disease. Of course, thyroid atrophy and fibrosis can also occur in HT, but in long-standing disease, not at the time of clinical presentation. Moreover, the natural course of some patients initially hyperthyroid with Graves' disease may be the development of hypothyroidism related to thyroiditis (7). Hypothyroidism induced in Graves' patients by radioactive iodine or stable iodine is also unrelated to TSHR autoantibodies.
A few reports address the question of why the pendulum may swing between the two extremes of autoantibody-mediated thyroid dysfunction, namely TBAb-induced hypothyroidism and TSAb-induced hyperthyroidism. The goal of the present review is to summarize the information available on these rare cases to seek an insight into potential factors that may contribute to this swing of the pendulum.
TSHR Antibody Definitions and Antibody Assays
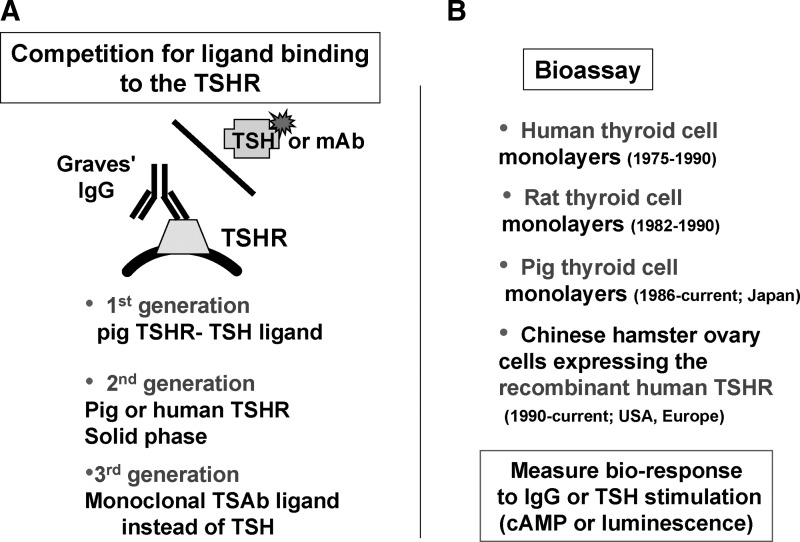
In order to interpret the literature regarding the foregoing issues, it is important to understand the information that can be provided by the wide variety of TSHR autoantibody assays employed in different studies. These assays can be separated into two groups, namely bioassays using intact cells in tissue culture, and assays involving competition for ligand binding to TSHR preparations (Fig. 1). Beginning ∼40 years ago, each type of assay has undergone extensive modifications.
FIG. 1.
Two types of thyrotropin receptor (TSHR) antibody assays. (A) Assays employing competition by TSHR autoantibodies for ligand (TSH or monoclonal antibody) binding to the TSHR. The ligand may be radio-labeled or tagged with an enzyme or fluorescent dye. The three generations of such assays are described in the text. (B) Bioassays involving cultured thyroid cells or nonthyroidal cells expressing the recombinant human TSHR.
The “first generation” of competition assays involved inhibition by TSHR antibodies for radiolabeled TSH binding to porcine thyroid membranes (8) or membrane extracts (9), extended later to the use of recombinant human TSHR (rhTSHR) extracted from transfected non-human cells (10). In “second generation” TSH binding inhibition (TBI) assays, porcine (11) or rhTSHR (12) preparations were used in solid phase rather than in solution, along with nonradioactive TSH ligand tagged with a reagent to provide a fluorescent or color signal. TSHR autoantibody competition for a tagged human monoclonal TSHR autoantibody represents a “third generation” assay (13). Both second- and third-generation TBI assays provide comparable excellent sensitivity and specificity.
Bioassays for TSAb initially employed human thyroid cell monolayers and measured the ability of TSHR autoantibodies or TSH to increase intracellular cAMP levels (14,15). A rat thyroid cell line (16) and porcine thyroid cells (primarily in Japan) (17) have also been used for this purpose. The sensitivity of the cultured thyroid cells assays was increased by using immunoglobulin G (IgG) or serum diluted in hypotonic medium (18) or in medium containing polyethylene glycol (19). In recent years, human thyroid cell assays have been replaced by Chinese hamster ovary (CHO) cells expressing the rhTSHR (20). In some assays, cAMP is indirectly detected by means of a light-generating reporter molecule (21). Since both TSH-blocking antibodies (TBAb) and TSAb will be positive in the TBI assay, only the bioassay can be used to specifically detect the former type of autoantibody. The recent use of a bioassay with CHO cells expressing a chimeric (TSH–LH) recombinant receptor to specifically detect TSAb and exclude TBAb (22) does not have a theoretical basis for such a property (23,24), as confirmed in practice (25).
The nomenclature of the TSHR competition and bioassays has been confusing and deserves comment. In our opinion, the competition assays are most simply described as TBI assays, rather than TSH-binding inhibitory Ig or TSHR antibody assays. The former is unnecessarily complex, and the latter does not distinguish the competition assays from the bioassays. It is true that TSH is no longer used in some TBI assays, but the term TBI can still describe competition for a TSHR autoantibody. With regard to the bioassays, we use the term TSAb (thyroid-stimulating antibody) rather than the older term TSI (thyroid-stimulating Ig) for consistency with TBAb (TSH-blocking antibodies). In our view, the commonly used term TSBAb (TSH or thyroid-stimulating blocking antibody) is redundant and a tongue twister; TSH is inherently a stimulator.
Most important in assessing reports on TBAb activity is whether TSAb activity is also detected in the same serum. Although both types of autoantibodies have been proved to coexist in the same blood sample from a hypothyroid patient (5), this concurrence in other patients may be difficult to establish on the basis of the TBAb bioassay alone. The TBAb bioassay, by necessity, requires inhibition of a low TSH concentration, and the affinities of TSHR autoantibodies and TSH are comparable. Due to the large degree of overlap between the TSH- and TSAb-binding sites, all TSAb will compete for TSH binding. However, the inhibition of TSHR activation after TSH binding may be masked to a greater or lesser extent depending on the potency of the TSAb. As stated in Goodman and Gilman, “A partial agonist…can compete with a ‘full’ agonist for binding to the receptor. However, increasing concentrations of a partial agonist will inhibit (the) response to a finite level characteristic of the drug's intrinsic efficacy; a competitive antagonist will reduce the response to zero.”(26)
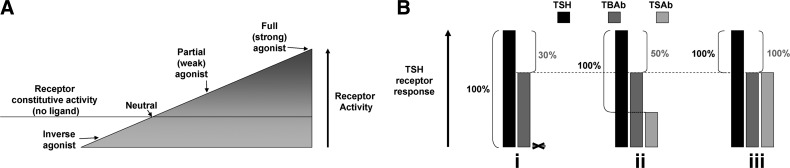
Some background information is helpful to understanding this concept. The TSHR, similar to many receptors, has a degree of ligand-independent or constitutive activity (27,28). A pharmacological principle is that a partial (or weak) agonist (Fig. 2A) is also an antagonist. Therefore, a TSAb that is not a full (or strong) agonist may also be an antagonist for TSH and can be measured as a TBAb in the bioassay. A positive TBAb assay in a goitrous hyperthyroid patient may, rather, reflect the presence of TSAb. In our opinion, unless TSAb activity is absent, detection of TBAb in serum can only be established definitively if the donor has hypothyroidism with an atrophic thyroid gland (absence of TSH activity). Assuming acceptance of this logic, many reports describing the concurrent presence of TSAb and TBAb activities in the same serum are open to question.
FIG. 2.
