Abstract
The endoribonuclease RNase-L is the terminal component of an interferon-regulated RNA decay pathway known as the 2′-5′-oligoadenylate (2–5A) system, whose established functions include antimicrobial and tumor suppressive activities. RNase-L activity requires binding of the small molecule 2–5A, leading to RNase-L dimerization and cleavage of single-stranded RNA. RNase-L expression is controlled post-transcriptionally by its 3′-untranslated region (3′ UTR), which exerts a strong negative effect on RNase-L levels. MicroRNAs (miRNAs) are a class of small noncoding RNAs that repress expression of target genes by binding to regions of complementarity often in the 3′ UTR. The miR-29 family acts as a tumor suppressor in several cancers, including acute and chronic myelogenous leukemia (CML), and has many oncogenic targets. We report that the miR-29 family represses RNase-L protein expression across several cell types. Using a luciferase reporter, we showed that miR-29 acts via 4 target sites within the RNASEL 3′ UTR. Mutation of all sites is required for abrogation of miR-29 repression. In light of the reported tumor suppressive role of miR-29 in K562 CML cells and miR-29 repression of RNase-L in these cells, we generated K562 cells with stable RNase-L knockdown and demonstrated that loss of RNase-L inhibits proliferation in vitro as well as tumor growth in a xenograft model. Our findings identify a previously unknown miRNA regulator of RNase-L expression and support a novel oncogenic role for RNase-L in CML and potentially other hematopoietic malignancies.
Introduction
The endoribonuclease RNase-L was originally discovered as the terminal component of an RNA cleavage pathway that serves as an important mediator of interferon (IFN)-induced antiviral activity (Zhou and others 1993; Zhou and others 1997; Ezelle and Hassel 2012). RNase-L enzymatic activity requires binding of its allosteric activator, 2′-5′-oligoadenylate (2–5A). 2–5A is a class of small 2′,5′-linked oligoadenylate molecules [p35′A(2′p5′A)n, n ≥2] that is produced by IFN-regulated 2′,5′-oligoadenylate synthetase enzymes in the presence of double-stranded RNA. Binding of 2–5A to latent cytoplasmic RNase-L induces a conformational change that results in its dimerization and catalytic activity to cleave single-stranded RNA with a preference for UU and UA dinucleotides (Silverman 2007a). RNase-L activity is attenuated by cellular phosphatases and a 2′-phosphodiesterase that degrades 2–5A (Kubota and others 2004) and through interaction with a cellular RNase-L inhibitor protein (Bisbal and others 1995). The control of RNase-L activation is clearly an important mechanism in determining its biologic activities; however, less is known about the regulation of RNase-L expression. RNase-L is expressed at low basal levels in most cell types, and analysis of the human RNase-L promoter did not find transcription to be a primary level of regulation (Zhou and others 2005). In contrast, RNase-L is subject to robust post-transcriptional regulation. We previously reported that the 3′-untranslated region (3′ UTR) of RNase-L mRNA exerts a strong negative effect on its expression (Li and others 2007); the role of this regulation in RNase-L biologic activities is an area of active investigation. RNase-L is protective against a variety of viruses (Silverman 2007b) and plays a broader role in the innate immune response (Malathi and others 2007; Li and others 2008) and in antiproliferative/tumor suppressive activities, including apoptosis and senescence (Hassel and others 1993; Castelli and others 1997; Andersen and others 2007). These activities are thought to occur through the cleavage of cellular RNAs (Malathi and others 2005; Salehzada and others 2009; Ezelle and Hassel 2012), many of which remain to be identified. At the same time, the cleavage products of viral and cellular RNAs can themselves exert secondary functions, activating cytosolic innate immune receptors to amplify IFN-β production and the antiviral response (Malathi and others 2007; Malathi and others 2010).
MicroRNAs (miRNAs) are small noncoding RNAs that bind to regions of partial complementarity in target mRNAs to inhibit translation or enhance transcript turnover (Bartel 2004). A single miRNA can regulate multiple targets, and multiple miRNAs may regulate a single mRNA; thus, modulation of the cellular miRNA/target profile provides a potent mechanism to post-transcriptionally alter the gene expression program in distinct biologic settings. Indeed, miRNAs have emerged as critical regulators of virtually all physiologic and pathologic processes, including cancer (Bartel 2004; Croce 2012). The miR-29 family has been extensively studied in the context of human cancer, where it was shown to mediate either tumor suppressive or oncogenic functions in distinct malignancies (Pekarsky and Croce 2010; Kriegel and others 2012). The miR-29 family is comprised of 3 isoforms arranged in 2 clusters: the miR-29b-1/miR-29a cluster located at chromosome 7q32 and the miR-29b-2/miR-29c cluster at chromosome 1q23 (Kriegel and others 2012). Decreased expression of miR-29 members has been reported in many cancers, including rhabdomyosarcoma, cholangiocarcinoma, acute myelogenous leukemia (AML), lung cancer, nasopharyngeal carcinoma, and the aggressive form of chronic lymphocytic leukemia characterized by high ZAP-70 expression and unmutated IgH V(H) (Pekarsky and others 2006; Fabbri and others 2007; Mott and others 2007; Sengupta and others 2008; Wang and others 2008; Garzon and others 2009). Restoration of miR-29 sensitized cholangiocarcinoma and AML cells to apoptotic stimuli (Mott and others 2007; Garzon and others 2009) and inhibited growth of rhabdomyosarcoma, lung cancer, and chronic myelogenous leukemia (CML) xenografts in nude mice (Fabbri and others 2007; Wang and others 2008; Garzon and others 2009). Consistent with a tumor suppressor role for miR-29, confirmed targets of miR-29 repression include prosurvival and proproliferation factors such as the antiapoptotic protein myeloid cell leukemia sequence 1 (Mcl-1) (Mott and others 2007), the oncogenic protein T-cell leukemia/lymphoma 1 (Tcl1) (Pekarsky and others 2006), and cyclin-dependent kinase 6 (CDK6) (Zhao and others 2010), as well as other targets relevant to tumorigenesis, such as the DNA methyltransferases 3A and 3B (DNMT3A and B) (Fabbri and others 2007). In contrast, miR-29 upregulation is observed in a subset of malignancies, including breast cancer, colorectal cancer, prostate cancer, pancreatic cancer, and the indolent form of chronic lymphocytic leukemia characterized by low ZAP-70 expression and mutated IgH V(H) (Volinia and others 2006; Gebeshuber and others 2009; Santanam and others 2010). In this context, miR29 may exhibit oncogenic functions such as promotion of epithelial-to-mesenchymal transition in breast cancer cells (Gebeshuber and others 2009). Thus, understanding the roles of miR-29 in specific cancers and identifying the relevant mRNA targets that mediate its tumor suppressor or oncogenic activities are essential to develop miR-29 as a therapeutic target.
