Abstract
Background
Serum thyroglobulin (Tg) is the most sensitive biomarker for recurrence of differentiated thyroid cancer (DTC). We have assessed the changing pattern of stimulated Tg (sTg) and the clinical course of patients with no structural evidence of disease (NSED), based on imaging studies such as neck ultrasonography (US), fluorodeoxyglucose positron emission tomography, and/or chest computed tomogram (CT). We sought to determine if, in patients with DTC who had been treated with bilateral thyroidectomy and remnant ablation with radioactive iodine, sTg 1 year (sTg1) after initial treatment and repeated sTg measurements, 1–2 years after sTg1, helped predict the long-term outcome with respect to structural recurrence and biochemical remission (BR), which is defined as sTg <1 ng/mL.
Methods
We retrospectively assessed the records of patients with DTC who had been treated with bilateral thyroidectomy and remnant ablation with radioactive iodine between 1995 and 2004. The study included 186 patients who had NSED with sTg1 ≥2 ng/mL and subsequent sTg measurements (sTg2) without additional treatment. Patients were classified into three groups based on their sTg1 measurements: Group A, 2–4.9 ng/mL; Group B, 5–19.9 ng/mL; and Group C, ≥20 ng/mL. Patients were also classified into two groups based on whether sTg2, 1–2 years after sTg1, had decreased by ≥50% (Group 1) or had either decreased by <50% or increased (Group 2). sTg was measured every 1–2 years until structural recurrence or BR.
Results
Patients remaining in NSED showed a decrease in serial sTg. Of patients in Groups A, B, and C, 41%, 17%, and 1%, respectively, achieved BR, and there was a significant difference in the BR rate between Groups 1 and 2 (p<0.001). In patients with structural recurrence, serial sTg generally did not decrease from sTg1. There was a significant difference in the recurrence rate among Groups A, B, and C (p=0.005) and between Groups 1 and 2 (p<0.001).
Conclusions
We found that 41% of patients with sTg1 in the range 2–5 ng/mL achieved BR, and that sTg1 and percent change of subsequent sTg were predictive of BR. Repeated sTg measurements are useful for predicting patient prognosis in patients with DTC.
Introduction
Differentiated thyroid cancer (DTC) is the most common endocrine malignancy with a rapidly increasing incidence worldwide. Although DTC is generally an indolent tumor and cancer-specific mortality is low, persistent/recurrent disease is common and is associated with significant morbidity and long-term mortality. Therefore, evaluation of strategies for the detection and monitoring of recurrent and/or persistent disease are needed to reduce progression and mortality, while minimizing unnecessary procedures for patients with low risk of recurrence.
Serum thyroglobulin (Tg) is the most sensitive and established biomarker for DTC recurrence/persistence, especially in patients who have undergone thyroid surgery and radioactive iodine remnant ablation (RRA). This is because the only possible source of Tg is thyroid tissue, which may be normal or neoplastic (1,2).
Serum Tg concentrations after thyroid hormone withdrawal during the first year after surgery and RRA is a highly sensitive and specific indicator of future recurrence (1,3). Recently, new methods for serum Tg measurement with functional sensitivities <0.1 ng/mL have become available (4,5). However, a recent article reported that these ultrasensitive assays can decrease the need for thyrotropin (TSH) stimulation, but cannot entirely replace it (6). The American Thyroid Association guidelines recommend that patients with sTg >2 ng/mL undergo diagnostic whole-body scans (DxWBSs) and further testing to localize the source of Tg (7–10). Some patients with detectable sTg have no structurally evident disease. Empirical radioactive iodine (RAI) therapy in some patients with elevated sTg and a negative DxWBS reduced the serum Tg concentration (11–13), but did not improve survival (12,14,15). However, other studies have found that sTg can decrease even in the absence of further treatment (12,16–18).
