Abstract
Background
In the past 3 decades, the incidence of thyroid cancer in the United States has been increasing. There has been debate on whether the increase is real or an artifact of improved diagnostic scrutiny. Our hypothesis is that both improved detection and a real increase have contributed to the increase.
Methods
Because socioeconomic status (SES) may be a surrogate for access to diagnostic technology, we compared thyroid cancer incidence trends between high- and low-SES counties within the Surveillance, Epidemiology, and End Results 9 (SEER 9) registries. The incidence trends were assessed using joinpoint regression analysis.
Results
In high-SES counties, thyroid cancer incidence increased moderately (annual percentage change 1 [APC1]=2.5, p<0.05) before the late 1990s and more pronounced (APC2=6.3, p<0.05) after the late 1990s. In low-SES counties, incidence increased steadily with an APC of 3.5 (p<0.05) during the entire study period (1980–2008). For tumors ≤4.0 cm, incidence was higher in high-SES counties, and APC was higher for high- than low-SES counties after the late 1990s. For tumors >4.0 cm, high- and low-SES counties had similar increasing incidence trends. Similarly, for tumors ≤2.0 cm, the incidence trends differed between counties that are in or adjacent to metropolitan areas and counties that are in rural areas, whereas for tumors >2.0 cm, all counties regardless of area of residence had similar increasing trends.
Conclusions
Enhanced detection likely contributed to the increased thyroid cancer incidence in the past decades, but cannot fully explain the increase, suggesting that a true increase exists. Efforts should be made to identify the cause of this true increase.
Introduction
In the past three decades, the incidence of thyroid cancer in the United States has more than doubled, from 4.3 cases per 100,000 individuals in 1980 to 12.9 cases per 100,000 individuals in 2008 (1). The reasons for the increasing incidence are still under investigation.
In 2006, Davies and Welch, as well as Kent et al. in 2007, proposed that rather than representing a true increase in the occurrence of thyroid cancer, the increasing incidence may be an artifact of improved sensitivity of diagnostic tests (2,3). Physical examination was previously the chief method of detecting thyroid nodules, but it has a low sensitivity and typically detects only larger nodules (4). Sensitivity of physical examination increases with nodule size. Thyroid ultrasonography and fine-needle aspiration were widely adopted in the 1990s. Ultrasonography, which has a much greater sensitivity in the diagnosis of thyroid nodules, is increasingly performed in a physician's office (2). Stanicić et al. reported that the prevalence of thyroid nodules on palpation and ultrasonography were 0.5–6.5% and 13.4–46%, respectively (5). Therefore, the increase in thyroid cancer incidence in recent decades may be due to improved detection of small, asymptomatic carcinomas (2). More than 50 years ago, pathologists reported thyroid cancer to be a common autopsy finding in patients not with the disease before they died, suggesting that many thyroid cancers are incidental and never cause symptoms during a person's life (6). Later, Arem et al. reported in a review of autopsy studies published between year 1952 and 1998 that the prevalence of thyroid carcinomas ≤1.5 cm among people who died of unrelated causes was ∼5–10% (7). The prevalence varied by geographic area and was highest in Finland, where a prevalence of occult papillary carcinoma has been reported to be as high as 36% (8). These findings, together with the stable thyroid cancer mortality rate of ∼0.5 deaths per 100,000 individuals per year over the past three decades (1), support the hypothesis that the increase in incidence may be solely an artifact of improved detection.
However, other researchers argue that improved diagnostic technologies may not fully explain the increase (9–12). For example, Chen et al. found an increase in tumors >4 cm and tumors diagnosed at distant stage, both unlikely to be incidental diagnoses (9), suggesting a true increase in thyroid cancer incidence. Morris and Myssiorek also found significant increases in the incidences of large cancers (including those >4 and >6 cm) and cancers with significant pathological adverse features in the past 3 decades (10). To date, the well-established risk factors for thyroid cancer are exposure to ionizing radiation and a history of benign thyroid nodules and goiter. A birth cohort analysis suggested that increased environmental exposures (such as exposures to diagnostic radiography and polybrominated diphenyl ethers) might have contributed to the observed increase during the past 3 decades (12). It is also possible that an increase in exposure to some unknown risk factors or factors accounts for the increase in incidence (13).
Many reports suggest that socioeconomic status (SES) is highly associated with access to health care (14–17). People with low SES may have less access to medical services such as screening, preventive care, and treatment (14–17). Therefore, if the observed increase in thyroid cancer is due to enhanced detection, patients with higher SES, who generally have better access to health care—including diagnostic tests—would be expected to have a greater increase in thyroid cancer incidence than patients with lower SES. Several studies have indicated that higher SES is indeed associated with higher thyroid cancer incidence (18–20), but there is limited understanding of changing trends in thyroid cancer incidence over time by SES or how tumor size might factor into these associations. In an effort to elucidate the contribution of enhanced detection to the recent increases in thyroid cancer incidence, we compared thyroid cancer incidence trends between low- and high-SES counties in the United States over the past three decades. To perform these analyses, we linked the U.S. Surveillance, Epidemiology, and End Results 9 (SEER 9) registry database with the 2000 U.S. Census database.
