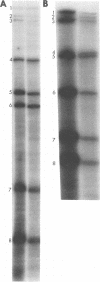
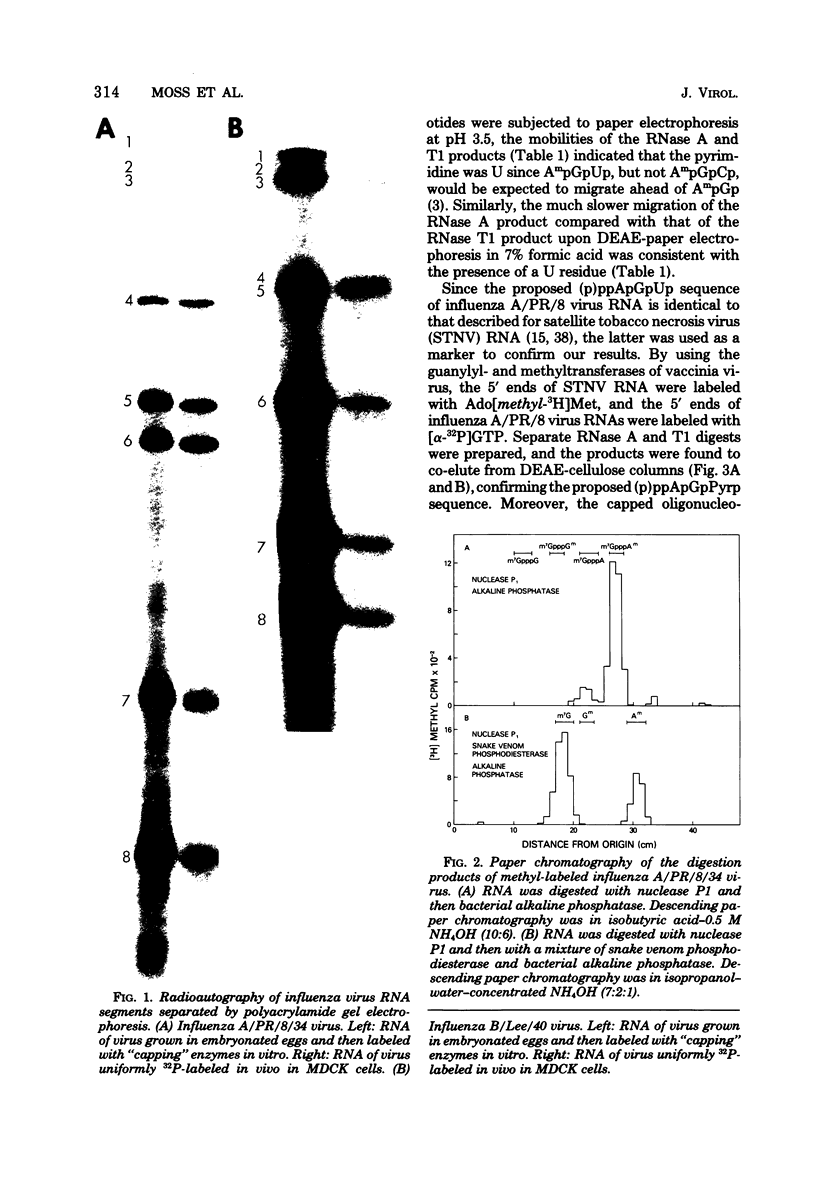
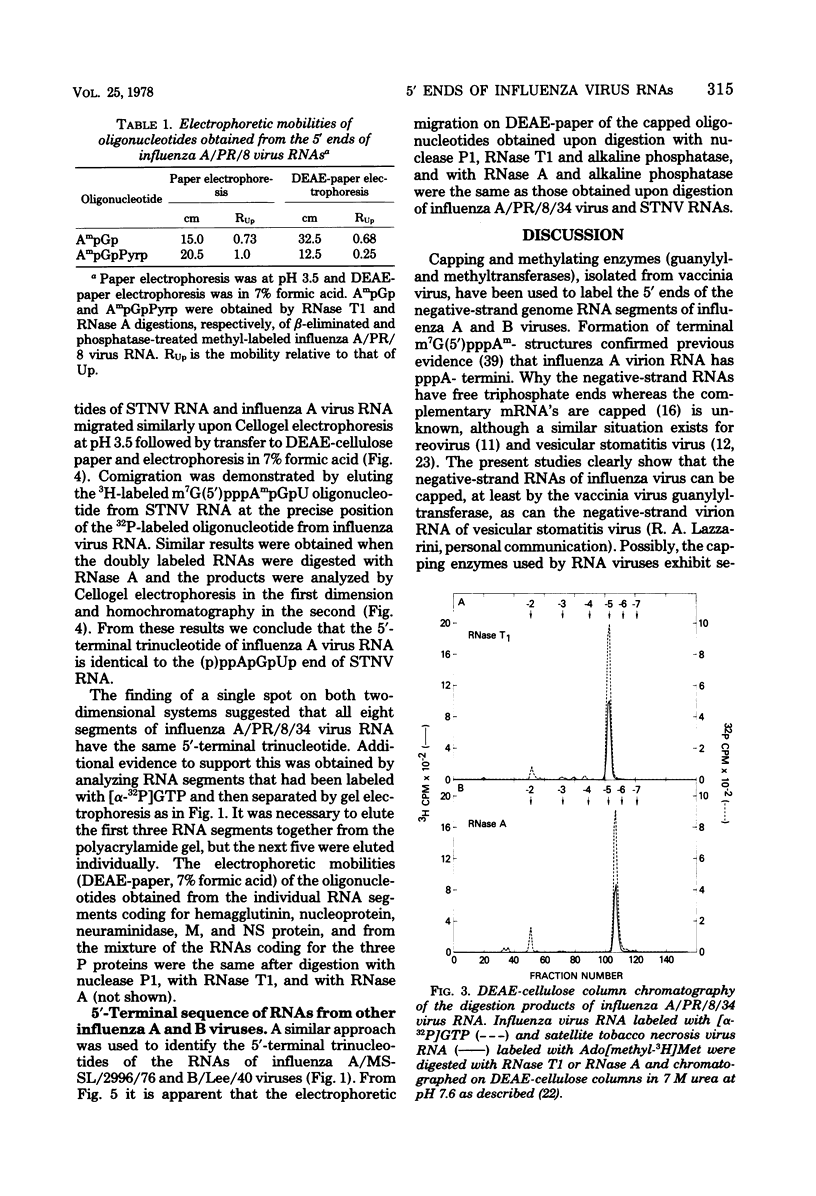
Abstract
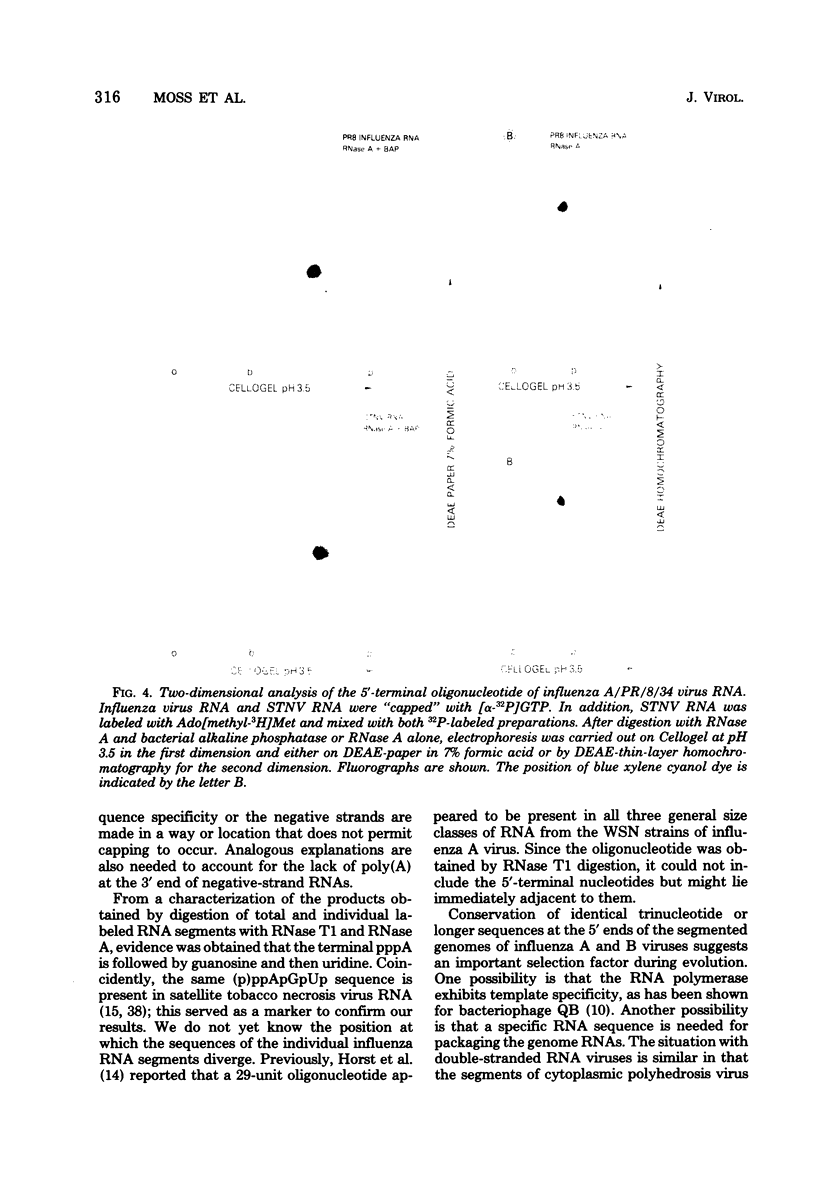
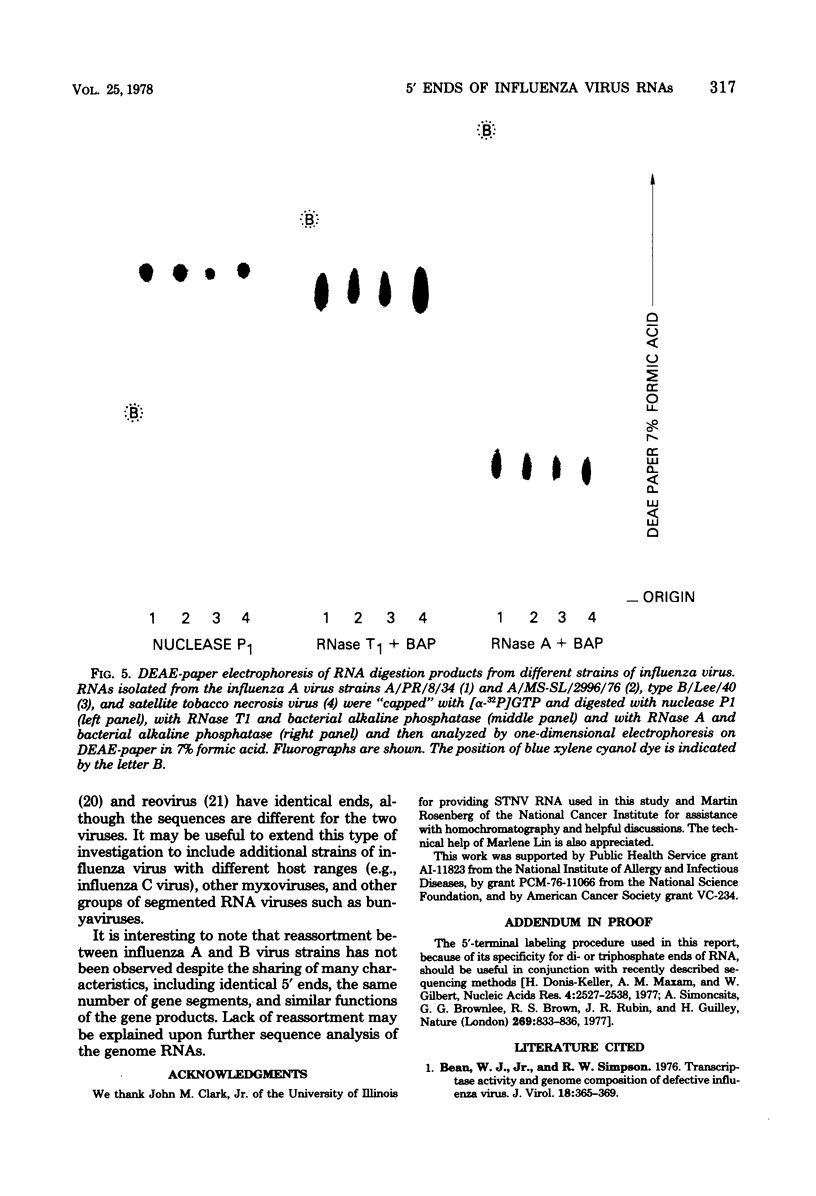
Guanylyl- and methyltransferases, isolated from purified vaccinia virus, were used to specifically label the 5′ ends of the genome RNAs of influenza A and B viruses. All eight segments were labeled with [α-32P]guanosine 5′-triphosphate or S-adenosyl[methyl-3H]methionine to form “cap” structures of the type m7G(5′)pppNm-, of which unmethylated (p)ppN- represents the original 5′ end. Further analyses indicated that m7G(5′)pppAm, m7G(5′)pppAmpGp, and m7G(5′)pppAmpGpUp were released from total and individual labeled RNA segments by digestion with nuclease P1, RNase T1, and RNase A, respectively. Consequently, the 5′-terminal sequences of most or all individual genome RNAs of influenza A and B viruses were deduced to be (p)ppApGpUp. The presence of identical sequences at the ends of RNA segments of both types of influenza viruses indicates that they have been specifically conserved during evolution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean W. J., Jr, Simpson R. W. Transcriptase activity and genome composition of defective influenza virus. J Virol. 1976 Apr;18(1):365–369. doi: 10.1128/jvi.18.1.365-369.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare A. S., Sherwood J. E., Callow K. A., Craig J. W. Selection of influenza B virus recombinants and their testing in humans for attenuation and immunogenicity. Infect Immun. 1977 Feb;15(2):347–353. doi: 10.1128/iai.15.2.347-353.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Bishop D. H., Meier-Ewert H. Structural components of influenza C virions. J Virol. 