Abstract
It is increasingly recognized that gastric cancer is a heterogeneous disease which may be divided into subgroups based on histological, anatomical, epidemiological and molecular classifications. Distinct molecular drivers and tumor biology, and thus different treatment targets and predictive biomarkers, may be implicated in each subtype. However, there is little evidence in the literature regarding the correlation among these different classifications, and particularly the molecular aberrations present in each subtype. In this review, we approach advanced gastric cancer (AGC) by presenting aberrant molecular pathways and their potential therapeutic targets in gastric cancer according to histological and anatomical classification, dividing gastric cancer into proximal nondiffuse, distal nondiffuse and diffuse disease. Several pathways are involved predominantly, although not exclusively, in different subtypes. This may help to explain the disappointing results of many published AGC trials in which study populations were heterogeneous regardless of clinicopathological characteristics of the primary tumor. Histological and anatomical classification may provide insights into tumor biology and facilitate selection of an enriched patient population for targeted agents in future studies and in the clinic. However, some molecular pathways implicated in gastric cancer have not been studied in correlation with histological or anatomical subtypes. Further studies are necessary to confirm the suggestion that such classification may predict tumor biology and facilitate selection of an enriched patient population for targeted agents in future studies and in the clinic.
Keywords: Advanced gastric cancer, angiogenic pathway, biomarker, epidermal growth factor pathway, targeted therapy
Introduction
Modest efficacy and considerable toxicities associated with chemotherapy in advanced gastric cancer (AGC) has prompted the pursuit of novel systemic treatment strategies. Aberrant genetic and molecular alterations in gastric carcinogenesis represent logical treatment targets. However, the enthusiasm in developing such therapy has not been met with great success thus far. Trastuzumab is currently the only approved targeted agent for the subgroup of human epidermal growth factor receptor (HER)-2-positive AGC, based on results of the phase III trastuzumab for gastric cancer (ToGA) trial [Bang et al. 2010]. Although an overall survival (OS) advantage was demonstrated, the majority of patients did not respond to the combination of chemotherapy and trastuzumab in the first-line setting despite having HER2-positive disease. However, the addition of bevacizumab to chemotherapy did not result in significant OS benefit in the phase III Avastin in Gastric Cancer (AVAGAST) trial [Kang et al. 2010]; more recently the results of the Randomized EOX for advanced and locally advanced esophagogastric cancer 3 (REAL-3) study in abstract form reported an inferior OS with the addition of panitumumab to chemotherapy [Wadell et al. 2012]. Data on other agents are still awaited.
The difficulties encountered in the development of targeted therapy in AGC are caused by the lack of biomarkers to guide patient management. In the clinic to date, except for HER2, there are no established biomarkers predictive of tumor response to targeted agents. Few potential biomarkers are pending clinical validation, including amplification of MET [Lennerz et al. 2011] and fibroblast growth factor receptor 2 (FGFR2) [Deng et al. 2012], while others are more controversial. Moreover, the process of gastric carcinogenesis is complex [Wu et al. 2009; Yin, et al. 2009]. Rather than predominantly addicted to a particular oncogene and its associated signaling pathway, gastric cancer may be driven by multiple essential pathways with their cross talks poorly understood, preventing effective targeting by single agents. It is also increasingly recognized that gastric cancer is a heterogeneous disease which may be divided into subgroups based on histological [Lauren, 1965], anatomical [Blot et al. 1991], epidemiological [Crew and Neugut, 2006], and more recently, genomic or molecular classifications [Tay et al. 2003; Ooi et al. 2009; Shah et al. 2011; Deng et al. 2012]. Distinct molecular drivers and tumor biology, and thus different treatment targets and predictive biomarkers, may be implicated in each subtype. However, there is little evidence in the literature regarding the correlation between these different classifications, and particularly the molecular aberrations present in each subtype. Also, the classifications currently have a limited role in the prospective selection of patients with AGC in the clinic or trials, and no consensus classification exists.
In this review, we approach AGC by describing molecular aberrations predominantly implicated in according histological-anatomical subtypes of gastric cancer, namely proximal nondiffuse, distal nondiffuse and diffuse disease (Figure 1). While providing a brief account of clinical trials of targeted agents in AGC, which have been summarized in detail elsewhere [Wong and Yau, 2012], we also focus on preclinical developments.
Figure 1.