Materials and Methods
Data sources and variables
The SEER Program is the primary source of cancer incidence statistics in the United States. In this study, we used the SEER 9 Registries, which cover Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, and Utah. Other SEER registries beyond these original nine began contributing cases in only 1992 or 2000, and thus could not provide the incidence data for the earlier years of our study. Since the steady increase in thyroid cancer incidence began before 1992 (1), we chose to use the SEER 9 registries for this analysis. We included patients with thyroid cancer of all histologic types, excluding lymphomas. The overwhelming majority of thyroid cancers in the SEER database are of the papillary type (82%) with only 2% being of the medullary type. While the increasing incidence trends appear driven chiefly by the papillary histologic type (2), screening bias could influence early detection of other types. To improve the stability of the analysis by maximizing sample size and accounting for potential histologic misclassification among thyroid carcinoma subtypes in such a large database, we chose to include all histologic types rather than the papillary type alone.
Because the SEER database lacks individual-level SES data, we linked the SEER database with the 2000 U.S. Census database, which provides county-level SES data. Incidence was calculated for each calendar year from 1980 through 2008. For each incidence calculation, the numerator consisted of cases diagnosed from January 1 to December 31 within the catchment area of the SEER 9 registries. The denominator consisted of the population size estimates for the counties in the SEER 9 Registries. Both the numerator and the denominator data were obtained from the SEER database. Incidence was age-adjusted to the 2000 U.S. standard population.
County-level data on median household income, proportion of residents with at least a high school education, and area of residence were obtained from the 2000 U.S. Census county attributes via SEER*Stat software. The insurance data were extracted from the 2000 estimates of health insurance coverage for all counties, which were released by the Census Bureau's Small Area Health Insurance Estimates program (21). To eliminate the effects of cost-of-living differences between different counties in the United States, we used the cost-of-living-adjusted median household income data from SEER*Stat, which were adjusted by the cost-of-living index developed on the basis of the Economic Policy Institute's Basic Family Budget (22). On the basis of the Rural–Urban Continuum Code issued by the U.S. Census, counties of residence were classified into two groups in regard of healthcare accessibility: in or adjacent to a metropolitan area and not adjacent to a metropolitan area.
Data analysis
On the basis of the distribution of cost-of-living-adjusted median household income and number of residents, we divided all U.S. counties into quartiles. We divided the counties in SEER 9 Registries into two categories: low income, which consisted of the counties in the lowest quartile, and high income, which consisted of the counties in the top three quartiles. The education and insurance variables were also categorized as lowest quartile versus top three quartiles.
Thus far, there is no widely accepted standard of measuring county-level SES, but many investigators have used composite indices of single-SES indicators. Robert et al. divided SES indicators into quintiles and coded them to create a composite index of community SES in which four single-SES indicators were equally weighed and combined (23). While this method has been validated in several studies, including a SEER-Medicare study, there is no consensus regarding which socioeconomic indicators are most appropriate (24,25). Researchers have created composite indices of SES indicators using different single-SES indicators according to the availability of data, the specific aims, or the salient findings of previous studies. In this study, we created a composite index of county SES that included three county SES indicators: cost-of-living-adjusted median household income, percentage of population with at least a high school education, and percentage of population with health insurance. We coded the three indicators with normal scores based on their quartile ranking and combined them into a composite SES score by weighing the three single-SES indicators equally (23). We assigned counties in SEER 9 registries into two categories: low SES, consisting of the counties in the lowest quartile, and high SES, consisting of the counties in the top three quartiles. We decided to use a ratio of 1:3 to divide counties into low- and high-SES groups, because we wanted to see if the counties with the lowest SES differed from the rest.
To describe the incidence trends, we first computed the annual age-standardized incidence stratified by income, education, insurance, or area of residence using SEER*Stat software, version 7.0.5 (1). To evaluate and compare the thyroid cancer incidence trends, we used Joinpoint Regression software, version 3.5.1, to fit joinpoint regression models (26,27). A joinpoint regression model describes the trends by a continuous, piecewise exponential function (27,28). Adjacent segments join at points called joinpoints, which denote statistically significant changes in the time trend (p<0.05). The Monte Carlo permutation method was used in joinpoint regression analysis to select the best-fitted model with the range of each segment and the number of joinpoints (27). The segments are connected on the basis of the assumption that rates generally change smoothly. Both the joinpoints and annual percentage changes (APCs) were calculated to summarize incidence trends. This was done first for thyroid cancer incidence overall and then for thyroid cancer incidence according to tumor size and SES.
