Abstract
Persistent controversy exists as to whether there are worthwhile beneficial effects of early, rapid lowering of elevated blood pressure (BP) in acute stroke. Elevated BP or ‘hypertension’ (i.e. systolic >140 mmHg) is common in stroke, especially in patients with pre-existing hypertension and large strokes, due to variable ‘autonomic stress’ and raised intracranial pressure. While positive associations between BP levels and poor outcomes are evident across a range of studies, very low BP levels and large reductions in BP have also been shown to predict death and dependence, more so for ischaemic stroke (IS) than intracerebral haemorrhage (ICH). Accumulating evidence indicates that early BP lowering can reduce haematoma expansion in ICH, but there is uncertainty over whether this translates into improved clinical outcomes, particularly since such an effect was not evident from haemostatic therapy in clinical trials. Guidelines generally recommend control of high systolic BP (>180 mmHg), but recent evidence indicates that even more modest elevation (>140 mmHg) increases risks of cerebral oedema and haemorrhagic transformation following thrombolysis in IS. Thus, any potential benefits of rapid BP lowering in acute stroke, particularly in IS, must be balanced against the potential risks of worsening cerebral ischaemia from altered autoregulation/perfusion. This paper explores current knowledge regarding the management of hypertension in acute stroke and introduces ongoing clinical trials aimed at resolving such a critical issue in the care of patients with acute stroke.
Keywords: acute stroke, blood pressure, clinical trials, guidelines
Introduction
Stroke, as the second most common cause of death [Donnan et al. 2008] and the fourth leading cause of global disease burden, was estimated to result in 16 million first-ever events, 62 million survivors, 51 million disability-adjusted life years (DALYs) lost and 5.7 million deaths worldwide in 2005 [Strong et al. 2007]. These figures are enormous in their own right, but they clearly underestimate other important sequelae of stroke such as cognitive loss, depression, seizures, family caregiver burden and economic hardship. It is likely that the burden of stroke will increase substantially in view of ageing and other demographic changes, and unhealthy lifestyles of populations all over the world over coming decades.
Prevention is undoubtedly the most effective strategy to reduce the burden of stroke, but major therapeutic changes are also possible to improve outcomes. In the past 25 years, the management of acute stroke has moved radically from one of passive supportive (possibly nihilistic) care to that of active, well-coordinated, multidisciplinary care. This radical shift has occurred because careful observational studies and sophisticated neuroimaging modalities have improved our understanding of pathophysiological processes, and randomised controlled trials (RCTs) have demonstrated the potential for avoidance (and even reversal) of serious brain injury from early, appropriately targeted, therapy.
Chronically elevated blood pressure (BP), so-called ‘hypertension’, is the predominant underlying risk factor for stroke [O’Donnell et al. 2010], with population-wide changes (i.e. Rose’s left shifting of the whole distribution curve [Rose 1985]) and the ‘high-risk approach’ for both primary and secondary prevention of stroke being well-established and complimentary approaches, that are now supported by substantial evidence [Zhang et al. 2006]. However, there is continued controversy as to the optimal approach to the management of elevated BP in patients with acute stroke; in part due to concerns arising from long-established ‘neurological dogma’ that BP lowering compromises cerebral perfusion in the context of acute stroke; and in part because of the substantial effort and complexities to generating reliable evidence for a therapeutic strategy with likely modest (but still clinically worthwhile as a standard of care) potential benefits and risks.
A large and increasing number of studies using with varied methodologies have shown that elevated BP in the acute phase of stroke is associated with poor outcomes [Leonardi-Bee et al. 2002]. In addition, high BP has been shown to be associated with an increased risk of intracranial bleeding in patients receiving thrombolytic treatment [Ahmed et al. 2009] and an increased risk of haematoma expansion and subsequent neurological deterioration in patients with intracerebral haemorrhage (ICH) [Qureshi et al. 2007]. This paper reviews the acute hypertensive response, guidelines for the management of hypertension in ischaemic stroke (IS) and ICH, rationale for early BP lowering as a therapeutic modality and the potential impact on clinical practice from positive results of ongoing RCTs in the area.
