Abstract
Background/Purpose
The purpose of this study is to determine if patients with osteosarcoma (OS) with metachronous metastatic pulmonary disease presenting with a single pulmonary nodule (SPN) on computed tomography (CT) were found to have other lesions at the time of thoracotomy.
Methods
Data were collected retrospectively on consecutive patients with OS treated at our institution from 1982 to 2007. Patients with no evidence of disease at the end of initial therapy who subsequently relapsed in the lung were identified.
Results
In our study, 16 (8%) of 198 patients with OS with metachronous metastatic pulmonary disease presented with a SPN on CT scan. In all patients, only 1 metastatic nodule for OS was found at the time of thoracotomy. The median time between diagnosis and first lung relapse was 23.8 months (range, 4–80 months). Eleven patients (68.7%) subsequently had a second lung relapse, but only 3 patients had involvement of the ipsilateral lung (mean time interval between first and second pulmonary relapses of 17 months; range, 2–44 months). Five-year overall survival from diagnosis was 56.2%. Seven patients (43.8%) died of disease progression.
Conclusions
In our experience, patients with OS with metachronous metastatic pulmonary disease presenting with a SPN on CT were not found to have additional malignant lesions at the time of thoracotomy. Consideration should be given in this group of selected patients to use a minimally invasive approach to nodule removal with image-guided localization, if needed, rather than open thoracotomy because ipsilateral metastases are not likely to be found.
Keywords: Osteosarcoma, Lung nodule, Thoracotomy, Computed tomography
Osteosarcoma (OS) is the most common malignant bone tumor arising in children and adolescents and has an annual incidence of approximately 400 cases in children younger than 20 years [1]. The combination of chemotherapy, local surgical control, and aggressive pulmonary metastasectomies in patients with metastatic disease has improved long-term survival of patients with OS. Fifteen to 20% of patients will have radiographically detectable pulmonary metastases at diagnosis (synchronous metastatic pulmonary disease). Among patients with nonmetastatic disease at diagnosis, 20% to 25% will relapse, usually in the lungs (metachronous metastatic pulmonary disease). Depending on the time of metastasis detection (early on therapy vs late off therapy), 20% to 45% of patients can be salvaged by resection of the pulmonary lesions, even if multiple operative procedures are required [2]. Complete resection of all disease is required for cure in patients with OS, even in patients with pulmonary metatastatic disease [3]. Because computed tomographic (CT) scans of the chest have been shown to underestimate the number of metastatic pulmonary nodules found at surgery [4], open thoracotomy, not thoracoscopy, is recommended for patients with metachronous pulmonary metastases (single or multiple) to permit thorough palpation of the pulmonary parenchyma and identification of all subcentimeric (<3 mm) nodules. We conducted this retrospective study to test our observation that patients with OS having a metachronous single pulmonary nodule (SPN) detected on CT 2 months or longer after completion of therapy do not appear to have additional lesions at the time of thoracotomy and may be able to avoid open thoracotomy. Open thoracotomy can result in substantial pleural and pulmonary scarring, which can complicate subsequent thoracotomies that are often needed in these patients. With improvement in minimally invasive surgery, most single pulmonary lesions depending on the nodule location can be completely resected by thoracoscopy, with or without preoperative image-guided localization. To verify this observation, we reviewed our experience treating patients with OS who presented with a metachronous SPN on CT.