About 30%–68% of patients with elevated sTg 1 year after RRA have a subsequent decline in sTg concentration or an undetectable sTg (11,12,16). Those studies, however, only included small numbers of patients, and did not show natural courses that change in clinical state and patterns of spontaneous reduction in the sTg level specifically in patients who did not receive additional treatment. We therefore assessed the changing pattern of stimulated Tg (sTg) and the clinical course of patients with no structural evidence of disease (NSED), based on imaging studies such as neck ultrasonography (US), fluorodeoxyglucose positron emission tomography (FDG-PET), and/or chest computed tomogram (CT). We sought to determine if, in patients with DTC who had been treated with bilateral thyroidectomy and remnant ablation with RAI, sTg1 after initial treatment, and repeated sTg measurements 1–2 years after sTg1, would help predict the long-term outcome with respect to structural recurrence and biochemical remission (BR), defined as sTg<1 ng/mL.
Materials and Methods
Definition of BR and clinical evidence of disease
BR was defined as sTg<1 ng/mL, and absence of BR as sTg≥1 ng/mL. Structural recurrence was defined as the appearance of pathologically proven malignant tissue and/or the appearance of metastatic lesions in the lungs, bones, and/or in the brain. NSED was defined as no evidence of disease, based on physical examination, neck US or neck CT scan and any other imaging performed as part of the clinical evaluation, at the end of follow-up, regardless of serum Tg concentrations.
Study population
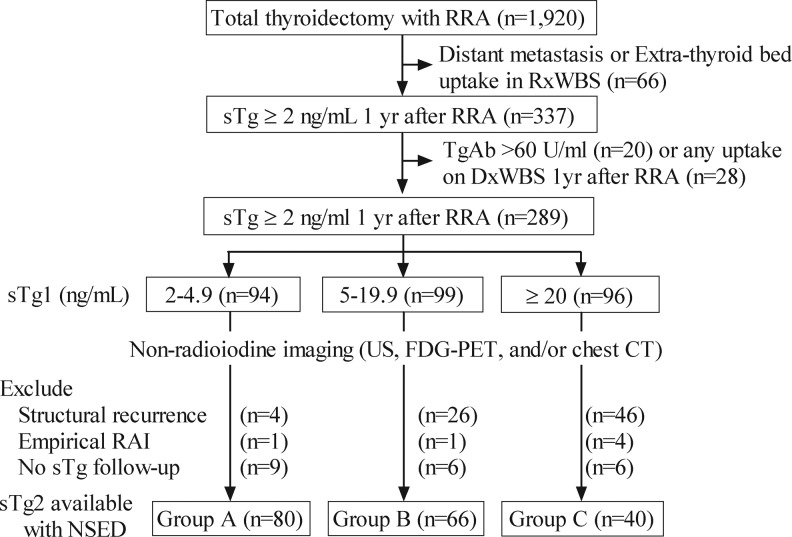
We retrieved the medical records of a total of 1920 consecutive patients with DTC who underwent bilateral thyroidectomy and remnant ablation with RAI at the Asan Medical Center (Seoul, Korea) between 1995 and 2004. A flow diagram describing eligible patients is shown in Figure 1. We excluded 66 patients who presented with clinical evidence of distant metastases or who had radioactive iodine uptake outside the thyroid bed on initial post-treatment whole-body scan.
FIG. 1.
Description of the study cohort. This algorithm demonstrates how the final inclusion of patients was determined. Seventy-six patients with structural recurrences detected on neck US or CT scan and any other imaging performed as part of the clinical evaluation 1 year after remnant ablation, 6 who received empirical RAI therapy and 21 without available follow-up sTg were excluded. Patients were classified into three groups according to sTg1 concentrations: (A) 2–4.9 ng/mL, (B) 5–19.9 ng/mL, (C) ≥20 ng/mL. RRA, radioactive iodine remnant ablation; sTg, stimulated thyroglobulin; RxWBs, post-treatment whole-body scan; TgAb, anti-thyroglobulin antibody; sTg1, sTg measured 1 year after total thyroidectomy and remnant ablation; sTg2, sTg measured 1–2 years after sTg1; US, ultrasonography; FDG-PET, fluorodeoxyglucose positron emission tomography; CT, computed tomogram; RAI, radioactive iodine; BR, biochemical remission; NSED, no structural evidence of disease.