Acute hypertensive response: frequency and mechanisms
The acute hypertensive response of stroke, defined by the International Society of Hypertension (ISH) and World Health Organisation (WHO) as a systolic BP level of >140 mmHg and diastolic BP >90 mmHg, or levels above established premorbid baseline levels [Bath et al. 2003; Chobanian et al. 2003], is a well-established independent predictor of poor outcome despite generally being transient and self-limiting [Leonardi-Bee et al. 2002; Qureshi, 2008]. Its frequency varies across studies due to variability in patient characteristics, settings and criteria used to define hypertension, but a systematic review of 18 observational studies reported a 53% frequency of hypertension (systolic BP, 150–200 mmHg) in the setting of acute stroke [Willmot et al. 2004]. More impressive was a study which reported a 63% overall frequency of systolic BP >140 mmHg among 563,704 patients with acute stroke attending emergency departments across the United States [Qureshi et al. 2007]. In addition, the frequency of hypertension varied from 67% for IS, 75% for ICH to 100% for subarachnoid haemorrhage.
A complex array of mechanisms likely underlie the acute hypertensive response in acute stroke. As well as acute sympathetic response to the stress of a critical illness and hospitalisation, there are a wide range of other influencing factors such as dehydration, pain/discomfort and the direct involvement of the ischaemic lesion on autonomic pathways in the brain. Patients with established chronic hypertension, more so in those where it is previously unrecognized, appear particularly prone to an acute hypertensive response, while the well-recognized Cushing response of elevated BP from the mass effect of cerebral oedema is another potential mechanism. To further complicate matters, patients with pre-existing hypertension appear to have their cerebral autoregulation shifted to a higher level, potentially making the injured brain more vulnerable to rapid and intensive lowering of BP [Qureshi, 2008].
Current guideline recommendations for BP-lowering treatment in acute IS
In the absence of definitive randomized evidence, it should be recognized that guidelines for the early management of high BP in patients with acute IS are based in the consensus opinion of experts. As shown in Table 1, the American Heart Association (AHA)/American Stroke Association (ASA) [Adams et al. 2007], like other organisations around the world, recommend that antihypertensive medication should be withheld unless BP levels are very high (>220/120 mmHg), or there is ‘organ dysfunction’, or that a patient is otherwise eligible for thrombolytic therapy. In the latter situation, the recommendations change to that of achieving a systolic BP ≤185/110 mmHg before the use of intravenous recombinant tissue plasminogen activator (rtPA), and to maintain the BP <180/105 mmHg thereafter. While such guidelines acknowledge there are significant gaps in knowledge and that more randomised evidence is necessary, they often make the establishment (i.e. approval of ethics committees and regulatory agencies) and conduct (i.e. numbers of participating sites and recruited patients, and the degree of BP separation between randomised groups) particularly challenging due to the recommendations being taken at face value as the ‘best standard of care’.
Table 1.
Recommendations for blood pressure (BP) lowering in patients with acute ischaemic stroke.
| Patients who are not candidates for recombinant tissue plasminogen activator | ||
|---|---|---|
| Agency | Threshold BP (systolic/diastolic) for starting treatment | Target BP level |
| AHA | >220/120 mmHg | Reduce 15–20% |
| ESO | >220/120 mmHg | |
| NSF (Australia) | >220/120 mmHg | Reduce by <20% |
| Patients who are candidates for recombinant tissue plasminogen activator | ||
| Agency | Threshold BP (systolic/diastolic) for starting treatment | Target BP level |
| AHA | >185/110 mmHg | <180/105 mmHg |
| ESO | >185/110 mmHg | <185/110 mmHg |
| NSF (Australia) | >185/110 mmHg | <180/105 mmHg |
AHA, American Heart Association; ESO, European Stroke Organisation; NSF, National Stroke Foundation.
Association of BP and outcome in acute IS
The relationship between BP levels and outcomes has been well described in secondary observation analysis from the first International Stroke Trial (IST) [Leonardi-Bee et al. 2002] which involved 17,398 patients within 48 hours of acute IS. Figure 1 shows the U-shaped association between systolic BP levels and death at 14 days, and death and dependency at 6 months, in the IST population. It should be noted, though, that these relationships were mediated in part by increased rates of early stroke recurrence and deaths in patients with the highest BP and fatal coronary heart disease in those with low BP levels, and that the best outcomes were observed in patients with systolic BP 150–160 mmHg. Such a relationship, therefore, is not necessarily causal.
Figure 1.