1. Material and methods
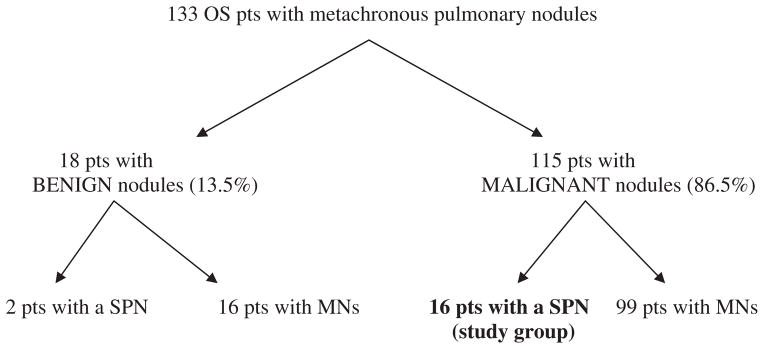
A search of the solid tumor database of all patients with OS treated at St Jude Children’s Research Hospital between 1982 and 2007 found 16 patients who had a SPN on follow-up CT imaging and then underwent thoracotomy (Figure). One pediatric radiologist (MBM) reviewed the CT imaging and confirmed the presence of a SPN. From 1982 to 1987, CTs were performed on a GE 8800 axial scanner (GE Medical Systems, Milwaukee, WI); from 1987 to 1992, on a Siemens DRH axial scanner (Siemens, Erlangen, Germany); from 1991 to 1992, on a Siemens Plus-S single-detector helical scanner; from 1992 to 2000, on a Siemens Plus-4 4-detector helical scanner; from 2000 to 2007, on a GELightspeed Ultra 8-detector helical scanner; and in 2007, on a GE VCT 64-detector helical scanner. Computed tomographic examinations performed after 1993 were available for review in our picture archive and communication system. All other CTs were reviewed as hard-copy film. Data regarding patient demographics, treatment, lung relapses, surgical findings at relapse, and survival status were collected. Surgical findings were analyzed qualitatively. Chemotherapy and surgical management of the primary tumor varied over the study period. All patients were treated on institutional protocols (OST-77, OS-86, OS-91, and OS-99) with chemotherapeutic agents that included cyclophosphamide, cisplatin, doxorubicin, methotrexate, carboplatin, and ifosfamide [5–7]. Patients were monitored at regular intervals to detect pulmonary metastases with CT scan. Between 1982 and 1999, follow-up evaluation after completion of therapy consisted of plain radiography of the chest every 6 to 8 weeks and CT of the chest every 4 months for the first 2 years. After this point, patients were evaluated by clinical assessment and plain radiography of the chest unless symptoms suggested tumor recurrence. After 1999, patients were monitored by plain radiography or CT of the chest every 2 to 3 months for the first year. Subsequently, patients underwent radiography or CT of the chest every 3 to 6 months for at least 4 years after completion of therapy. After 2006, patients were monitored with CT chest and not chest radiographs. All patients with isolated pulmonary metastases had surgical resection via open thoracotomy. This retrospective review was approved by our institutional review board.
Figure. Patient’s distribution.
pts: patients, SPN: single pulmonary nodule, MNs: multiple nodules
2. Results
Patient distribution and characteristics are shown in the Figure and Table 1. One hundred thirty-three patients with OS developed metachronous pulmonary nodules. Eighteen (13.5%) of these patients had nonmalignant pulmonary nodules confirmed on pathology after excision. Among them, 2 patients had a benign SPN, and 16 had multiple benign nodules. Among the identified 115 cases with OS and metachronous metastatic lung disease, 16 (13.9%) presented with a SPN. Therefore, among 133 patients with OS, 18 presented with a SPN during follow-up; 16 (88.8%) of the nodules were malignant, and 2 (11.2%) were benign. For the study group of 16 patients having a SPN that was found to be malignant, median age at diagnosis of primary OS was 11.5 years (range, 3–23 years). There were 9 male patients (56%). At diagnosis, 15 patients had localized OS without radiographic evidence of pulmonary metastasis, and 1 patient had metastatic lung disease that cleared after neoadjuvant chemotherapy. Primary tumor location was distal femur (n = 9), proximal tibia (n = 5), proximal humerus (n = 1), and iliac bone (n = 1).
Table 1.
