Abstract
Interferon alpha (IFNalpha) is a type I interferon that plays a major role in host defense. There are 13 different IFNalpha genes in humans, but much of the work concerning their role in viral defense has been limited to studying either subtype 2 or pan IFNalpha due to the inability to distinguish between highly similar genetic and amino acid sequences. Because of recent advances in molecular and biochemical techniques, it is possible to study the regulation of individual subtypes. It has been reported that HIV/SIV infection results in impaired IFNalpha responses in certain tissues. Using a pigtailed macaque SIV model, we examined the subtype response during acute infection in 3 tissues that are known to be infected with HIV/SIV, but whose IFNalpha subtype response has not been extensively studied: the brain, spleen, and lung. We found that the expression and regulation of specific subtypes occur in a tissue-specific manner. There was more limited IFNalpha subtype expression in the lung and brain, where predominantly macrophages are infected compared to the spleen, which contains both infected CD4+ lymphocytes and macrophages. Understanding the IFNalpha subtype response in tissues known to be infected with HIV/SIV can help tailor adjunctive treatment regimens to highly active antiretroviral therapy.
Introduction
Type I interferons are potent antiviral cytokines that are the first responders to viral infection. While there is only one IFNbeta gene and protein, there exists 13 different IFNalpha genes encoding 12 different functional proteins in humans and macaques. Each IFNalpha gene is intronless and regulated by its own promoter region equipped with distinct transcription factor binding sites (Civas and others 2006). In humans, each subtype exhibits highly conserved amino acid and genetic sequences, ranging between 78% and 99% homology (Antonelli 2008; Szubin and others 2008). Macaques exhibit comparable conservation between subtypes, ranging between 71% and 97% homology. It is thought that the various alpha genes are a result of gene duplications that have occurred over time, although they have been reported to serve nonredundant functions (Antonelli 2008; Manry and others 2011). Different subtypes have been reported to exhibit varying degrees of antiviral potency in response to viral infections. For example, in human cell lines, alpha subtype 10 has been shown to be the most active isoform against rhinovirus and vesicular stromatatis virus, while alpha 2 has been shown to be most effective against HIV-1 (Sperber and others 1992, 1993).
IFNalpha dysregulation has been implicated in several diseases, including HIV-1. HIV-infected monocytes are reported to have an impaired ability to produce total IFNalpha, but not beta, when exposed to another infectious stimulus (Meltzer and others 1991). In vitro experiments in T cells and macrophages demonstrated the effectiveness of IFNalpha in restricting HIV replication. This led to the treatment of patients with different IFNalpha isoforms, such as recombinant IFNalpha-2b, as well as a mixture of IFNalpha subtypes produced from Sendai virus-treated leukocytes (Lane and others 1990; Skillman and others 1996). Conversely, clinical trials in which IFNalpha production was neutralized were conducted due to reports that claimed IFNalpha was toxic to HIV-infected individuals (Gringeri and others 1994). Both types of treatments were eventually discontinued due to either ineffectiveness or toxicity. It is possible that the failure of these types of studies was due to lack of understanding of the effects of individual IFNalpha subtypes in this particular disease, since highly specific gene amplification techniques that could distinguish between the different genes were not available. It is known now that not only do different subtypes exhibit different degrees of antiviral potency against different pathogens, but also that the induction pattern of IFNalpha subtypes varies depending on target cell (Baig and Fish 2008). Therefore, an understanding of the effects and induction of IFNalpha subtypes in response to HIV infection is needed before evidence-based therapy can be implemented.
It has been found that using an MT-2 cell line of all the subtypes, IFNalpha 2 has the strongest antiviral potency against HIV (Sperber and others 1992). However, examining the effect of interferons in HIV-infected cell lines is not physiologically relevant and does not take into account cell type specificity of the IFNalpha proteins. Current studies using advanced biochemical techniques in primary human macrophages have found that rather than using individual subtypes, structural hybrids of different subtypes have been most successful in limiting viral replication without the inflammatory side effects against HIV (Vazquez and others 2011). While it is extremely useful to study the antiviral effects of alpha subtypes in primary cells, it is also necessary to study the subtype response in vivo in different tissues, since these are also sites, where ongoing HIV and SIV replication occurs. Depending on the tissue, many different cellular populations will be present, which will affect the subtype response in these microenvironments. For example, in Hepatitis C infection, 98% of the IFNalpha subtypes expressed in liver is subtype 5, while in peripheral blood mononuclear cells, the major subtypes are 5, 14, and 21 (Castelruiz and others 1999). This indicates expression of the IFNalpha subtypes can differ within different tissues.
HIV and SIV replication occurs in many tissues, including the brain, lung, and spleen in humans and macaques (van't Wout and others 1998; Mankowski and others 2002; Barber and others 2004; Barber and others 2006b). In both the brain and the lung, the macrophage is the major cell type harboring HIV/SIV replication, which has been reported to occur as early as 4 days p.i. in both tissues (van't Wout and others 1998; Barber and others 2006a; Alammar and others 2011). However, AIDS-related pathologies due to virus and virus-associated inflammation do not occur until the late stage of infection, so it is necessary to study the induction of specific arms of the innate immune response, how they initially control viral replication and how they eventually fail to control virus replication. Unlike the lung which is exposed to soluble factors circulating in the blood, the brain is compartmentalized from the blood via the blood– brain barrier, making these 2 tissues extremely different despite the macrophage being the major cell in which virus replicates. The spleen on the other hand, is a secondary lymphoid organ that contains both lymphocytes and macrophages, where the lymphocytes are more abundant.
We have developed and characterized an accelerated, consistent SIV pigtailed macaque model of HIV. Accelerated disease is based on the coinoculation of the macaques with immunnosuppressive swarm, SIV/B670, a biological isolate that consists of over 20 different viral strains and is responsible for the immunosuppresion of the macaques and this virus swarm contains both CD4+ T cell and macrophage-tropic SIV viruses. The neurovirulent clone, SIV/17E-Fr, replicates in macrophages in the periphery as well as the brain and is similar to the macrophage-tropic strains that are known to transmit HIV from one individual to another. Using this model, we studied the IFNalpha subtype response in 3 tissues, the brain, lung, and spleen, to determine if different cellular/tissue environments would express different IFNalpha genes in response to SIV infection in vivo. We found that different IFNalpha subtype expression patterns do exist within each tissue, demonstrating that the IFNalpha response to SIV infection is tissue specific, and this is highly relevant to decisions about treatment with IFNalpha for HIV and other viral diseases.
Materials and Methods
Animal studies
Pigtailed macaques were intravenously dual inoculated with an immunosuppressive swarm of SIV [SIV/DeltaB670 50 animal infectious dose 50 (AID50)] and a neurovirulent clone (SIV/17E-Fr 10,000 AID50) as previously described (Zink and others 1999). Macaques were sacrificed either before infection (controls) or at 7 or 42 days postinfection in accordance with federal guidelines and approved by the institutional review board. Upon necropsy, the brain, spleen, and lung tissue were snap frozen for subsequent RNA isolation.
