Abstract
Tocilizumab is a humanized antihuman interleukin-6 (IL-6) receptor monoclonal antibody. It was developed in Japan as the first biological disease-modifying antirheumatic drug targeting IL-6 receptors. Many large-scale global studies have demonstrated its efficacy and safety, and in April 2008 it was approved in Japan for use in the treatment of rheumatoid arthritis, sooner than in other countries. In this paper, I review the efficacy and safety of tocilizumab in the light of front-line clinical data and data from large-scale studies, and I consider the place of tocilizumab treatment in real clinical practice.
Keywords: biological disease-modifying antirheumatic drug, interleukin-6, rheumatoid arthritis, tocilizumab
Introduction
Rheumatoid arthritis is a systemic inflammatory disease characterized by swelling and pain of the joints [Harris, 1990]. The bone or joints become damaged due to the persistent inflammation, restricting the patient’s physical function and activities of daily living. The inflammatory cytokines interleukin-1 (IL-1), interleukin-6 (IL-6) and tumour necrosis factor α (TNFα) are heavily implicated in the aetiology of rheumatoid arthritis [Firestein et al. 1990]. IL-6 contributes to inflammation and antibody production through its action on T cells, B cells, monocytes and neutrophils, and it is also reported to be involved in the activation of osteoclasts [Kishimoto, 2006].
Tocilizumab (TCZ) is a biological disease-modifying antirheumatic drug (DMARD) that targets IL-6 receptors and blocks signalling from them. A range of trials as well as studies in real clinical practice have served to confirm its efficacy and safety profile in the treatment of rheumatoid arthritis. In this paper, I consider the place of TCZ in the treatment of rheumatoid arthritis in the light of the findings from trials and other studies of this agent in the clinical setting.
Efficacy of tocilizumab in patients whose condition responds poorly to other DMARDs (including methotrexate)
Methotrexate is the key drug worldwide in the treatment of rheumatoid arthritis. If the patient fails to respond adequately to methotrexate, the current standard approach is to add other synthetic or biological DMARDs to the treatment regimen. The therapeutic efficacy and safety of biological DMARDs targeting TNFα, namely TNF inhibitors, have also been established in a number of studies, and up to five TNF inhibitors are available for use in the treatment of rheumatoid arthritis. Combining a TNF inhibitor with methotrexate is clinically more effective than treatment with a TNF inhibitor alone in patients with methotrexate-resistant disease, and as long as the patient can tolerate methotrexate, a better response can be expected with methotrexate combination therapy [Klareskog et al. 2004; Breedveld et al. 2006].
Two phase III studies of TCZ have taken place in Japan (the SATORI and SAMURAI studies), each of which demonstrated the effectiveness of TCZ monotherapy compared with the control group [Nishimoto et al. 2007, 2009] (Table 1). The SATORI study was a randomized double-blind study of TCZ monotherapy (8 mg/kg, every 4 weeks) and methotrexate monotherapy in patients with rheumatoid arthritis whose condition had failed to respond adequately to methotrexate at 8 mg/week. With TCZ, 80% of patients achieved the American College of Rheumatology (ACR) ACR20 criteria [Felson et al. 1995] at 24 weeks (the primary study endpoint), which was significantly higher than the 25% of patients receiving methotrexate who achieved ACR20 (p < 0.001) [Nishimoto et al. 2009]. The SAMURAI study was an open-label study in which patients with disease duration of less than 5 years were randomized to a TCZ monotherapy arm (8 mg/kg, every 4 weeks) or to a synthetic DMARD arm. In that study, suppression of joint destruction at 52 weeks was assessed by using van der Heijde modified Sharp scores, with change in bone erosion scores at 52 weeks as the primary endpoint. TCZ monotherapy significantly suppressed bone erosion with a score of 0.9 compared with 3.2 for the controls (p < 0.001) [Nishimoto et al. 2007]. A change of up to 0.5 in the total modified Sharp score over 52 weeks was seen in 39% of the controls as opposed to 56% in those on TCZ (p < 0.01), demonstrating the protection against joint damage provided by TCZ monotherapy. These studies indicated that switching to TCZ monotherapy is extremely effective in patients with rheumatoid arthritis who are refractory to treatment with DMARDs including methotrexate.
Table 1.