Of these remaining patients, 337 were found to have sTg concentrations 1 year after remnant ablation (sTg1) of ≥2 ng/mL. We excluded 20 patients with anti-Tg antibody (TgAb) concentrations >60 U/mL because this high titer may interfere with reliable measurements of serum Tg, and also 28 patients with uptake on DxWBS 1 year after remnant ablation. We also excluded 76 patients with structural disease detected on neck US or CT scan and any other imaging performed as part of the clinical evaluation 1 year after remnant ablation, 6 who received empirical RAI therapy and 21 without available follow-up sTg. Thus, our patient cohort consisted of 186 patients who had sTg1 ≥2 ng/mL, NSED at 1 year after remnant ablation, and who subsequently had measurements of Tg. The study protocol was approved by the local Ethics Committee.
Measurements of Tg and anti-Tg antibody
TSH was stimulated in all patients by thyroid hormone withdrawal; when TSH was >30 mU/L, sTg, TgAb, and TSH were measured, as described (19).
Follow-up protocol
Thyroid hormone treatment was initiated just after remnant ablation to decrease serum TSH to subnormal levels without producing clinical thyrotoxicosis. Physical examinations were performed regularly, and serum Tg was measured in all patients every 6–12 months.
The thyroid hormone dose was titrated every 6 months according to serum-free T4 and TSH levels. One year after remnant ablation and thyroid hormone withdrawal, DxWBS with 148 MBq RAI was performed and sTg1 was measured. Patients with sTg1 >2 ng/mL underwent further examinations, such as neck US, fluorodeoxyglucose positron emission tomography (FDG-PET), and/or chest CT to evaluate the recurrent/persistent disease. The sTg measurement 1–2 years after sTg1 was defined as sTg2; sTg was measured every 1 or 2 years until patients achieved BR or structural recurrence. In patients lost to follow-up or administered empirical RAI treatment, subsequent data were censored.
Categorization of patients according to sTg1 and sTg slope (Fig. 1)
Patients were classified into three groups according to their sTg1 measurements: Group A, 2–4.9 ng/mL; Group B, 5–19.9 ng/mL, and Group C, ≥20 ng/mL. Percent change in sTg was defined as the relative change between sTg1 and sTg2. Patients were also classified into two groups based on whether sTg2 had decreased by ≥50% from sTg1 (Group 1) or had either decreased by <50% or increased (Group 2).
Statistics
Categorical variables are presented as numbers and percentages. Continuous variables are expressed as mean±SD or median with range. Associations between variables were analyzed using contingency tables and chi-square tests. Analysis of variance (ANOVA) models were used to evaluate continuous data. When the data were not normally distributed, nonparametric tests were used. The Kaplan-Meier method was used to construct survival curves for BR, which were compared using the log-rank test. All p-values were two-sided; p<0.05 was considered statistically significant. R version 2.13 and R libraries Survival, Car, and Cairo were used to analyze data and to draw survival curves (20).
Results
Baseline characteristics
We identified 186 patients, 34 men (18%) and 152 women (82%), with elevated sTg1 and with sTg2 measurements. Mean patient age was 42±12 years; their baseline clinical characteristics are shown in Table 1. Tumor size (p=0.002) and the number of pathologically proven malignant lymph nodes (p=0.004) were significantly greater in Group C than in Groups A and B, and patients in Group C also underwent more extensive neck dissection than those in the other two groups (p=0.002). However, there were no significant differences among the three groups in age, sex distribution, pathology, AJCC Tumor/Node/Metastasis (TNM) stage (21), and the number of removed lymph nodes.
Table 1.