RNA isolation from tissue
RNA was isolated from the brain (parietal cortex), spleen, and lung as previously described (Alammar and others 2011).
Cloning of interferon alpha subtypes to make standards
Pigtailed macaque DNA from brain was isolated as previously described (Barber and others 2006b). Forward and reverse primers were designed that spanned the 5′ and 3′ noncoding regions of each alpha subtype based on the annotated rhesus macaque (Macacca mulatta) genome. Separate polymerase chain reactions (PCR) were run on DNA with each primer set using high-fidelity Taq polymerase (Invitrogen) under the following parameters: Step 1: 94°C for 2 min, step 2: 94°C for 30 s, 55°C for 30 s, 72°C for 1 min, step 3: 72°C for 8 min, with step 2 repeated 25 times. Amplification products were run on a 1% agarose gel and the appropriate band was gel purified using the PCR purification kit (Invitrogen). Purified products were cloned into the pcr 2.1 vector using the TOPO TA cloning kit (Invitrogen), and then transformed into TOP10 cells (Invitrogen). At least 10 colonies were checked by colony PCR, and then inoculated in LB media + kanamycin for 18 h at 37°C. Mini preps using the Qiagen Plasmid Mini kit (Qiagen) were performed the next day, and then sequencing analysis was performed to obtain the correct pigtailed macaque gene sequence. Maxi preps were then performed on 1 positive plasmid from each subtype using TOP10 cells and the Qiagen Plasmid Maxi kit (Qiagen), and sequencing analysis was done to confirm the correct sequence of the gene.
Specificity assay for real-time PCR primers and probes
Real-time (RT)-PCR reactions were done on each alpha subtype standard (106 copies/reaction) using primer/probe sets for alphas 1/13, 2, 4, 6, 8, 17, and 14 in separate reactions according to the following protocol: step 1: 95°C for 10 min, step 2: 95°C for 15 s, 55°C for 15 s, 60°C for 30 s, with step 2 repeated 45 times. Reactions were run in duplicate wells together with no-RT and no template controls.
RT-PCR for total interferon alpha in tissues
Protocol for cDNA synthesis, SYBR green RT-PCR reactions, and primer sequences are fully detailed in (Alammar and others 2011). All samples are normalized to 18 s ribosomal RNA and expressed as a fold induction over the average of uninfected controls.
RT-PCR for interferon alpha subtypes and viral RNA
0.1–0.2 μg of RNA isolated from tissue was reversed transcribed into cDNA using Superscript III reverse transcriptase (Invitrogen) according to the manufacturer's protocol. Total sample was then used for RT-PCR using Multiplex NoRox supermix (Qiagen) and multiplexed with primers/probe for 18 s ribosomal RNA according to the following protocol: step 1: 95°C for 10 min, step 2: 95°C for 15 s, 55°C for 15 s, 60°C for 30 s, with step 2 repeated 45 times. Samples were run in triplicate with no reverse transcriptase controls and no template controls. 2–3 independent experiments were run. Using Ct values, samples are expressed as fold inductions over uninfected controls. For alpha subtype 21, where uninfected animals did not express any measurable amount, a Ct value of 45 was designated for uninfected controls to calculate fold induction. SIV viral RNA was quantitated using primers in the gag region according to the same protocol listed above. A standard curve for this particular gene product was generated to obtain the virus copy number.
Statistical analysis
The data from the qRT-PCR analysis were analyzed using the nonparametric Mann–Whitney test.
Results
Determination of pigtailed macaque interferon alpha subtype sequences
Our pigtailed macaque model was used for this study rather than a rhesus macaque model because the pigtailed macaques develop neurological manifestations of SIV infection with a higher frequency (Zink and others 1997). In our model, pigtailed macaques develop AIDS (i.e., immunosuppression as measured by CD4+ T cells levels) in 84 days, with 90% of the infected animals having some form of neurological disease (Zink and others 1999). While the human and rhesus macaque genomes have been annotated, the pigtailed macaque genome has not been sequenced. Because we wanted to distinguish between genes with very few nucleotide differences, it was important that we use the correct species-specific genomic sequences for IFNalpha subtypes. DNA from pigtailed macaque brain was isolated and used to clone 9 IFNalpha subtypes based on the rhesus macaque sequence: 1, 2, 4, 6, 8, 13, 14, 17, and 21. Primers that spanned the 3′ and 5′ untranslated regions outside of the genes, except for alpha 6, whose 3′ UTR was too short for a primer, were used (Table 1), so the entire coding region of each gene (except alpha 6) were included within the amplification product. The primers used to clone alpha 4 also included the 5 alpha 4-like sequences in the macaque genome, since all 5 of those sequences were too similar to be easily distinguished from each other. PCR amplicons were cloned into the vector pcr 2.1 and at least 10 colonies were used for sequence analysis. We found that there were very few nucleotide differences between rhesus and pigtailed macaque IFNalpha subtype sequences (data not shown). Using these primers, plasmid constructs for each subtype were made and used as standards for subsequent experiments.
Table 1.
Primers Sequences Used to Clone Subtypes Based on Rhesus Macaque Genome
| Alpha subtype | F (5′-3′) | R (5′-3′) |
|---|---|---|
| 13 | AGCCTGGATAACAGGAAGA | CATGAAAGTGTGAGCTGGT |
| 2 | CTAACATTTAGGCTCACCCA | AATGGCAGATCATAAAAAGG |
| 6 | GTTCCCTATTTAAGACCTACACA | CATGATTTCTGCTCTGACAA |
| 8 | TCATCCATCTGAACCAGC | CCCATACAGATGAACAGGAT |
| 14 | GTTACTCCTCATCAACCAGC | AATTTTGATTCAACTCGTGG |
| 21 | CAAGGTTACCCACCTCAGTA | AGTCAAGGAAGTGTGGAATG |
| 17 | GCAATATTTGCAACATCCCAAT | AAGAGTCATGAGCCTTTGAAATGG |
| 1 | GCAAAAACAGAAATGGAAAG | AGAGTCTTTGAAATGGCAGA |
| 4 | GGTTCAACGTTACCCACCTCAA | ATTACCCACAGTGTAAAGGTACACATG |
All primers are located in 5′ and 3′ UTR (except for alpha 6), so clones contain entire alpha gene.