Overview of studies involving tocilizumab monotherapy, and efficacy.
| Study | Subjects | Assessment period | Treatment group | ACR20 | ACR50 | ACR70 | DAS28<2.6 |
|---|---|---|---|---|---|---|---|
| SAMURAI | DMARDs IR | 52 weeks | TCZ | 78%* | 64%* | 44%* | 59%* |
| DMARDs | 34% | 13% | 6% | 3% | |||
| SATORI | MTX IR | 24 weeks | TCZ | 80%* | 49%* | 30%* | 43%* |
| MTX | 25% | 11% | 6% | 2% | |||
| AMBITION | MTX naïve | 24 weeks | TCZ | 70%* | 44%$ | 28%* | 34% |
| MTX | 53% | 34% | 15% | 12% | |||
| ACT-RAY | MTX IR | 24 weeks | TCZ | 71% | 41% | 26% | 35% |
| TCZ+MTX | 72% | 45% | 25% | 40% |
p < 0.001; $p < 0.01.
DMARD, disease-modifying antirheumatic drug; IR, inadequate response; MTX, methotrexate; TCZ, tocilizumab.
The AMBITION study was a global study comparing the efficacy of using methotrexate monotherapy and the efficacy of TCZ monotherapy (8 mg/kg, every 4 weeks) in patients who had either never used methotrexate or who had not used it in the previous 6 months even though they had not been classed as having an inadequate response to methotrexate [Jones et al. 2010]. A significantly higher percentage, 70% of the patients on TCZ monotherapy achieved an ACR20 response at 24 weeks as against 52% for those on methotrexate monotherapy. In terms of the European League Against Rheumatism (EULAR) 28-joint Disease Activity Scores (DAS28), significantly superior efficacy was also evident for TCZ monotherapy, with 34% in that group achieving remission (DAS28 < 2.6) versus 12% in the methotrexate monotherapy group.
In a study comparing the efficacy of biological DMARDs other than TCZ and the efficacy of methotrexate in methotrexate-naïve patients with rheumatoid arthritis, monotherapy with a biological DMARD was not found to be superior to methotrexate monotherapy [Klareskog et al. 2004; Breedveld et al. 2006]. TCZ is currently the only biological DMARD for rheumatoid arthritis as its performance is superior to that of methotrexate used alone.
There are also some papers documenting combination therapy with TCZ and another DMARD or methotrexate. In each of these studies, combining TCZ with a synthetic DMARD gave higher levels of efficacy and safety than were achieved in the control group [Genovese et al. 2008; Smolen et al. 2008; Kremer et al. 2011]. In the OPTION study, the efficacy of TCZ with methotrexate was confirmed in patients whose condition responded poorly to methotrexate. ACR20 response at 24 weeks was achieved by 59% in the group receiving TCZ 8 mg/kg every 4 weeks plus methotrexate versus 26% in the methotrexate monotherapy group, demonstrating the significant superiority of the combination therapy over methotrexate alone (p < 0.0001) [Smolen et al. 2008].
The ACT-RAY study examined whether or not higher levels of efficacy could be achieved by using methotrexate concomitantly with TCZ [Dougados et al. 2012]. That study compared the efficacy of TCZ (8 mg/kg every 4 weeks) plus placebo (TCZ monotherapy group) with TCZ (8 mg/kg, every 4 weeks) plus methotrexate (combination therapy group) in patients with moderate to severe active rheumatoid arthritis whose condition had failed to respond adequately to methotrexate and who had no history of biological DMARD use. DAS28 remission at 24 weeks was 35% in the TCZ monotherapy group compared with 40% in the combination therapy group and no significant difference was identified.
No firm conclusions have yet emerged as to whether TCZ should be used on its own or in conjunction with other DMARDs. However, insofar as can be judged from these various papers, the concomitant use of methotrexate with TCZ does not seem to have any greater effect on efficacy than using a biological DMARD targeting TNFα.
In addition to anticipating higher levels of response upon switching from synthetic DMARDs including methotrexate to TCZ monotherapy, adding TCZ as the first biological DMARD to methotrexate therapy in patients whose condition has shown a poor response to methotrexate can also provide considerable improvements in efficacy [Genovese et al. 2008]. TCZ is thus a useful drug that may be regarded as the first-line choice among biological DMARDs.
Methotrexate is indispensable in the treatment of rheumatoid arthritis, but due to adverse reactions to methotrexate itself or patient comorbidities, some patients find it difficult to use. TCZ monotherapy would thus seem to occupy a significant position when dealing with patients who face problems with methotrexate treatment itself.