Difficulty in measuring TSH-blocking autoantibodies (TBAb) in the presence of thyroid-stimulating autoantibodies (TSAb). (A) Schematic depiction of a range of agonists of increasing potency. The TSHR, similar to many receptors, has a degree of ligand-independent or constitutive activity (27,28), as shown by the horizontal line through the wedge. TSHR ligands (TSH or TSAb) further increase receptor activity (not shown to scale). An inverse agonist suppresses constitutive activity, whereas a full agonist maximally activates the receptor. Ligands of intermediate activity are either neutral agonists (no activation of the receptor or suppression of constitutive activity) or partial agonists or inverse agonists. Ligands can also be an antagonist for another ligand depending on their relative affinities and binding sites. Therefore, a TSAb that is a partial (not a full) agonist can also be an antagonist for TSH. If a serum displays both TSAb and TBAb activity, unless the former is very weak and the latter is very strong, it cannot be assumed that there are two separate antibodies. (B) Difficulty in quantifying TBAb activity in the presence of TSAb. All TBAb bioassay data are expressed as the percent decrease in TSHR activation induced by TBAb relative to a baseline denominator, the latter being the activity of a standard, constant, and low TSH concentration. However, if TSAb activity is also present, different reports in the literature either do, or do not, subtract this TSAb value from the baseline denominator. This variation can have a major effect on deciding whether a TBAb is positive. For example, in a serum lacking TSAb activity (i), a patient's IgG or serum may inhibit TSH activity by 30%. Since many reports require 30%–40% inhibition of TSH activity to establish TBAb positivity, this serum would be regarded as TBAb negative. However, if weak intrinsic TSAb activity (ii) is present in the same serum and is subtracted to establish the “100%” TSH denominator, a 30% suppression of TSH activity represents a calculated TBAb activity of 50%, which is now positive. In an extreme example (iii), a stronger TSAb serum occupying most TSHR on the cell surface is a partial agonist, generating a 70% signal of that for TSH. This serum suppresses TSH activity by the same 30% but has a calculated TBAb of 100% after TSAb subtraction despite the absence of TBAb. In our view, therefore, it is preferable not to subtract TSAb activity in calculating the TBAb assay data.
Another factor that obfuscates measuring TBAb in the presence of TSAb is the formula used to calculate TBAb activity. All TBAb bioassay data are expressed as the percent inhibition of the activity of a standard, constant, and low TSH concentration measured in the absence of an antibody. However, if TSAb activity is also present, different reports in the literature use a different control denominator to quantitate TBAb activity. At face value (and as used in some reports), it seems logical to subtract intrinsic TSAb activity from control TSH activity to establish the baseline denominator to calculate TBAb activity. However, this maneuver may introduce a major quantitative error. For example, in a serum lacking TSAb activity, a patient's IgG or serum may inhibit TSH activity by 30% (Fig. 2B-i). Since many reports require 30%–40% inhibition of TSH activity to establish TBAb positivity, this serum would be regarded as TBAb negative. However, if weak intrinsic TSAb activity (Fig. 2B-ii) is present in the same serum and is subtracted to establish the “100%” TSH denominator, a 30% suppression of TSH activity represents a calculated TBAb activity of 50%, which is now positive. In an extreme example, a stronger TSAb serum that occupies the bulk of TSHR on the cell surface and is a partial agonist generates a signal 70% of that for TSH (Fig. 2B-iii). This serum suppresses TSH activity by the same 30% but has a calculated TBAb of 100% despite the absence of TBAb. In our view, therefore, it is preferable not to subtract TSAb activity in calculating the TBAb assay data. (Even better is not to measure TBAb in serum with TSAb activity, although in some circumstances it may be necessary.) We have applied these criteria to interpreting the TBAb literature, as described in the next few sections.
TBAb-Positive Hypothyroid Patients That Switch to TSAb and Hyperthyroidism
TSAb and hyperthyroidism develop unexpectedly in some patients with hypothyroidism that is caused by TBAb (Table 1) (29–33). This shift in thyroid function occurs only rarely in a disease that is, in itself, rare. Thus, in the largest series to date of TBAb-induced hypothyroid patients studied over a period of 10 years, Takasu and Matsushita reported that only 5.9% (2 of 34) of such patients developed TSAb and became hyperthyroid (33). A small number of patients with hypothyroid HT without TBAb later develop hyperthyroidism associated with the appearance of TSAb, as well as with increased titers of serum TgAb and/or TPOAb (Table 2) (31,34).
Table 1.
Switch from Hypothyroidism Involving Thyrotropin Receptor-Blocking Antibodies to Hyperthyroidism Caused by Thyroid-Stimulating Antibodies
| |
|
Thyroid autoantibodies |
|
|||
|---|---|---|---|---|---|---|
| Sex, age (years) | Status | TBAb | TSAb | TgAb | TPOAb | Ref. |
| F, 48 | Hypo | 96 | 93 | 6×106 | 105 | (29) |
| Eu | 77 | 114 | 6400 | 1600 | ||
| Hyper | 31 | 163 | 6400 | 6400 | ||
| M, 40a | Hypo | 89 | 92 | Neg | 100 | (30) |
| Eu | 100 | 100 | ||||
| Hyper | 12 | 2703 | ||||
| F, 38b | Hypo | 96 | 92 | 802 | (31) | |
| Hyper | 2 | 1490 | ||||
| Eu | Neg | Neg | ||||
| F, 55 | Hypo | (32) | ||||
| Hyper | Neg | 576 | Neg | Neg | ||
| Eu | 18 | 275 | ||||
| Hyper | 48 | 593 | ||||
| F, 45 | Hypo | 98 | 110 | (33) | ||
| Hyper | 20 | 900 | ||||
| M, 38 | Hypo | 97 | 98 | (33) | ||
| Hyper | 1100 | 1100 | ||||
All patients were treated with LT4. Thyroid status at two or more time points is indicated. Patients are characterized by sex, age, TSAb, TBAb, TgAb, and TPOAb. Values for all thyroid autoantibodies are TSAb μU/mL bTSH equivalent; Neg, negative (undetectable); blanks, data not reported. TgAb and TPOAb are reciprocal titers. Bold indicates positive TSAb or TBAb values.
Concomitant myasthenia gravis (30).
Only one of seven patients (#5) described by Takasu et al. (31) had hypothyroidism due to TBAb and a switch to TSAb during hyperthyroidism.
Eu, euthyroidism; F, female; hyper, hyperthyroidism; hypo, hypothyroidism; LT4, levothyroxine; M, male; TBAb, TSH blocking autoantibodies; TgAb, autoantibodies to thyroglobulin; TPOAb, autoantibodies to thyroid peroxidase; TSAb, thyroid-stimulating autoantibodies.
Table 2.
Unexpected Development of Hyperthyroidism Caused by Thyroid-Stimulating Autoantibodies in Hypothyroid Patients with Hashimoto's Thyroiditis Lacking Thyrotropin-Blocking Autoantibodies
| |
|
Thyroid autoantibodies |
|||
|---|---|---|---|---|---|
| Sex, age (years) | Status | TBAb | TSAb | TgAb | TPOAb |
| F, 23 | Hypo | 3202 | 802 | ||
| Hyper | Neg | 1900 | 6402 | 3202 | |
| Eu | Neg | 100 | 802 | Neg | |
| F, 53 | Hypo | 6402 | 3202 | ||
| Hyper | Neg | 780 | 6402 | 3202 | |
| Eu | Neg | 96 | 1602 | 802 | |
| Hyper | Neg | 1050 | 6402 | 3202 | |
| Eu | Neg | 100 | 1602 | 802 | |
| F, 28 | Hypo | (80) | (160) | ||
| Hyper | Neg | (900) | 6402 | 1602 | |
| F, 42 | Hypo | pos | pos | ||
| Hyper | 802 | 1602 | |||
| F, 38 | Hypo | 6402 | 6402 | ||
| Hyper | Neg | 12802 | 12802 | ||
| F, 80 | Neg | 1583a | 1300 units | ||
First five patients from Ref. (31); sixth patient from Ref. (34). Values for all thyroid autoantibodies are TSAb μU/mL bovine thyrotropin (bTSH) equivalent; Neg, negative (undetectable); blanks, data not reported. TgAb and TPOAb are reciprocal titers. Bold indicates positive values for TSAb or TBAb, or increased values for TgAb or TPOAb after LT4. Parentheses indicate values estimated from the figures in Ref. (31) for TgAb, TPOAb, or TSAb activity.
% Stimulation of cAMP.
Hyperthyroid Patients That Switch from TSAb to TBAb and Hypothyroidism
In Graves' disease, the spontaneous change from hyperthyroidism to hypothyroidism (without ablative intervention with radioactive iodine or overtreatment with anti-thyroid drugs) may occur in two ways: by the unexpected development of TBAb (Group I, Table 3); or because the process of thyroid damage reflected in chronic lymphocytic thyroiditis overcomes the stimulatory effects of TSAb and, eventually, TSH (Group II, Table 3). Our focus is on Group I, namely TSAb-positive hyperthyroid Graves' patients who develop TBAb-induced hypothyroidism after treatment with anti-thyroid drugs. In the first unequivocal report of this syndrome by Tamai et al. (35), TSAb activity disappeared and was replaced by potent TBAb activity in a patient in parallel with the transition from hyper- to hypothyroidism. In a subsequent study by this group, approximately one quarter (6 of 26; 23%) of hyperthyroid Graves' patients developed hypothyroidism in association with TBAb and a marked decrease in goiter size after withdrawal of anti-thyroid drugs (36). This proportion is slightly lower than that reported in the latter study (31%), because we have excluded two patients whose TBAb activity was equivocal owing to high concurrent TSAb activity (see above). Similar reports on smaller numbers of patients have appeared over the past 25 years (e.g., 5,32,33,37,38). In some unusual patients, thyroid function can fluctuate between hyper- and hypothyroidism in accordance with alterations in TBAb and TSAb detected in serum (32,38).