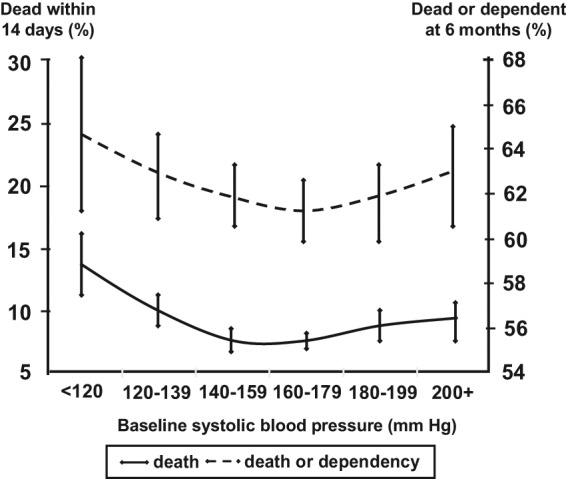
Proportion of patients who died within 14 days (solid lines) or were dead or dependent at 6 months (dashed lines) by baseline systolic blood pressure. 95% confidence intervals are represented by T bars.
BP levels and ICH after rtPA
Modern therapy for acute IS is based on the premise that early vessel recanalisation and reperfusion are both essential for the preservation of brain function and promotion of satisfactory outcome. Intravenous rtPA is currently the only approved treatment for carefully selected patients with IS [Hacke et al. 1998, 2004, 2008], but it is complicated by severe ICH (large parenchymal haematoma formation accompanied by neurological deterioration) in about 5% of cases [Hacke et al. 2004; Lindley et al. 2004]. In a prospective multicentre study of 1136 patients, parenchymal haematoma, and not the more frequent reperfusion-related petechial or patchy haemorrhagic transformation of the cerebral infarct, was a major predictor of death and disability [Paciaroni et al. 2008].
A recent analysis of the first European Cooperative Acute Stroke Study (ECASS) of 615 patients showed that high baseline BP and reduced variability in diastolic BP over the subsequent 72 hours was associated with better outcome at 90 days [Yong et al. 2005]. The association of baseline BP and symptomatic ICH, mortality and independency was evaluated in a multivariable regression analysis of 6483 patients registered with the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SISTS-MOST) and 464 patients pooled from several clinical trials. High initial systolic BP was a significant predictor of ICH in patients treated with rtPA, and a diastolic BP >90 mmHg was associated with lower levels of independence and higher mortality at 3 months [Wahlgren et al. 2008]. These findings were consistent with results of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET), where a logistic regression model of the baseline data showed an increase risk of parenchymal ICH for every 10 mmHg increase in systolic BP [Butcher et al. 2010].
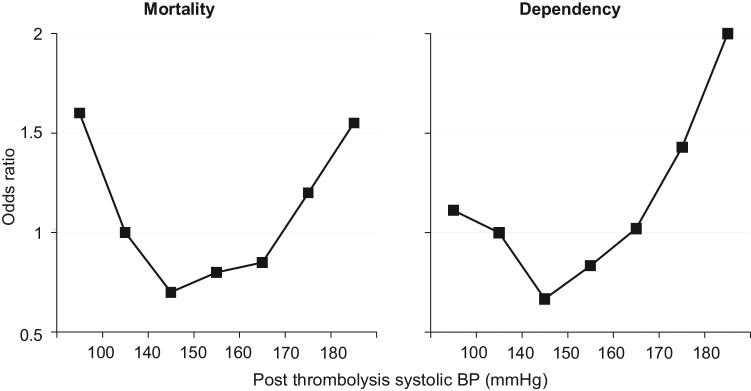
The relationship between BP and symptomatic ICH, mortality and independency was evaluated in the larger, Safe Implementation of Thrombolysis in Stroke–International Stroke Thrombolysis Register (SITS-ISTR) of 10,812 IS patients treated with rtPA, who had data on BP levels at baseline and 2–24 hours, and history of hypertension and use of antihypertensive drugs over 7 days after rtPA [Ahmed et al. 2009]. In a multivariable-adjusted model, high systolic BP (2–24 hours after rtPA) as a continuous variable was associated with worse outcome (p < 0.001), and as a categorical variable had a linear association with symptomatic ICH (Figure 2), and a U-shaped association with mortality and dependence at 3 months (Figure 3). The best 3-month outcome was in patients with mean systolic BP levels of between 140 and 150 mmHg.