Results
Characteristics of the 49,819 patients with thyroid cancer during 1980–2008 and included in the SEER 9 Registries database are summarized in Table 1. Overall, there were similar proportions of patients <45 and ≥45 years of age. The female-to-male ratio was 3:1. About 86% of the patients were White, and 6% of the patients were Black. Three-fifths of the patients were married, one-fifth was single, and one-fifth was separated, divorced, or widowed. Three percent of the patients were from counties in the lowest quartile of median household income in the United States, whereas more than half of the patients were from counties in the highest quartile of median household income. Only 2% of the patients were from counties in the lowest quartile of percentage of residents with at least a high school education, whereas approximately half of the patients were from counties in the highest quartile of this indicator. Approximately 10% of the patients were from counties in the lowest quartile of percentage of the population covered by any type of health insurance, whereas approximately one-third of patients were from counties in the top quartile. Four percent of the patients were from counties in the lowest quartile of composite SES. The distribution of SES indicators suggested that thyroid cancer patients in the SEER 9 Registries tended to reside in the areas that have higher SES than the average U.S. population. Among all the patients with thyroid cancer, ∼95% were from counties that are in or adjacent to metropolitan areas.
Table 1.
Characteristics of Patients with Thyroid Cancer: SEER 9 Registries Database, 1980–2008
| Characteristic | No. of cases (%) |
|---|---|
| Total | 49,819 |
| Age | |
| <45 years | 22,619 (45%) |
| ≥45 years | 27,200 (55%) |
| Sex | |
| Male | 12,456 (25.0%) |
| Female | 37,363 (75.0%) |
| Race | |
| White | 42,265 (85.5%) |
| Black | 2925 (5.9%) |
| Others or unspecified | 4271 (8.6%) |
| Marital status | |
| Single (never married) | 9220 (19%) |
| Married | 31,376 (66%) |
| Separated, divorced, or widowed | 6863 (14%) |
| Median county household incomea | |
| Q1 ($16,920–$38,310) | 1477 (3.0%) |
| Q2 ($38,320–$43,480) | 9778 (19.6%) |
| Q3 ($43,490–$48,010) | 12,848 (25.8%) |
| Q4 ($48,020–$80,410) | 25,716 (51.6%) |
| Percent having at least a high school education | |
| Q1 (34.7–75.75%) | 1020 (2.0%) |
| Q2 (75.76–81.77%) | 7172 (14.4%) |
| Q3 (81.78–85.45%) | 18,621 (37.4%) |
| Q4 (85.46–96.96%) | 23,006 (46.2%) |
| Percent with health insurance | |
| Q1 (62.0–82.9%) | 4810 (9.7%) |
| Q2 (83.0–86.3%) | 13,795 (27.7%) |
| Q3 (86.4–89.2%) | 16,898 (33.9%) |
| Q4 (89.3–96.2%) | 14,316 (28.7%) |
| County-level composite SES | |
| Q1 | 2003 (4.0%) |
| Q2 | 7605 (15.3%) |
| Q3 | 11,684 (23.4%) |
| Q4 | 28,527 (57.3%) |
| Area of residence | |
| In or adjacent to metropolitan area | 47,238 (94.8%) |
| Not adjacent to metro area | 2581 (5.2%) |
Cost-of-living adjusted.
SES, socioeconomic status; SEER, Surveillance, Epidemiology, and End Results; Q1–Q4, quartiles (first through fourth).
Table 2 presents the results of joinpoint analyses of thyroid cancer incidence trends by socioeconomic factors or area of residence from 1980 to 2008. For the counties with income ranked in the top three quartiles, there was a moderate increase (APC1=2.6, p<0.05) in thyroid cancer incidence between 1980 and 1997 and a more pronounced increase (APC2=6.6, p<0.05) between 1997 and 2008. For the counties with income ranked in the lowest quartile, there was no joinpoint in the incidence trends; there was a steady increase in incidence (APC=4.0, p<0.05) over time. A similar pattern was observed when incidence trends were analyzed by proportion of county residents with at least a high school education. For the counties with insurance coverage in the lowest quartile, the incidence was stable from 1980 to 1987, but steadily increased after 1987, with an APC2 of 4.9 (p<0.05). For counties with insurance coverage ranked in the top three quartiles, the incidence increased between 1980 and 1997 (APC2=2.5, p<0.05), but increased more pronouncedly between 1997 and 2008 (APC2=6.7, p<0.05). In counties with a high composite SES score, the increase in incidence was moderate during the 1980s and 1990s (APC1=2.5, p<0.05), but more pronounced after 1996 (APC2=6.3, p<0.05). In contrast, in counties with a low composite SES score, the incidence increased moderately and steadily over the entire study period (APC=3.5, p<0.05). The rate of increase in thyroid cancer incidence since the 2000s was greater in metropolitan areas or adjacent than in counties that were not adjacent to metropolitan areas (APC, 6.7 vs. 4.1).
Table 2.