Table 3.
Thyroid Autoantibodies at the Time Hypothyroidism Developed in Graves' Patients Treated with Anti-Thyroid Drugs
| |
Autoantibodies |
|
|||
|---|---|---|---|---|---|
| Sex, age (years) | TSAb | TBAb | TgAb | TPOAb | Ref. |
| Group I | |||||
| F, 35 | 80 | 85 | 640 | 72,900 | (37) |
| F, 20 | 80 | 83 | 212 | 212 | (36) |
| F, 24 | 358 | 57 | 27 | (36) | |
| F, 18 | 68 | 97 | 212 | (36) | |
| F, 39 | 102 | 87 | 24 | 212 | (36) |
| M, 36 | 97 | 92 | 22 | 210 | (36) |
| F, 62 | 104 | 90 | 212 | (36) | |
| F, 42 | 1252 | 55 | 22 | 26 | (36) |
| M, 29 | 101 | 98 | 212 | (36) | |
| F, 55a | (32) | ||||
| B | 576 | Neg | Neg | Neg | |
| Eu | 275 | 18 | |||
| A | 593 | 48 | |||
| F, 40 | (38) | ||||
| B | 295 | 58 | 100 | 6400 | |
| Eu | 76 | 97 | 100 | 400 | |
| A | 484 | 45 | 400 | 1600 | |
| F, 54b | 313 | 82 | Neg | 500 | (5) |
| F, 40 | (33) | ||||
| B | 1625 | 2 | |||
| A | ∼150 | ∼98 | |||
| F, 48 | (33) | ||||
| B | 800 | 5 | |||
| A | ∼200 | ∼98 | |||
| Group II | |||||
| F, 48 | <140 | 10.0 | >104 | >105 | (37) |
| F, 54 | 101 | −2.0 | >104 | >106 | (37) |
| F, 30 | 902 | 6.8 | 210 | 212 | (36) |
| F, 52 | 182 | −17.4 | 28 | 212 | (36) |
| F, 47 | 432 | −12.4 | 28 | 212 | (36) |
| F, 25 | 279 | −4.0 | 24 | 212 | (36) |
| F, 25 | 190 | 6.7 | 212 | (36) | |
| F, 33 | 11,429 | ND | 210 | (36) | |
| F, 27 | 312 | −10.9 | 24 | 28 | (36) |
| F, 42 | 1290 | 22.0 | 26 | 210 | (36) |
Group I patients: hypothyroidism due to the appearance of TBAb; Group II patients: hypothyroidism due to thyroiditis. Where available, data are included before (B), when euthyroid (Eu), and after (A) development of hypothyroidism. Values for all thyroid autoantibodies are TSAb μU/mL bTSH equivalent; Neg, negative (undetectable); blanks, data not reported; ND, TSAb too high for reliable TBAb measurement. TgAb and TPOAb are reciprocal titers. Bold indicates values positive for TSAb or TBAb.
Patient initially hypothyroid without detectable TBAb subsequently fluctuated between hyper- and hypothyroidism with associated TSAb and TBAb (32).
Patient from whom monoclonal TBAb and TSAb were derived (5).
Pregnancy and Postpartum TSAB/TBAb Switches
As mentioned in the Introduction section, dilution analysis of sera from a mother and her two children who developed delayed neonatal hyperthyroidism indicated the presence of TSAb and an inhibitory IgG (presumably TBAb) (6). The hyperthyroidism emerged as the blocking IgG concentration diminished. TSHR antibodies are not generated in the neonates but are present because of passive transfer from the mother. Therefore, any change in the balance between TSAb and TBAb should occur as the antibodies are cleared from the neonate's blood and their concentrations diminish. There are two possible explanations for this phenomenon. First, both TSAb and TBAb are cleared at the same rate, but TSAb are initially at a higher concentration or have a higher affinity than TBAb. However, present evidence suggests that TBAb concentrations sufficient to cause hypothyroidism are far higher than TSAb levels that induce hyperthyroidism (e.g., Refs. 39,40), and both types of antibodies are of very high affinity.
The second possible explanation for a shift in the functional antibody balance is that TBAb are cleared more rapidly than TSAb. It is difficult to envisage how such a difference in clearance rate could occur unless the former were of subclass IgG3, which has a shorter half-life than IgG1, IgG2, or IgG4 (41). Serum TSAb are of subclass IgG1 (42), sometimes IgG4 (43), and the monoclonal human TSAb and TBAb isolated to date are IgG1 (5,44,45). Although IgG1 was the predominant subclass, in some patients, TBAb activity was detected in subclasses IgG2, 3, and 4 (46). Consequently, it is possible that TBAb activity of IgG3 subclass could play a role in the shift from blocking to stimulating activity.
The difficulty in interpreting the data for TBAb in the presence of TSAb activity is worth reiterating. It should also be pointed out that these TSHR antibodies are measured in vitro at concentrations (typically dilutions) different from those occurring in vivo. Nevertheless, regardless of the difficulties in explaining the phenomenon, the thyroid function resulting from these autoantibodies is most informative. Thus, transient hyper- or hypothyroidism in a neonate indicates the in vivo balance of TSAb or TSAb, even when questions arise as to the validity of assaying both antibodies in the same serum. For example, even though both TSAb and TBAb were detected in the sera of three infants found to be hypothyroid at birth (neonatal screening), TBAb activity was more potent (47). These TSHR antibody levels reflected those in the mothers, who were hypothyroid on thyroid hormone replacement.
In contrast to passively transferred TSHR autoantibodies, alterations in the TSAb/TBAb balance have also been studied during the course of pregnancy, reflecting possible changes in both antibody generation and clearance. In a group of 13 euthyroid pregnant Graves' patients (6 not requiring treatment and 7 on low-dose antithyroid drugs discontinued during the second trimester), TBAb activity increased and TSAb activity decreased from the second to the third trimester (48). Although data are not presented for individual patients, the shift in the TSAb/TBAb balance in the group is convincing. Possible contributing factors include the amelioration of Graves' disease that occurs in pregnancy or the discontinuation of anti-thyroid drugs, as will be discussed. Two other case reports are of interest: (i) transient Graves' disease and TSAb developed during pregnancy in a patient with Hashimoto's hypothyroidism treated with levothyroxine (LT4) (49); and (ii) maternal transient hypothyroidism caused by TBAb developing in the postpartum period (50).
Switch from TSAb to TBAb in an Animal Model of Graves' Disease
Graves' disease can be induced in susceptible mouse strains by repeated immunization over 9–12 weeks with cDNA encoding the full-length TSHR or the TSHR A-subunit using plasmid or adenoviral vectors (reviewed in Ref. 51). In order to break self-tolerance in the mouse, the human TSHR or its A-subunit is usually employed. Sera from TSHR or A-subunit immunized mice contain both TSAb and TBAb activity (e.g., Ref. 52). Importantly, in order for the mice to develop hypothyroidism, TSAb induced by immunization against the human TSHR should cross react with the mouse TSHR (53). Five or more months after immunization, TSAb activity gradually declines. Although some mice remain hyperthyroid (54), most return to the euthyroid state (55).
Recently, approaches have been developed in order to investigate the outcome of immunization with the mouse TSHR A-subunit, which is similar to, but not identical with, the human A-subunit. Self-tolerance prevents antibody induction in wild-type mice. Consequently, a two-step approach is required. First, TSHR knockout mice (which lack tolerance to the mouse TSHR) develop TSHR antibodies when immunized with adenovirus expressing the mouse TSHR A-subunit (56). Second, primed immune cells from these animals are adoptively transferred to athymic mice to determine their effect on thyroid function (57). The transferred immune cells do not reconstitute the defective immune system of athymic mice but instead generate TSHR antibodies for extended time periods. In the recipient mice, both TSAb and TBAb activity are detectable 4–8 weeks after transfer but after 24 weeks, TSAb activity and hyperthyroidism switch to TBAb activity and hypothyroidism (57).