Initial Clinical Characteristics
| |
Stimulated thyroglobulin (ng/mL) |
|
||
|---|---|---|---|---|
| 2.0–4.9 (n=80) | 5.0–19.9 (n=66) | ≥20 (n=40) | p | |
| Age (years) | 41±11 | 42±13 | 42±13 | 0.830 |
| Sex, male | 14 (18%) | 9 (14%) | 11 (28%) | 0.196 |
| Tumor size (cm) | 2.1±1.3 | 2.3±1.1 | 3.1±1.7 | 0.002 |
| Pathology | 0.749 | |||
| PTC | 79 (99%) | 65 (99%) | 40 (100%) | |
| FTC | 1 (1%) | 1 (1%) | 0 | |
| Initial operation extent | 0.002 | |||
| Total thyroidectomy | 10 (13%) | 3 (5%) | 2 (5%) | |
| Plus CND | 56 (70%) | 50 (76%) | 19 (48%) | |
| Plus MRND | 14 (18%) | 13 (20%) | 19 (48%) | |
| Remove LN | 10 (0–75) | 9 (0–63) | 11 (0–105) | 0.051 |
| Malignant LN | 4 (0–25) | 4 (0–26) | 7 (0–44) | 0.004 |
| T stage | 0.013 | |||
| T1 | 21 (26%) | 8 (12%) | 5 (13%) | |
| T2 | 3 (4%) | 9 (14%) | 4 (10%) | |
| T3 | 55 (69%) | 46 (70%) | 26 (65%) | |
| T4 | 1 (1%) | 3 (5%) | 3 (13%) | |
| N stage | 0.002 | |||
| N0 | 22 (28%) | 7 (11%) | 2 (5%) | |
| N1a | 43 (54%) | 43 (65%) | 21 (53%) | |
| N1b | 15 (19%) | 16 (24%) | 17 (43%) | |
| AJCC TNM stage | 0.278 | |||
| I/II | 54 (68%) | 41 (62%) | 21 (53%) | |
| III/IV | 26 (33%) | 25 (38%) | 19 (48%) | |
| Ablation RAI dose | 0.131 | |||
| 30 mCi | 6 (8%) | 1 (2%) | 1 (3%) | |
| 75 mCi | 10 (13%) | 6 (9%) | 1 (3%) | |
| 150 mCi | 64 (80%) | 59 (89%) | 38 (95%) | |
The numbers in parentheses are percentages or ranges.
PTC, papillary thyroid cancer; FTC, follicular thyroid cancer; CND, central neck dissection; MRND, modified radical neck dissection; LN, lymph node; RAI, radioactive iodine.
Time trends of BR
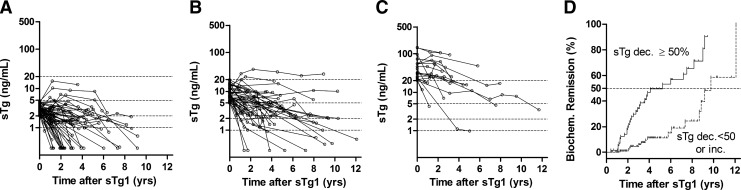
During follow-up, 61 patients (76%) in Group A, 46 (70%) in Group B, and 20 (50%) in Group C remained in NSED. Their serial sTg concentrations generally decreased from sTg1 (Fig. 2A–C): 33 patients (41%) in Group A, 11 (17%) in Group B, and 1 (3%) in Group C achieved spontaneous BR (log-rank statistics=28.5, df=2, p<0.001). The median times from sTg1 to BR in Groups A, B, and C were 69 months, 106 months, and undefined, respectively.
FIG. 2.
(A–C) Patterns of change in sTg concentration in patients with NSED and sTg1 of (A) 2–4.9 ng/mL, (B) 5–19.9 ng/mL, (C) ≥20 ng/mL. (D) Biochemical remission rate in patients with a ≥50% decrease in sTg and those with a <50% decrease or an increase in sTg. The open dotted lines represent the serial sTg concentrations of each patient, measured every one or two years until biochemical or structural recurrence. Percent sTg change was defined as the relative change between sTg1 and sTg2.
We observed a significant difference in BR rate between Groups 1 and 2, classified by sTg slope (log-rank statistics=28.0, df=1, p<0.001), with median times from sTg1 to BR in Groups 1 and 2 of 63 months and 117 months, respectively (Fig. 2D).
Time trends of structural recurrence
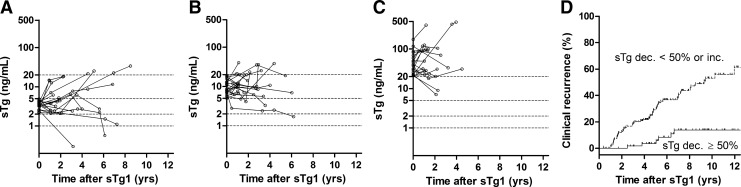
During follow-up, 19 patients (24%) in Group A, 20 (30%) in Group B, and 20 (50%) in Group C had recurrent/persistent disease, despite having NSED 1 year after remnant ablation. Their serial sTg concentrations generally did not decrease compared with sTg1 (Fig. 3A–C). There was a significant difference in the recurrence rate among Groups A, B, and C (log-rank statistics=10.8, df=2, p=0.005), with a median time to recurrence in Group C of 84 months.