Figure 2.
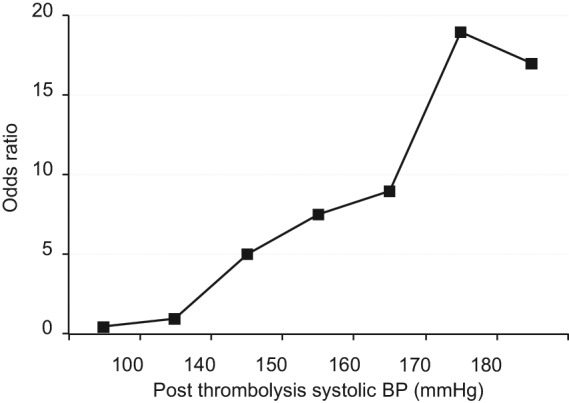
Adjusted odds ratio (midpoints) of symptomatic intracerebral haemorrhage (ICH) by categories of average postrecombinant tissue plasminogen activator systolic blood pressure (BP) within 24 hours, derived from multivariable analysis. ICH was defined was defined by ≥4 points deterioration in National Institutes of Health Stroke Scale score over 24 hours.
Figure 3.
Adjusted odds ratios (midpoints) for key outcomes (death and dependency) by categories of average postrecombinant tissue plasminogen activator systolic blood pressure (BP) within 24 hours, derived from multivariable analysis. Mortality and independence, defined by modified Rankin Scale scores of ‘6’ and ‘0 to 2’, respectively, at 3 months.
The level of risk of ICH associated with very high (‘uncontrolled’) hypertension in the context of rtPA is uncertain; such patients are excluded from RCTs and trial protocols are extrapolated into clinical practice. The guidelines recommendations (Table 1) of patients having their systolic BP levels <180 mmHg prior to receiving any rtPA is re-emphasized by a study showing that such violation, which occurred in 12.4% of 510 patients at a single centre, was independently associated to an increased risk of symptomatic ICH [Tsivgoulis et al. 2009].
The association within BP levels and proportion of recanalization has been evaluated in a study of 149 patients with acute IS treated with intra-arterial thrombolysis [Mattle et al. 2005]. In patients with successful recanalisation, the systolic BP declined significantly faster than among those where recanalisation failed, indicating a inverse relationship between BP levels and outcome, and potentially a more cautious approach to lowering BP in large ISs.
Effects of BP-lowering treatment in acute IS
Post hoc analysis of the 624 patients who participated in the pivotal National Institutes of Neurological Diseases and Stroke (NINDS) trial of rtPA indicated that those initially hypertensive patients who received BP-lowering therapy had less favourable outcomes than the patients who did not receive any such despite of also having high BP levels [Brott et al. 1998]. However, as emphasised by the authors, the significance of the observation is unclear due to the small numbers and nonrandomised use of BP-lowering therapy.
A large systematic review involving 10,892 patients with acute IS and primary ICH from 32 studies has shown that high baseline BP (systolic BP range 150 to 200 mmHg) was significantly associated with death, the combined endpoint cluster of death and dependency, and early stroke recurrence [Willmot et al. 2004]. Another review involving the meta-regression technique on 9008 patients recruited within 6 to 120 hours of stroke (IS or ICH) onset in 37 randomized trials found a clear U-shaped relationship between poor outcome and variability of systolic BP [Geeganage and Bath, 2009]. Moreover, large therapeutic reductions and any increase of BP were also associated with poor outcomes. A systolic BP difference of approximately 15 mmHg between randomised groups was associated with the lowest odds of death and dependency, with reverse or larger differences being associated with worst outcomes.
Most recently, the Scandinavian Candesartan Acute Stroke Trial (SCAST), which involved 2029 patients with acute stroke (IS and ICH) and a systolic BP >140 mmHg within 30 hours (mean time 18 hours) of symptom onset, randomly allocated to candesartan or placebo, achieved a modest mean systolic BP difference of 5 mmHg (p < 0.0001) between groups over 7 days but no difference in functional outcome or vascular events between groups at 6 months [Sandset et al. 2011]. An accompanying meta-analysis of the SCAST results with those of 10 other trials showed no evidence of any effect of BP lowering in acute stroke. Taken together with the neutral result in a substudy involving patients (n = 1360) who were enrolled within 72 hours of the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) study, the largest ever secondary stroke prevention study which compared extended-release dipyridamole plus aspirin versus clopidogrel and telmisartan on top of standard antihypertensive treatment (excluding an angiotensin receptor blocker) [Bath et al. 2010], there does not appear to be any clear beneficial effects of routinely lowering BP by modest levels in the subacute phase (<72 hours) of acute stroke.