Mechanisms Potentially Involved in Changes from TBAb to TSAb (or Vice Versa)
A number of mechanisms may be involved in switching from TBAb to TSAb or vice versa. These include effects on thyroid autoantibody responses and immune responses in general, as well as inherent characteristics of TSAb and TBAb themselves.
Effects of therapy for autoimmune thyroid diseases on the immune response
Treatment with LT4
The effect of LT4 treatment on thyroid autoantibody levels is an old “war-horse,” with innumerable reports with variable results spanning more than 50 years. More recent longitudinal studies suggest that LT4 therapy has little or no effect on thyroid microsomal/TPO or Tg autoantibodies in euthyroid individuals (58,59) or in patients with subclinical hypothyroidism (60). On the other hand, LT4 therapy is reported to decrease the levels of these antibodies in hypothyroid patients (61,62). TSHR antibody levels (TBI assay) were reduced in patients with agoitrous, but not goitrous, hypothyroidism treated with LT4 (63). Total IgG levels, which are elevated in hypothyroid patients, decrease after LT4 administration (64), although such changes may reflect alterations in plasma volume and IgG clearance (as for many other plasma components).
In contrast to LT4 treatment decreasing thyroid autoantibody levels, other studies have reported increased levels of autoantibodies to the TSHR (TBI and TSAb assays; Table 1) and to TPO (Table 2). Significantly, from the perspective of the present review on the shift in TSHR autoantibody functional activities, examples of a LT4 associated increase in TSAb and the development of hyperthyroidism in hypothyroid patients is clearly depicted by Takasu and Matsushita (33). In another patient who swung between hypo- and hyperthyroidism, TSAb activity was first detected after LT4 therapy for hypothyroidism (32). Taken together, the data from these case reports show that, at least in a select group of patients, LT4 treatment is associated with an increase in ongoing thyroid autoantibody responses as well as in the de novo appearance of TSAb in HT patients with or without TBAb.
What is the basis for the increase in thyroid autoantibodies after LT4 treatment? One possibility is that this association is simply fortuitous. Overt hypothyroidism is always treated with thyroid hormones, and it would be unethical to follow appropriate controls, that is, what hypothyroid patients followed over time without therapy. However, there is no doubt that thyroid hormones have complex effects on the innate and adaptive immune responses (reviewed in Ref. 65). Based on studies by other researchers, we hypothesize that the following mechanisms could play a role. First, dendritic cells are potent antigen-presenting cells, and regulatory T cells (Treg) control responses to both autoantigens and conventional antigens. Elevated thyroid hormone levels influence the phenotype and function of dendritic cells, including increased expression of costimulatory molecules required to initiate antibody production (66). Second, hyperthyroidism polarizes dendritic cells, which leads to impaired functional Treg, changes that potentially permit the emergence of pathogenic autoantibodies (67) such as TSAb. It should be appreciated that the range of thyroid hormones is narrower for a particular individual than the normal range for a population (68). Consequently, despite the goal of treatment to “normalize” thyroid hormone levels, serum T4 may be elevated for a particular individual, and the resulting hyperthyroidism will adversely impact dendritic cell and Treg function.
Effects of antithyroid drugs
Treatment with anti-thyroid drugs, in particular methimazole, decreases TPO autoantibodies and TSHR autoantibodies measured as TBI or TSAb activity as illustrated in a 5 year survey (69). Studies of peripheral blood lymphocytes in vitro suggested that methimazole, at least at high concentrations, inhibited thyroid autoantibody synthesis, paralleling the in vivo effects of the drug (70). An alternative hypothesis suggests that hyperthyroidism worsens thyroid autoimmunity, in agreement with the deleterious effects of elevated T4 effects mentioned earlier, and that restoring euthyroidism (as achieved by anti-thyroid drugs) reduces thyroid autoimmunity (71).
Pregnancy, postpartum, and neonatal disease
Thyroid autoantibody levels usually decrease during pregnancy due to immunosuppression and/or hemodilution and are lowest shortly before delivery. In the postpartum period, thyroid autoantibody levels rebound and may exceed the levels detected in early pregnancy. Apart from changes in the mother, maternally transferred IgG-containing thyroid autoantibodies may be present in newborns, and the levels of these antibodies disappear as the maternal IgG is metabolized.
TSAb to TBAb changes in a mouse model of Graves' disease
The observations from a mouse model are relevant because of their similarity to transient thyroid dysfunction in neonates born to mothers with TSAb and TBAb activity in their sera. Both neonates and athymic mice receive immune response components with “term limits.” In neonates, maternally transferred antibodies have defined half-lives; in athymic recipient mice, immune cells undergo differentiation, including plasma cell secretion, but no additional immune cells are recruited to perpetuate the immune response. Thus, in terms of TSHR antibodies and thyroid function, the athymic mouse recipients of splenocytes from mouse-TSHR immunized donors (57) resemble human neonates with maternally transferred TSAb and TBAb in which the blocking activity predominates (47).
Strength of the antigenic stimulus to TSHR antibody generation
Although no data are available for spontaneous Graves' disease occurring in humans, evidence from an induced mouse model of Graves'-like hyperthyroidism suggests that the strength of immunization alters the balance between TSAb and TBAb generation. Thus, immunization with decreasing doses of adenovirus expressing the TSHR shifts the balance of TSHR antibody generation toward TSAb (and hyperthyroidism) away from TBAb (52).
Inherent properties of TSAb and TBAb
The concentrations of TSAb and TBAb in vivo and their affinities for the TSHR play a critical role in the balance of the two activities. Theoretically, the clinical status of a patient with TSHR autoantibodies reflects the “algebraic sum” at a particular time of a pool of TSAb and TBAb of varying concentrations and affinities for antigen. The concentrations of these autoantibodies may change through therapeutic intervention (e.g., LT4, antithyroid drugs), normal physiological changes (pregnancy), or through clearance from plasma (reduction in antibody generation or after passive transfer in neonates, as discussed earlier). As concentrations of a mixed pool of TSAb and TBAb decline (assuming equivalent clearance rates), those at a higher concentration and of a higher affinity are likely to predominate and may alter the clinical presentation.
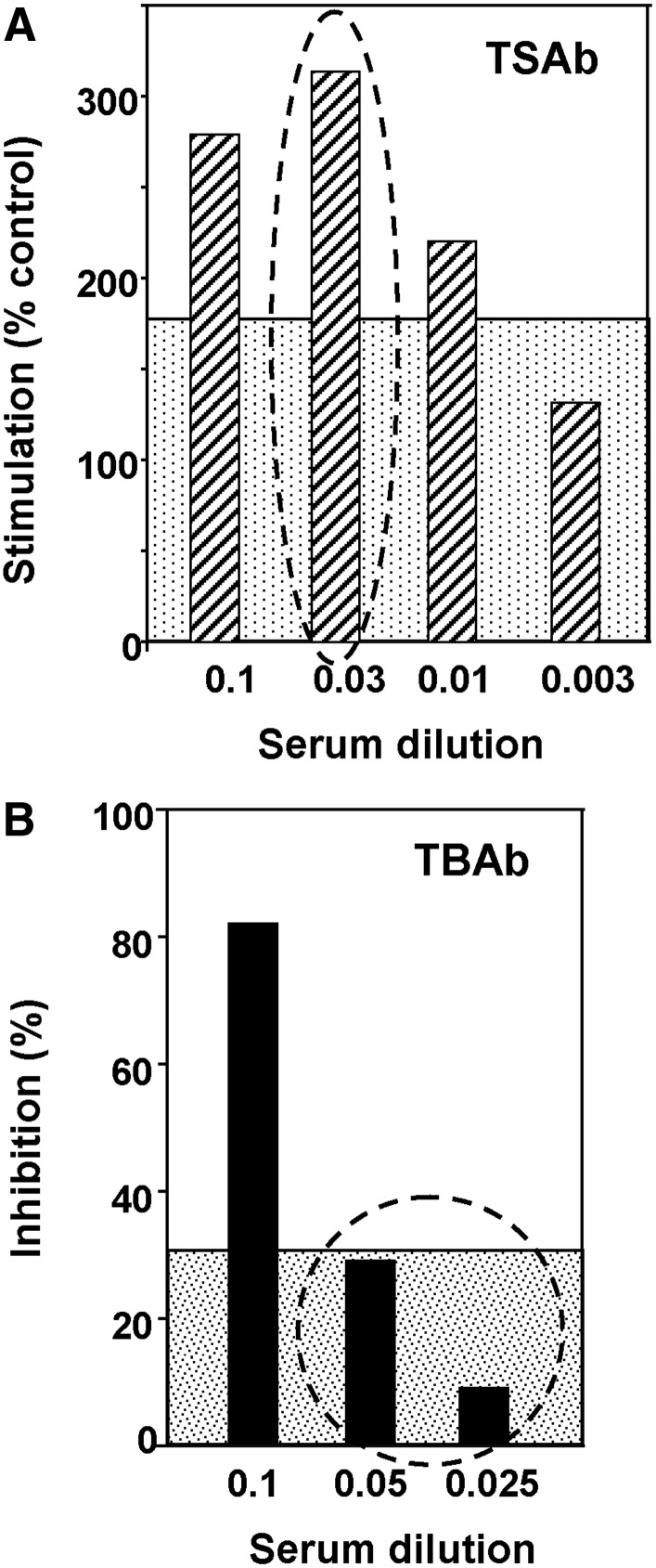
Assays of TSAb and TBAb activities in serial dilutions of a serum can illustrate the foregoing in vivo phenomenon. A recent instructive example is dilution of a serum from a patient with TBAb-induced hypothyroidism that also contains TSAb activity (5). Notwithstanding the difficulties in detecting TBAb in the presence of TSAb (see above), the isolation of a monoclonal TBAb (K1–70) and TSAb (K1–18) from the same blood samples proves the simultaneous presence of such antibodies. Although the patient is hypothyroid, at a serum dilution of 1:33 TSAb remains clearly positive; whereas TBAb activity at similar dilutions of 1:20 and 1:40 is no longer detectable (Fig. 3).