Our previous study demonstrating that RNase-L expression is post-transcriptionally downregulated via elements in the 3′ UTR of its mRNA (Li and others 2007) and the established role of miRNAs in repressing gene expression through sites in the 3′ UTR of target mRNAs suggested that miRNAs may negatively regulate RNase-L expression and modulate its biologic activities. Analysis of the RNase-L 3′ UTR with multiple miRNA target prediction algorithms identified several potential miRNA target sites. Among these, miR-29 has 4 predicted target sites in the RNase-L 3′ UTR and represented the best-candidate miRNA. Ectopic expression of miR-29 downregulated RNase-L, miR-29 knockdown upregulated RNase-L, and RNase-L was inversely correlated with miR-29 expression in several cell types indicating that endogenous RNase-L is regulated by miR-29. A luciferase reporter containing the RNase-L 3′ UTR exhibited miR-29-dependent downregulation, and mutation of miR-29 sites rescued luciferase expression, demonstrating functional regulation of the RNase-L 3′ UTR by miR-29. Consistent with our identification of RNase-L as a miR-29 target, RNase-L was identified as a candidate miR-29-regulated transcript in a study of miR-29-mediated tumor suppression in myelogenous leukemias (Garzon and others 2009), and we validated this regulation in K562 CML cells. The miR-29-dependent repression of RNase-L expression in the context of a tumor suppressor phenotype suggested a previously undescribed oncogenic role for RNase-L. To determine if RNase-L represented an important target of miR-29 tumor suppressor activity independent of other miR-29 targets, we stably knocked down RNase-L expression in K562 cells and analyzed the proliferative phenotype. Remarkably, RNase-L knockdown reduced K562 proliferation in culture, and more strikingly, inhibited tumorigenesis in nude mouse xenografts. Thus, RNase-L knockdown phenocopied the tumor suppressive activity of miR-29 in K562 xenografts, revealing a novel tumorigenic role for RNase-L in this setting. These findings suggest that RNase-L functions to promote tumorigenesis in some malignancies, and thus RNase-L inhibition may represent a viable strategy for therapeutic intervention.
Materials and Methods
Plasmids and miRNA precursors and inhibitors
The ZC5 and ZC5+ RNase-L constructs have been previously described (Zhou and others 1993; Li and others 2007). To generate the RNase-L 3′ UTR reporter, the firefly luciferase gene was cloned into the NheI and XhoI sites of the pcDNA3.1(+) plasmid after digestion with the appropriate restriction enzymes. The RNase-L ZC5+ construct was then partially digested with EcoRI, and the 3′ UTR fragment was gel-purified and cloned into the EcoRI site downstream of firefly luciferase. The resulting chimeric transcript contains both luciferase and the RNase-L 3′ UTR and utilizes the pcDNA3.1-encoded polyadenylation signal. All restriction enzymes were purchased from New England Biolabs. The Renilla luciferase plasmid was generously provided by Dr. Myriam Gorospe (National Institute on Aging, Baltimore). miR-29-site mutations were created using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) using primers that deleted the 7 nucleotides predicted to bind the seed region (nt2–8) of the miR-29 family. Primers used for mutagenesis were as follows: site A (forward 5′-GCACTTTATAAATTTATGATTGGTACCTCTCATTTGGGC-3′, reverse 5′-GCCCAAATGAGAGGTACCAATCATAAATTTATAAAGTGC-3′); site B (forward 5′-CCAGACAAAAATATCAAGAGGTTGAGAAAACCTGAC-3′, reverse 5′-GTCAGGTTTTCTCAACCTCTTGATATTTTTGTCTGG-3′); site C (forward 5′-CTGTCTTACGTTTTTCTTATAATGTATACATTACATCTGAG-3′, reverse 5′-CTCAGATGTAATGTATACATTATAAGAAAAACGTAAGACAG-3′); site D (forward 5′-CTTGATTTGAACAAATTTTCAAGTCTGATGTTCTTTCCATG-3′, reverse 5′-CATGGAAAGAACATCAGACTTGAAAATTTGTTCAAATCAAG-3′). Constructs were verified by sequencing (Biopolymer/Genomics Core Facility, University of Maryland, Baltimore). pGIPZ-encoded nonspecific and RNase-L short-hairpin RNAs (shRNAs) were purchased from Open Biosystems. miR-29 Pre-miR miRNA precursors and mirVana miRNA inhibitors were purchased from Ambion.
Cell culture, transfection, and transduction
293T, HeLa, and MDA-MB-231 cells were passaged in the Dulbecco's modified Eagle's medium (Cellgro) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals), antibiotic/antimycotic (Invitrogen), and 2.5 μg/mL Plasmocin (InvivoGen). K562 cells were passaged in RPMI 1640 (Cellgro) with l-Glutamine supplemented with 10% (FBS), antibiotic/antimycotic and Plasmocin. All cell lines were maintained in a humidified incubator at 37°C and 95% balanced air plus 5% CO2. 293T cells were transfected using Lipofectamine2000 (Invitrogen), and all other cell types were reverse-transfected using Lipofectamine RNAiMax (Invitrogen) according to the manufacturer's recommendations. K562 cells with stable knockdown of a nonspecific sequence (shNS) or RNase-L (shRNL) were generated by lentiviral transduction. Virus-containing pGIPZ-encoded shRNA constructs were generated using the Trans-Lentiviral shRNA packaging system from Open Biosystems according to the manufacturer's instructions. Forty-eight hours after transduction, cells were moved into complete RPMI supplemented with 4 μg/mL puromycin (Sigma). Seventy-two hours after transduction, cells were counted and seeded into 96-well plates at varying dilutions. Dilutions in which fewer than 10% of wells gave rise to colonies were considered likely to represent single-cell clones, and individual wells were expanded for testing of RNase-L expression. Pools and selected clones were subsequently passaged in complete RPMI with 1 μg/mL puromycin. All cells were originally obtained from the American Type Culture Collection.