FIG. 3.
(A–C) Patterns of change in stimulated thyroglobulin (sTg) concentration in patients with structural recurrence and sTg1 of (A) 2–4.9 ng/mL, (B) 5–19.9 ng/mL, (C) ≥20 ng/mL. (D) Structural recurrence rate in patients with a ≥50% decrease in sTg and those with a <50% decrease or an increase in sTg. The open dotted lines represent the serial sTg concentrations of each patient, measured every 1 or 2 years until biochemical or structural recurrence. Percent sTg change was defined as the relative change between sTg1 and sTg2.
We also observed a significant difference in the recurrence rate in Groups 1 and 2 (log-rank statistics=18.7, df=1, p<0.001), with a median time to recurrence from sTg1 of 111 months in Group 1 (Fig. 3D).
Discussion
We assessed the natural course of DTC in patients without structural evidence of disease after total thyroidectomy and remnant ablation. Patients who remained in NSED showed a general decrease in sTg level concentrations from sTg1. Of patients with sTg1 in the range 2–4.9 ng/mL, 41% achieved spontaneous BR at a median 69 months. We also observed a significant difference in the BR rate according to the sTg slope. Patients with a ≥50% decrease in sTg achieved BR at a median of 63 months, whereas those with a <50% decrease or an increase in sTg achieved BR at a median of ∼10 years. We also found that 32% of our patients developed structural recurrences, despite having NSED 1 year after remnant ablation.
Our findings indicate that the Tg concentration >2 ng/mL 1 year after thyroid hormone withdrawal following initial surgery and RRA is a good marker for identifying patients with recurrent/persistent disease. Previous consensus reports have shown that this elevated sTg concentration occurs in about 20% of low-risk patients, with one third developing persistent/recurrent disease (1). These findings suggest that patients with sTg >2 ng/mL require further testing to localize the source of the Tg. However, there is no established consensus on follow-up of the two thirds who are free of clinical disease 1 year after initial surgery and RRA due to the absence of reliable studies of large numbers of patients with long-term follow-up.
To our knowledge, this study is the first to show changing patterns in serial sTg concentrations in patients with NSED and elevated sTg. Previous studies have reported that sTg concentrations decreased or were undetectable in some patients who were followed-up without treatment (12,16). That study, however, included a small patient population and only one follow-up sTg measurement, thus not determining the pattern of change in sTg and the duration required to observe BR. In contrast, we found that serial sTg concentrations generally decreased in patients with NSED, with 35% of patients who remained NSED at the end of the study achieving spontaneous BR. Specifically, we found that approximately 41% patients with sTg1 of 2–4.9 ng/mL achieved BR at a median of 5 years, suggesting that watchful waiting with periodic sTg measurements is a reasonable follow-up strategy in these patients.
In contrast, we found that serial sTg concentrations in patients who experienced tumor recurrence generally increased or remained constant, a finding made possible by the relatively long follow-up of our patients. Repeated measurements of sTg are useful in determining whether or not to perform meticulous local and systemic evaluations.
BR rates in patients with NSED and elevated sTg1 after total thyroidectomy and RRA have been found to differ. For example, of 28 patients with NSED and detectable sTg, 19 (68%) had undetectable sTg (<3 ng/mL) at the end of the study (12). In contrast, an evaluation of 37 patients with sTg >1 ng/mL found that 11 (30%) achieved spontaneous BR (16). Among our patients with sTg ≥2 ng/mL 1 year after bilateral thyroidectomy and RRA, 24% achieved spontaneous BR (<1 ng/mL sTg). These differences in cutoff values for undetectable sTg and the enrollment of patients with various sTg may explain the differences in spontaneous BR rates among these studies.