1977 Feb;21(2):658–665. doi: 10.1128/jvi.21.2.658-665.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Content J., Duesberg P. H. Base sequence differences among the ribonucleic acids of influenza virus. J Mol Biol. 1971 Dec 14;62(2):273–285. doi: 10.1016/0022-2836(71)90427-x. [DOI] [PubMed] [Google Scholar]
- Cox N. J., Kendal A. P. Presence of a segmented single-stranded RNA genome in influenza C virus. Virology. 1976 Oct 1;74(1):239–241. doi: 10.1016/0042-6822(76)90147-1. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. The RNA of influenza virus. Proc Natl Acad Sci U S A. 1968 Mar;59(3):930–937. doi: 10.1073/pnas.59.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Martin S. A., Paoletti E., Moss B. Modification of the 5'-terminus of mRNA by soluble guanylyl and methyl transferases from vaccinia virus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2525–2529. doi: 10.1073/pnas.72.7.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Muthukrishnan S., Shatkin A. J. 5'-Terminal m-7G(5')ppp(5')G-m-p in vivo: identification in reovirus genome RNA. Proc Natl Acad Sci U S A. 1975 Feb;72(2):742–745. doi: 10.1073/pnas.72.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRST G. K. Genetic recombination with Newcastle disease virus, polioviruses, and influenza. Cold Spring Harb Symp Quant Biol. 1962;27:303–309. doi: 10.1101/sqb.1962.027.001.028. [DOI] [PubMed] [Google Scholar]
- Hefti E., Bishop D. H. The 5' sequence of VSV viral RNA and its in vitro transcription product RNA. Biochem Biophys Res Commun. 1975 Sep 16;66(2):785–792. doi: 10.1016/0006-291x(75)90578-1. [DOI] [PubMed] [Google Scholar]