FIG. 3.
(A) TSAb and (B) TBAb at different dilutions in the TBAb-positive hypothyroid patient from whom monoclonal TSAb and TBAb were isolated (5). Data from Table 1 are plotted.
However, a detailed analysis of these data, the first on human monoclonal TSHR autoantibodies and polyclonal antibodies from the same patient, reveals surprising complexity. Thus, monoclonal TBAb K1–70 has an affinity for the TSHR which is fivefold higher than that of TSAb K1–18 (5). Moreover, in previous studies on whole serum, TBAb concentrations associated with hypothyroidism are far higher than TSAb concentrations that can cause hyperthyroidism (39,40). Therefore, if the concentration and affinity of TBAb K1–70 is higher than TSAb K1–18, the latter should dilute out more rapidly, the reverse of what is observed. A possible explanation for this apparent discrepancy is that monoclonal K1–70 and K1–18 are not representative of polyclonal TSAb and TBAb at varying stages of affinity maturation present in the patient's serum. Indeed, K1–18 has a slightly lower affinity for the TSHR than human monoclonal TSAb M22 isolated from another patient (72). It should also be appreciated that steric hindrance to TSAb (but not TBAb) binding to the TSHR may alter the apparent affinity determined in vitro (39,73).
It should be emphasized that mutations in the Ig receptors of B cells generate antibodies of the same specificity but with higher affinity. The de novo appearance of TSAb in an HT patient represents the appearance of a B cell clone with a new combination of heavy and light chains.
Other Characteristics of Patients Who “Switch” from TBAb to TSAb or the Reverse
Are there other characteristics of patients who switch from TBAb to TSAb (or vice versa)? The relatively sparse available information for this unusual group of patients is related to age, sex, and ethnicity (5,29–33,36–38,47–50,74). The majority of patients with TBAb/TSAb switches (or the reverse) are women, and they have the same age range as other patients with thyroid autoimmune disease (Table 4). In terms of genetic background, most reports of TBAb to TSAb (or the reverse) come from Japan, although individual patients exhibiting TSAb/TBAb changes have been reported from China, Israel, and the United Kingdom. The Asian bias may be attributed to the propensity of physicians in Japan, unlike those in North America, to routinely test thyroid autoantibodies for diagnosis and follow-up. Despite the rarity of patients who undergo changes in thyroid function consequent to a shift in the balance between TSAb and TBAb, it may be possible and informative to perform whole genome screens in this unique set of patients compared with relevant patient controls.
Table 4.
Sex, Age, and Ethnicity of Patients Who Switched from Thyrotropin-Blocking to Thyroid-Stimulating Autoantibodies or the Reverse
| |
|
|
|
Ethnicityc |
|
|||
|---|---|---|---|---|---|---|---|---|
| n | Sex (F:M)a | Age (years)b | As | W | Af | O | Ref. | |
| TBAb to TSAb | 6 | 4:2 | 44±7 | 5 | 1 | (29–33) | ||
| HT to TSAb | 6 | 6:0 | 44±21 | 5 | (31,34) | |||
| TSAb to TBAb | 14 | 12:2 | 39±13 | 12 | 1 | 1 | (5,32,33,36–38) | |
| Relevant disease populations | ||||||||
| TBAb(+) hypothyroidism | 34 | 24:10 | 43±18 | 100% | (33) | |||
| TSAb(+) hyperthyroidism | 98 | 74:24 | 43±17 | 100% | (33) | |||
| Hashimoto's disease | 87 | 87:0 | 23–50 | 26% | 60% | 14% | (74) | |
| Graves' disease | 87 | 87:0 | 23–50 | 66% | 13% | 22% | (74) | |
| Pregnancy | ||||||||
| TSAb fall, TBAB constant | 10 | 10:0 | 10 | (48) | ||||
| Neonate hyper, mother hypo | 1 | 1 | (47) | |||||
| Neonate hypo, mother hyper | 3 | 3 | (47) | |||||
| TSAb, 34th week gestation | 1 | 1 | (49) | |||||
| TSAb postpartum | 1 | 1 | (50) | |||||
Information is also included for Hashimoto's thyroiditis (HT) patients without TBAb who developed TSAb, relevant disease populations, and cases involving TBAb/TSAb switches in pregnancy.
Number indicates number of patients of the specified sex.
Values reported as mean patient age±SD, or as age range of patients.
Ethnicity values are reported as number of patients or percent of population. Abbreviations for ethnicity (presumed from authors' country of origin): As, Asian (Japanese, Chinese); W, white; Af, African American; O, other.
Summary
(i) A small proportion of TBAb-positive hypothyroid patients treated with LT4 switch to TSAb and hyperthyroidism; conversely, some Graves' patients treated with anti-thyroid drugs switch from TSAb to TBAb-induced hypothyroidism.
(ii) LT4 therapy, often associated with decreased thyroid autoantibodies, in a select patient group induces or enhances thyroid autoantibody levels. In contrast, antithyroid drug treatment usually decreases thyroid autoantibody levels.
(iii) Hyperthyroidism is responsible for polarization of dendritic cells (potent antigen-presenting cells), leading to the impaired development of regulatory T cells, thereby compromising control of autoimmune responses.
(iv) Pregnancy usually involves decreasing thyroid autoantibody levels and a rebound in the postpartum period. Maternally transferred IgG transiently impacts thyroid function until maternal IgG is metabolized.
(v) A Graves' disease model induced by immunizing TSHR knockout mice with mouse TSHR-adenovirus and transfer of TSHR antibody-secreting splenocytes to athymic mice demonstrates the TSAb to TBAb shift and parallels the outcome of maternally transferred “term limited” TSHR antibodies to neonates.
(vi) Finally, as illustrated by analyses of patient sera, TSHR antibody characteristics including concentrations and affinities play a critical role in the switch between TSAb and TBAb in vivo functional activity.
Synthesis and Conclusions
The rare cases of switching between TBAb and TSAb (or vice versa) occurs in unusual patients after LT4 therapy for hypothyroidism or anti-thyroid drug treatment for Graves' disease. These changes arise because of differences in individual patients of the relative concentrations, affinities, or potencies of TBAb and TSAb. Thus, anti-thyroid drugs or immune suppression and/or hemodilution in pregnancy may reduce low TSAb levels, leading to TBAb dominance. In contrast, emergence of TSAb, even at low levels, after LT4 administration may be sufficient to counteract TBAb inhibition. It is also conceivable that, under conditions responsible for diminishing thyroid autoantibody levels, TBAb activity may fall below the “cut-off” point while the stimulatory effect of potent TSAb persists. The occurrence of functional TSHR antibody “switching” emphasizes the need for careful patient monitoring and the difficulties sometimes encountered in regulating thyroid function. Finally, whole genome screening of relatively rare “switch” patients and appropriate Graves' and Hashimoto's controls could provide unexpected and valuable information regarding the basis for thyroid autoimmunity.