Thyroid Cancer Incidence Trends by Socioeconomic Factors or Area of Residence
| |
Joinpoint analysis: 1980–2008 |
|||
|---|---|---|---|---|
| |
Trend 1 |
Trend 2 |
||
| Years | APC [CI] | Years | APC [CI] | |
| Median household incomea | ||||
| Q1 | 1980–2008 | 4.0 [2.8–5.1]b | ||
| Q2–Q4 | 1980–1997 | 2.6 [2.3–2.9]b | 1997–2008 | 6.6 [6.0–7.2]b |
| Percent having at least a high school education | ||||
| Q1 | 1980–2008 | 3.4 [2.1–4.8]b | ||
| Q2–Q4 | 1980–1997 | 2.6 [2.3–2.9]b | 1997–2008 | 6.6 [6.0–7.2]b |
| Percent with health insurance | ||||
| Q1 | 1980–1987 | −0.8 [−5.1–3.6] | 1987–2008 | 4.9 [4.0–5.7]b |
| Q2–Q4 | 1980–1997 | 2.5 [2.2–2.8]b | 1997–2008 | 6.7 [6.1–7.3]b |
| Composite SES | ||||
| Q1 | 1980–2008 | 3.5 [2.6–4.5]b | ||
| Q2–Q4 | 1980–1996 | 2.5 [2.2–2.7]b | 1996–2008 | 6.3 [5.9–6.8]b |
| Area of residence | ||||
| Not adjacent to metro area | 1980–2008 | 4.1 [−3.3–4.9]b | ||
| In or adjacent to metropolitan area | 1980–1997 | 2.6 [2.2–3.0]b | 1997–2008 | 6.7 [6.0–7.3]b |
Cost-of-living adjusted.
p<0.05, significant APC.
APC, annual percentage change; CI, 95% confidence interval.
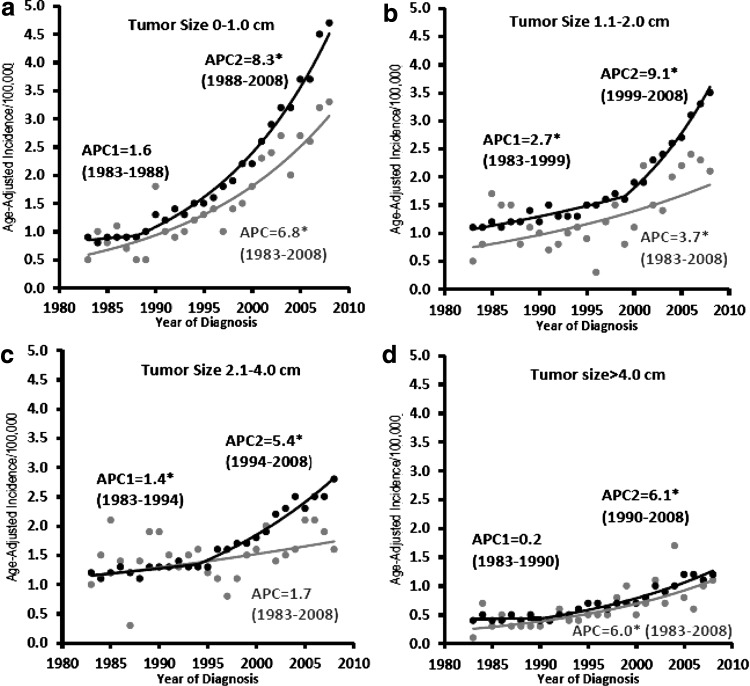
Figure 1 shows the incidence trends for high- and low-SES counties by tumor size. Overall, the positive slopes of the incidence trends decreased with increasing tumor size. In low-SES counties, the incidence increased steadily without any joinpoints during the study period, whereas in high-SES counties, the increases in incidence were moderate before the 1990s and more pronounced after the 1990s. Additionally, for tumors ≤4.0 cm, incidence was greater in high-SES counties than in low-SES counties, whereas for tumors >4.0 cm, incidence was approximately equal in low- and high-SES counties. As shown in Figure 1a–c, for tumors ≤4.0 cm, before the late 1990s the APCs of high- and low-SES counties were similar, but after the late 1990s the incidence in high-SES counties increased pronouncedly while there was no significant change in the incidence trends in low-SES counties. As shown in Figure 1d, for tumors >4.0 cm, high- and low-SES counties had a similar increase in thyroid cancer incidence throughout the study period.
FIG. 1.
Thyroid cancer incidence trends by socioeconomic status (SES, high [black] and low [gray]) and tumor size: (a) 0–1.0 cm; (b) 1.1–2.0 cm; (c) 2.1–4.0 cm; (d) >4.0 cm. Tumor size was not well documented in the Surveillance, Epidemiology, and End Results (SEER) database until 1983; thus, the incidence trends by tumor size were analyzed from 1983. *Statistical significance (p<0.05) was found in incidence trends as indicated.