- Horst J., Content J., Mandeles S., Fraenkel-Conrat H., Duesberg P. Distinct oligonucleotide patterns of distinct influenza virus RNA's. J Mol Biol. 1972 Aug 21;69(2):209–215. doi: 10.1016/0022-2836(72)90226-4. [DOI] [PubMed] [Google Scholar]
- Horst J., Fraenkel-Conrat H., Mandeles S. Terminal heterogeneity at both ends of the satellite tobacco necrosis virus ribonucleic acid. Biochemistry. 1971 Dec 7;10(25):4748–4752. doi: 10.1021/bi00801a022. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Morgan M. A., Shatkin A. J. Influenza viral mRNA contains internal N6-methyladenosine and 5'-terminal 7-methylguanosine in cap structures. J Virol. 1976 Oct;20(1):45–53. doi: 10.1128/jvi.20.1.45-53.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski L. J., Content J., Leppla S. H. Characterization of the subunit structure of the ribonucleic acid genome of influenza virus. J Virol. 1971 Nov;8(5):701–707. doi: 10.1128/jvi.8.5.701-707.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- McGeoch D., Fellner P., Newton C. Influenza virus genome consists of eight distinct RNA species. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3045–3049. doi: 10.1073/pnas.73.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Watanabe K., Sugiura M. 5'-Terminal nucleotide sequences of the double-stranded RNA of silkworm cytoplasmic polyhedrosis virus. J Mol Biol. 1974 Jun 15;86(1):31–48. doi: 10.1016/s0022-2836(74)80005-7. [DOI] [PubMed] [Google Scholar]
- Miura K., Watanabe K., Sugiura M., Shatkin A. J. The 5'-terminal nucleotide sequences of the double-stranded RNA of human reovirus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3979–3983. doi: 10.1073/pnas.71.10.