Acknowledgments
This work was supported by National Institutes of Health Grants DK54684 (S.M.M.) and DK19289 (B.R). The authors are also grateful for contributions by Dr. Boris Catz, Los Angeles, CA.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Rapoport B. Chazenbalk GD. Jaume JC. McLachlan SM. The thyrotropin receptor: interaction with thyrotropin and autoantibodies. Endocr Rev. 1998;19:673–716. doi: 10.1210/edrv.19.6.0352. [DOI] [PubMed] [Google Scholar]
- 2.Orgiazzi J. Williams DE. Chopra IJ. Solomon DH. Human thyroid adenyl cyclase-stimulating activity in immunoglobulin G of patients with Graves' disease. J Clin Endocrinol Metab. 1976;42:341–354. doi: 10.1210/jcem-42-2-341. [DOI] [PubMed] [Google Scholar]
- 3.Endo K. Kasagi K. Konishi J. Ikekubo K. Tatsuyo O. Takeda Y. Mori T. Torizuka K. Detection and properties of TSH-binding inhibitor immunoglobulins in patients with Graves' disease and Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1978;46:734–739. doi: 10.1210/jcem-46-5-734. [DOI] [PubMed] [Google Scholar]
- 4.Matsuura N. Yamada Y. Nohara Y. Konishi J. Kasagi K. Endo K. Kojima K. Wataya K. Familial neonatal transient hypothyroidism due to maternal TSH-binding inhibitor immunoglobulins. N Engl J Med. 1980;303:738–741. doi: 10.1056/NEJM198009253031306. [DOI] [PubMed] [Google Scholar]
- 5.Evans M. Sanders J. Tagami T. Sanders P. Young S. Roberts E. Wilmot J. Hu X. Kabelis K. Clark J. Holl S. Richards T. Collyer A. Furmaniak J. Smith BR. Monoclonal autoantibodies to the TSH receptor, one with stimulating activity and one with blocking activity, obtained from the same blood sample. Clin Endocrinol (Oxf) 2010;73:404–412. doi: 10.1111/j.1365-2265.2010.03831.x. [DOI] [PubMed] [Google Scholar]
- 6.Zakarija M. McKenzie JM. Munro DS. Immunoglobulin G inhibitor of thyroid-stimulating antibody is a cause of delay in the onset of neonatal Graves' disease. J Clin Invest. 1983;72:1352–1356. doi: 10.1172/JCI111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood LC. Ingbar SH. Hypothyroidism as a late sequela in patient with Graves' disease treated with antithyroid agents. J Clin Invest. 1979;64:1429–1436. doi: 10.1172/JCI109601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rees Smith B. Hall R. Thyroid-stimulating immunoglobulins in Graves' disease. Lancet. 1974;ii:427–431. doi: 10.1016/s0140-6736(74)91815-7. [DOI] [PubMed] [Google Scholar]
- 9.Southgate K. Creagh F. Teece M. Kingswood C. Rees SB. A receptor assay for the measurement of TSH receptor antibodies in unextracted serum. Clin Endocrinol (Oxf) 1984;20:539–548. doi: 10.1111/j.1365-2265.1984.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 10.Ludgate M. Perret J. Parmentier M. Gerard C. Libert F. Dumont JE. Vassart G. Use of the recombinant human thyrotropin receptor (TSH-R) expressed in mammalian cell lines to assay TSH-R autoantibodies. Mol Cell Endocrinol. 1990;73:R13–R18. doi: 10.1016/0303-7207(90)90050-i. [DOI] [PubMed] [Google Scholar]
- 11.Bolton J. Sanders J. Oda Y. Chapman C. Konno R. Furmaniak J. Rees SB. Measurement of thyroid-stimulating hormone receptor autoantibodies by ELISA. Clin Chem. 1999;45:2285–2287. [PubMed] [Google Scholar]
- 12.Costagliola S. Morgenthaler NG. Hoermann R. Badenhoop K. Struck J. Freitag D. Poertl S. Weglohner W. Hollidt JM. Quadbeck B. Dumont JE. Schumm-Draeger PM. Bergmann A. Mann K. Vassart G. Usadel KH. Second generation assay for thyrotropin receptor antibodies has superior diagnostic sensitivity for Graves' disease. J Clin Endocrinol Metab. 1999;84:90–97. doi: 10.1210/jcem.84.1.5415. [DOI] [PubMed] [Google Scholar]
- 13.Zophel K. Roggenbuck D. von Landenberg P. Wunderlich G. Gruning T. Kotzerke J. Lackner KJ. Rees SB. TSH receptor antibody (TRAb) assays based on the human monoclonal autoantibody M22 are more sensitive than bovine TSH based assays. Horm Metab Res. 2010;42:65–69. doi: 10.1055/s-0029-1241196. [DOI] [PubMed] [Google Scholar]
- 14.Toccafondi R. Aterini S. Medici MA. Rotella CM. Tanini A. Zonefrati R. Thyroid-stimulating antibody (TSAb) detected in sera of Graves' patients using human thyroid cell cultures. Clin Exp Immunol. 1980;40:532–539. [PMC free article] [PubMed] [Google Scholar]
- 15.Hinds WE. Takai N. Rapoport B. Filetti S. Clark OH. Thyroid-stimulating immunoglobulin bioassay using cultured human thyroid cells. J Clin Endocrinol Metab. 1981;52:1204–1210. doi: 10.1210/jcem-52-6-1204. [DOI] [PubMed] [Google Scholar]
- 16.Vitti P. Valente WA. Ambesi-Impiombato FS. Fenzi GF. Pinchera A. Kohn LD. Graves' IgG stimulation of continuously cultured rat thyroid cells: a sensitive and potentially useful clinical assay. J Endocrinol Invest. 1982;5:179–182. doi: 10.1007/BF03349476. [DOI] [PubMed] [Google Scholar]
- 17.Kasagi K. Konishi J. Arai K. Misaki T. Iida Y. Endo K. Torizuka K. A sensitive, practical assay for thyroid-stimulating antibodies using crude immunoglobulin fractions precipitated with polyethylene glycol. J Clin Endocrinol Metab. 1986;62:855–862. doi: 10.1210/jcem-62-5-855. [DOI] [PubMed] [Google Scholar]
- 18.Kasagi K. Konishi J. Iida Y. Ikekubo K. Mori T. Kuma K. Torizuka K. A new in vitro assay for human thyroid stimulator using cultured thyroid cells: effect of sodium chloride on adenosine 3′,5′-monophosphate increase. J Clin Endocrinol Metab. 1982;54:108–114. doi: 10.1210/jcem-54-1-108. [DOI] [PubMed] [Google Scholar]
- 19.Kouki T. Inui T. Yamashiro K. Hachiya T. Ochi Y. Kajita Y. Takasu N. Sato Y. Nagata A. Demonstration of fragments with thyroid stimulating activity from thyroid stimulation blocking antibodies—IgG molecules by papain digestion. Clin Endocrinol. 1997;47:693–698. doi: 10.1046/j.1365-2265.1997.3191139.x. [DOI] [PubMed] [Google Scholar]
- 20.Vitti P. Elisei R. Tonacchera M. Chiovato L. Mancusi F. Rago T. Mammoli C. Ludgate M. Vassart G. Pinchera A. Detection of thyroid-stimulating antibody using Chinese hamster ovary cells transfected with cloned human thyrotropin receptor. J Clin Endocrinol Metab. 1993;76:499–503. doi: 10.1210/jcem.76.2.8094393. [DOI] [PubMed] [Google Scholar]
- 21.Watson PF. Ajjan RA. Phipps J. Metcalfe R. Weetman AP. A new chemiluminescent assay for the rapid detection of thyroid stimulating antibodies in Graves' disease [In Process Citation] Clin Endocrinol (Oxf) 1998;49:577–581. doi: 10.1046/j.1365-2265.1998.00619.x. [DOI] [PubMed] [Google Scholar]
- 22.Lytton SD. Li Y. Olivo PD. Kohn LD. Kahaly GJ. Novel chimeric thyroid-stimulating hormone-receptor bioassay for thyroid-stimulating immunoglobulins. Clin Exp Immunol. 2010;162:438–446. doi: 10.1111/j.1365-2249.2010.04266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagayama Y. Wadsworth HL. Russo D. Chazenbalk GD. Rapoport B. Binding domains of stimulatory and inhibitory thyrotropin (TSH) receptor autoantibodies determined with chimeric TSH- lutropin/chorionic gonadotropin receptors. J Clin Invest. 1991;88:336–340. doi: 10.1172/JCI115297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz-Lauer L. Chazenbalk G. McLachlan SM. Ochi Y. Nagayama Y. Rapoport B. Evidence for a simplified view of autoantibody interactions with the TSH receptor. Thyroid. 2002;12:115–120. doi: 10.1089/105072502753522347. [DOI] [PubMed] [Google Scholar]