Patient characteristics, treatment protocol, and outcome
| Patient | Sex | Age at diagnosis (y) | Treatment protocol | Outcome (time from diagnosis) |
|---|---|---|---|---|
| 1 | Female | 23 | OS 86 | DOD 3 y |
| 2 | Female | 3 | OST 77 | NED 28 y |
| 3 | Male | 16 | OST 77 | DOD 2.1 y |
| 4 | Male | 13 | OST 77 | DOD 2.1 y |
| 5 | Male | 4 | OST 77 | DOD 4.1 y |
| 6 | Male | 12 | OST 77 | DOD 9 y |
| 7 | Female | 17 | OS 99 | NED 11 y |
| 8 | Male | 12 | OS 99 | NED 10 y |
| 9 | Male | 16 | OS 91 | NED 12 y |
| 10 | Female | 10 | OS 86 | DOD 4 y |
| 11 | Male | 9 | OST 77 | DOD 1.6 y |
| 12 | Male | 16 | OST 77 | NED 30 y |
| 13 | Male | 11 | OST 77 | NED 26 y |
| 14 | Female | 10 | OS 91 | NED 13 y |
| 15 | Female | 6 | OS 91 | NED 14 y |
| 16 | Female | 7 | OS 99 | NED 5 y |
Abbreviations: DOD, died of disease; NED, no evidence of disease.
The median time to first lung relapse was 23.8 months (range, 4–80); 3 (18.8%) of these 16 patients developed a SPN within the first year of diagnosis. Single pulmonary nodule location was left upper lobe (n = 6), right lower lobe (n = 5), left lower lobe (n = 3), right upper lobe (n = 1), and right middle lobe (n = 1). All patients underwent thoracotomy, and the entire lung was manually explored; no additional lesions were found either manually or by pathologic identification in the specimen. Pulmonary wedge resection was performed in 14 patients, and lobectomy, in 2 patients. A second lung relapse was observed in 11 (68.7%) of the 16 patients with involvement of the ipsilateral lung in 3 patients (median time interval between first and second pulmonary relapse was 17 months; range, 2–44 years) and contralateral lung in 8 patients (median time interval between first and second pulmonary relapses was 16 months; range, 1–43 months). The surgical approach for the second relapse was via open thoracotomy in all cases. Details of nodule location, surgical approach, and time interval between relapses are summarized in Table 2. Nine patients (56%) are alive without evidence of disease at follow-up of 2 to 27 years (median time, 12.4 years). Seven patients died of disease progression.
Table 2.
Location of pulmonary nodules, pathology, surgical approach, and time interval to relapse
| Patient | First relapse (months between diagnosis and first relapse)/pathology | Surgical approach | Second relapse (months between first and second relapse) |
|---|---|---|---|
| 1 | LUL (16)/malignant SPN | Thoracotomy, wedge resection | LLL (5) |
| 2 | LUL (6)/malignant SPN | Thoracotomy, wedge resection | RLL (2) |
| 3 | LUL (20)/malignant SPN | Thoracotomy, wedge resection | Left pleural massive involvement (2) |
| 4 | RUL (13)/malignant SPN | Thoracotomy, wedge resection | LUL (1) |
| 5 | LUL (18)/malignant SPN | Thoracotomy, lobectomy | RLL (15) |
| 6 | ML (28)/malignant SPN | Thoracotomy, wedge resection | Left diaphragm massive involvement (12) |
| 7 | RLL (21)/malignant SPN | Thoracotomy, wedge resection | LUL (36) |
| 8 | RLL (22)/malignant SPN | Thoracotomy, wedge resection | RML (44) |
| 9 | LLL (12)/malignant SPN | Thoracotomy, wedge resection | RML (43) |
| 10 | RLL (17)/malignant SPN | Thoracotomy, wedge resection | LLL (11) |
| 11 | LLL (4)/malignant SPN | Thoracotomy, wedge resection | RLL, RUL, ML (9) |
| 12 | LUL (15)/malignant SPN | Thoracotomy, wedge resection | No second lung relapse |
| 13 | LLL (80)/malignant SPN | Thoracotomy, wedge resection | No second lung relapse |
| 14 | RLL (27)/malignant SPN | Thoracotomy, wedge resection | No second lung relapse |
| 15 | LUL (50)/malignant SPN | Thoracotomy, wedge resection | No second lung relapse |
| 16 | RLL (38)/malignant SPN | Thoracotomy, lobectomy | No second lung relapse |
Abbreviations: RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
3. Discussion
Osteosarcoma accounts for approximately 5% of childhood cancers. At diagnosis, 20% of patients will have radiographically detectable metastases, with the lung being the most common site [1]. Factors that predict a better outcome in patients with pulmonary metastatic disease include fewer pulmonary nodules, unilateral pulmonary metastases, and longer intervals between primary tumor resection and metastases [2]. Hawkins and Arndt [3] also identified that solitary pulmonary nodules at recurrence, more than 24 months between the initial diagnosis and first disease recurrence, and achievement of a second complete response were positive factors for improving survival rates. Five-year overall survival is 65% to 75% for patients without lung metastases; in contrast, only 10% to 30% of patients with detectable metastatic OS at diagnosis will become long-term disease-free survivors [8,9]. The ability to achieve a complete resection of recurrent disease is the most important prognostic factor at first relapse, with a 5-year survival rate of 20% to 45% after complete resection of metastatic pulmonary disease. Treatment for patients with OS with pulmonary metastases remains a significant challenge at present [10–14], and the efficacy of systemic chemotherapy is variable using regimens based on combinations of ifosfamide, etoposide, cyclophosphamide, gemcitabine, and docetaxel [15–18]. The ability to detect pulmonary nodules has increased by the use of refined helical CT that permits thinner axial sections and with picture archive communications systems that provide magnification and window/level to improve lesion conspicuity and characterization [19–21].