However, two ongoing trials are set to provide reliable data on the balance of potential benefits and risks of early BP lowering in acute stroke. The Efficacy of Nitric Oxide in Stroke (ENOS) trial aims to assess the effects of treatment with 5 mg transdermal glyceryl trinitrate (GTN) over 7 days in hypertensive patients with acute stroke (IS and ICH) presenting within 48 hours of symptom onset [The ENOS Investigators, 2006]. In addition, a subgroup of patients previously on antihypertensive treatment will also be randomised into temporarily stopping or continuing antihypertensive medication. The results of this study, with over 3000 of the required sample size of 3500 patients currently included, will provide valuable information on the effects of acute BP management in patients with an acute stroke. In the context of use of rtPA in IS, the ENhanced Control of Hypertension ANd Thrombolysis strokE stuDy (ENCHANTED), which will evaluate the effectiveness of intensive (systolic target 140–150 mmHg) versus standard (<180 mmHg) BP lowering as well as low (0.6 mg/kg) versus standard (0.9 mg/kg) dose rtPA in approximately 5000 patients recruited from more than 100 sites in 20 countries, has just commenced recruitment and is funded by the National Health and Medical Research Council (NHMRC) of Australia.
BP lowering in previously hypertensive patients
As many patients who present with IS have already been on antihypertensive therapy yet have persistently elevated BP levels, an obvious question is whether to continue such treatment and/or introduce more aggressive BP lowering. However, observational data indicate that chronically hypertensive patients appear to have their cerebral autoregulation shifted to a higher level, which could potentially make rapid BP lowering more hazardous as cerebral perfusion is more directly related to systemic BP [Tikhonoff et al. 2009]. However, the SITS-ISTR database suggests that withholding antihypertensive therapy in such patients is associated with higher risks of symptomatic ICH, death and dependency [Ahmed et al. 2009]. Although terminated early due to poor recruitment, the Continue Or Stop post-Stroke Antihypertensives Collaborative randomised controlled Study (COSSACS) in 763 patients (recruitment target was 2900 patients) gives us some guidance about whether to continue or stop usual antihypertensive therapy within 48 hours of suspected stroke [Robinson et al. 2010]. In this study, where the mean BP difference between the ‘continue’ and ‘stop’ randomised groups was 13/8 mmHg at 2 weeks, there were no beneficial (or adverse) effects.
BP lowering in ICH
Spontaneous ICH is one of the most serious pathological subtypes of stroke, which accounts for about 10% of strokes in ‘white Caucasian’ populations, but between 20% and 50% of strokes in other ethnic groups, and has a 30-day case fatality of between 20% and 40% [Broderick et al. 1993]. As described previously, BP is commonly elevated after ICH, and is either a marker or contributor to active haematoma growth within the first few hours after onset. While the role of BP management for the secondary prevention of stroke once patients are clinical stable is well defined [Chapman et al. 2004], there is uncertainty over the appropriate approach to BP control in the hyperacute setting. Haematoma growth is an important determinant of prognosis in ICH, with a meta-analysis involving 218 patients from clinical trials and a population-based study indicating that as little as 10% increase in the size of haematoma is associated with a worse outcome including death [Davis et al. 2006; Dowlatshahi et al. 2011]. Recently, attention has been drawn to the role of the ‘spot sign’, a small area of contrast extravasation within the haematoma on computerized tomography (CT) angiogram, as the stronger predictor of active bleeding and haematoma growth [Delgado Almandoz et al. 2009].
The goal of the BP lowering in ICH is slightly different to that in IS. Instead of the avoidance on cerebral oedema and haemorrhagic transformation, the biological rationale is to reduce or even tapenade ongoing bleeding and reduce the size of the haematoma in the brain. A small retrospective series of 76 patients with likely hypertensive-related ICH, those with systolic BP >160 mmHg had more haematoma growth than those with BP <150 mmHg [Ohwaki et al. 2004]. Such treatment appears safer in ICH as the perihaematomal region appears devoid of an ischaemic penumbra [Butcher et al. 2004] as in IS and therefore less compromised by alteration in cerebral perfusion through surrounding collateral vessels or as is the potential in IS [Bath et al. 2010]. Thus, compared with IS, more rapid and tighter control of BP appears safer to be undertaken in ICH.