In conclusion, we found that 41% of patients with an sTg1 of 2–4.9 ng/mL achieved spontaneous BR, at a median of 69 months. We also found that both sTg1 and percent change in sTg may be good markers for predicting the clinical course of these patients, suggesting that repeated measurements of sTg are useful for predicting the prognosis of patients with elevated sTg after initial treatment for DTC.
Acknowledgment
This study was supported by the Research Grant Number CB-2011-03-02 of the Korean Foundation for Cancer Research, Seoul, Korea.
Disclosure Statement
Young Kee Shong is a consultant for Bayer and Genzyme. All other authors declare no competing financial interests exist.
References
- 1.Mazzaferri EL. Robbins RJ. Spencer CA. Braverman LE. Pacini F. Wartofsky L. Haugen BR. Sherman SI. Cooper DS. Braunstein GD. Lee S. Davies TF. Arafah BM. Ladenson PW. Pinchera A. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:1433–1441. doi: 10.1210/jc.2002-021702. [DOI] [PubMed] [Google Scholar]
- 2.Spencer CA. LoPresti JS. Fatemi S. Nicoloff JT. Detection of residual and recurrent differentiated thyroid carcinoma by serum thyroglobulin measurement. Thyroid. 1999;9:435–441. doi: 10.1089/thy.1999.9.435. [DOI] [PubMed] [Google Scholar]
- 3.Eustatia-Rutten CFA. Smit JWA. Romijn JA. Van Der Kleij-Corssmit EPM. Pereira AM. Stokkel MP. Kievit J. Diagnostic value of serum thyroglobulin measurements in the follow-up of differentiated thyroid carcinoma, a structured meta-analysis. Clin Endocrinol (Oxf) 2004;61:61–74. doi: 10.1111/j.1365-2265.2004.02060.x. [DOI] [PubMed] [Google Scholar]
- 4.Iervasi A. Iervasi G. Ferdeghini M. Solimeo C. Bottoni A. Rossi L. Colato C. Zucchelli GC. Clinical relevance of highly sensitive Tg assay in monitoring patients treated for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2007;67:434–441. doi: 10.1111/j.1365-2265.2007.02907.x. [DOI] [PubMed] [Google Scholar]
- 5.Spencer C. Fatemi S. Singer P. Nicoloff J. Lopresti J. Serum Basal thyroglobulin measured by a second-generation assay correlates with the recombinant human thyrotropin-stimulated thyroglobulin response in patients treated for differentiated thyroid cancer. Thyroid. 2010;20:587–595. doi: 10.1089/thy.2009.0338. [DOI] [PubMed] [Google Scholar]
- 6.Castagna MG. Tala Jury HP. Cipri C. Belardini V. Fioravanti C. Pasqui L. Sestini F. Theodoropoulou A. Pacini F. The use of ultrasensitive thyroglobulin assays reduces but not abolishes the need for TSH stimulation in patients with differentiated thyroid carcinoma. J Endocrinol Invest. 2011;34:e219–e223. doi: 10.3275/7571. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 8.Pacini F. Molinaro E. Lippi F. Castagna MG. Agate L. Ceccarelli C. Taddei D. Elisei R. Capezzone M. Pinchera A. Prediction of disease status by recombinant human TSH-stimulated serum Tg in the postsurgical follow-up of differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:5686–5690. doi: 10.1210/jcem.86.12.8065. [DOI] [PubMed] [Google Scholar]
- 9.Pacini F. Capezzone M. Elisei R. Ceccarelli C. Taddei D. Pinchera A. Diagnostic 131-iodine whole-body scan may be avoided in thyroid cancer patients who have undetectable stimulated serum Tg levels after initial treatment. J Clin Endocrinol Metab. 2002;87:1499–1501. doi: 10.1210/jcem.87.4.8274. [DOI] [PubMed] [Google Scholar]
- 10.Haugen BR. Pacini F. Reiners C. Schlumberger M. Ladenson PW. Sherman SI. Cooper DS. Graham KE. Braverman LE. Skarulis MC. Davies TF. DeGroot LJ. Mazzaferri EL. Daniels GH. Ross DS. Luster M. Samuels MH. Becker DV. Maxon HR. Cavalieri RR. Spencer CA. McEllin K. Weintraub BD. Ridgway EC. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999;84:3877–3885. doi: 10.1210/jcem.84.11.6094. [DOI] [PubMed] [Google Scholar]