Antibodies and Western blotting
Cell lysates were prepared using a radioimmunoprecipitation assay buffer (Millipore) plus a protease inhibitor cocktail (Sigma). Protein concentrations were determined using the Bradford protein assay (Bio-Rad), and equal amounts of protein per sample were separated on 10% SDS-PAGE gels (Bio-Rad). Proteins were then electrotransferred to Immobilon-P membranes (Millipore). Membranes were blocked for 1 h at room temperature in a TBST buffer (10 mM Tris, pH 8.0, 150 mM NaCl, and 0.1% Tween 20) plus 10% FBS (RNase-L antibody only) or 5% nonfat milk (all other antibodies). Anti-human RNase-L mouse monoclonal antibody (clone 2E9; Alexis Biochemicals) was used at a 1:1,000 dilution in TBS-T plus 5% bovine serum albumin. All other antibodies were diluted in TBS-T plus 5% nonfat milk. Anti-human MCL-1 mouse monoclonal antibody (clone 22; BD Biosciences) was used at 2 μg/mL. Anti-human DNMT3A rabbit polyclonal antibody (Cell Signaling) was used at 1:1,000. Anti-human β-actin (clone AC15) and α-tubulin (clone B-5-1-2) mouse monoclonal antibodies (Sigma) were used at 1:10,000 dilutions. Membranes were incubated with a primary antibody at 4°C overnight and then incubated with horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch) at 1:10,000 dilution in TBST plus 5% nonfat milk for 1 h at room temperature. Membranes were washed again and visualized using a SuperSignal West Pico Chemiluminescent Substrate (Pierce) and Hyblot CL autoradiography film (Denville Scientific).
Quantitative real-time polymerase chain reaction
Total RNA was prepared using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Concentration and 260/280 ratios were determined on a Tecan Infinite 200 Promultimode reader. mRNA quantitative real-time polymerase chain reaction (qRT-PCR) analysis was carried out on a CFX96 Touch Real-Time PCR Detection System using the iScript One-Step RT-PCR Kit with SYBR Green (Bio-Rad) and 0.5μg total RNA per triplicate reaction. Primers for human DICER were as follows: forward 5′-AGTGCCAGTCCTGCAGTAGTTGAT-3′; reverse 5′-ATGTGATTCACCAACATGCCAGCC-3′. Primers for human ribosomal protein L13a (rpl13a) have been previously described (Li and others 2007). Primers for human RNase-L were as follows: forward 5′-CAGGATCTGCAACCACAAAA-3′; reverse 5′-CCCACTTGATGCTCTTATCAAA-3′. Primers for human hydroxymethylbilane synthase were designed by Vandesompele and others (2002): forward 5′-GGCAATGCGGCTGCAA-3′; reverse 5′-GGGTACCCACGCGAATCAC-3′. Cycling conditions were as follows: 50°C for 5 min; 95°C for 10 min; and 40 cycles of 95°C for 10 s and 58°C for 30 s. miRNAs and control U6 small RNAs were detected by qRT-PCR using TaqMan miRNA Assays (Applied Biosystems) according to the manufacturer's instructions.
Luciferase assays
293T cells were seeded in 12-well plates at 350,000 cells per well and allowed to adhere overnight. Cells were then transfected with a combination of firefly luciferase reporter (0.5 μg per well) and Renilla internal control (5 ng per well) plasmids, along with the indicated miRNA mimetics at 25 nM. Cells were harvested 24 h later and analyzed for luciferase activity on a PerkinElmer Victor X3 Multilabel Plate Reader using the Dual Luciferase Reporter Assay (Promega) according to the manufacturer's instructions. Within each sample, firefly was normalized to Renilla luciferase, and for each reporter construct, the firefly/Renilla ratio of the nonspecific control-transfected sample was set to 1.
Proliferation studies
K562 cell proliferation was measured using the Promega Cell Titer 96 Nonradioactive Cell Proliferation MTT assay according to the manufacturer's instructions. For proliferation studies, 1000 K562 cells were seeded in triplicate in 100 μL complete RPMI per well in 96-well plates. MTT dye was added to a new plate and absorbance measured at 570 nM daily over 4 days. For plates measured on later days, cells were spun down, and 25 μL of media was replaced daily.
Xenograft studies
All mouse experiments were carried out by the University of Maryland Translational Core Laboratory (Baltimore, MD). Mice were maintained in accordance with the protocols approved by the Institutional Animal Care and Use Committee at the University of Maryland. For xenograft studies, 6-week-old female nude (nu/nu) mice were subcutaneously injected in the flank with K562 cells. Cells were washed and reconstituted in phosphate-buffered saline (PBS; Cellgro), and 100 μL of PBS containing 10 million cells was mixed with an equal amount of Matrigel (BD Biosciences) before injection. Each mouse was injected with a matched pair of K562 cell lines, the shNS cells in the left flank and the shRNL cells in the right flank. Each pair of cells was tested in 10 mice (n=10). Mice were monitored every 2 to 3 days for weight loss and tumor size. Tumor length and width measurements were taken with calipers, and the tumor volume was calculated according to the following formula: (volume=width2×length/2). Mice were euthanized and tumors were weighed and frozen for protein analysis when tumor volume on either side exceeded 1,500 mm3.
Results
miRNA-dependent repression of RNase-L by the miR-29 family
Our previous report of the post-transcriptional, 3′ UTR-dependent repression of RNase-L expression suggested that this regulation may be mediated by miRNAs. To test the hypothesis that miRNAs are involved in RNase-L repression, we used shRNAs to knockdown the essential miRNA-processing enzyme DICER in 293T cells. DICER knockdown was confirmed by qRT-PCR and resulted in an increase in RNase-L protein (Fig. 1A), indicating that production of mature miRNAs is necessary for RNase-L repression. This approach does not rule out the possibility of indirect regulation (i.e., miRNAs targeting a regulator of RNase-L); therefore, we used miRNA target prediction algorithms to identify miRNAs that may directly interact with the RNase-L message. Analysis of the RNase-L 3′ UTR (NM_021133) using TargetScan (version 5.1) (Lewis and others 2003) and microRNA.org (September 2008 release) (Betel and others 2008) identified 114 predicted miRNA-binding sites that were common to both algorithms. Notable among these was the miR-29 family, which had the highest number of predicted sites (Fig. 1B); each site was predicted to bind all 3 miR-29 isoforms. Therefore, we assessed the ability of miR-29 family members to repress endogenous RNase-L expression by transfecting synthetic miR-29 precursors into 293T cells. All 3 miR-29 family members potently repressed expression of RNase-L protein as well as the previously identified miR-29 target, Mcl-1 (Mott and others 2007), while no repression was observed after transfection with a nonspecific miRNA control (Fig. 2A). Expression of the mature miR-29 family members was confirmed by qRT-PCR (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/jir). miR-29-mediated repression of RNase-L was also observed in K562 and HeLa cells (Fig. 2B and Supplementary Fig. S1B). At 25 nM, miR-29 transfection decreased RNase-L protein without changing steady-state RNase-L mRNA (Supplementary Fig. S1C), suggesting that miR-29 inhibits RNase-L translation. However, a reduction in RNase-L mRNA was observed at higher miR-29 concentrations and later time points (Supplementary Fig. S1D). This finding implies that miR-29 can influence both RNase-L mRNA stability and translation, though the 2 processes may occur with different kinetics and efficiencies depending on miRNA concentration. Consistent with the repression of RNase-L by ectopically expressed miR-29, knockdown of endogenous miR-29 using antisense oligonucleotides in MDA-MB-231 breast cancer cells (which express high levels of miR-29) resulted in an upregulation of RNase-L protein (Fig. 2C). Thus, the inverse relationship between miR-29 and RNase-L observed after miR-29 overexpression or knockdown suggests that RNase-L is an authentic miR-29 target. In agreement with this prediction, expression of miR-29 and RNase-L was inversely correlated in several cell lines examined (Fig. 2D).