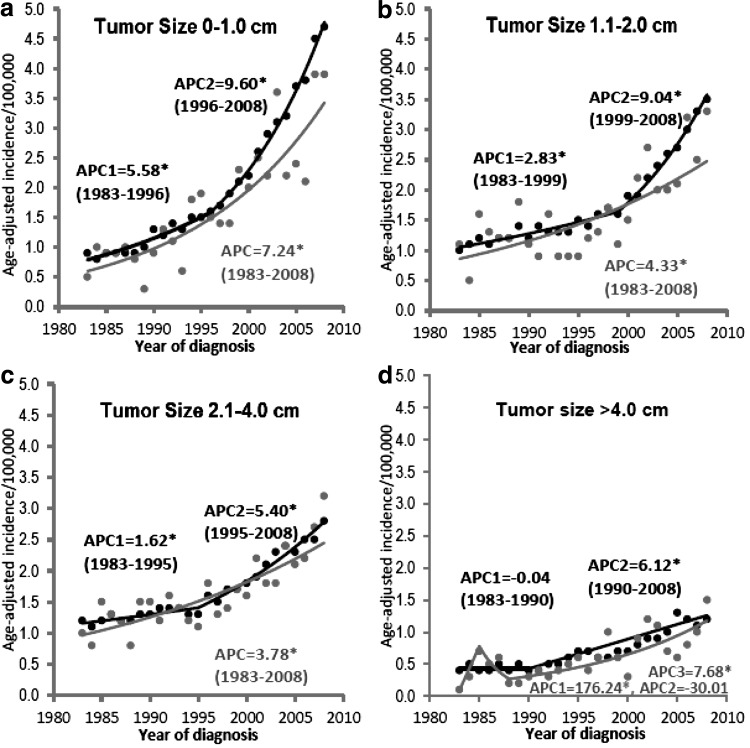
Figure 2 shows the incidence trends for counties that are in or adjacent to metropolitan areas and counties that are not adjacent to metropolitan areas segregated by tumor size. For both tumors 0–1.0 and 1.1–2.0 cm, the incidence in counties that are in or adjacent to metropolitan areas has a higher APC after 2000s than before. However, the incidence in counties that are not adjacent to metropolitan areas had a relatively stable increase. For tumors 2.1–4.0 and >4.0 cm, the incidence trends are quite similar for the two groups.
FIG. 2.
Thyroid cancer incidence trends by area of residence (counties in or adjacent to [black] or not adjacent to [gray] a metropolitan area) and tumor size: (a) 0–1.0 cm; (b) 1.1–2.0 cm; (c) 2.1–4.0 cm; (d) >4.0 cm. Tumor size was not well documented in the SEER database until 1983; thus, the incidence trends by tumor size were analyzed from 1983. *Statistical significance (p<0.05) was found in incidence trends as indicated.
Discussion
We found that the thyroid cancer incidence in high-SES counties increased moderately before the late 1990s and more pronounced afterward, whereas the incidence in low-SES counties increased moderately and steadily during the years 1980–2008. For tumors ≤4.0 cm, the incidence trends differed between high- and low-SES counties, whereas for tumors >4.0 cm, high- and low-SES counties had similar increasing incidence trends. Similarly, for tumors ≤2.0 cm, the incidence trends differed between counties that are in or adjacent to metropolitan areas and counties that are in rural areas, whereas for tumors >2.0 cm, all counties regardless of the area of residence had similar increasing incidence trends. These findings suggest that enhanced detection likely contributed to the observed increase in thyroid cancer incidence over the past decades, but cannot fully explain the increasing incidence, suggesting that a true increase also exists.
Our findings have shown that the distribution of the cases was skewed toward the higher quartiles compared with the national distribution, which suggest that patients with thyroid cancer in the SEER 9 Registries on average had higher SES than the average SES for the U.S. population. Admittedly, a skewed distribution and over-representation of a high-SES population could affect the validity of our study. However, this registry provides the largest nationwide data from a population-based cancer registry, and the results we report still provide evidence of the impact of enhanced detection on the increase in thyroid cancer incidence and lead to further investigation and discussion. Our findings are in agreement with other studies that have suggested that higher SES is associated with higher thyroid cancer incidence (18–20). Morris et al. reported a lower incidence of thyroid cancer diagnosis among individuals residing in poorer versus wealthier zip code areas (18). Morris et al. also reported a lower incidence of thyroid cancer among uninsured individuals than insured individuals (18). Levi et al. found in a study conducted in Switzerland that patients with thyroid cancer tended to be better educated (odds ratio=2.1 [95% confidence interval 1.1–4.1] for ≥14 vs. ≤8 years of education) (29).
Our findings regarding the relationship between SES and thyroid cancer incidence trends suggest that enhanced diagnosis may have played a role in the observed recent increase in thyroid cancer incidence. Joinpoint analyses of thyroid cancer incidence trends in counties in the top three SES quartiles showed a moderate increase (APC1=2.5, p<0.05) in the incidence of thyroid cancer between 1980 and 1997 and a more pronounced increase (APC2=6.3, p<0.05) between 1997 and 2008, whereas analysis of counties in the bottom SES quartile showed no joinpoint (which indicates no significant change in incidence trend) and a moderate increase in the incidence of thyroid cancer (APC=3.5, p<0.05). The difference in incidence trends between high- and low-SES counties may reflect the effect of improved thyroid cancer detection since the late 1990s. Since people with high SES are more likely to be aware of their health status, have insurance coverage, and thus use health services more frequently than people with low SES, the elevated APC in high-SES counties since the late 1990s is likely to be a result of widespread use of ultrasonography and fine-needle aspiration since the 1990s. These technologies have made it possible to detect small, asymptomatic carcinomas, many of which were found in patients having undergone imaging for another health concern. For instance, in a population-based study in Australia, among 452 patients with newly diagnosed thyroid cancer, 60% had their cancer detected incidentally during a medical encounter (30). Therefore, the comparison of incidence trends between groups with different SES shown in Table 2 suggested the effect of improved diagnostic technology.