Design of real-time PCR specificity assay
The real-time PCR specificity assay was designed using the same principles as outlined in Szubin and others 2008 for human sequences. Primer and probe sequences were made based on the pigtailed macaque sequences and were located within the gene product amplified by the cloning primers for subtypes 1, 13, 2, 6, 8, 14, 21, 17, and 4. The alpha 4 gene locus is comprised of 5 different alpha 4-like genes with extremely similar sequences that are unable to be distinguished by PCR. Therefore, these 5 sequences will henceforth be referred to solely as alpha 4. For subtypes 1 and 13, the same primer/probe set was used, since these 2 genes have identical coding regions. Primer sequences were made such that in at least one of the primers, the 3′ most nucleotide always formed a mismatch with all genes except the specific gene it was amplifying. To ensure specificity, the third to last nucleotide in the primer sequence was deliberately changed to form another mismatch with the nontarget gene sequences as indicated by the amplification refractory mutations system for identifying single-nucleotide polymorphisms (Newton and others 1989; Szubin and others 2008). This states that if there is a strong mismatch at the 3′ most nucleotide, then the nucleotide at the −3 position is changed to form a weak mismatch with nontarget sequences, and vice versa, where strong and weak interactions are determined by the extent of destabilization between the mismatched nucleotides. Moderate mismatches at the 3′ most end were paired with moderate mismatches at the −3 position. Probes were made using sequences that had the most nucleotide differences between subtypes (Table 2).
Table 2.
Real-Time Polymerase Chain Reaction Primer and Probe Sequences Based on Pigtailed Macaque Alpha Sequences
| Alpha subtype | F (5′-3′) | R (5′-3′) | Probe |
|---|---|---|---|
| 1/13 | CCATCTCTGTCCTCCATGTGC (56) | GCTTCCAAGTCATTCAGCTGCT (55) | AGACTCATCTGCTGCTTGGGATGAGGAC (63) |
| 2 | CTGAAGGACAGACATGACTTGGA (55) | TCTCATGGAGGACAGGGATTGT (55) | CCCAGGAGGAGTTTGGCAACCAGT (63.3) |
| 6 | ATGAGGTGATCCAGCAGACGTA (55) | TTCAGTGTAGAGTTTGTCTAGAACCA (55) | CAGCACAAAGGACTCATCTGCTGCTTGG (63) |
| 8 | CCTGAAGGACAGACATGACTTGA (55) | CTGGTCAAGTTCGATGTAGAATGCA (56) | CCCCAGGAGGAGTTTGATGACAAAAACTTCC (62) |
| 21 | TCCCCCAGGAGGAGTTTGATG (56) | ATTCAGTTGCTGGTAAAGTCCCGT (56) | CTGCTGCTTGGGAACACAGCCTCCTAGAA (65) |
| 17 | CTCTGGGCTGTGATCCTACC (56) | CAAAGTCATGTCTGTCCTTCCGA (55) | CTGGGTCATAGGAGGGCCTTGATACTCC (63) |
| 14 | CCTGAAGGACAGAAATGACTTCGA (56) | TTCCAAGTCATTCAGTTGCTCGA (55) | CAGCACAAAGGACTCATCTGCTGCTTGG (63) |
| 4 | CTCTCTGGGCTGTGATCTTACT (54) | CCTGGGGGAATCCAAAGTAAC (55) | CTGGGTCATAGGAGGGCCTTGATACTCC (63) |
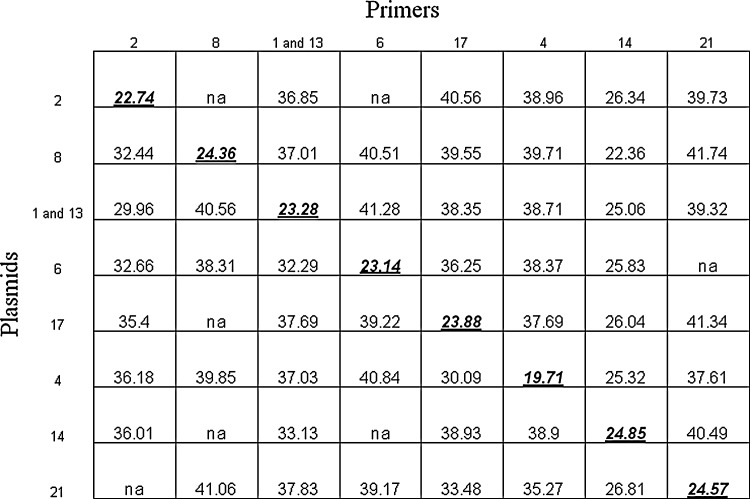
Each primer/probe set was run in separate RT-PCR reactions with each subtype molecular clone to determine the specificity of amplification. For all subtype reactions, (except alpha 14), each primer/probe set maintained specificity toward the specific gene it was amplifying, as indicated by Ct values that were at least 10–15 cycles lower than reactions with nontargeting non-subtype plasmids (Fig. 1). Because IFNalpha subtype 14 RNA could not be found in macaque brain through cloning and sequencing analysis, this subtype was omitted from the panel of subtypes analyzed.
FIG. 1.
Real-time polymerase chain reaction (RT-PCR) was conducted for each set of primers on all interferon alpha plasmid constructs (106 copies/reaction) to test the specificity of the assays. The numbers on the top of the chart represent primer/probe sets for each subtype, while numbers on the left side of the chart represent subtype plasmids. Ct values are shown, with primer/target gene Ct in bold print and underlined. Because of lack of specificity, alpha 14 primers/probe were not used. All PCR reactions were done with duplicate technical replicates in 2 independent experiments.
Total interferon alpha expression differs between brain, lung, and spleen
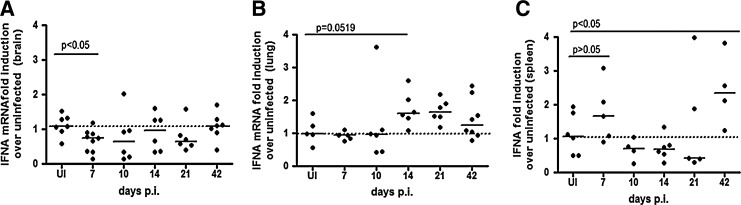
We examined total IFNalpha mRNA in the brain, lung, and spleen using primers that spanned conserved regions of all IFNalpha subtypes during acute SIV infection, 7–21 days postinoculation (p.i.) to determine when during SIV early infection it was appropriate to quantitate the IFNalpha subtype expression. We chose to examine acute infection because this is the time when type I interferons as well as other innate immune regulators are coordinately regulated to mount a defense against viral replication (Witwer and others 2009). At least 6 animals were analyzed in uninfected, 7, 10, 14, 21 days p.i. as well as 42 days p.i. in the spleen. In brain, there is a significant downregulation of IFNalpha mRNA at 7 days p.i. (P<0.05) as compared to uninfected controls (Fig. 2A), while in the lung, there is an upregulation of IFNalpha by day 14 p.i. that trends toward significance (P=0.0519) (Fig. 2B). The spleen shows about a 2-fold upregulation of IFNalpha mRNA at 7 days p.i., although not significantly (P=0.329) (Fig. 2C). We chose to evaluate IFNalpha subtype expression in tissues at the time point, where there was the greatest magnitude of change from uninfected control tissue. In the brain and spleen, it was 7 days p.i, and in the lung, 14 days p.i.