FIG. 1.
miRNA-mediated repression of RNase-L and identification of miR-29 family target sites in the RNase-L 3′ UTR. (A) 293T cells were transiently transfected with shRNA against a nonspecific sequence (shNS) or DICER (shDICER). Cells were harvested at 24 and 72 h for RNase-L immunoblot and DICER qRT-PCR. (B) Diagram of RNase-L mRNA with 4 miR-29-binding sites (designated A–D) in the 3′ UTR predicted by microRNA.org (September 2008 release). Numbers above each target site indicate putative seed-binding nucleotides, while predicted alignments of RNase-L mRNA and the miR-29 family are shown below. Sites B–D were also identified by TargetScan.org (version 5.1). miRNA, microRNAs; 3′ UTR, 3′-untranslated region; qRT-PCR, quantitative real-time polymerase chain reaction; shRNA, short-hairpin RNA.
FIG. 2.
RNase-L is regulated by the miR-29 family in multiple cell types. (A) 293T cells were transfected with 25 nM miRNA mimetics and harvested for protein immunoblot after 24 h. (B) K562 cells were transfected with 50 nM miRNA mimetics and harvested for protein immunoblot after 24 h. (C) MDA-MB-231 cells were transfected with antisense miRNA inhibitors and harvested for protein immunoblot after 24 h. Mcl-1 was included as a known target of miR-29 (A–C). (D) A panel of cell lines was analyzed for RNase-L protein by immunoblot and miR-29 isoforms by qRT-PCR. miR-29 signal was normalized to U6 small nuclear RNA.
miR-29 represses RNase-L via multiple target sites in its 3′ UTR
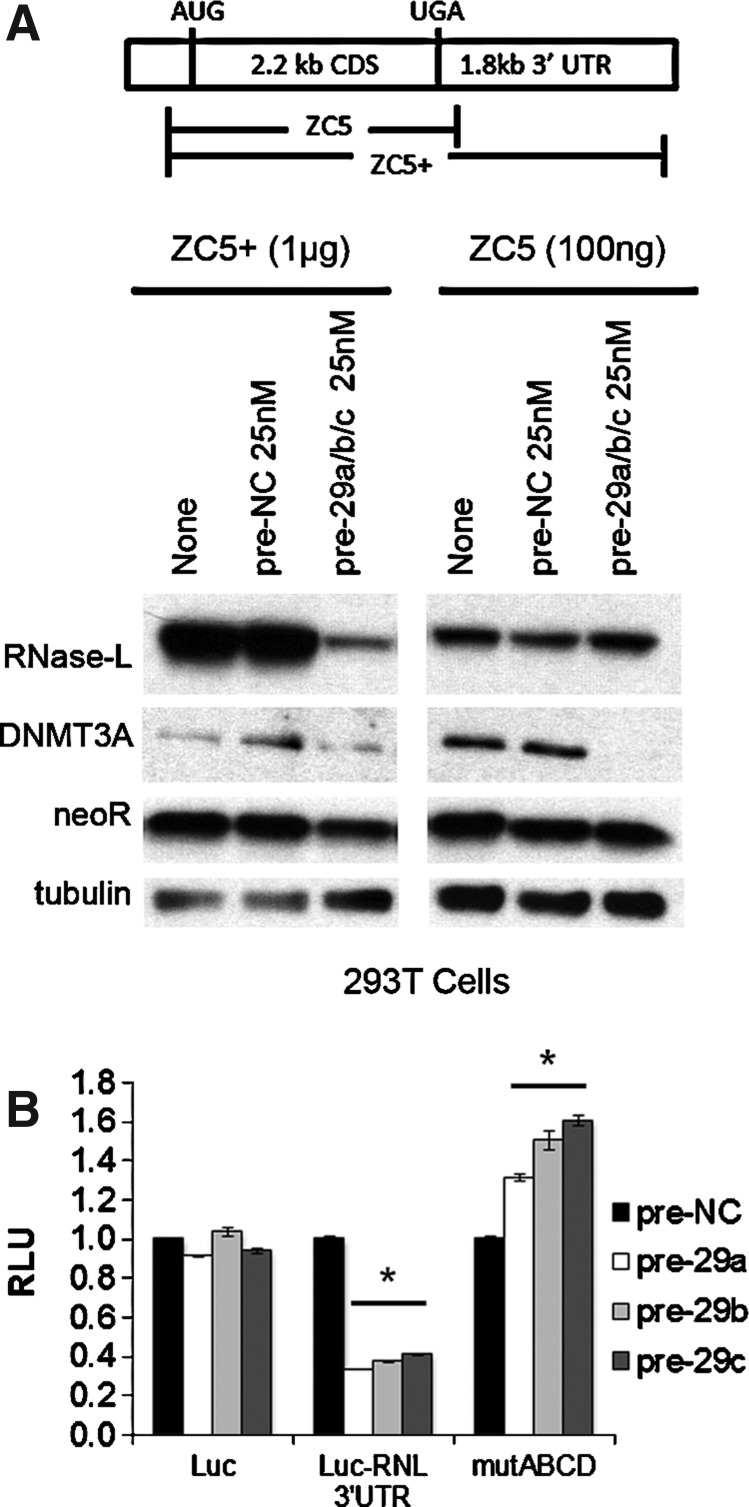
Given that multiple miR-29 sites were identified in the RNase-L 3′ UTR, we expected that deletion of the 3′ UTR would abrogate miR-29-mediated regulation. Accordingly, a combination of pre-miR-29a/b/c was cotransfected with an RNase-L expression construct that contained (ZC5+) or lacked (ZC5) the 3′ UTR (Zhou and others 1993; Li and others 2007). Consistent with the presence of miR-29-binding sites in the RNase-L 3′ UTR, miR-29 potently repressed RNase-L expression from the ZC5+ construct, but did not markedly alter expression from ZC5 (Fig. 3A). To quantify the contributions of individual target sites to miR-29-mediated RNase-L repression, the human RNase-L 3′ UTR was cloned downstream of the firefly luciferase reporter gene. Transfection of the reporter construct and pre-miR-29 family members into 293T cells resulted in a significant inhibition of luciferase activity that was not observed in the absence of the 3′ UTR; thus, the RNase-L 3′ UTR conferred miR-29-dependent regulation by all 3 family members (Fig. 3B). Mutation of miR-29 target sites that are critical for RNase-L repression is predicted to rescue expression and luciferase activity. Therefore, we generated mutations in which the seed regions (nt2–8) of predicted miR-29 target sites (designated A–D) were deleted (Fig. 1B). Mutation of all 4 target sites (mutABCD) resulted in the complete loss of miR-29-mediated repression and full rescue of luciferase activity (Fig. 3B), while deletion of between 1 and 3 target sites led to partial repression that was proportional to the number of intact target sites (Supplementary Fig. S2). Target site A was the weakest, as it was neither necessary for complete repression (mutBCD), nor capable of mediating repression on its own (mutA). Nonetheless, site A exhibited some functionality, as its presence enhanced repression by other more robust target sites (e.g., mutABD versus mutBD). Together, these findings identify miR-29-mediated repression as a novel mechanism regulating RNase-L expression.