Guideline recommendations for BP lowering in ICH
Expert-derived guidelines, such as the recent statement from the AHA/ASA [Broderick et al. 2007] acknowledge the importance of controlling BP according to individual patient factors such as initial BP level, presumed cause, age, and whether there is any elevation of intracranial pressure (ICP) after ICH. BP lowering is recommended where systolic levels are >180 mmHg to a target of 160/90 mmHg. However, uncertainty over the most appropriate level is also well recognized.
Effects of BP-lowering treatment in ICH
The best evidence regarding the role of BP management in ICH comes from the pilot phase, Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT), which involved 404 patients who were randomised to early intensive BP lowering (systolic target 140 mmHg) versus guideline recommended BP management (target <180 mmHg) within 6 hours of onset. The treatment was associated with reduced haematoma growth (14% versus 36% relative growth), although this was not significant after adjustment for initial haematoma volume [Anderson et al. 2008]. However, in contrast to the popular view at the time, intensive BP lowering was feasible to implement and was not associated with excessive risks of adverse events. The ongoing main phase, INTERACT2, is currently nearing completion in assessing the early intensive BP-lowering protocol in nearly 3000 patients with ICH, with results due in 2013. Similarly, the ongoing main phase, Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH II) Trial uses a similar BP-lowering regime but evaluating specifically use of intravenous nicardipine on clinical outcomes when administered within 3 hours of supratentorial ICH.
Clinical implications
While there is insufficient evidence to prescribe the most appropriate management of BP to produce the best outcomes for patients in the acute phase of stroke, certain sensible recommendations can now be made on the basis of current knowledge with incomplete data. Given the consistency of the epidemiological data, there is merit in careful rapid BP lowering in patients with either IS or ICH who have co-occurring very high systolic BP levels, that is >200 mmHg. Even more aggressive BP lowering to systolic levels of 140–150 mmHg is probably safe in ICH, but until the results of INTERACT2 are available, there is uncertainty as to whether the effort (and cost) of such management will translate into improved clinical outcomes. For patients with IS, there is no necessity for routinely being aggressive in the management of BP except unless rtPA is being used, in which case achieving systolic levels <180 mmHg immediately before and for the next 24 hours after rtPA seems sensible. Whether or not a policy of more aggressive control of BP (140–150 mmHg systolic target) after thrombolysis is effective and safe requires results from carefully conducted, internationally cooperative, clinical trials as there is real concern that such a policy could comprise cerebral perfusion from collaterals and worsen the ischaemic penumbra and clinical outcome. Finally, it seems reasonable to continue pre-existing oral antihypertensive medication where possible, and to commence such treatment when patients are clinically stable, generally when they have commenced mobilising out of bed after acute stroke.
Should the results of INTERACT2 prove positive for ICH, it is likely then that the benefits will be greatest when BP control is achieved as early as possible after onset, early intensive BP lowering could be a treatment that is implemented in the prehospital ambulance phase of acute stroke care, without the need for CT. While ICH accounts for only a minority of strokes in the ‘white Caucasian’ populations, this proportion may be as high as 10–20% among early stroke calls, with the benefits of BP control overlapping in cases of IS by avoiding any treatment delay in the use of rtPA.
Guidelines recommendations for common and important conditions, such as the long-standing debate regarding BP control in acute stroke, that are based solely on expert opinion highlight the necessity for randomised evidence. Until this evidence is available, BP-lowering therapy should be used carefully and on an individual patient basis during the acute phase of stroke in patients with high BP.
Footnotes
Funding: This research is supported by grants from the NHMRC of Australia.
Conflict of interest statement: CSA is the Principal Investigator for the INTERACT and ENCHANTED studies, and receives project and fellowship grant support from the NHMRC of Australia.
Contributor Information
Sully Xiomara Fuentes Patarroyo, The George Institute for Global Health and Neurology Department, Royal Prince Alfred Hospital, The University of Sydney, Sydney, NSW, Australia.
Craig Anderson, The George Institute for Global Health, PO Box M201, Missenden Road, NSW 2050, Australia.