FIG. 2.
Total interferon alpha expression patterns are different in (A) brain (parietal cortex), (B) lung, and (C) spleen. RT-PCR was done with interferon alpha primers that spanned conserved regions among all alpha subtypes on uninfected animals, as well as animals sacrificed at 7, 10, 14, and 21 days postinfection, with at least 5 animals per group. All values are normalized to 18 s ribosomal RNA and expressed as a fold induction over uninfected controls. Solids lines represent medians. All reactions were done with duplicate technical replicates in 3 independent experiments. One representative experiment is shown.
The interferon subtype expression patterns differ between brain, lung, and spleen
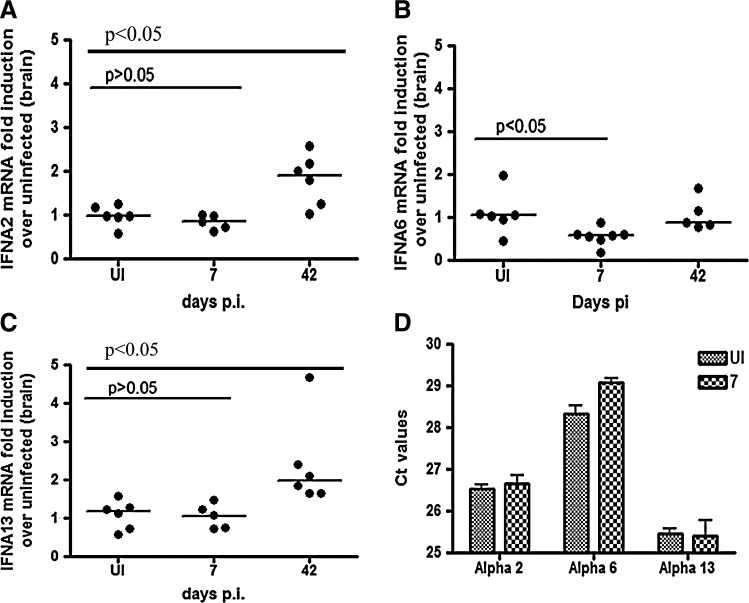
The IFNalpha subtype expression pattern was assessed in uninfected macaques, as well was macaque sacrificed at 7 days p.i. 4–6 animals were studied in each group and each value is expressed as a fold induction over uninfected controls. In brain, the only subtypes expressed in both uninfected and SIV-infected animals at 7 days p.i. were 2, 6, and 13 (Fig. 3A–C). Interestingly, subtype 6 was the only IFNalpha gene that was significantly downregulated in brain by almost 50% at 7 days p.i as compared to uninfected controls (P<0.05). The fold change from uninfected for IFNalphas 2 and 13 did not change significantly during acute infection. To ensure changes in subtypes 2 and 13 could be detected with our method, mRNA was measured at 42 days p.i as a positive control, since this is when clinical manifestations of encephalitis appear (Mankowski and others 2002). At this time point, we did see a significant upregulation (P<0.05). An analysis of the Ct values for each gene suggests that IFNalpha 13 is the most abundant subtype in brain in uninfected and infected animals, as suggested by the lowest Ct value, followed by alpha 2, and then alpha 6 (Fig. 3D).
FIG. 3.
Interferon alpha subtype expression in brain. RT-PCR assays for all interferon alpha subtypes were run in the parietal cortex in uninfected, 7 and 42 days animals, with alphas (A) 13, (B) 2, and (C) 6 being expressed. At least 4 animals were run per group. All values are normalized to 18 s ribosomal RNA and expressed as a fold induction over uninfected controls. Solid lines represent medians. All samples were run with duplicate technical replicates in 3 independent experiments. One representative experiment is shown. (D) Ct values for all uninfected (solid gray bars) and 7-day (shaded bars) animals are graphed for alphas 6, 13, and 2.
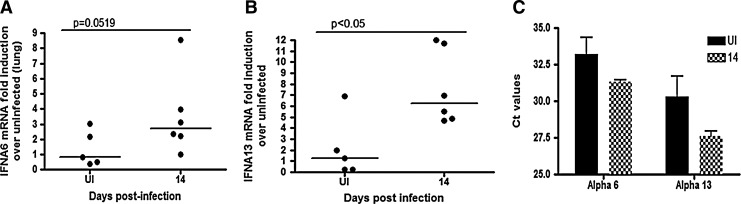
IFNalpha subtype expression was assessed in the lung at 14 days p.i. The only subtypes that were found to be expressed were IFNalphas 6 and 13 in both infected and uninfected animals (Fig. 4A, B). IFNalpha Subtype 6 was upregulated about 3-fold with a trend toward significance (P=0.0519), while subtype 13 was significantly upregulated 6-fold (P<0.05). IFNalpha 13 was more abundant in both uninfected and infected animals based on the lower Ct value (Fig. 4C).
FIG. 4.
Interferon alpha subtype expression in lung. RT-PCR assays for all interferon subtypes were run in the lung of uninfected and 14-day animals with alphas (A) 6 and (B) 13 being expressed. At least 5 animals were used per groups. All values are normalized to 18s ribosomal RNA and expressed as a fold induction over uninfected controls. Solid lines represent medians. All samples were run with duplicate technical replicates in 3 independent experiments. (C) Ct values for all uninfected (solid black bars) and 14-day (shaded bars) animals are graphed for alphas 6 and 13.