- 25.Li Y. Kim J. Larrimer A. Klasen R. Kanitz M. Olivo PD. Kahaly GJ. A chimeric TSHR expressing cell line is highly sensitive for detecting thyroid blocking antibody. Program, American Thyroid Association 81st Annual Meeting, abstract P227.2011. [Google Scholar]
- 26.Blumenthal DK. Garrison JC. Pharmacodynamics: molecular mechanisms of drug action. In: Brunton L, editor; Chabner B, editor; Knollman B, editor. Goodman and Gilman: The Pharmacological Basis of Therapeutics. 12th. McGraw-Hill; New York: 2011. p. 46. [Google Scholar]
- 27.Van Sande J. Swillens S. Gerard C. Allgeier A. Massart C. Vassart G. Dumont J. In Chinese hamster ovary K1 cells dog and human thyrotropin receptors activate both the cyclic AMP and the phosphatidylinositol 4,5-bisphosphate cascades in the presence of thyrotropin and the cyclic AMP cascade in its absence. Eur J Biochem. 1995;229:338–343. [PubMed] [Google Scholar]
- 28.Chazenbalk GD. Kakinuma A. Jaume JC. McLachlan SM. Rapoport B. Evidence for negative cooperativity among human thyrotropin receptors overexpressed in mammalian cells. Endocrinology. 1996;137:4586–4591. doi: 10.1210/endo.137.11.8895321. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K. Takamatsu J. Kasagi K. Sakane S. Ikegami Y. Isotani H. Majima T. Majima M. Kitaoka H. Iida Y. Ikekubo K. Konishi J. Mozai T. Development of hyperthyroidism following primary hypothyroidism: a case report with changes in thyroid-related antibodies. Clin Endocrinol. 1988;28:341–344. doi: 10.1111/j.1365-2265.1988.tb03664.x. [DOI] [PubMed] [Google Scholar]
- 30.Cho BY. Shong YK. Lee HK. Koh C-S. Min HK. Graves' hyperthyroidism following primary hypothyroidism: sequential changes in various activities of thyrotropin receptor antibodies. Acta Endocrinol (Copenh) 1989;120:447–450. doi: 10.1530/acta.0.1200447. [DOI] [PubMed] [Google Scholar]
- 31.Takasu N. Yamada T. Sato A. Nakagawa M. Komiya I. Nagasawa Y. Asawa T. Graves' disease following hypothyroidism due to Hashimoto's disease: studies of eight cases. Clin Endocrinol (Oxf) 1990;33:687–698. doi: 10.1111/j.1365-2265.1990.tb03906.x. [DOI] [PubMed] [Google Scholar]
- 32.Kraiem Z. Baron E. Kahana L. Sadeh O. Sheinfeld M. Changes in stimulating and blocking TSH receptor antibodies in a patient undergoing three cycles of transition from hypo to hyper-thyroidism and back to hypothyroidism. Clin Endocrinol (Oxf) 1992;36:211–214. doi: 10.1111/j.1365-2265.1992.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 33.Takasu N. Matsushita M. Changes of TSH-stimulation blocking antibody (TSBAb) and thyroid stimulating antibody (TSAb) over 10 years in 34 TSBAb-positive patients with hypothyroidism and in 98 TSAb-positive Graves' patients with hyperthyroidism: reevaluation of TSBAb and TSAb in TSH-receptor-antibody (TRAb)-positive patients. J Thyroid Res. 2012;2012:182176. doi: 10.1155/2012/182176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamath C. Young S. Kabelis K. Sanders J. Adlan MA. Furmaniak J. Rees SB. Premawardhana LD. Thyrotrophin receptor antibody characteristics in a woman with long-standing Hashimoto's who developed Graves' disease and pretibial myxoedema. Clin Endocrinol (Oxf) 2012;77:465–470. doi: 10.1111/j.1365-2265.2012.04397.x. [DOI] [PubMed] [Google Scholar]
- 35.Tamai H. Hirota Y. Kasagi K. Matsubayashi S. Kuma K. Iida Y. Konishi J. Okimura MC. Walter RM. Kumagai LF. The mechanism of spontaneous hypothyroidism in patients with Graves' disease after antithyroid drug treatment. J Clin Endocrinol Metab. 1987;64:718–722. doi: 10.1210/jcem-64-4-718. [DOI] [PubMed] [Google Scholar]
- 36.Tamai H. Kasagi K. Takaichi Y. Takamatsu J. Komaki G. Matsubayashi S. Konishi J. Kuma K. Kumagai LF. Nagataki S. Development of spontaneous hypothyroidism in patients with Graves' disease treated with antithyroidal drugs: clinical, immunological, and histological findings in 26 patients. J Clin Endocrinol Metab. 1989;69:49–53. doi: 10.1210/jcem-69-1-49. [DOI] [PubMed] [Google Scholar]
- 37.Shigemasa C. Mitani Y. Taniguch T. Adachi T. Ueta Y. Urabe K. Miyazaki S. Tanaka T. Yoshida A. Mashiba H. Three patients who spontaneously developed persistent hypothyroidism during or following treatment with antithyroid drugs for Graves' hyperthyroidism. Arch Int Med. 1990;150:1105–1109. [PubMed] [Google Scholar]
- 38.Kasagi K. Hidaka A. Endo K. Miyamoto S. Takeuchi R. Misaki T. Sakahara H. Konishi J. Fluctuating thyroid function depending on the balance between stimulating and blocking types of TSH receptor antibodies: a case report. Thyroid. 1993;3:315–318. doi: 10.1089/thy.1993.3.315. [DOI] [PubMed] [Google Scholar]
- 39.Chazenbalk GD. Pichurin P. Chen CR. Latrofa F. Johnstone AP. McLachlan SM. Rapoport B. Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor. J Clin Invest. 2002;110:209–217. doi: 10.1172/JCI15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakatake N. Sanders J. Richards T. Burne P. Barrett C. Pra CD. Presotto F. Betterle C. Furmaniak J. Smith BR. Estimation of serum TSH receptor autoantibody concentration and affinity. Thyroid. 2006;16:1077–1084. doi: 10.1089/thy.2006.16.1077. [DOI] [PubMed] [Google Scholar]
- 41.Morell A. Terry WD. Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest. 1970;49:673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weetman AP. Yateman ME. Ealey PA. Black CM. Reimer CB. Williams RC., Jr. Shine B. Marshall NJ. Thyroid-stimulating antibody activity between different immunoglobulin G subclasses. J Clin Invest. 1990;86:723–727. doi: 10.1172/JCI114768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latrofa F. Chazenbalk GD. Pichurin P. Chen CR. McLachlan SM. Rapoport B. Affinity-enrichment of thyrotropin receptor autoantibodies from Graves' patients and normal individuals provides insight into their properties and possible origin from natural antibodies. J Clin Endocrinol Metab. 2004;89:4734–4745. doi: 10.1210/jc.2003-032068. [DOI] [PubMed] [Google Scholar]
- 44.Sanders J. Chirgadze DY. Sanders P. Baker S. Sullivan A. Bhardwaja A. Bolton J. Reeve M. Nakatake N. Evans M. Richards T. Powell M. Miguel RN. Blundell TL. Furmaniak J. Smith BR. Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid. 2007;17:395–410. doi: 10.1089/thy.2007.0034. [DOI] [PubMed] [Google Scholar]
- 45.Sanders J. Evans M. Betterle C. Sanders P. Bhardwaja A. Young S. Roberts E. Wilmot J. Richards T. Kiddie A. Small K. Platt H. Summerhayes S. Harris R. Reeve M. Coco G. Zanchetta R. Chen S. Furmaniak J. Rees Smith B. A human monoclonal autoantibody to the thyrotropin receptor with thyroid stimulating blocking activity. Thyroid. 2008;18:735–746. doi: 10.1089/thy.2007.0327. [DOI] [PubMed] [Google Scholar]
- 46.Kraiem Z. Cho BY. Sadeh O. Shong MH. Pickerill P. Weetman AP. The IgG subclass distribution of TSH receptor blocking antibodies in primary hypothyroidism. Clin Endocrinol. 1992;37:135–140. doi: 10.1111/j.1365-2265.1992.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 47.Zakarija M. McKenzie JM. Eidson MS. Transient neonatal hypothyroidism: characterization of maternal antibodies to the thyrotropin receptor. J Clin Endocrinol Metab. 1990;70:1239–1246. doi: 10.1210/jcem-70-5-1239. [DOI] [PubMed] [Google Scholar]