FIG. 3.
miR-29 represses RNase-L via multiple target sites in the 3′ UTR. (A) Control or miR-29 mimetics were cotransfected into 293T cells with plasmid-encoded RNase-L, including (ZC5+) or lacking (ZC5) the 3′ UTR, and protein expression was analyzed by immunoblot. As absence of the RNase-L 3′ UTR leads to greatly enhanced expression, only 100 ng of the ZC5 construct was transfected, with empty vector used to equalize total plasmid DNA. neoR was used as a transfection control. All ZC5+ and ZC5 panels are from the same blot at identical exposure times. At these exposures, endogenous RNase-L was not detectable (data not shown). (B) 293T cells were cotransfected with the indicated miR-29 mimetics, Renilla luciferase vector and firefly luciferase constructs containing the wild-type or mutated RNase-L 3′ UTR. Cells were harvested at 24 h and assayed for luciferase activity. All readings were normalized to Renilla and reported as relative luciferase units (RLU) with the nonspecific control set to 1. Luc, luciferase-only vector; Luc-RNL 3′ UTR, wild-type RNase-L 3′ UTR reporter; mutABCD, RNase-L 3′ UTR with deletion of all miR-29 seed regions as designated in Fig. 1. miR-29-transfected cells were compared with control-transfected cells using Student's t-test; *P<0.0005.
miR-29-dependent regulation reveals a novel role for RNase-L in tumorigenesis
Our data demonstrating the miR-29-dependent regulation of RNase-L in cell culture systems suggested that RNase-L is an important miR-29 target and contributes to its biologic functions in physiologic and pathologic settings. Consistent with this prediction, a study of miR-29 tumor suppressor activity in myelogenous leukemias identified RNase-L as the most highly repressed transcript after miR-29 transfection of K562 CML cells (Garzon and others 2009). We demonstrated that miR-29 transfection also downregulated RNase-L protein, validating their inverse relationship in K562 cells (Fig. 2B). The role of RNase-L in antiproliferative and tumor suppressor activities is well established (Hassel and others 1993; Castelli and others 1997; Andersen and others 2007), so its identification as a target of miR-29 repression and tumor suppressor activity was surprising, and suggested that RNase-L may serve a novel oncogenic function in this setting. miR-29 regulates the expression of many transcripts that may contribute to its tumor suppressor activity (e.g., Mcl-1, Tcl1, CDK6, DNMT3A, and 3B) (Pekarsky and others 2006; Fabbri and others 2007; Mott and others 2007; Zhao and others 2010); to examine the role of RNase-L in miR-29-mediated tumor suppressor activities independent of other miR-29 targets, we stably knocked down RNase-L in K562 cells (Fig. 4A). An 80-kDa immunoreactive band that migrates just below the authentic 83-kDa RNase-L is observed in some conditions and cell types, but is not consistently repressed by shRNA directed against RNase-L, thus demonstrating its nonspecific nature. RNase-L knockdown resulted in a modest reduction in the proliferation of cultured cells (42% in the pools and 31% in the clones, Fig. 4B), suggesting that RNase-L functions to stimulate proliferation in K562 cells. Similar results were observed in all knockdown clones tested (data not shown). More strikingly, RNase-L knockdown dramatically inhibited tumorigenesis in nude mouse xenografts, whereas nonspecific shRNA-transfected control cells exhibited robust tumor growth (Fig. 4C). All control shRNA K562 cells formed detectable tumors by 11 days postinjection, whereas RNase-L knockdown cells failed to produce tumors in the majority of mice even at 26 days postinjection. Furthermore, RNase-L knockdown cells failed to produce tumors in several mice that were monitored through day 46 postinjection (data not shown). In the few small tumors generated from RNase-L knockdown cells, functional knockdown of RNase-L expression was maintained at the time of tumor harvest (Supplementary Fig. S3A). Thus, RNase-L knockdown phenocopied the tumor suppressive activity of miR-29 in K562 xenografts, revealing a novel tumorigenic role for RNase-L in this setting. This remarkable phenotype provides the first evidence of a tumorigenic role for RNase-L, and suggests that RNase-L mRNA is an important target of miR-29 tumor suppressor activity. Consistent with this finding, RNase-L activity is increased in CML patient samples (Hubbell and others 1994), and was among the top 10% of upregulated genes in a large microarray analysis of multiple leukemias, including CML and B-cell acute lymphocytic leukemia (Rhodes and others 2004; Haferlach and others 2010). However, further studies are required to determine the specific settings in which RNase-L mediates oncogenic functions and the molecular mechanisms involved in these activities.
FIG. 4.
RNase-L promotes K562 proliferation and xenograft growth. (A) K562 cells were transfected with shRNA against a nonspecific sequence (shNS) or RNase-L (shRNL) and grown in puromycin for selection of stable shRNA expression. Clones were isolated by limiting dilution in 96-well plates, and RNase-L expression was assessed by protein immunoblot. Note that RNase-L expression is the top band in (A), while the bottom band is nonspecific. (B) Proliferation of K562 cell lines was measured by daily MTT readings. Error bars are derived from triplicate wells, and data are representative of 3 independent experiments. (C) K562 cells were injected subcutaneously into nude mice, and xenograft growth was monitored daily. Each mouse was injected with an shRNL cell line on the right flank and an shNS cell line on the left flank. Error bars are representative of 10 mice per cell pair (n=30). (D) Tumor weight of shNS and shRNL tumors. Mice were euthanized and tumors weighed when either tumor exceeded a predetermined volume. Results are from left and right side tumors harvested from 5 mice (n=5) on the same day.