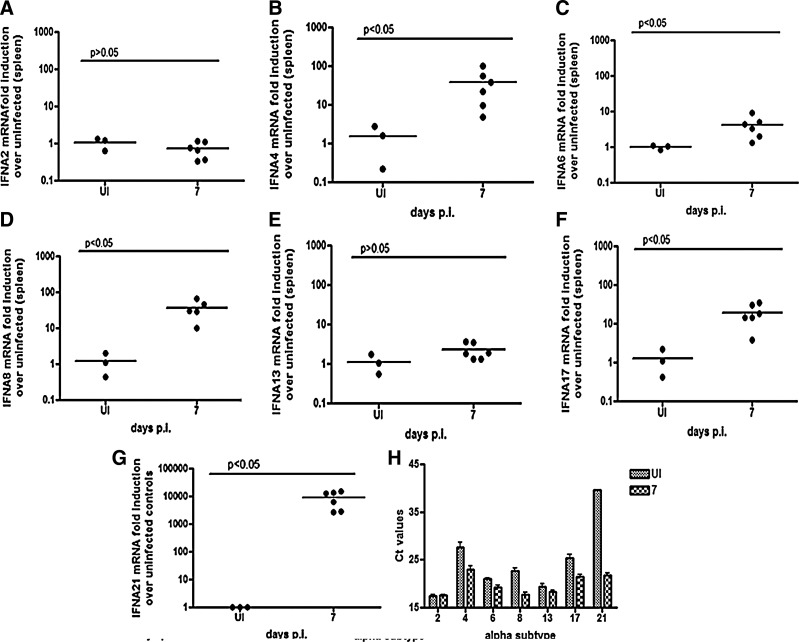
Subtype expression was assessed in the spleen at 7 days p.i. Unlike the brain and lung, all subtypes tested were expressed in the spleen of the SIV-infected macaques. IFNalpha subtypes 2 and 13 did not change significantly at 7 days p.i (Fig. 5A, E). IFNalpha subtype 6 shows the smallest fold induction with a median of about 4-fold (P<0.05) (Fig. 5C), whereas there is about a 16-fold induction of IFNalpha subtype 17 (P<0.05) (Fig. 5F). IFNalpha subtypes 8 and 4 show close to a 100-fold induction in the spleen (P<0.05 for both) (Fig. 5B, D). IFNalpha subtype 21 shows by far the highest fold induction, which is about 10,000-fold (P<0.05) (Fig. 5G). Analyses of the RT-PCR Ct values suggest that IFNalpha subtypes 21, 17, and 4 are the least abundant IFNalpha subtypes measured in the spleen in SIV-infected animals and IFNalpha subtypes 2, 8, and 13 are the most abundant. IFNalpha subtype 6 is intermediate between the least and most abundant subtypes (Fig. 5H). This could explain why when total IFNalpha mRNA was quantitated at 7 days p.i., there is only a trend toward upregulation of total IFNalpha. In other words, the subtypes that are present in the highest amounts show the least fold induction, thus overshadowing the fold inductions of subtypes present at relatively low levels.
FIG. 5.
Interferon alpha subtype expression in spleen. RT-PCR assays were run on RNA isolated from spleen of uninfected and 7-day animals. 3 uninfected animals and 6 infected animals were used. Subtypes (A) 2, (B) 13, (C) 6, (D) 17, (E) 8, (F) 4, and (G) 21 were measured. For IFNalpha subtype 21, there were undetectable levels of mRNA in uninfected animals, so a Ct value of 45 was assigned for the purposes of calculating fold induction. All values are normalized to 18 s ribosomal RNA and expressed as a fold induction over uninfected controls. All samples were run with duplicate technical replicates in 2 independent experiments. One representative experiment is shown. (H) Ct values for uninfected (shaded bars) and infected (checkered bars) are graphed for all subtypes expressed.
Viral loads in brain, lung, and spleen correlate with IFNalpha subtype expression
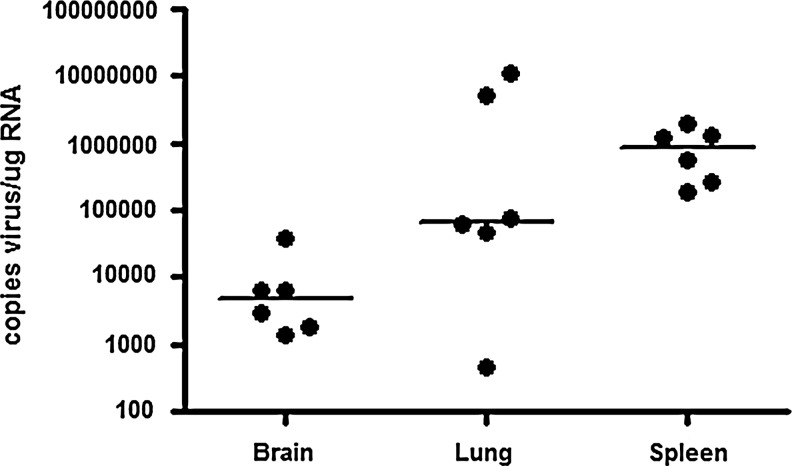
To determine if IFNalpha subtype expression correlates with virus replication, viral loads were measured in the brain and spleen at 7 days p.i., and in the lung at 14 days p.i., since this is when IFNalpha subtype expression was measured (Fig. 6). The viral load is lowest in brain, where there is less than 103 logs viral copies/μg brain RNA, and highest in the spleen, which contains >106 copies of virus. The lung has an intermediate viral load of 105 logs of viral copies. These values correlate with the extent of IFNalpha mRNA induction and expression profile, since in the spleen there is the highest induction of subtypes, while in brain there is the least.
FIG. 6.
Viral loads in brain, lung, and spleen. Copies of viral RNA were determined on RNA isolated from brain (7 days p.i.), lung (14 days p.i.), and spleen (7 days p.i.). Values are expressed as copies of virus/μg of tissue RNA.
Discussion
IFNalpha is comprised of 13 different genes in humans and macaques. Because of the gene and amino acid similarity between subtypes, until recently it has been difficult to differentiate the multiple subtypes. We developed a real-time PCR assay based on the IFNalpha sequences in pigtailed macaques using the same principles as the human and rhesus macaque RT-PCR assays (Szubin and others 2008; Easlick and others 2010) to quantitate IFNalpha subtypes for the first time in the brain, lung, and spleen during acute SIV infection. The lack of commercially available antibodies that distinguish between the IFNalpha subtype proteins limits our ability to identify the specific cells in tissues that express each particular protein. However, without such tools examining the transcriptional regulation of interferon alpha subtypes provides valuable information in understanding the signaling pathways taking place during acute infection. There are dramatic differences in the expression of the IFNalpha subtypes in the tissues. Expression of IFNalpha subtypes are limited in the brain and lung, where only 3 subtypes are expressed in brain and only 2 in the lung, and in both these tissues SIV infection is mainly in tissue macrophages. In contrast, in the spleen, both CD4+ lymphocytes and macrophages are infected with SIV and all IFNalpha subtypes 2, 4, 6, 8, 13, 17, and 21 are detected.
The differences in both magnitude of IFNalpha fold change and subtype expression profile between the 3 tissues correlates with SIV replication. In the spleen, during acute infection, SIV replication is more productive (>106 logs of viral RNA copies/μg spleen) than in either the brain or lung (Barber and others 2006b; Witwer and others 2009) (Ravimohan and others, 2012), which may both stimulate and require more robust interferon responses to control infection. In the lung, where there is also an upregulation of IFNalpha, but fewer subtypes are expressed, there is about a log decrease of SIV viral replication (>105 logs of viral RNA copies/μg lung) as compared to the spleen. This may be due to the fact that in the spleen there is a much more diverse population of infected and uninfected cells that are able to mount an interferon response in an autocrine and paracrine manner than in the lung. Finally, in brain, where there is a downregulation of IFNalpha, virus replication is lower than the other tissues (>103 logs viral copies/μg brain). In brain, IFNb is the major antiviral cytokine that is attributed to controlling SIV replication, which may explain why lower IFNalpha levels do not result in high viral titres (Barber and others 2006a).