Efficacy of tocilizumab in patients whose condition responds poorly to tumour necrosis factor inhibitors
Substantial advances in the treatment of rheumatoid arthritis have been made since the appearance of TNF inhibitors, and physicians can now not only improve symptoms but also control the progression of joint destruction. However, some 20–40% of patients fail to respond satisfactorily to TNF inhibitors and others are forced to switch treatment due to adverse events; therefore, establishing treatment strategies for such patients is a major topic of concern.
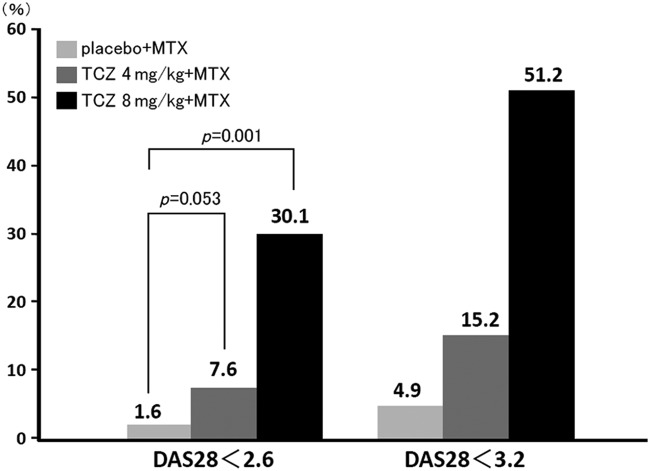
The RADIATE study documented the efficacy of TCZ in patients with rheumatoid arthritis whose condition responded inadequately to TNF inhibitors [Emery et al. 2008] (Figure 1). Patients whose condition responded poorly to one or more TNF inhibitors were randomized to receive TCZ (8 or 4 mg/kg once every 4 weeks) or placebo, each plus methotrexate (10–25 mg/week), and their response was evaluated at 24 weeks. An ACR20 response at 24 weeks was achieved by significantly more patients treated with TCZ, 50.0% at 8 mg/kg and 30.1% at 4 mg/kg, than by those on placebo (only 10.1%). The DAS28 remission rates were likewise significantly higher with TCZ, 30.1% at 8 mg/kg and 7.6% at 4 mg/kg compared with 1.6% for placebo. No significant difference in treatment response was identified with respect to the number or types of TNF inhibitor used prior to treatment with TCZ.
Figure 1.
Percentages of patients achieving DAS28 remission (<2.6) and low disease activity (<3.2) at 24 weeks in the RADIATE study [Emery et al. 2008]. DAS28, 28-joint Disease Activity Score; MTX, methotrexate; TCZ, tocilizumab.
At the level of clinical practice, there are the findings of the REACTION study, which examined the efficacy of TCZ in a clinical setting in Japan [Takeuchi et al. 2011]. That study described the therapeutic outcomes of 232 patients treated with TCZ at three specialist rheumatoid arthritis centres in Japan. Of the patients enrolled for that study, 62.8% had been receiving a TNF inhibitor before being treated with TCZ. After 52 weeks on TCZ, their DAS28 calculated using erythrocyte sedimentation rate (DAS28-ESR), health assessment questionnaire disability index (HAQ-DI), and modified Sharp scores had each improved considerably, and no significant difference in efficacy was found depending on whether they had or had not previously received a TNF inhibitor.
These reports suggest that the efficacy of TCZ is little affected by the patient’s response to the TNF inhibitor that was used first. However, if patients resistant to one TNF inhibitor are switched to another one, the percentage of those showing treatment response is lower than when the first agent was used [Yazici and Erkan, 2004; Bombardieri et al. 2007; Karlsson et al. 2008). Data from the UK National Biologics Register indicate that when one TNF inhibitor is discontinued due to lack of response and the patient is put onto a second one, the likelihood of the second agent being discontinued due to lack of response is higher than the likelihood of discontinuation due to an adverse event [hazard ratio for discontinuation due to lack of response 2.7, 95% confidence interval (CI) 2.1–3.4; hazard ratio for discontinuation due to adverse event 1.1, 95% CI 0.9–5.5] [Hyrich et al. 2007].
In Japan, TCZ is already widely used as a first-line biological DMARD in the same way as TNF inhibitors, but clinically it also occupies a significant position as a second- and third-line biological DMARD. If a TNF inhibitor proves ineffective, changing the treatment target is a sensible decision, and although there are no directly comparative studies, it is possible that the percentage of patients achieving treatment response would be higher than had they been switched to another TNF inhibitor. In fact, disease activity can be controlled by TCZ in many patients whose condition has failed to respond to TNF inhibitors. TCZ is also highly rated as a second-line biological DMARD and is widely used in clinical practice.