Our findings regarding differences in thyroid cancer incidence trends by tumor size also support an effect of improved diagnostic technology. The joinpoint analyses of incidence trends by tumor size showed that generally APC decreased with increasing tumor size. For tumors ≤4.0 cm, the APCs of high- and low-SES counties were similar to each other before the late 1990s. After the late 1990s, however, the incidence in high-SES counties increased markedly whereas the incidence in low-SES counties essentially remained the same. The pronounced increase in the incidence of small tumors in high-SES counties after the 1990s (Fig. 1a–c) is consistent with the findings from previous research suggesting that improved detection has contributed to the increase in thyroid cancer incidence via increased detection of small, asymptomatic carcinomas (2). In contrast, the relatively steady increases in thyroid cancer incidence in low-SES counties suggest the possibility of less access to such improved technologies.
Our findings regarding differences in thyroid cancer incidence trends by tumor size also support a real increase in thyroid cancer incidence. For large tumors (>4.0 cm), high- and low-SES counties had a similar steady increase in incidence. This similarity in incidence trends, compared with the patterns observed for smaller tumors (<4.0 cm), suggest that enhanced detection barely played a role in the increase in the incidence of large tumors. If the enhanced detection was the sole reason for the increase, we would expect a decrease, rather than an increase, in the incidence of large tumors, since through improved screening, many tumors would have been detected when they were still asymptomatic and of smaller size. However, we observed an increase in the incidence of large tumors, which suggests the existence of a real increase. While certainly more thyroid cancers are being detected, it is also possible that more large thyroid cancers are being recorded simply due to some misclassification bias by which in more recent years there is better documentation of the true tumor size. At the same time, there has been a rise in obesity in the population, and thus a potential delay in the clinical diagnosis of the proportion of patients with thyroid cancer presenting with a palpable or visible thyroid mass.
This real increase in thyroid cancer incidence might be attributed to the increase in the general population's constant exposure to environmental radiation, such as radiation from medical diagnostic tests and therapies, cosmic radiation, and other exposures (13,31,32). It was reported in a pooled analysis that external medical radiation increases the thyroid cancer risk by 7.7 times per Gy, especially among children (32). An increased cancer risk observed among airline crews has been attributed to increased exposure to cosmic radiation, as such crews travel at high altitudes (31). There also might be an increase in exposure to other undetermined risk factors such as environmentally abundant chemicals, certain solvents, and pesticides (13). We encourage greater efforts to identify the causes of the observed increase in thyroid cancer incidence and to minimize the increased exposure to radiation in the general population as well as to protect high-risk populations.
Our finding that the incidence increase in metropolitan or adjacent areas (which have higher physician density and advanced medical equipment) was greater than the incidence increase in counties not adjacent to metropolitan areas (rural areas) is consistent with the finding from a previous study that people living in metropolitan areas were more likely than people living in rural areas to be incidentally found to have thyroid cancer (30). This suggests that the accessibility to healthcare affects how thyroid cancer is diagnosed and thus the incidence trends. Our results of joinpoint analysis by tumor size further revealed the effect of enhanced detection by showing a more pronounced increase of smaller tumors (≤2.0 cm) in metropolitan or adjacent areas versus rural areas, whereas a similar increase of tumors >2.0 cm in both groups.
Our study has several strengths. Previous research on thyroid cancer and SES mostly compared the cumulative incidence during a time frame between groups with different SES or studied the correlation between SES and thyroid cancer incidence (18–20). Our study focused on time trends and compared the APC between high- and low-SES counties over the course of the study period, during which ultrasonography and fine-needle aspiration were introduced and adopted into widespread use. This approach was a different way of evaluating the contribution of enhanced detection to the increase in incidence. Additionally, in contrast to previous research, which was mostly citywide or statewide (20,33,34), our nationwide study, conducted with the SEER 9 Registries data, which cover almost 10% of the U.S. population, offers greater generalizability and reliability.
Our study also has limitations. Because the SEER database does not contain socioeconomic data on individual or census-block group level, we used county-level socioeconomic data, which constrained our analyses to the county level. Counties can cover large areas, and these county-level estimates mask individual-level variability in SES, which likely plays a role in access to healthcare and care received. Thus, the results and findings of this study could be affected by ecological bias and residual confounding due to lack of SES data at the individual level. However, research has suggested that the patterns of socioeconomic inequities in healthcare detected through individual-level socioeconomic measures are similar to those detected through area-level economic measures (35–38). A second limitation of our study is the relatively small sample size in the low-SES group, which may, to some extent, limit the power to detect changes in the incidence trends. Given that 12.2% of population in the United States live below poverty line, we attempt to see the difference in incidence trends between the lowest group and the rest (39). To maintain an adequate sample size, we did not segregate by sex or ethnicity in our analysis of incidence trends. While the APCs of incidence might be affected by sex or ethnicity, we would expect that county-level analyses would offset the differential in sex or ethnicity distribution. Additionally, because of the deficiency of environmental radiation exposure data, we were not able to assess the difference in the environmental radiation exposures in association with the incidence of thyroid cancer between high- and low-SES counties. Future research can focus on controlling for the differences in the environmental radiation exposures and evaluating their effect on thyroid cancer incidence. Finally, the use of SES data from a single year (the 2000 U.S. Census) to examine trends by SES over 2 decades is a potential limitation, because county SES characteristics might have changed over the time period covered by the study.