The abundance of each IFNalpha subtype in the 3 tissues is important to compare as well as the fold inductions, since the IFNalpha subtypes that are present in lower amounts will show more dramatic fold inductions. For example, in the spleen, IFNalpha subtype 21 is induced by infection almost 10,000-fold. However, it is also one of the least abundant gene products of the IFNalpha subtype genes, especially in uninfected animals, where almost none is detectable, so very small changes in expression of this gene will lead to a greater fold change than the same change in a gene that is more abundant. Abundance of genes also accounts for the very minor fold induction of total IFNalpha in the spleen. mRNA levels of 2 of the 3 subtypes that are the most abundant, IFNalpha subtype 2 and 13, do not change upon infection, thus blunting the effect of the changes in IFNalpha genes that are less abundant.
The abundance of IFNalpha in control animals, as opposed to fold inductions in infected animals, is also an important factor to take into account. It is known that tonic expression of type I interferons have a variety of functions. For example, constitutive interferon beta expression has been shown to protect cells against viral infection by serving as a surveillance modulator, regulate hematopoietic stem cells niches, and maintain homeostasis of macrophages and NK cells (Gough and others 2012). It is probable that constitutive protein levels of individual IFNalpha subtypes have certain biological functions as well, but exactly what these functions are and which subtype they can be attributed to are not yet known.
The tissue-specific IFNalpha subtype expression pattern we observed in vivo is most likely due to the different cell types that are present in each tissue, as well as the different soluble factors that resident tissue cells are exposed to. Different cell types have been reported to induce different IFNalpha subtype expression patterns to the same virus (Baig and Fish 2008). In macrophages that were infected with Newcastle disease virus, alphas 2, 4, 6, 9, and 1 were predominantly expressed, while fibroblasts infected with the same virus only expressed alphas 2 and 4 (Hoss-Homfeld and others 1989). In addition to the interferon response in individual cell types, interactions between cell types also affect the overall tissue-specific IFNalpha subtype response. Soluble factors released from various types of neighboring cells can prime infected cells differently, resulting in a different interferon response or level of response to infection. For example, the interferon response in SIV-infected macrophages when cultured alone is different than macrophages cultured with supernatants from SIV-infected astrocytes (Zaritsky and others, in press). Therefore, the particular resident cell types in each tissue greatly influence the IFNalpha subtype and level of the interferon response.
The brain contains unique cell types, such as astrocytes and neurons, which are able to induce an interferon response to viral infection (Roberts and others 2003). The brain is also sheltered from cells involved in a systemic immune response via the blood–brain barrier. It is known that IFNalpha production in brain is neurotoxic and highly inflammatory (Akwa and others 1998). For example, the most common reported side effects of IFNalpha 2b therapy in Hepatitis C patients are neuropsychiatric (Dieperink and others 2000). Two genetic neurological disorders, Aicardi-Goutierres syndrome and Cree encephalitis, which are characterized by neuroinflammatory lesions, are associated with elevated levels of IFNalpha in brain (Crow and others 2003; van Heteren and others 2008). Therefore, it is important that resident CNS cells efficiently control expression of IFNalpha to protect nonrenewable neurons. This suggests that downmodulating IFNalpha production in brain during acute infection is extremely important and could account for the specific IFNalpha response in brain, as compared to peripheral tissue, such as the lung and spleen (Alammar and others 2011). Even at 42 days p.i., which is when encephalitic pathologies occur, the highest fold induction of a particular subtype is 2-fold, which is much less than in the lung or spleen. The specific IFNalpha subtypes that lead to toxicity are not yet well characterized. The specific downregulation of IFNalpha 6 in brain during acute infection suggests it is controlled and correlates with the downregulation of other inflammatory cytokines in the SIV-infected brain at this time (Witwer and others 2009; Alammar and others 2011), suggesting that this subtype may be associated with inflammatory toxicities.
The spleen is a secondary lymphoid tissue, where lymphocyte activation and clonal expansion occurs. Antigen presenting cells in peripheral blood filter through the spleen, where they interact with lymphocytes and mobilize them to control infection (Tarantino and others 2011). One of the major cells responsible for mobilizing an adaptive immune response is the plasmacytoid dendritic cell (pDC). pDCs are known to be the major source of IFNalpha in blood, and migrate to the spleen during SIV infection in rhesus macaques (Easlick and others 2010). It has been shown that the frequency of pDCs in the infected spleen is comparable to that in the lymph nodes, where pDCs are known to migrate during SIV/HIV infection (Malleret and others 2008; Easlick and others 2010; Lehmann and others 2010). The IFNalpha subtype expression pattern has been characterized in the lymph nodes of SIV-infected macaques, and has been attributed to the infiltration of pDCs (Easlick and others 2010). The subtype expression pattern in lymph nodes most similarly mirrors the expression pattern we found in the spleen, where IFNalpha subtypes 2, 4, 6, 8, 13, 17, and 21 are all expressed. This expression pattern differs greatly from that in the lung and brain, where, to our knowledge, there have been no reports of significant pDC infiltration during SIV/HIV infection. Therefore, it is probable that the particular IFNalpha subtype expression pattern we observe in the spleen is greatly influenced by pDC infiltration, as it is in lymph nodes. IFNalpha is thought to affect T cell proliferation and activation, making it an important cytokine in organs, where lymphocyte priming occurs (Long and Stoddart 2012).
The lung, like the spleen, is a peripheral organ that is exposed to blood and lymphatics. In noninflammatory conditions, about 80% of the cells in the lung are macrophages, with the remaining 20% being lymphocytes and dendritic cells (von Garnier and others 2005). The dendritic cells present are mainly of myeloid origin, with only a fraction of the cells being of plasmacytoid origin (Jahnsen and others 2001; von Garnier and others 2005; Blank and others 2008). Although the lung differs from brain in the sense that it is involved in a peripheral immune response and has continuous exposure to blood, one major similarity between these 2 tissues is that very few IFNalpha subtypes are expressed. In brain, alphas 2, 13, and 6 are expressed, while in the lung, only alphas 6 and 13 are expressed. The macrophage is the major cell type responsible for HIV/SIV replication in the brain and lung, suggesting that the subtype response in the macrophage may play a large role in the overall tissue response. However, alpha 2 is not expressed in the lung at all, while it is one of the more abundant subtypes in brain, indicating that the infected cell alone does not completely regulate expression levels of the IFNalpha subtypes. It is important to note the lack of expression of IFNalpha subtype 2 in the lung, since this was the major isoform that was used in the treatment of HIV.