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Utilization of the guanylyltransferase and methyltransferases of vaccinia virus to modify and identify the 5'-terminals of heterologous RNA species. Biochem Biophys Res Commun. 1977 Jan 24;74(2):374–383. doi: 10.1016/0006-291x(77)90314-x. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Abraham G., Adler R., Banerjee A. K. Methylated and blocked 5' termini in vesicular stomatitis virus in vivo mRNAs. Cell. 1975 May;5(1):59–67. doi: 10.1016/0092-8674(75)90092-6. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B. Live attenuated influenza virus vaccines. Strains with temperature-sensitive defects in P3 protein and nucleoprotein. Virology. 1977 May 1;78(1):183–191. doi: 10.1016/0042-6822(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L., Kilbourne E. D. Genetic composition of a high-yielding influenza A virus recombinant: a vaccine strain against "Swine" influenza. Science. 1976 Oct 15;194(4262):334–335. doi: 10.1126/science.968486. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2142–2146. doi: 10.1073/pnas.73.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Pons M. W. A reexamination of influenza single-and double-stranded RNAs by gel electrophoresis. Virology. 1976 Feb;69(2):789–792. doi: 10.1016/0042-6822(76)90508-0. [DOI] [PubMed] [Google Scholar]
- Pons M. W., Hirst G. K. Polyacrylamide gel electrophoresis of influenza virus RNA. Virology. 1968 Feb;34(2):385–388. doi: 10.1016/0042-6822(68)90257-2. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Kilbourne E. D. RNAs of influenza A, B, and C viruses. J Virol. 1976 May;18(2):738–744. doi: 10.1128/jvi.18.2.738-744.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Difference in protein patterns of influenza A viruses. Virology. 