Our results suggest that patients with OS who present with a SPN on CT 2 months or longer after completion of therapy (metachronous metastasis) could be possibly spared an open thoracotomy; because this procedure is associated with pulmonary and pleural scarring that can complicate subsequent thoracotomies, this is a potentially important observation. This patient population frequently required multiple thoracotomies, and two-thirds of our patients had a second pulmonary relapse with a significant portion being ipsilateral.
Repeated thoracotomy with resection of pulmonary tissue can lead to a significant decrease in pulmonary function, thereby increasing the risk of postoperative respiratory complications. Although several adult studies document increased postoperative morbidity in patients with diminished pulmonary function, there is little information in the pediatric population. Tobias et al [22] reviewed the postoperative courses of 19 patients who underwent thoracotomy who preoperatively had diminished pulmonary function. They concluded that aggressive surgical treatment of metastatic pulmonary disease is indicated even in this group of patients, although aggressive perioperative management is suggested. Preoperative teaching, incentive spirometry, early ambulation, and effective pain management are recommended. Further prospective studies in pediatric patients who need repeated lung resections focusing on the perioperative and postoperative management are needed to optimize their care. Our observation for patients with OS with a SPN that is amenable to thoracoscopy may help to decrease the number of thoracotomies in this selected group of patients.
Video-assisted thoracoscopic surgery (VATS) has gained widespread acceptance as a method of resecting pulmonary metastases in pediatric patients with cancer. Advantages of the thoracoscopic approach include shortened hospital stay, decreased postoperative pain, improved cosmetic results, shorter convalescence, faster return to normal activity, and reduced pulmonary adhesions [23,24]. This is particularly important for patients with OS who are at risk to experience multiple metachronous pulmonary metastases and may need repeated thoracic surgeries. The better visualization of the pleura and the pulmonary surface owing to the magnification by the optic system permits the identification of nodules on the pleural surface [25]. The major disadvantage of VATS is that the surgeon cannot palpate the lung parenchyma, so that identification of deeper lesions is difficult. A few localization techniques have been described such as CT-guided hook-wire localization with or without injection of methylene blue, radioisotope marking under CT guidance using a handheld gamma probe during VATS for localizing pulmonary lesions, and intrathoracoscopic ultrasound [26]. Waldhausen et al [27] reported 3 pediatric cases using CT-guided needle localization, methylene blue staining, and VATS. Partrick et al [25] confirmed this technique as excellent for approaching pulmonary nodules less than 1 cm in size or those greater than 0.5 cm deep to the pleural surface in a group of 11 children. In 2008, Gow et al [28] published the use of thoracoscopic ultrasound for localization of pulmonary nodules in 7 children and concluded that this is a real-time imaging tool that helps isolate small pulmonary lesions that may otherwise be difficult to see intraoperatively. These techniques may be helpful to facilitate thoracoscopic resection of deeper seated SPNs, but they do not overcome the missed detection of additional subcentimeric nodules. Treatment for the patients with OS with pulmonary metastases remains a significant challenge. Repeated resections of pulmonary recurrences can lead to extended disease control and possible cure for some patients. In our experience, patients who presented with single metachronous pulmonary lesions on CT did not have other malignant nodules at the time of thoracotomy. This finding may help to reduce the number of thoracotomies performed on this select group of patients. Our study is limited by the small number of patients, and prospective clinical trials are needed to confirm this retrospective observation.