Why different cell types exhibit different IFNalpha subtype responses could be due to the concentrations of certain transcription factors present within each cell type. Interferon regulatory factors 3 and 7 (IRF3 and IRF7) are the main transcription factors activated by pattern recognition receptor stimulation that lead to IFN production (Honda and others 2006). The promoter regions of the IFNalpha subtypes contain transcription factor binding sites for the family of IRFs. However, the specific sequence domains in each promoter exhibit differential affinity to these transcription factors, leading to a stronger induction of some subtypes over others (Au and Pitha 2001; Civas and others 2006). Because binding affinity is dependent on the concentration of protein, increasing the concentrations of transcription factors can lead to activation of subtypes that have weaker binding sequences in their promoter regions. For example, in humans, 2 nucleotide differences that exist between the IFNalpha 1 and IFNalpha 2 promoters are responsible for the increased affinity of the alpha 1 promoter to bind IRF7 during Sendai virus infection. However, this decreased IFNalpha 2 induction could be rescued by overexpression of IRF7 (Au and Pitha 2001). In addition, manipulating the amounts of IRF3 and IRF7 in human cell lines by ribozyme-mediated degradation has been shown to alter the expression profile of IFNalpha subtypes (Yeow and others 2001). These studies indicate that differences between the relative levels of IRF3 and IRF7 in different cell types will affect the expression patterns of interferon subtypes (Hiscott and others 1984; Dent and others 1996; Au and Pitha 2001). Other factors, such as methylation status and chromatin structure of the particular subtype genes could also differ between cell types, thus leading to differing expression patterns.
Because IFNalpha is used to treat many diseases, it is important to fully understand the IFNalpha subtype response, from its regulation to the specific antiviral effects. In vivo IFNalpha subtype expression and antiviral affects will be different depending on tissue, so an examination of the tissue-specific response is necessary. Both in vitro and in vivo work need to be done to study the interferon response in different physiological microenvironments to gain full advantage of the benefits of specific IFNalpha subtype treatment regimens.
Acknowledgments
We would like to thank all the members of the retrovirus laboratory for their thought provoking discussions and valuable insights. This project was supported by the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Number P40 OD013117.
Author Disclosure Statement
No competing financial interests exist.
References
- Akwa Y. Hassett DE. Eloranta ML. Sandberg K. Masliah E. Powell H. Whitton JL. Bloom FE. Campbell IL. Transgenic expression of IFN-alpha in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol. 1998;161(9):5016–5026. [PubMed] [Google Scholar]
- Alammar L. Gama L. Clements JE. Simian immunodeficiency virus infection in the brain and lung leads to differential type I IFN signaling during acute infection. J Immunol. 2011;186(7):4008–4018. doi: 10.4049/jimmunol.1003757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli G. Biological basis for a proper clinical application of alpha interferons. New Microbiol. 2008;31(3):305–318. [PubMed] [Google Scholar]
- Au WC. Pitha PM. Recruitment of multiple interferon regulatory factors and histone acetyltransferase to the transcriptionally active interferon a promoters. J Biol Chem. 2001;276(45):41629–41637. doi: 10.1074/jbc.M105121200. [DOI] [PubMed] [Google Scholar]
- Baig E. Fish EN. Distinct signature type I interferon responses are determined by the infecting virus and the target cell. Antivir Ther. 2008;13(3):409–422. [PubMed] [Google Scholar]
- Barber SA. Gama L. Dudaronek JM. Voelker T. Tarwater PM. Clements JE. Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus-macaque model. J Infect Dis. 2006a;193(7):963–970. doi: 10.1086/500983. [DOI] [PubMed] [Google Scholar]
- Barber SA. Gama L. Li M. Voelker T. Anderson JE. Zink MC. Tarwater PM. Carruth LM. Clements JE. Longitudinal analysis of simian immunodeficiency virus (SIV) replication in the lungs: compartmentalized regulation of SIV. J Infect Dis. 2006b;194(7):931–938. doi: 10.1086/507429. [DOI] [PubMed] [Google Scholar]
- Barber SA. Herbst DS. Bullock BT. Gama L. Clements JE. Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J Neurovirol. 2004;10(Suppl 1):15–20. doi: 10.1080/753312747. [DOI] [PubMed] [Google Scholar]
- Blank F. von Garnier C. Obregon C. Rothen-Rutishauser B. Gehr P. Nicod L. Role of dendritic cells in the lung: in vitro models, animal models and human studies. Expert Rev Respir Med. 2008;2(2):215–233. doi: 10.1586/17476348.2.2.215. [DOI] [PubMed] [Google Scholar]
- Castelruiz Y. Larrea E. Boya P. Civeira MP. Prieto J. Interferon alfa subtypes and levels of type I interferons in the liver and peripheral mononuclear cells in patients with chronic hepatitis C and controls. Hepatology. 1999;29(6):1900–1904. doi: 10.1002/hep.510290625. [DOI] [PubMed] [Google Scholar]
- Civas A. Genin P. Morin P. Lin R. Hiscott J. Promoter organization of the interferon-A genes differentially affects virus-induced expression and responsiveness to TBK1 and IKKepsilon. J Biol Chem. 2006;281(8):4856–4866. doi: 10.1074/jbc.M506812200. [DOI] [PubMed] [Google Scholar]
- Crow YJ. Black DN. Ali M. Bond J. Jackson AP. Lefson M. Michaud J. Roberts E. Stephenson JB. Woods CG. Lebon P. Cree encephalitis is allelic with Aicardi-Goutieres syndrome: implications for the pathogenesis of disorders of interferon alpha metabolism. J Med Genet. 2003;40(3):183–187. doi: 10.1136/jmg.40.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent CL. Macbride SJ. Sharp NA. Gewert DR. Relative transcriptional inducibility of the human interferon-alpha subtypes conferred by the virus-responsive enhancer sequence. J Interferon Cytokine Res. 1996;16(2):99–107. doi: 10.1089/jir.1996.16.99. [DOI] [PubMed] [Google Scholar]
- Dieperink E. Willenbring M. Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. Am J Psychiatry. 2000;157(6):867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- Easlick J. Szubin R. Lantz S. Baumgarth N. Abel K. The early interferon alpha subtype response in infant macaques infected orally with SIV. J Acquir Immune Defic Syndr. 2010;55(1):14–28. doi: 10.1097/QAI.0b013e3181e696ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough DJ. Messina NL. Clarke CJ. Johnstone RW. Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36(2):166–174. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringeri A. Santagostino E. Mannucci PM. Tradati F. Cultraro D. Buzzi A. Criscuolo M. David A. Guillemot L. Barre-Sinoussi F, et al. A randomized, placebo-controlled, blind anti-AIDS clinical trial: safety and immunogenicity of a specific anti-IFN alpha immunization. J Acquir Immune Defic Syndr. 1994;7(9):978–988. [PubMed] [Google Scholar]