Discussion
In this study, we demonstrate that RNase-L expression is post-transcriptionally regulated by the miR-29 family of miRNAs via 4 target sites in its 3′ UTR. The RNase-L 3′ UTR-mediated repression of a luciferase reporter required multiple miR-29 sites, as deletion of at least 3 sites was necessary to rescue expression (Fig. 3 and Supplementary Fig. S2). Consistent with this finding, 3 of the miR-29 sites occur in a region that we previously reported to negatively regulate RNase-L expression (Li and others 2007). At the same time, binding of miR-29 to the RNase-L 3′ UTR may impact the activities of additional 3′ UTR regulatory elements and their cognate binding factors (van Kouwenhove and others 2011). Indeed, our prior study identified AU-rich elements (AREs) in the RNase-L 3′ UTR that interact with ARE-binding proteins (AREBPs) to modulate mRNA stability and translation (Li and others 2007). Interestingly, the fourth miR-29 site in the RNase-L 3′ UTR is adjacent to a binding site for the AREBP HuR that enhances RNase-L expression in response to stress stimuli; therefore, miR-29 and HuR may mediate antagonistic effects on RNase-L expression in conditions where they are expressed together. In addition to the several miR-29-binding sites, other potential miR-binding sites were identified in the RNase-L 3′ UTR; the presence of multiple candidate miRNA- and RNABP-binding sites in the RNase-L 3′ UTR provides a platform for complex regulatory interactions to mediate rapid modulation of RNase-L expression. Identification of the specific cis-elements and trans-acting regulators that control RNase-L expression in distinct biologic settings may reveal strategies to modulate its expression for therapeutic applications.
The strong miR-29-dependent regulation of RNase-L involving multiple target sites suggests that it is an important target in miR-29-mediated biologic functions. Consistent with this prediction, miR-29 family members and RNase-L are implicated in an overlapping set of physiologic and pathologic activities, including antiviral immunity (Silverman 2007b; Ahluwalia and others 2008; Bandyopadhyay and others 2011), tumorigenesis (Casey and others 2002; Pekarsky and others 2006; Volinia and others 2006; Fabbri and others 2007; Liu and others 2007; Mott and others 2007; Madsen and others 2008; Sengupta and others 2008; Wang and others 2008), and myogenesis (Wang and others 2008; Salehzada and others 2009; Winbanks and others 2011). In this study, we focused on the regulation of RNase-L in the context of a tumor suppressor function for miR-29 in myelogenous leukemias, as RNase-L was identified as a candidate miR-29 target in this system (Garzon and others 2009). miR-29 transfection of K562 CML cells reduced proliferation, enhanced apoptosis, and inhibited tumorigenesis; therefore, our validation of RNase-L as a miR-29-repressed transcript in K562 cells suggested that RNase-L serves a novel oncogenic function in this setting. Consistent with this interpretation, stable knockdown of RNase-L, independent of other miR-29 targets, decreased K562 proliferation and inhibited tumorigenesis in a xenograft model (Fig. 4). However, RNase-L knockdown did not phenocopy the enhanced sensitivity to proapoptotic chemotherapeutic agents observed after ectopic expression of miR-29 (Mott and others 2007; Garzon and others 2009), indicating that other miR-29 targets (e.g., Mcl-1) also contribute to its tumor suppressor phenotype (Supplementary Fig. S2B). Importantly, endogenous RNase-L is functional in K562 cells (Castelli and others 1998); therefore, a mutation in RNase-L is unlikely to account for the tumorigenic phenotype. These findings provide the first evidence of a functional role for RNase-L in tumorigenesis and are supported by previous reports of cancer-associated increases in RNase-L activity and expression in CML and colon cancer (Hubbell and others 1994; Wang and others 1995). Furthermore, microarray analyses revealed that RNase-L is upregulated in diverse types of leukemia (Rhodes and others 2004; Haferlach and others 2010). RNase-L, however, was not altered in a separate analysis of AML (Garzon and others 2009), suggesting that it contributes to the oncogenic phenotype in a subset of hematopoietic malignancies. Further work is required to determine the extent to which RNase-L functions in proliferation and tumorigenesis in other types of cancer.
Our data indicating a tumorigenic role for RNase-L contrasts with the more-established antiproliferative and tumor suppressor activities of RNase-L (Hassel and others 1993; Castelli and others 1997; Andersen and others 2007) and suggest that it mediates context-specific tumor suppressor and oncogenic activities as has been reported for other proteins and regulatory RNAs (e.g., c-myc and miR-29) (Larsson and Henriksson 2010; Pekarsky and Croce 2010). This dual functionality is often observed for regulators of gene expression and is thought to reflect distinct profiles of targets or cofactors that promote the expression of tumor suppressor or oncogenic gene products. We hypothesize that RNABPs expressed in different cell types or cancers target RNase-L cleavage to specific RNAs and, in turn, dictate its function as an oncogene or tumor suppressor. Indeed, the dysregulation of RNABPs has been reported in multiple malignancies (Abdelmohsen and Gorospe 2010; Baou and others 2011); these and other cancer-associated alterations may lead to aberrant substrate recognition and cleavage by RNase-L. Consistent with this model, microarray analyses revealed distinct profiles of RNase-L-regulated RNAs in different cell types and conditions (Malathi and others 2005; Salehzada and others 2009; Ezelle and Hassel 2012). A comparison of RNase-L substrates in healthy and cancer tissues will provide insights into potential mechanisms by which RNase-L contributes to tumorigenic activity. In this regard, the diminished tumor growth in K562 RNase-L knockdown xenografts did not provide sufficient material for analysis of RNase-L targets in the context of tumorigenesis. Therefore, future studies will employ a conditional RNase-L knockdown approach in a mouse model of leukemogenesis to analyze the impact of RNase-L on gene expression and disease progression.
The discovery that RNase-L serves a novel oncogenic function as a target of miR-29 regulation has important clinical implications. Elevated levels of RNase-L may serve as a biomarker of miR-29 downregulation and compromised tumor suppressor activity. From a therapeutic standpoint, modulation of miRNA expression has shown efficacy in animal models and is an area of intense investigation (van Rooij and others 2012). Furthermore, the direct targeting of clinically relevant miRNA targets provides an alternate strategy that may increase specificity and potency. Therefore, as suggested by the antitumor activity of RNase-L knockdown, RNase-L inhibitors may represent a novel class of therapeutic agent for tumors in which RNase-L functions to promote malignancy. In this regard, sunitinib, an ATP-competitive inhibitor that is an FDA-approved chemotherapeutic agent, was recently shown to inhibit RNase-L activity (Jha and others 2011); thus, sunitinib may have novel indications as an RNase-L-targeting agent. Our findings demonstrate that RNase-L is an important target of miR-29 and identify a previously unknown role for RNase-L in tumorigenesis, opening a door for further investigations into the mechanisms behind its dual function.