In conclusion, our findings suggest that enhanced detection likely contributed to the increasing thyroid cancer incidence in the recent decades, but cannot fully explain the increasing incidence, suggesting that a true increase also exists. Efforts should be made to identify the cause of this observed increase in incidence. This study is not meant to imply that early detection or treatment of thyroid cancer is unnecessary; however, the efficacy of detection and management of smaller thyroid cancers should be carefully studied.
Acknowledgments
This work was supported by the National Institutes of Health through grant U01 DE019765-01 (to Dr. Adel K. El-Naggar, Department of Pathology, M.D. Anderson Cancer Center, University of Texas; E.M.S. is project 2 leader), and M.D. Anderson's Cancer Center Support Grant, CA016672. The authors thank Dr. Dejian Lai (Division of Biostatics, School of Public Health, Health Science Center, University of Texas) and Dr. James Hixson (Human Genetics Center, School of Public Health, Health Science Center, University of Texas) for suggestions on manuscript revision. The authors also thank Stephanie P. Deming (Department of Scientific Publications, M.D. Anderson Cancer Center, University of Texas) for manuscript editing.
Disclosure Statement
The authors declare that no conflicts of interest exist.
References
- 1.Surveillance, Epidemiology, and End Results Program 2011 SEER*Stat Database: SEER 9 Registry Research Data, (1973–2008) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. www.seer.cancer.gov www.seer.cancer.gov
- 2.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Kent WD. Hall SF. Isotalo PA. Houlden RL. George RL. Groome PA. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ. 2007;177:1357–1361. doi: 10.1503/cmaj.061730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiest PW. Hartshorne MF. Inskip PD. Crooks LA. Vela BS. Telepak RJ. Williamson MR. Blumhardt R. Bauman JM. Tekkel M. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med. 1998;17:487–496. doi: 10.7863/jum.1998.17.8.487. [DOI] [PubMed] [Google Scholar]
- 5.Stanicić J. Prpic M. Jukic T. Boric M. Kusic Z. Thyroid nodularity—true epidemic or improved diagnostics. Acta Clin Croat. 2009;48:413–418. [PubMed] [Google Scholar]
- 6.Vanderlaan WP. The occurrence of carcinoma of the thyroid gland in autopsy material. N Engl J Med. 1947;237:221. doi: 10.1056/NEJM194708142370703. [DOI] [PubMed] [Google Scholar]
- 7.Arem R. Padayatty SJ. Saliby AH. Sherman SI. Thyroid microcarcinoma: prevalence, prognosis, and management. Endocr Pract. 1999;5:148–156. doi: 10.4158/EP.5.3.148. [DOI] [PubMed] [Google Scholar]
- 8.Harach HR. Franssila KO. Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen AY. Jemal A. Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 10.Morris LG. Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg. 2010;200:454–461. doi: 10.1016/j.amjsurg.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enewold L. Zhu K. Ron E. Marrogi AJ. Stojadinovic A. Peoples GE. Devesa SS. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomark Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu C. Zheng T. Kilfoy BA. Han X. Ma S. Ba Y. Bai Y. Wang R. Zhu Y. Zhang Y. A birth cohort analysis of the incidence of papillary thyroid cancer in the United States, 1973–2004. Thyroid. 2009;19:1061–1066. doi: 10.1089/thy.2008.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leux C. Guenel P. Risk factors of thyroid tumors: role of environmental and occupational exposures to chemical pollutants. Rev Epidemiol Sante Publique. 2010;58:359–367. doi: 10.1016/j.respe.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Katz SJ. Hofer TP. Socioeconomic disparities in preventive care persist despite universal coverage: breast and cervical screening in Ontario and the United States. JAMA. 1994;272:530–534. [PubMed] [Google Scholar]
- 15.Alter DA. Naylor CD. Austin P. Tu JV. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med. 1999;341:1359–1367. doi: 10.1056/NEJM199910283411806. [DOI] [PubMed] [Google Scholar]
- 16.Kapral MK. Wang H. Mamdani M. Tu JV. Effect of socioeconomic status on treatment and mortality after stroke. Stroke. 2002;33:268–73. doi: 10.1161/hs0102.101169. [DOI] [PubMed] [Google Scholar]
- 17.Katz SJ. Hofer TP. Manning WG. Hospital utilization in Ontario and the United States: the impact of socioeconomic status and health status. Can J Public Health. 1996;87:253–256. [PubMed] [Google Scholar]
- 18.Morris LG. Sikora AG. Myssiorek D. DeLacure MD. The basis of racial differences in the incidence of thyroid cancer. Ann Surg Oncol. 2008;15:1169–1176. doi: 10.1245/s10434-008-9812-6. [DOI] [PubMed] [Google Scholar]