- 48.Kung AW. Jones BM. A change from stimulatory to blocking antibody activity in Graves' disease during pregnancy. J Clin Endocrinol Metab. 1998;83:514–518. doi: 10.1210/jcem.83.2.4598. [DOI] [PubMed] [Google Scholar]
- 49.Lu R. Burman KD. Jonklaas J. Transient Graves' hyperthyroidism during pregnancy in a patient with Hashimoto's hypothyroidism. Thyroid. 2005;15:725–729. doi: 10.1089/thy.2005.15.725. [DOI] [PubMed] [Google Scholar]
- 50.Hara T. Tamai H. Mukuta T. Fukata S. Kuma K. Sugawara M. Transient postpartum hypothyroidism caused by thyroid-stimulation-blocking antibody. Lancet. 1990;336:946. doi: 10.1016/0140-6736(90)92321-8. [DOI] [PubMed] [Google Scholar]
- 51.Nagayama Y. Graves' animal models of Graves' hyperthyroidism. Thyroid. 2007;17:981–988. doi: 10.1089/thy.2007.0161. [DOI] [PubMed] [Google Scholar]
- 52.Chen C-R. Pichurin P. Chazenbalk GD. Aliesky H. Nagayama Y. McLachlan SM. Rapoport B. Low-dose immunization with adenovirus expressing the thyroid-stimulating hormone receptor A-subunit deviates the antibody response toward that of autoantibodies in human Graves' disease. Endocrinology. 2004;145:228–233. doi: 10.1210/en.2003-1134. [DOI] [PubMed] [Google Scholar]
- 53.Rapoport B. Williams RW. Chen C-R. McLachlan SM. Immunoglobulin heavy chain variable region genes contribute to the induction of thyroid stimulating antibodies in recombinant inbred mice. Genes Immun. 2010;3:254–263. doi: 10.1038/gene.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao SX. Tsui S. Cheung A. Douglas RS. Smith TJ. Banga JP. Orbital fibrosis in a mouse model of Graves' disease induced by genetic immunization of thyrotropin receptor cDNA. J Endocrinol. 2011;210:369–377. doi: 10.1530/JOE-11-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLachlan SM. Aliesky H. Chen CR. Rapoport B. Role of self-tolerance and chronic stimulation in the long-term persistence of adenovirus-induced thyrotropin receptor antibodies in wild-type and transgenic mice. Thyroid. 2012;22:931–937. doi: 10.1089/thy.2012.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakahara M. Mitsutake N. Sakamoto H. Chen CR. Rapoport B. McLachlan SM. Nagayama Y. Enhanced response to mouse thyroid-stimulating hormone (TSH) receptor immunization in TSH receptor-knockout mice. Endocrinology. 2010;151:4047–4054. doi: 10.1210/en.2010-0315. [DOI] [PubMed] [Google Scholar]
- 57.Nakahara M. Johnson K. Eckstein A. Taguchi R. Yamada M. Abiru N. Nagayama Y. Adoptive transfer of antithyrotropin receptor (TSHR) autoimmunity from TSHR knockout mice to athymic nude mice. Endocrinology. 2012;153:2034–2042. doi: 10.1210/en.2011-1846. [DOI] [PubMed] [Google Scholar]
- 58.Karges B. Muche R. Knerr I. Ertelt W. Wiesel T. Hub R. Neu A. Klinghammer A. Aufschild J. Rapp A. Schirbel A. Boehm BO. Debatin KM. Heinze E. Karges W. Levothyroxine in euthyroid autoimmune thyroiditis and type 1 diabetes: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1647–1652. doi: 10.1210/jc.2006-2493. [DOI] [PubMed] [Google Scholar]
- 59.Padberg S. Heller K. Usadel KH. Schumm-Draeger PM. One-year prophylactic treatment of euthyroid Hashimoto's thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249–255. doi: 10.1089/105072501750159651. [DOI] [PubMed] [Google Scholar]
- 60.Ozen S. Berk O. Simsek DG. Darcan S. Clinical course of Hashimoto's thyroiditis and effects of levothyroxine therapy on the clinical course of the disease in children and adolescents. J Clin Res Pediatr Endocrinol. 2011;3:192–197. doi: 10.4274/jcrpe.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiovato L. Marcocci C. Mariotti S. Mori A. Pinchera A. L-thyroxine therapy induces a fall of thyroid microsomal and thyroglobulin antibodies in idiopathic myxedema and in hypothyroid, but not in euthyroid Hashimoto's thyroiditis. J Endocrinol Invest. 1986;9:299–305. doi: 10.1007/BF03346932. [DOI] [PubMed] [Google Scholar]
- 62.Guclu F. Ozmen B. Kirmaz C. Kafesciler SO. Degirmenci PB. Taneli F. Hekimsoy Z. Down-regulation of the auto-aggressive processes in patients with hypothyroid Hashimoto's thyroiditis following substitutive treatment with L-thyroxine. Eur Cytokine Netw. 2009;20:27–32. doi: 10.1684/ecn.2009.0147. [DOI] [PubMed] [Google Scholar]
- 63.Khoo DH. Eng PH. Ho SC. Fok AC. Differences in the levels of TSH-binding inhibitor immunoglobulins in goitrous and agoitrous autoimmune thyroiditis after twelve months of L-thyroxine therapy. Clin Endocrinol (Oxf) 1999;51:73–79. doi: 10.1046/j.1365-2265.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 64.Yamauchi K. Yamada T. Sato A. Inazawa K. Aizawa T. Elevation of serum immunoglobulin G in Hashimoto's thyroiditis and decrease after treatment with L-thyroxine in hypothyroid patients. Intern Med. 2010;49:267–271. doi: 10.2169/internalmedicine.49.2154. [DOI] [PubMed] [Google Scholar]
- 65.De Vito P. Incerpi S. Pedersen JZ. Luly P. Davis FB. Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21:879–890. doi: 10.1089/thy.2010.0429. [DOI] [PubMed] [Google Scholar]
- 66.Dedecjus M. Stasiolek M. Brzezinski J. Selmaj K. Lewinski A. Thyroid hormones influence human dendritic cells' phenotype, function, and subsets distribution. Thyroid. 2011;21:533–540. doi: 10.1089/thy.2010.0183. [DOI] [PubMed] [Google Scholar]
- 67.Mao C. Wang S. Xiao Y. Xu J. Jiang Q. Jin M. Jiang X. Guo H. Ning G. Zhang Y. Impairment of regulatory capacity of CD4+CD25+ regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves' disease. J Immunol. 2011;186:4734–4743. doi: 10.4049/jimmunol.0904135. [DOI] [PubMed] [Google Scholar]
- 68.Andersen S. Pedersen KM. Bruun NH. Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 69.Laurberg P. Wallin G. Tallstedt L. Abraham-Nordling M. Lundell G. Torring O. TSH-receptor autoimmunity in Graves' disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur J Endocrinol. 2008;158:69–75. doi: 10.1530/EJE-07-0450. [DOI] [PubMed] [Google Scholar]
- 70.McGregor AM. Petersen MM. McLachlan SM. Rooke P. Smith BR. Hall R. Carbimazole and the autoimmune response in Graves' disease. N Engl J Med. 1980;303:302–307. doi: 10.1056/NEJM198008073030603. [DOI] [PubMed] [Google Scholar]
- 71.Laurberg P. Remission of Graves' disease during anti-thyroid drug therapy. Time to reconsider the mechanism? Eur J Endocrinol. 2006;155:783–786. doi: 10.1530/eje.1.02295. [DOI] [PubMed] [Google Scholar]
- 72.Sanders J. Evans M. Premawardhana LD. Depraetere H. Jeffreys J. Richards T. Furmaniak J. Rees SB. Human monoclonal thyroid stimulating autoantibody. Lancet. 2003;362:126–128. doi: 10.1016/s0140-6736(03)13866-4. [DOI] [PubMed] [Google Scholar]
- 73.Mizutori Y. Chen CR. Latrofa F. McLachlan SM. Rapoport B. Evidence that shed TSH receptor A-subunits drive affinity maturation of autoantibodies causing Graves' disease. J Clin Endocrinol Metab. 2009;94:927–935. doi: 10.1210/jc.2008-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hutfless S. Matos P. Talor MV. Caturegli P. Rose NR. Significance of prediagnostic thyroid antibodies in women with autoimmune thyroid disease. J Clin Endocrinol Metab. 2011;96:E1466–E1471. doi: 10.1210/jc.2011-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]