Supplementary Material
Acknowledgments
The authors would like to thank Sarah Brennan-Laun, Tiha Long, and Kristina Pedersen for critical discussion of the manuscript. This study was funded by an NIH grant AI077556 (BAH) and a VA Merit Award (BAH). TYL is supported by an NIH grant T32 AI07540 (JBK) and the University of Maryland School of Medicine MSTP program.
Author Disclosure Statement
BAH is an inventor on patents relating to RNase-L licensed to Alios BioPharma. No competing financial interests exist for all other authors.
References
- Abdelmohsen K. Gorospe M. Posttranscriptional regulation of cancer traits by HuR. Wiley Interdiscip Rev RNA. 2010;1(2):214–229. doi: 10.1002/wrna.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia JK. Khan SZ. Soni K. Rawat P. Gupta A. Hariharan M. Scaria V. Lalwani M. Pillai B. Mitra D. Brahmachari SK. Human cellular microRNA hsa-miR-29a interferes with viral nef protein expression and HIV-1 replication. Retrovirology. 2008;5:117. doi: 10.1186/1742-4690-5-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JB. Li XL. Judge CS. Zhou A. Jha BK. Shelby S. Zhou L. Silverman RH. Hassel BA. Role of 2–5A-dependent RNase-L in senescence and longevity. Oncogene. 2007;26(21):3081–3088. doi: 10.1038/sj.onc.1210111. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S. Friedman RC. Marquez RT. Keck K. Kong B. Icardi MS. Brown KE. Burge CB. Schmidt WN. Wang Y. McCaffrey AP. Hepatitis C virus infection and hepatic stellate cell activation downregulate miR-29: miR-29 overexpression reduces hepatitis C viral abundance in culture. J Infect Dis. 2011;203(12):1753–1762. doi: 10.1093/infdis/jir186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baou M. Norton JD. Murphy JJ. AU-rich RNA binding proteins in hematopoiesis and leukemogenesis. Blood. 2011;118(22):5732–5740. doi: 10.1182/blood-2011-07-347237. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Betel D. Wilson M. Gabow A. Marks DS. Sander C. The microRNA.org. resource: targets and expression. Nucleic Acids Res. 2008;36:D149–D153. doi: 10.1093/nar/gkm995. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisbal C. Martinand C. Silhol M. Lebleu B. Salehzada T. Cloning and characterization of a RNAse L inhibitor. A new component of the interferon-regulated 2–5A pathway. J Biol Chem. 1995;270(22):13308–13317. doi: 10.1074/jbc.270.22.13308. [DOI] [PubMed] [Google Scholar]
- Casey G. Neville PJ. Plummer SJ. Xiang Y. Krumroy LM. Klein EA. Catalona WJ. Nupponen N. Carpten JD. Trent JM. Silverman RH. Witte JS. RNASEL Arg462Gln variant is implicated in up to 13% of prostate cancer cases. Nat Genet. 2002;32(4):581–583. doi: 10.1038/ng1021. [DOI] [PubMed] [Google Scholar]
- Castelli JC. Hassel BA. Maran A. Paranjape J. Hewitt JA. Li XL. Hsu YT. Silverman RH. Youle RJ. The role of 2′-5′ oligoadenylate-activated ribonuclease L in apoptosis. Cell Death Differ. 1998;5(4):313–320. doi: 10.1038/sj.cdd.4400352. [DOI] [PubMed] [Google Scholar]
- Castelli JC. Hassel BA. Wood KA. Li XL. Amemiya K. Dalakas MC. Torrence PF. Youle RJ. A study of the interferon antiviral mechanism: apoptosis activation by the 2–5A system. J Exp Med. 1997;186(6):967–972. doi: 10.1084/jem.186.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. Introduction to the role of microRNAs in cancer diagnosis, prognosis, and treatment. Cancer J. 2012;18(3):213–214. doi: 10.1097/PPO.0b013e31825efb41. [DOI] [PubMed] [Google Scholar]
- Ezelle HJ. Hassel BA. Pathologic effects of RNase-L dysregulation in immunity and proliferative control. Front Biosci (Schol Ed) 2012;4:767–786. doi: 10.2741/s298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M. Garzon R. Cimmino A. Liu Z. Zanesi N. Callegari E. Liu S. Alder H. Costinean S. Fernandez-Cymering C. Volinia S. Guler G. Morrison CD. Chan KK. Marcucci G. Calin GA. Huebner K. Croce CM. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104(40):15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon R. Heaphy CE. Havelange V. Fabbri M. Volinia S. Tsao T. Zanesi N. Kornblau SM. Marcucci G. Calin GA. Andreeff M. Croce CM. MicroRNA 29b functions in acute myeloid leukemia. Blood. 2009;114(26):5331–5341. doi: 10.1182/blood-2009-03-211938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebeshuber CA. Zatloukal K. Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep. 2009;10(4):400–405. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haferlach T. Kohlmann A. Wieczorek L. Basso G. Kronnie GT. Bene MC. De Vos J. Hernandez JM. Hofmann WK. Mills KI. Gilkes A. Chiaretti S. Shurtleff SA. Kipps TJ. Rassenti LZ. Yeoh AE. Papenhausen PR. Liu WM. Williams PM. Foa R. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: report from the International Microarray Innovations in Leukemia Study Group. J Clin Oncol. 2010;28(15):2529–2537. doi: 10.1200/JCO.2009.23.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel BA. Zhou A. Sotomayor C. Maran A. Silverman RH. A dominant negative mutant of 2–5A-dependent RNase suppresses antiproliferative and antiviral effects of interferon. EMBO J. 1993;12(8):3297–3304. doi: 10.1002/j.1460-2075.1993.tb05999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell HR. Kariko K. Suhadolnik RJ. Brodsky I. RNase L and increased endoribonuclease activities in the mononuclear cells of patients with chronic myelogenous leukemia. Anticancer Res. 1994;14(2A):341–346. [PubMed] [Google Scholar]
- Jha BK. Polyakova I. Kessler P. Dong B. Dickerman B. Sen GC. Silverman RH. Inhibition of RNase L and RNA-dependent protein kinase (PKR) by sunitinib impairs antiviral innate immunity. J Biol Chem. 2011;286(30):26319–26326. doi: 10.1074/jbc.M111.253443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel AJ. Liu Y. Fang Y. Ding X. Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics. 2012;44(4):237–244. doi: 10.1152/physiolgenomics.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K. Nakahara K. Ohtsuka T. Yoshida S. Kawaguchi J. Fujita Y. Ozeki Y. Hara A. Yoshimura C. Furukawa H. Haruyama H. Ichikawa K. Yamashita M. Matsuoka T. Iijima Y. Identification of 2′-phosphodiesterase, which plays a role in the 2–5A system regulated by interferon. J Biol Chem. 2004;279(36):37832–37841. doi: 10.1074/jbc.M400089200. [DOI] [PubMed] [Google Scholar]