Safety of tocilizumab
As TCZ is currently the only drug targeting IL-6 that has been approved for use in patients with rheumatoid arthritis, it is important for clinicians to be aware of how its adverse reaction profile differs from that of other biological DMARDs. Postmarketing surveillance began after TCZ was approved for treatment of rheumatoid arthritis in Japan, earlier than the rest of the world, and has so far assembled safety data on 3881 patients [Koike et al. 2011] (Table 2). The most commonly reported adverse events were abnormal laboratory values (35.46/100 patient years) comprising rises in the hepatic function markers alanine transaminase and aspartate aminotransaminase, decreased white cell count, decreased platelet count and rises in blood cholesterol levels. Hepatic dysfunction appeared at a frequency of 15.00/100 patient years. Multiple logistic regression analysis indicated concurrent hepatic dysfunction and concomitant methotrexate use as risk factors for this, and particular attention should be paid to the monitoring of hepatic function markers (risk of hepatic dysfunction with concurrent hepatic dysfunction: odds ratio 2.546, 95% CI 1.681–3.857; risk of hepatic dysfunction with concomitant methotrexate use: odds ratio 2.174, 95% CI 1.705–2.771). Levels of low-density and high-density lipoprotein cholesterol may rise immediately after TCZ has been administered; however, the atherogenic index remains steady [Kawashiri et al. 2011] and it is unknown whether or not rises in cholesterol directly increase the risk of cardiovascular events. Routine cholesterol control should be practiced [Nishimoto et al. 2010]. However, the exposure to simvastatin is significantly reduced by administration of TCZ [Schmitt et al. 2011]. Caution should be exercised when starting simvastatin in patients who are receiving TCZ therapy. In Japan, hypertension occurred at a frequency of 5% or more in clinical studies, but at a lower frequency of 0.47% (37 patients) in subsequent postmarketing surveillance. Although the increased blood pressure is attributed to increased blood consistency due to elevated haemoglobin levels associated with improvement in inflammation, the exact mechanism is unknown. When it occurs, hypertension must be treated (e.g. using antihypertensive medications).
Table 2.
Frequency of main infections seen in the post marketing surveillance of tocilizumab for the treatment of rheumatoid arthritis in Japan .
| Adverse events | Frequency (/100 patient years) |
|---|---|
| Infections | 30.8 |
| Serious infection | 9.1 |
| Pneumonia | 2.6 |
| Cellulitis | 1.1 |
| Herpes zoster | 0.6 |
| Tuberculosis | 0.2 |
Infections occurred at a frequency of 30.8/100 patient years, with serious infections at 9.1/100 patient years [Koike et al. 2011]. This differed little from the findings of postmarketing surveillance of TNF inhibitors conducted in Japan [Takeuchi et al. 2008; Koike et al. 2009]. The incidence of serious infections is comparable to that of other biological DMARDs in the 2011 Cochrane Database of Systematic Reviews (odds ratio 0.84, 95% CI 0.20–3.56) [Singh et al. 2011].
Gut perforation occurred as seven events per 100 patient years according to postmarketing surveillance in Japan [Koike et al. 2011], and as three events per 2188 patient years according to Nishimoto and colleagues [Nishimoto et al. 2010]. Although gut perforation is a rare event, TCZ should be used with caution in patients with previous or current diverticulitis.
While TCZ is a potent anti-inflammatory, it also has the singular characteristic of normalizing C-reactive protein (CRP) levels due to its property of inhibiting IL-6. Nishimoto and colleagues claim that if free TCZ not bound to IL-6 receptors is present in the blood at levels of 1 μg/ml or over, it will be bound to at least 95% of the soluble IL-6 receptors, and CRP in the blood will normalizes [Nishimoto et al. 2008]. There have even been instances in which CRP levels associated with infection have failed to rise when a sufficient concentration of TCZ was present in the blood [Fujiwara et al. 2009]. Trying to detect infection in the early stages without relying solely on laboratory data is a vital point in terms of stopping infections from becoming more serious.
Three-year follow up of the patients from the postmarketing surveillance is currently taking place in Japan, and we await information on the frequency of events such as malignancies, intestinal perforation and cardiac function disorders.