Acknowledgments
The authors thank Valerie McPherson, CCRP, for data management.
Footnotes
Supported in part by Cancer Center Support grants CA21765 and CA23099 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities.
References
- 1.Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin North Am. 1997;44:973–89. doi: 10.1016/s0031-3955(05)70540-x. [DOI] [PubMed] [Google Scholar]
- 2.Goorin AM, Shuster JJ, Baker A, et al. Changing pattern of pulmonary metastases with adjuvant chemotherapy in patients with osteosarcoma: results from the Multiinstitutional Osteosarcoma Study. J Clin Oncol. 1991;9:600–5. doi: 10.1200/JCO.1991.9.4.600. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins DS, Arndt CA. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. 2003;98:2447–56. doi: 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- 4.Kayton ML, Huvos AG, Casher J, et al. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J Pediatr Surg. 2006;41:200–6. doi: 10.1016/j.jpedsurg.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Pratt CB, Champion JE, Fleming ID, et al. Adjuvant chemotherapy for osteosarcoma of the extremity. Long-term results of two consecutive prospective protocol studies. Cancer. 1990;65:439–45. doi: 10.1002/1097-0142(19900201)65:3<439::aid-cncr2820650311>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Meyer WH, Pratt CB, Poquette CA, et al. Carboplatin/ifosfamide window therapy for osteosarcoma: results of the St Jude Children’s Research Hospital OS-91 trial. J Clin Oncol. 2001;19:171–82. doi: 10.1200/JCO.2001.19.1.171. [DOI] [PubMed] [Google Scholar]
- 7.Daw NC, Neel MD, Rao BN, et al. Frontline treatment of localized osteosarcoma without methotrexate: results of the St. Jude Children’s Research Hospital OS99 trial. Cancer. 2011 doi: 10.1002/cncr.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martini N, Huvos AG, Mike V, et al. Multiple pulmonary resections in the treatment of osteogenic sarcoma. Ann Thorac Surg. 1971;12:271–80. doi: 10.1016/s0003-4975(10)65124-7. [DOI] [PubMed] [Google Scholar]
- 9.Spanos PK, Payne WS, Ivins JC, et al. Pulmonary resection for metastatic osteogenic sarcoma. J Bone Joint Surg [A] 1976;58:624–8. [PubMed] [Google Scholar]
- 10.Telander RL, Pairolero PC, Pritchard DJ, et al. Resection of pulmonary metastatic osteogenic sarcoma in children. Surgery. 1978;84:335–41. [PubMed] [Google Scholar]
- 11.Rosenberg SA, Flye MW, Conkle D, et al. Treatment of osteogenic sarcoma. II. Aggressive resection of pulmonary metastases. Cancer Treat Rep. 1979;63:753–6. [PubMed] [Google Scholar]
- 12.Schaller RT, Haas J, Schaller J, et al. Improved survival in children with osteosarcoma following resection of pulmonary metastases. J Pediatr Surg. 1982;17:546–50. doi: 10.1016/s0022-3468(82)80106-1. [DOI] [PubMed] [Google Scholar]
- 13.Putnam JB, Roth JA, Wesley MN, et al. Survival following aggressive resection of pulmonary metastases from osteogenic sarcoma: analysis of prognostic factors. Ann Thorac Surg. 1983;36:516–23. doi: 10.1016/s0003-4975(10)60679-0. [DOI] [PubMed] [Google Scholar]