References
- Adams H.P., Jr, del Zoppo G., Alberts M.J., Bhatt D.L., Brass L., Furlan A., et al. (2007) Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38: 1655–1711 [DOI] [PubMed] [Google Scholar]
- Ahmed N., Wahlgren N., Brainin M., Castillo J., Ford G.A., Kaste M., et al. (2009) Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR). Stroke 40: 2442–2449 [DOI] [PubMed] [Google Scholar]
- Anderson C.S., Huang Y., Wang J.G., Arima H., Neal B., Peng B., et al. (2008) Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol 7: 391–399 [DOI] [PubMed] [Google Scholar]
- Bath P., Chalmers J., Powers W., Beilin L., Davis S., Lenfant C., et al. (2003) International Society of Hypertension (ISH): statement on the management of blood pressure in acute stroke. J Hypertens 21: 665–672 [DOI] [PubMed] [Google Scholar]
- Bath P.M., Cotton D., Martin R.H., Palesch Y., Yusuf S., Sacco R., et al. (2010) Effect of combined aspirin and extended-release dipyridamole versus clopidogrel on functional outcome and recurrence in acute, mild ischemic stroke: PRoFESS subgroup analysis. Stroke 41: 732–738 [DOI] [PubMed] [Google Scholar]
- Broderick J., Connolly S., Feldmann E., Hanley D., Kase C., Krieger D., et al. (2007) Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 38: 2001–2023 [DOI] [PubMed] [Google Scholar]
- Broderick J.P., Brott T.G., Duldner J.E., Tomsick T., Huster G. (1993) Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 24: 987–993 [DOI] [PubMed] [Google Scholar]
- Brott T., Lu M., Kothari R., Fagan S.C., Frankel M., Grotta J.C., et al. (1998) Hypertension and its treatment in the NINDS rt-PA Stroke Trial. Stroke 29: 1504–1509 [DOI] [PubMed] [Google Scholar]
- Butcher K., Baird T., MacGregor L., Desmond P., Tress B., Davis S. (2004) Perihematomal edema in primary intracerebral hemorrhage is plasma derived. Stroke 35: 1879–1885 [DOI] [PubMed] [Google Scholar]
- Butcher K., Christensen S., Parsons M., De Silva D.A., Ebinger M., Levi C., et al. (2010) Postthrombolysis blood pressure elevation is associated with hemorrhagic transformation. Stroke 41: 72–77 [DOI] [PubMed] [Google Scholar]
- Chapman N., Huxley R., Anderson C., Bousser M.G., Chalmers J., Colman S., et al. (2004) Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS Trial. Stroke 35: 116–121 [DOI] [PubMed] [Google Scholar]
- Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo J.L., Jr, et al. (2003) The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560–2572 [DOI] [PubMed] [Google Scholar]
- Davis S.M., Broderick J., Hennerici M., Brun N.C., Diringer M.N., Mayer S.A., et al. (2006) Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 66: 1175–1181 [DOI] [PubMed] [Google Scholar]
- Delgado Almandoz J.E., Yoo A.J., Stone M.J., Schaefer P.W., Goldstein J.N., Rosand J., et al. (2009) Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke 40: 2994–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnan G.A., Fisher M., Macleod M., Davis S.M. (2008) Stroke. Lancet 371: 1612–1623 [DOI] [PubMed] [Google Scholar]
- Dowlatshahi D., Demchuk A.M., Flaherty M.L., Ali M., Lyden P.L., Smith E.E. (2011) Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology 76: 1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeganage C.M., Bath P.M. (2009) Relationship between therapeutic changes in blood pressure and outcomes in acute stroke: a metaregression. Hypertension 54: 775–781 [DOI] [PubMed] [Google Scholar]
- Hacke W., Donnan G., Fieschi C., Kaste M., von Kummer R., Broderick J.P., et al. (2004) Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 363: 768–774 [DOI] [PubMed] [Google Scholar]
- Hacke W., Kaste M., Bluhmki E., Brozman M., Davalos A., Guidetti D., et al. (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359: 1317–1329 [DOI] [PubMed] [Google Scholar]
- Hacke W., Kaste M., Fieschi C., von Kummer R., Davalos A., Meier D., et al. (1998) Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European–Australasian Acute Stroke Study Investigators. Lancet 352: 1245–1251 [DOI] [PubMed] [Google Scholar]