Predicted efficacy of tocilizumab
There have been various attempts to predict TCZ efficacy. Takeuchi and colleagues reviewed 232 patients treated with TCZ in the clinical setting and noted the significance of a low baseline HAQ-DI as a factor making it possible to meet the requirements for clinical remission (DAS28-ESR4 < 2.6), structural remission (van der Heijde-modified Sharp score ≤ 0.5) and functional remission (HAQ-DI ≤ 0.5 after 52 weeks of treatment) [Takeuchi et al. 2011]. Kojima and colleagues analyzed the therapeutic outcomes at 52 weeks in 123 patients treated with TCZ in clinical practice, and noted the significance of low baseline disease activity index (DAS28-ESR < 5.23) and short disease duration (<4.8 years) as factors needed to satisfy the new remission criteria proposed by ACR/EULAR in 2011 (Boolean remission) [Kojima et al. 2012; Shahouri et al. 2011].
Such findings demonstrate that, with TCZ in the same way as with other biological DMARDs, positive treatment initiation at an early stage is important to achieving high therapeutic targets in rheumatoid arthritis.
Kaneko and colleagues [Kaneko et al. 2011] evaluated the efficacy of TCZ by using the Clinical Disease Activity Index [Shahouri et al. 2011] and found that CRP normalization by 12 weeks and improvement in matrix metalloproteinase 3 (MMP-3) levels by 24 weeks were useful predictive factors for a low clinical disease activity index (<10) at 52 weeks. TCZ directly inhibits acute phase proteins linked to IL-6, such as CRP (as mentioned above). At the same time, the trough concentration of the agent can be predicted by monitoring; by tracking its course during treatment, the subsequent treatment response can also be predicted. Because TCZ is a potent inhibitor of CRP and ESR, there is a downside in that efficacy can be awkward to assess by composite measures that include these parameters, and so it is also important to monitor efficacy by taking markers not directly affected by IL-6 inhibition, such as MMP-3.
Conclusions
TCZ is currently the only drug targeting IL-6 receptors, and more information from trials and real clinical experience is becoming available. In addition to providing high levels of efficacy as a first-line biological DMARD, TCZ is also significantly positioned as a promising treatment for patients with rheumatoid arthritis whose condition is poorly controlled by TNF inhibitors. It is becoming increasingly important to establish treatment strategies and to determine which biological DMARD is appropriate to treat the individual patient. We look forward to further studies seeking to predict the efficacy of TCZ in rheumatoid arthritis.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: A. Kaneko has received speaking fees (less than US$5000) from Abbot Japan Co. Ltd, Eisai Co. Ltd, Mitsubishi Tanabe Pharma Corporation, Pfizer Co. Ltd, Chugai Phamacoceutical Co. Ltd and Bristol-Myers Squibb Co Ltd.
References
- Bombardieri S., Ruiz A., Fardellone P., Geusens P., McKenna F., Unnebrink K., et al. (2007) Effectiveness of adalimumab for rheumatoid arthritis in patients with a history of TNF-antagonist therapy in clinical practice. Rheumatology (Oxford) 46: 1191–1199 [DOI] [PubMed] [Google Scholar]
- Breedveld F., Weisman M., Kavanaugh A., Cohen S., Pavelka K., van Vollenhoven R., et al. (2006) The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 54: 26–37 [DOI] [PubMed] [Google Scholar]
- Dougados M., Kissel K., Sheeran T., Tak P., Conaghan P., Mola E., et al. (2012) Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis 7 July (Epub ahead of print). DOI: 10.1136/annrheumdis-2011-201282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P., Keystone E., Tony H., Cantagrel A., van Vollenhoven R., Sanchez A., et al. (2008) IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 67: 1516–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson D., Anderson J., Boers M., Bombardier C., Furst D., Goldsmith C., et al. (1995) American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 38: 727–735 [DOI] [PubMed] [Google Scholar]
- Firestein G., Alvaro-Gracia J., Maki R. (1990) Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol 144: 3347–3353 [PubMed] [Google Scholar]
- Fujiwara H., Nishimoto N., Hamano Y., Asanuma N., Miki S., Kasayama S., et al. (2009) Masked early symptoms of pneumonia in patients with rheumatoid arthritis during tocilizumab treatment: a report of two cases. Mod Rheumatol 19: 64–68 [DOI] [PubMed] [Google Scholar]
- Genovese M., McKay J., Nasonov E., Mysler E., da Silva N., Alecock E., et al. (2008) Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 58: 2968–2980 [DOI] [PubMed] [Google Scholar]