- 14.Carter SR, Grimer RJ, Sneath RS, et al. Results of thoracotomy in osteogenic sarcoma with pulmonary metastases. Thorax. 1991;46:727–31. doi: 10.1136/thx.46.10.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabone MD, Kalifa C, Rodary C, et al. Osteosarcoma recurrences in pediatric patients previously treated with intensive chemotherapy. J Clin Oncol. 1994;12:2614–20. doi: 10.1200/JCO.1994.12.12.2614. [DOI] [PubMed] [Google Scholar]
- 16.Heij HA, Vos A, de Kraker J, et al. Prognostic factors in surgery for pulmonary metastases in children. Surgery. 1994;115:687–93. [PubMed] [Google Scholar]
- 17.Navid F, Willert JR, McCarville MB, et al. Combination of gemcitabine and docetaxel in treatment of children and young adults with refractory bone sarcoma. Cancer. 2008;113:419–25. doi: 10.1002/cncr.23586. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Galindo C, Daw NC, Kaste SC, et al. Treatment of refractory osteosarcoma with fractionated cyclophosphamide and etoposide. J Pediatr Hematol Oncol. 2002;24:250–5. doi: 10.1097/00043426-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 19.McCarville MB, Kaste SC, Cain AM, et al. Prognostic factors and imaging patterns of recurrent pulmonary nodules after thoracotomy in children with osteosarcoma. Cancer. 2001;91:1170–6. doi: 10.1002/1097-0142(20010315)91:6<1170::aid-cncr1114>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Absalon MJ, McCarville MB, Liu T, et al. Pulmonary nodules discovered during the initial evaluation of pediatric patients with bone and soft-tissue sarcoma. Pediatr Blood Cancer. 2008;50:1147–53. doi: 10.1002/pbc.21454. [DOI] [PubMed] [Google Scholar]
- 21.McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239:514–20. doi: 10.1148/radiol.2392050631. [DOI] [PubMed] [Google Scholar]
- 22.Tobias JD, Bozeman PM, Mackert PW, et al. Postoperative outcome following thoracotomy in the pediatric oncology patient with diminished pulmonary function. J Surg Oncol. 1993;52:105–9. doi: 10.1002/jso.2930520210. [DOI] [PubMed] [Google Scholar]
- 23.Shah RM, Spirn PW, Salazar AM, et al. Localization of peripheral pulmonary nodules for thoracoscopic excision: value of CT-guided wire placement. AJR. 1993;161:279–83. doi: 10.2214/ajr.161.2.8333361. [DOI] [PubMed] [Google Scholar]
- 24.Gonfiotti A, Davini F, Vaggelli L, De Francisci A, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg. 2007;32:843–7. doi: 10.1016/j.ejcts.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Partrick DA, Bensard DD, Teitelbaum DH, et al. Successful thoracoscopic lung biopsy in children utilizing preoperative CT-guided localization. J Pediatr Surg. 2002;37:970–3. doi: 10.1053/jpsu.2002.33820. [DOI] [PubMed] [Google Scholar]
- 26.Sugi K, Kaneda Y, Hirasawa K, et al. Radioisotope marking under CT guidance and localization using a handheld gamma probe for small or indistinct pulmonary lesions. Chest. 2003;124:155–8. doi: 10.1378/chest.124.1.155. [DOI] [PubMed] [Google Scholar]
- 27.Waldhausen JH, Shaw DW, Hall DG, et al. Needle localization for thoracoscopic resection of small pulmonary nodules in children. J Pediatr Surg. 1997;32:1624–5. doi: 10.1016/s0022-3468(97)90468-1. [DOI] [PubMed] [Google Scholar]
- 28.Gow KW, Saad DF, Koontz C, et al. Minimally invasive thoracoscopic ultrasound for localization of pulmonary nodules in children. J Pediatr Surg. 2008;43:2315–22. doi: 10.1016/j.jpedsurg.2008.08.031. [DOI] [PubMed] [Google Scholar]