1977 Jan;76(1):122–128. doi: 10.1016/0042-6822(77)90289-6. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMPSON R. W., HIRST G. K. Genetic recombination among influenza viruses. I. Cross reactivation of plaque-forming capacity as a method for selecting recombinants from the progeny of crosses between influenza A strains. Virology. 1961 Dec;15:436–451. doi: 10.1016/0042-6822(61)90111-8. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Harms E., Rohde W., Orlich M., Rott R. Correlation between RNA fragments of fowl plague virus and their corresponding gene functions. Virology. 1976 Oct 15;74(2):332–344. doi: 10.1016/0042-6822(76)90340-8. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- TOMLINSON R. V., TENER G. M. THE EFFECT OF UREA, FORMAMIDE, AND GLYCOLS ON THE SECONDARY BINDING FORCES IN THE ION-EXCHANGE CHROMATOGRAPHY OF POLYNUCLEOTIDES OF DEAE-CELLULOSE. Biochemistry. 1963 Jul-Aug;2:697–702. doi: 10.1021/bi00904a013. [DOI] [PubMed] [Google Scholar]
- Tobita K., Kilbourne E. D. Genetic recombination for antigenic markers of antigenically different strains of influenza B virus. J Virol. 1974 Feb;13(2):347–352. doi: 10.1128/jvi.13.2.347-352.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer E., Chang A. Y., Clark J. M., Jr, Reichmann M. E. Sequence studies of satellite tobacco necrosis virus RNA. Isolation and characterization of a 5'-terminal trinucleotide. J Mol Biol. 1968 Nov 28;38(1):59–73. doi: 10.1016/0022-2836(68)90128-9. [DOI] [PubMed] [Google Scholar]
- Young R. J., Content J. 5'-terminus of influenza virus RNA. Nat New Biol. 1971 Mar 31;230(13):140–142. doi: 10.1038/newbio230140a0. [DOI] [PubMed] [Google Scholar]