- Leonardi-Bee J., Bath P.M., Phillips S.J., Sandercock P.A. (2002) Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 33: 1315–1320 [DOI] [PubMed] [Google Scholar]
- Lindley R.I., Wardlaw J.M., Sandercock P.A., Rimdusid P., Lewis S.C., Signorini D.F., et al. (2004) Frequency and risk factors for spontaneous hemorrhagic transformation of cerebral infarction. J Stroke Cerebrovasc Dis 13: 235–246 [DOI] [PubMed] [Google Scholar]
- Mattle H.P., Kappeler L., Arnold M., Fischer U., Nedeltchev K., Remonda L., et al. (2005) Blood pressure and vessel recanalization in the first hours after ischemic stroke. Stroke 36: 264–268 [DOI] [PubMed] [Google Scholar]
- O’Donnell M.J., Xavier D., Liu L., Zhang H., Chin S.L., Rao-Melacini P., et al. (2010) Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 376: 112–123 [DOI] [PubMed] [Google Scholar]
- Ohwaki K., Yano E., Nagashima H., Hirata M., Nakagomi T., Tamura A. (2004) Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke 35: 1364–1367 [DOI] [PubMed] [Google Scholar]
- Paciaroni M., Agnelli G., Corea F., Ageno W., Alberti A., Lanari A., et al. (2008) Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke 39: 2249–2256 [DOI] [PubMed] [Google Scholar]
- Qureshi A.I. (2008) Acute hypertensive response in patients with stroke: pathophysiology and management. Circulation 118: 176–187 [DOI] [PubMed] [Google Scholar]
- Qureshi A.I., Ezzeddine M.A., Nasar A., Suri M.F., Kirmani J.F., Hussein H.M., et al. (2007) Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med 25: 32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T.G., Potter J.F., Ford G.A., Bulpitt C.J., Chernova J., Jagger C., et al. (2010) Effects of antihypertensive treatment after acute stroke in the Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS): a prospective, randomised, open, blinded-endpoint trial. Lancet Neurol 9: 767–775 [DOI] [PubMed] [Google Scholar]
- Rose G. (1985) Sick individuals and sick populations. Int J Epidemiol 14: 32–38 [DOI] [PubMed] [Google Scholar]
- Sandset E.C., Bath P.M., Boysen G., Jatuzis D., Korv J., Luders S., et al. (2011) The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet 377: 741–750 [DOI] [PubMed] [Google Scholar]
- Strong K., Mathers C., Bonita R. (2007) Preventing stroke: saving lives around the world. Lancet Neurol 6: 182–187 [DOI] [PubMed] [Google Scholar]
- The ENOS Investigators (2006) Glyceryl trinitrate vs. control, and continuing vs. stopping temporarily prior antihypertensive therapy, in acute stroke: rationale and design of the Efficacy of Nitric Oxide in Stroke (ENOS) trial (ISRCTN99414122). Int J Stroke 1: 245–249 [DOI] [PubMed] [Google Scholar]
- Tikhonoff V., Zhang H., Richart T., Staessen JA. (2008) Blood pressure as a prognostic factor after acute stroke. Lancet Neurol 8: 938–948 [DOI] [PubMed] [Google Scholar]
- Tsivgoulis G., Frey J.L., Flaster M., Sharma V.K., Lao A.Y., Hoover S.L., et al. (2009) Pre-tissue plasminogen activator blood pressure levels and risk of symptomatic intracerebral hemorrhage. Stroke 40: 3631–3634 [DOI] [PubMed] [Google Scholar]
- Wahlgren N., Ahmed N., Eriksson N., Aichner F., Bluhmki E., Davalos A., et al. (2008) Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST). Stroke 39: 3316–3322 [DOI] [PubMed] [Google Scholar]
- Willmot M., Leonardi-Bee J., Bath P.M. (2004) High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 43: 18–24 [DOI] [PubMed] [Google Scholar]
- Yong M., Diener H.C., Kaste M., Mau J. (2005) Characteristics of blood pressure profiles as predictors of long-term outcome after acute ischemic stroke. Stroke 36: 2619–2625 [DOI] [PubMed] [Google Scholar]
- Zhang H., Thijs L., Staessen J.A. (2006) Blood pressure lowering for primary and secondary prevention of stroke. Hypertension 48: 187–195 [DOI] [PubMed] [Google Scholar]