- Harris E., Jr(1990) Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med 322: 1277–1289 [DOI] [PubMed] [Google Scholar]
- Hyrich K., Lunt M., Watson K., Symmons D., Silman A.; British Society for Rheumatology Biologics Register (2007) Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum 56: 13–20 [DOI] [PubMed] [Google Scholar]
- Jones G., Sebba A., Gu J., Lowenstein M., Calvo A., Gomez-Reino J., et al. (2010) Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 69: 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Kida D., Saito K., Tsukamoto M., Sato T. (2011) Clinical results for tocilizumab over one year in the clinical setting as assessed by CDAI (clinical disease activity index): CRP at week 12 and MMP-3 at week 24 are predictive factors for CDAI. Rheumatol Int 30 November (Epub ahead of print). DOI: 10.1007/s00296-011-2256-5 [DOI] [PubMed] [Google Scholar]
- Karlsson J., Kristensen L., Kapetanovic M., Gülfe A., Saxne T., Geborek P. (2008) Treatment response to a second or third TNF-inhibitor in RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology (Oxford) 47: 507–513 [DOI] [PubMed] [Google Scholar]
- Kawashiri S., Kawakami A., Yamasaki S., Imazato T., Iwamoto N., Fujikawa K., et al. (2011) Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol Int 31: 451–456 [DOI] [PubMed] [Google Scholar]
- Kishimoto T. (2006) Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther 8(Suppl. 2): S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., van der Heijde D., de Jager J., Gough A., Kalden J., Malaise M., et al. (2004) Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 363: 675–681 [DOI] [PubMed] [Google Scholar]
- Koike T., Harigai M., Inokuma S., Inoue K., Ishiguro N., Ryu J., et al. (2009) Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 36: 898–906 [DOI] [PubMed] [Google Scholar]
- Koike T., Harigai M., Inokuma S., Ishiguro N., Ryu J., Takeuchi T., et al. (2011) Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 70: 2148–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T., Kaneko A., Hirano Y., Ishikawa H., Miyake H., Takagi H., et al. (2012) Early aggressive intervention with tocilizumab for rheumatoid arthritis increases remission rate defined using a Boolean approach in clinical practice. Mod Rheumatol 22: 370–375 [DOI] [PubMed] [Google Scholar]
- Kremer J., Blanco R., Brzosko M., Burgos-Vargas R., Halland A., Vernon E., et al. (2011) Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 63: 609–621 [DOI] [PubMed] [Google Scholar]
- Nishimoto N., Hashimoto J., Miyasaka N., Yamamoto K., Kawai S., Takeuchi T., et al. (2007) Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an X-ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis 66: 1162–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N., Ito K., Takagi N. (2010) Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol 20: 222–232 [DOI] [PubMed] [Google Scholar]
- Nishimoto N., Miyasaka N., Yamamoto K., Kawai S., Takeuchi T., Azuma J., et al. (2009) Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol 19: 12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N., Terao K., Mima T., Nakahara H., Takagi N., Kakehi T. (2008) Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112: 3959–3964 [DOI] [PubMed] [Google Scholar]
- Schmitt C., Kuhn B., Zhang X., Kivitz A., Grange S. (2011) Disease-drug-drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther 89: 735–740 [DOI] [PubMed] [Google Scholar]
- Shahouri S., Michaud K., Mikuls T., Caplan L., Shaver T., Anderson J., et al. (2011) Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum 63: 3204–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Wells G., Tanjong-Ghogomu E., Maxwell L., Macdonald J., Christensen R., et al. (2011) Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev (2): CD008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J., Beaulieu A., Rubbert-Roth A., Ramos-Remus C., Rovensky J., Alecock E., et al. (2008) Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371: 987–997 [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Tanaka Y., Amano K., Hoshi D., Nawata M., Nagasawa H., et al. (2011) Clinical, radiographic and functional effectiveness of tocilizumab for rheumatoid arthritis patients – REACTION 52-week study. Rheumatology (Oxford) 50: 1908–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T., Tatsuki Y., Nogami Y., Ishiguro N., Tanaka Y., Yamanaka H., et al. (2008) Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 67: 189–194 [DOI] [PubMed] [Google Scholar]
- Yazici Y., Erkan D. (2004) Do etanercept-naive patients with rheumatoid arthritis respond better to infliximab than patients for whom etanercept has failed? Ann Rheum Dis 63: 607–608 [PMC free article] [PubMed] [Google Scholar]