- 19.Soloway LE. Boscoe FP. Schymura MJ. Kahn AR. Weinstein AL. Qiao B. McLaughlin CC. Thyroid cancer incidence in highly observant Jewish neighborhoods in metropolitan New York City. Thyroid. 2011;21:1255–1261. doi: 10.1089/thy.2011.0091. [DOI] [PubMed] [Google Scholar]
- 20.Torres-Cintron M. Ortiz AP. Ortiz-Ortiz KJ. Figueroa-Vallés NR. Pérez-Irizarry J. Díaz-Medina G. De la Torre-Feliciano T. Suárez-Pérez E. Using a socioeconomic position index to assess disparities in cancer incidence and mortality, Puerto Rico, 1995–2004. Prev Chronic Dis. 2012;9:E15. [PMC free article] [PubMed] [Google Scholar]
- 21.The United States Census Bureau. Small Area Health Insurance Estimates. www.census.gov/did/www/sahie/index.html. [Jan 18;2012 ]. www.census.gov/did/www/sahie/index.html
- 22.Economic Policy Institute. Basic Family Budget. www.epi.org/resources/budget/ [Jan 20;2012 ]. www.epi.org/resources/budget/
- 23.Robert SA. Strombom I. Trentham-Dietz A. Hampton JM. McElroy JA. Newcomb PA. Remington PL. Socioeconomic risk factors for breast cancer: distinguishing individual- and community-level effects. Epidemiology. 2004;15:442–450. doi: 10.1097/01.ede.0000129512.61698.03. [DOI] [PubMed] [Google Scholar]
- 24.Du XL. Fang S. Coker AL. Sanderson M. Aragaki C. Cormier JN. Xing Y. Gor BJ. Chan W. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer. 2006;106:1276–1285. doi: 10.1002/cncr.21732. [DOI] [PubMed] [Google Scholar]
- 25.Du XL. Sun CC. Milam MR. Bodurka DC. Fang S. Ethnic differences in socioeconomic status, diagnosis, treatment, and survival among older women with epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18:660–669. doi: 10.1111/j.1525-1438.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- 26.Joinpoint Regression Program, version 3.5. Statistical Methodology and Applications Branch and Data Modeling Branch, Surveillance Research Program, National Cancer Institute, April 2011. http://surveillance.cancer.gov/joinpoint http://surveillance.cancer.gov/joinpoint
- 27.Kim HJ. Fay MP. Feuer EJ. Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. (correction: 20:655). [DOI] [PubMed] [Google Scholar]
- 28.National Cancer Institute Web site. Technical Notes: Joinpoint Regression Analysis of Cancer Trends. http://seer.cancer.gov/csr/1975_2008/technotes/joinpoint.html. [Jan 10;2012 ]. http://seer.cancer.gov/csr/1975_2008/technotes/joinpoint.html
- 29.Levi F. Franceschi S. La Vecchia C. Negri E. Gulie C. Duruz G. Scazziga B. Previous thyroid disease and risk of thyroid cancer in Switzerland. Eur J Cancer. 1991;27:85–88. doi: 10.1016/0277-5379(91)90069-p. [DOI] [PubMed] [Google Scholar]
- 30.Kahn C. Simonella L. Sywak M. Boyages S. Ung O. O'Connell D. Pathways to the diagnosis of thyroid cancer in New South Wales: A population-based cross-sectional study. Cancer Causes Control. 2012;23:35–44. doi: 10.1007/s10552-011-9852-2. [DOI] [PubMed] [Google Scholar]
- 31.Boice JD., Jr. Blettner M. Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576–584. doi: 10.1097/00004032-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Ron E. Lubin JH. Shore RE. Mabuchi K. Modan B. Pottern LM. Schneider AB. Tucker MA. Boice JD., Jr Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 33.Sprague BL. Warren Andersen S. Trentham-Dietz A. Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes Control. 2008;19:585–593. doi: 10.1007/s10552-008-9122-0. [DOI] [PubMed] [Google Scholar]
- 34.Haselkorn T. Bernstein L. Preston-Martin S. Cozen W. Mack WJ. Descriptive epidemiology of thyroid cancer in Los Angeles County, 1972–1995. Cancer Causes Control. 2000;11:163–170. doi: 10.1023/a:1008932123830. [DOI] [PubMed] [Google Scholar]
- 35.Krieger N. Chen JT. Waterman PD. Rehkopf DH. Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95:312–323. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rehkopf DH. Haughton LT. Chen JT. Waterman PD. Subramanian SV. Krieger N. Monitoring socioeconomic disparities in death: comparing individual-level education and area-based socioeconomic measures. Am J Public Health. 2006;96:2135–2138. doi: 10.2105/AJPH.2005.075408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieger N. Chen JT. Waterman PD. Soobader MJ. Subramanian SV. Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 38.Krieger N. Chen JT. Waterman PD. Decline in US breast cancer rates after the Women's Health Initiative: socioeconomic and racial/ethnic differentials. Am J Public Health. 2010;100:S132–S139. doi: 10.2105/AJPH.2009.181628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.United States Census Bureau Website. www.census.gov/compendia/statab/2012/tables/12s0709.pdf. [Aug 11;2012 ]. www.census.gov/compendia/statab/2012/tables/12s0709.pdf