- Larsson LG. Henriksson MA. The Yin and Yang functions of the Myc oncoprotein in cancer development and as targets for therapy. Exp Cell Res. 2010;316(8):1429–1437. doi: 10.1016/j.yexcr.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Lewis BP. Shih IH. Jones-Rhoades MW. Bartel DP. Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Li XL. Andersen JB. Ezelle HJ. Wilson GM. Hassel BA. Post-transcriptional regulation of RNase-L expression is mediated by the 3′-untranslated region of its mRNA. J Biol Chem. 2007;282(11):7950–7960. doi: 10.1074/jbc.M607939200. [DOI] [PubMed] [Google Scholar]
- Li XL. Ezelle HJ. Kang TJ. Zhang L. Shirey KA. Harro J. Hasday JD. Mohapatra SK. Crasta OR. Vogel SN. Cross AS. Hassel BA. An essential role for the antiviral endoribonuclease, RNase-L, in antibacterial immunity. Proc Natl Acad Sci U S A. 2008;105(52):20816–20821. doi: 10.1073/pnas.0807265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. Liang SL. Liu H. Silverman R. Zhou A. Tumour suppressor function of RNase L in a mouse model. Eur J Cancer. 2007;43(1):202–209. doi: 10.1016/j.ejca.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Madsen BE. Ramos EM. Boulard M. Duda K. Overgaard J. Nordsmark M. Wiuf C. Hansen LL. Germline mutation in RNASEL predicts increased risk of head and neck, uterine cervix and breast cancer. PLoS One. 2008;3(6):e2492. doi: 10.1371/journal.pone.0002492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi K. Dong B. Gale M., Jr. Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448(7155):816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi K. Paranjape JM. Bulanova E. Shim M. Guenther-Johnson JM. Faber PW. Eling TE. Williams BR. Silverman RH. A transcriptional signaling pathway in the IFN system mediated by 2′-5′-oligoadenylate activation of RNase L. Proc Natl Acad Sci U S A. 2005;102(41):14533–14538. doi: 10.1073/pnas.0507551102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi K. Saito T. Crochet N. Barton DJ. Gale M., Jr. Silverman RH. RNase L releases a small RNA from HCV RNA that refolds into a potent PAMP. RNA. 2010;16(11):2108–2119. doi: 10.1261/rna.2244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JL. Kobayashi S. Bronk SF. Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26(42):6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y. Croce CM. Is miR-29 an oncogene or tumor suppressor in CLL? Oncotarget. 2010;1(3):224–227. doi: 10.18632/oncotarget.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y. Santanam U. Cimmino A. Palamarchuk A. Efanov A. Maximov V. Volinia S. Alder H. Liu CG. Rassenti L. Calin GA. Hagan JP. Kipps T. Croce CM. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006;66(24):11590–11593. doi: 10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- Rhodes DR. Yu J. Shanker K. Deshpande N. Varambally R. Ghosh D. Barrette T. Pandey A. Chinnaiyan AM. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehzada T. Cambier L. Vu Thi N. Manchon L. Regnier L. Bisbal C. Endoribonuclease L (RNase L) regulates the myogenic and adipogenic potential of myogenic cells. PLoS One. 2009;4(10):e7563. doi: 10.1371/journal.pone.0007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanam U. Zanesi N. Efanov A. Costinean S. Palamarchuk A. Hagan JP. Volinia S. Alder H. Rassenti L. Kipps T. Croce CM. Pekarsky Y. Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc Natl Acad Sci U S A. 2010;107(27):12210–12215. doi: 10.1073/pnas.1007186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S. den Boon JA. Chen IH. Newton MA. Stanhope SA. Cheng YJ. Chen CJ. Hildesheim A. Sugden B. Ahlquist P. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci U S A. 2008;105(15):5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RH. A scientific journey through the 2–5A/RNase L system. Cytokine Growth Factor Rev. 2007a;18(5–6):381–388. doi: 10.1016/j.cytogfr.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RH. Viral encounters with 2′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J Virol. 2007b;81(23):12720–12729. doi: 10.1128/JVI.01471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kouwenhove M. Kedde M. Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer. 2011;11(9):644–656. doi: 10.1038/nrc3107. [DOI] [PubMed] [Google Scholar]
- van Rooij E. Purcell AL. Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110(3):496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- Vandesompele J. De Preter K. Pattyn F. Poppe B. Van Roy N. De Paepe A. Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volinia S. Calin GA. Liu CG. Ambs S. Cimmino A. Petrocca F. Visone R. Iorio M. Roldo C. Ferracin M. Prueitt RL. Yanaihara N. Lanza G. Scarpa A. Vecchione A. Negrini M. Harris CC. Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Garzon R. Sun H. Ladner KJ. Singh R. Dahlman J. Cheng A. Hall BM. Qualman SJ. Chandler DS. Croce CM. Guttridge DC. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell. 2008;14(5):369–381. doi: 10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Zhou A. Vasavada S. Dong B. Nie H. Church JM. Williams BR. Banerjee S. Silverman RH. Elevated levels of 2′,5′-linked oligoadenylate-dependent ribonuclease L occur as an early event in colorectal tumorigenesis. Clin Cancer Res. 1995;1(11):1421–1428. [PubMed] [Google Scholar]
- Winbanks CE. Wang B. Beyer C. Koh P. White L. Kantharidis P. Gregorevic P. TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J Biol Chem. 2011;286(16):13805–13814. doi: 10.1074/jbc.M110.192625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ. Lin J. Lwin T. Yang H. Guo J. Kong W. Dessureault S. Moscinski LC. Rezania D. Dalton WS. Sotomayor E. Tao J. Cheng JQ. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115(13):2630–2639. doi: 10.1182/blood-2009-09-243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A. Hassel BA. Silverman RH. Expression cloning of 2–5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell. 1993;72(5):753–765. doi: 10.1016/0092-8674(93)90403-d. [DOI] [PubMed] [Google Scholar]
- Zhou A. Molinaro RJ. Malathi K. Silverman RH. Mapping of the human RNASEL promoter and expression in cancer and normal cells. J Interferon Cytokine Res. 2005;25(10):595–603. doi: 10.1089/jir.2005.25.595. [DOI] [PubMed] [Google Scholar]
- Zhou A. Paranjape J. Brown TL. Nie H. Naik S. Dong B. Chang A. Trapp B. Fairchild R. Colmenares C. Silverman RH. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 1997;16(21):6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.