- 11.Pineda JD. Lee T. Ain K. Reynolds JC. Robbins J. Iodine-131 therapy for thyroid cancer patients with elevated thyroglobulin and negative diagnostic scan. J Clin Endocrinol Metab. 1995;80:1488–1492. doi: 10.1210/jcem.80.5.7744991. [DOI] [PubMed] [Google Scholar]
- 12.Pacini F. Agate L. Elisei R. Capezzone M. Ceccarelli C. Lippi F. Molinaro E. Pinchera A. Outcome of differentiated thyroid cancer with detectable serum Tg and negative diagnostic (131)I whole body scan: comparison of patients treated with high (131)I activities versus untreated patients. J Clin Endocrinol Metab. 2001;86:4092–4097. doi: 10.1210/jcem.86.9.7831. [DOI] [PubMed] [Google Scholar]
- 13.Koh J-M. Kim ES. Ryu JS. Hong SJ. Kim WB. Shong YK. Effects of therapeutic doses of 131I in thyroid papillary carcinoma patients with elevated thyroglobulin level and negative 131I whole-body scan: comparative study. Clin Endocrinol (Oxf) 2003;58:421–427. doi: 10.1046/j.1365-2265.2003.01733.x. [DOI] [PubMed] [Google Scholar]
- 14.Fatourechi V. Hay ID. Javedan H. Wiseman GA. Mullan BP. Gorman CA. Lack of impact of radioiodine rherapy in Tg-positive, diagnostic whole-body scan-negative patients with follicular cell-derived thyroid cancer. J Clin Endocrinol Metab. 2002;87:1521–1526. doi: 10.1210/jcem.87.4.8373. [DOI] [PubMed] [Google Scholar]
- 15.Kim WG. Ryu J-S. Kim EY. Lee JH. Baek JH. Yoon JH. Hong SJ. Kim ES. Kim TY. Kim WB. Shong YK. Empiric high-dose 131-iodine therapy lacks efficacy for treated papillary thyroid cancer patients with detectable serum thyroglobulin, but negative cervical sonography and 18F-fluorodeoxyglucose positron emission tomography scan. J Clin Endocrinol Metab. 2010;95:1169–1173. doi: 10.1210/jc.2009-1567. [DOI] [PubMed] [Google Scholar]
- 16.Baudin E. Do Cao C. Cailleux AF. Leboulleux S. Travagli JP. Schlumberger M. Positive predictive value of serum thyroglobulin levels, measured during the first year of follow-up after thyroid hormone withdrawal, in thyroid cancer patients. J Clin Endocrinol Metab. 2003;88:1107–1111. doi: 10.1210/jc.2002-021365. [DOI] [PubMed] [Google Scholar]
- 17.Kloos RT. Thyroid cancer recurrence in patients clinically free of disease with undetectable or very low serum thyroglobulin values. J Clin Endocrinol Metab. 2010;95:5241–5248. doi: 10.1210/jc.2010-1500. [DOI] [PubMed] [Google Scholar]
- 18.Chao M. Management of differentiated thyroid cancer with rising thyroglobulin and negative diagnostic radioiodine whole body scan. Clin Oncol (R Coll Radiol) 2010;22:438–447. doi: 10.1016/j.clon.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Yim JH. Kim WB. Kim EY. Kim WG. Kim TY. Ryu J-S. Gong G. Hong SJ. Shong YK. The outcomes of first reoperation for locoregionally recurrent/persistent papillary thyroid carcinoma in patients who initially underwent total thyroidectomy and remnant ablation. J Clin Endocrinol Metab. 2011;96:2038–2039. doi: 10.1210/jc.2010-2298. [DOI] [PubMed] [Google Scholar]
- 20.R Foundation for Statistical Computing., The R Project for Statistical Computing. Institute for Statistics and Mathematics, Vienna University of Economics and Business; Vienna, Austria: [Google Scholar]
- 21.Edge SB, editor; Byrd DR, editor; Compton CC, editor; Fritz AG, editor; Greene FL, editor; Trotti A, editor. AJCC Cancer Staging Manual. 7th. Springer; New York: 2010. [Google Scholar]