- Hiscott J. Cantell K. Weissmann C. Differential expression of human interferon genes. Nucleic Acids Res. 1984;12(9):3727–3746. doi: 10.1093/nar/12.9.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K. Takaoka A. Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25(3):349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Hoss-Homfeld A. Zwarthoff EC. Zawatzky R. Cell type specific expression and regulation of murine interferon alpha and beta genes. Virology. 1989;173(2):539–550. doi: 10.1016/0042-6822(89)90566-7. [DOI] [PubMed] [Google Scholar]
- Jahnsen FL. Moloney ED. Hogan T. Upham JW. Burke CM. Holt PG. Rapid dendritic cell recruitment to the bronchial mucosa of patients with atopic asthma in response to local allergen challenge. Thorax. 2001;56(11):823–826. doi: 10.1136/thorax.56.11.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane HC. Davey V. Kovacs JA. Feinberg J. Metcalf JA. Herpin B. Walker R. Deyton L. Davey RT., Jr. Falloon J, et al. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection. A randomized, placebo-controlled trial. Ann Intern Med. 1990;112(11):805–811. doi: 10.7326/0003-4819-112-11-805. [DOI] [PubMed] [Google Scholar]
- Lehmann C. Lafferty M. Garzino-Demo A. Jung N. Hartmann P. Fatkenheuer G. Wolf JS. van Lunzen J. Romerio F. Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients. PLoS One. 2010;5(6):e11110. doi: 10.1371/journal.pone.0011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long BR. Stoddart CA. Interferon alpha and HIV infection cause activation of human T cells in NSG-BLT mice. J Virol. 2012;86(6):3327–3336. doi: 10.1128/JVI.06676-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret B. Maneglier B. Karlsson I. Lebon P. Nascimbeni M. Perie L. Brochard P. Delache B. Calvo J. Andrieu T. Spreux-Varoquaux O. Hosmalin A. Le Grand R. Vaslin B. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112(12):4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- Mankowski JL. Clements JE. Zink MC. Searching for clues: tracking the pathogenesis of human immunodeficiency virus central nervous system disease by use of an accelerated, consistent simian immunodeficiency virus macaque model. J Infect Dis. 2002;186(Suppl 2):S199–S208. doi: 10.1086/344938. [DOI] [PubMed] [Google Scholar]
- Manry J. Laval G. Patin E. Fornarino S. Itan Y. Fumagalli M. Sironi M. Tichit M. Bouchier C. Casanova JL. Barreiro LB. Quintana-Murci L. Evolutionary genetic dissection of human interferons. J Exp Med. 2011;208(13):2747–2759. doi: 10.1084/jem.20111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer MS. Baca L. Turpin JA. Kalter DC. Dieffenbach C. Friedman RM. Gendelman HE. Regulation of cytokine and viral gene expression in monocytes infected with the human immunodeficiency virus. Pathobiology. 1991;59(4):209–213. doi: 10.1159/000163647. [DOI] [PubMed] [Google Scholar]
- Newton CR. Graham A. Heptinstall LE. Powell SJ. Summers C. Kalsheker N. Smith JC. Markham AF. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989;17(7):2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravimohan S. Gama L. Engle EL. Zink MC. Clements JE. Early emergence and selection of a SIV-LTR C/EBP site variant in SIV-infected macaques that increases virus infectivity. PLoS One. 2012;7(8):e42801. doi: 10.1371/journal.pone.0042801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ES. Zandonatti MA. Watry DD. Madden LJ. Henriksen SJ. Taffe MA. Fox HS. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003;162(6):2041–2057. doi: 10.1016/S0002-9440(10)64336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skillman DR. Malone JL. Decker CF. Wagner KF. Mapou RL. Liao MJ. Testa D. Meltzer MS. Phase I trial of interferon alfa-n3 in early-stage human immunodeficiency virus type 1 disease: evidence for drug safety, tolerance, and antiviral activity. J Infect Dis. 1996;173(5):1107–1114. doi: 10.1093/infdis/173.5.1107. [DOI] [PubMed] [Google Scholar]
- Sperber SJ. Gocke DJ. Haberzettl C. Kuk R. Schwartz B. Pestka S. Anti-HIV-1 activity of recombinant and hybrid species of interferon-alpha. J Interferon Res. 1992;12(5):363–368. doi: 10.1089/jir.1992.12.363. [DOI] [PubMed] [Google Scholar]
- Sperber SJ. Hunger SB. Schwartz B. Pestka S. Anti-rhinoviral activity of recombinant and hybrid species of interferon alpha. Antiviral Res. 1993;22(2–3):121–129. doi: 10.1016/0166-3542(93)90090-6. [DOI] [PubMed] [Google Scholar]
- Szubin R. Chang WL. Greasby T. Beckett L. Baumgarth N. Rigid interferon-alpha subtype responses of human plasmacytoid dendritic cells. J Interferon Cytokine Res. 2008;28(12):749–763. doi: 10.1089/jir.2008.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino G. Savastano S. Capone D. Colao A. Spleen: a new role for an old player? World J Gastroenterol. 2011;17(33):3776–3784. doi: 10.3748/wjg.v17.i33.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Wout AB. Ran LJ. Kuiken CL. Kootstra NA. Pals ST. Schuitemaker H. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. J Virol. 1998;72(1):488–496. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heteren JT. Rozenberg F. Aronica E. Troost D. Lebon P. Kuijpers TW. Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in Aicardi-Goutieres syndrome. Glia. 2008;56(5):568–578. doi: 10.1002/glia.20639. [DOI] [PubMed] [Google Scholar]
- Vazquez N. Schmeisser H. Dolan MA. Bekisz J. Zoon KC. Wahl SM. Structural variants of IFNalpha preferentially promote antiviral functions. Blood. 2011;118(9):2567–2577. doi: 10.1182/blood-2010-12-325027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Garnier C. Filgueira L. Wikstrom M. Smith M. Thomas JA. Strickland DH. Holt PG. Stumbles PA. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol. 2005;175(3):1609–1618. doi: 10.4049/jimmunol.175.3.1609. [DOI] [PubMed] [Google Scholar]
- Witwer KW. Gama L. Li M. Bartizal CM. Queen SE. Varrone JJ. Brice AK. Graham DR. Tarwater PM. Mankowski JL. Zink MC. Clements JE. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One. 2009;4(12):e8129. doi: 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeow WS. Au WC. Lowther WJ. Pitha PM. Downregulation of IRF-3 levels by ribozyme modulates the profile of IFNA subtypes expressed in infected human cells. J Virol. 2001;75(6):3021–3027. doi: 10.1128/JVI.75.6.3021-3027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC. Amedee AM. Mankowski JL. Craig L. Didier P. Carter DL. Munoz A. Murphey-Corb M. Clements JE. Pathogenesis of SIV encephalitis. Selection and replication of neurovirulent SIV. Am J Pathol. 1997;151(3):793–803. [PMC free article] [PubMed] [Google Scholar]
- Zink MC. Suryanarayana K. Mankowski JL. Shen A. Piatak M., Jr. Spelman JP. Carter DL. Adams RJ. Lifson JD. Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73(12):10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]