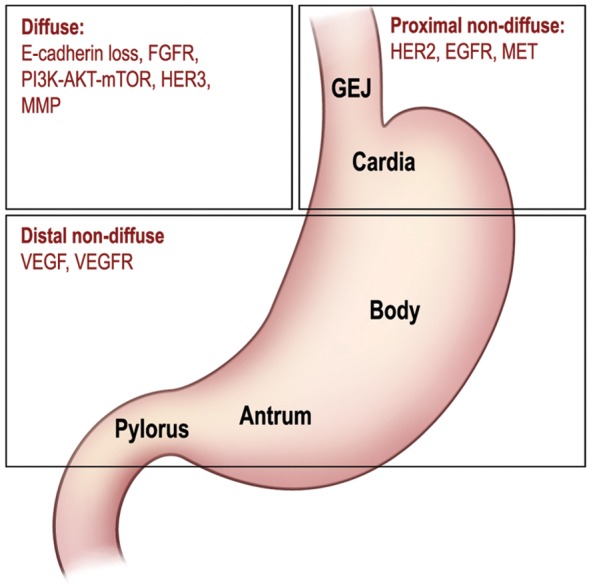
Subtypes of gastric cancer based on anatomical and histological classification, and important molecular targets implicated in each subtype. EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; GEJ, gastroesophageal junction; HER, human epidermal growth factor receptor; MMP, matrix metalloproteinase; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3 kinase; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor. (Illustration courtesy of Alessandro Baliani, Copyright © 2012.)
Proximal nondiffuse gastric cancer
Proximal nondiffuse gastric cancer is defined as tumor located in the gastric cardia which may extend up to the gastroesophageal junction (GEJ), where histopathology shows evidence of precursor glandular dysplasia or in situ carcinoma in the setting of chronic inflammation usually without atrophy [Shah et al. 2011]. In contrast to distal nondiffuse disease when chronic inflammation is more associated with Helicobacter pylori, in proximal tumors carcinogenic inflammation is often caused by gastric acid reflux [Blot et al. 1991; Crew and Neugut, 2006].
The overexpression of HER2 is more prevalent, though not exclusive, in intestinal and proximal gastric cancer compared with diffuse or distal disease [Koeppen et al. 2001; Tanner et al. 2005; Gravalos et al. 2007; Leon-Chong et al. 2007; Zhang et al. 2009]. HER2 overexpression is observed in 10–38% of gastric cancer tumor samples overall, in 16–34% of intestinal tumors and 2–7% of diffuse tumors [Jaehne et al. 1992; Koeppen et al. 2001; Tanner et al. 2005; Yano et al. 2006; Gravalos et al. 2007; Leon-Chong et al. 2007], and in 24–73% in the subgroup of GEJ and esophageal adenocarcinoma [Polkowski et al. 1999; Ross and McKenna, 2001; Zhang et al. 2009] (Table 1). HER2, a transmembrane tyrosine kinase receptor encoded by the ErbB2 gene, is a member of the HER family. Epidermal growth factor receptor (EGFR/HER1), HER3 and HER4 are all activated by ligand binding, while the HER2 receptor has no known ligand. Activation of these receptors leads to homo- or hetero-dimerization, which in turn initiates phosphorylation cascades and subsequent signaling pathways, including RAS– RAF–MEK–mitogen-activated protein kinase and phosphatidylinositol 3 (PI3K)–AKT–mammalian target of rapamycin (mTOR) pathways, for cancer cell proliferation and survival [Schlessinger, 2004; Dhanasekaran and Johnson, 2007] (Figure 2).
Table 1.
Comparison of molecular characteristics between intestinal and diffuse subtypes of gastric cancer.
| Characteristics | Molecular methods | Intestinal | Diffuse |
|---|---|---|---|
| HER2 amplification or overexpression | FISH/IHC | 16–34% | 2–7% |
| EGFR amplification or overexpression | FISH/ IHC | 30–60% | – (overall 27–44%) |
| MET amplification | FISH | 3% | – (overall 0–2%) |
| Mean VEGF staining intensity | IHC | 2.2 | 1.4 |
| Mean vessel count | Light microscopy | 37 | 26 |
| VEGFR overexpression | IHC | 39% | 15% |
| Loss of E-cadherin | IHC | 69% | 89% |
| FGFR2 amplification or overexpression | FISH/ IHC | 0% | 13–53% |
| Phospho-mTOR expression | IHC | 47–60% | 58–64% |
| HER3 overexpression | IHC | 5% | 26% |
| MMP (-1, -7) | IHC | 32–70% | 62–90% |
Note: The bold values signify the higher prevalence of particular molecular characteristics when comparing gastric subtypes. HER2-, EGFR-, MET- and VEGF-related signaling are predominantly implicated in the intestinal subtype, while loss of E-cadherin, FGFR2-, mTOR-, HER3- and MMP-related pathways are more frequently involved in the diffuse subtype. EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; FISH, fluorescence in situ hybridization; HER, human epidermal growth factor receptor; IHC, immunohistochemistry; MMP, matrix metalloproteinase; mTOR, mammalian target of rapamycin; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
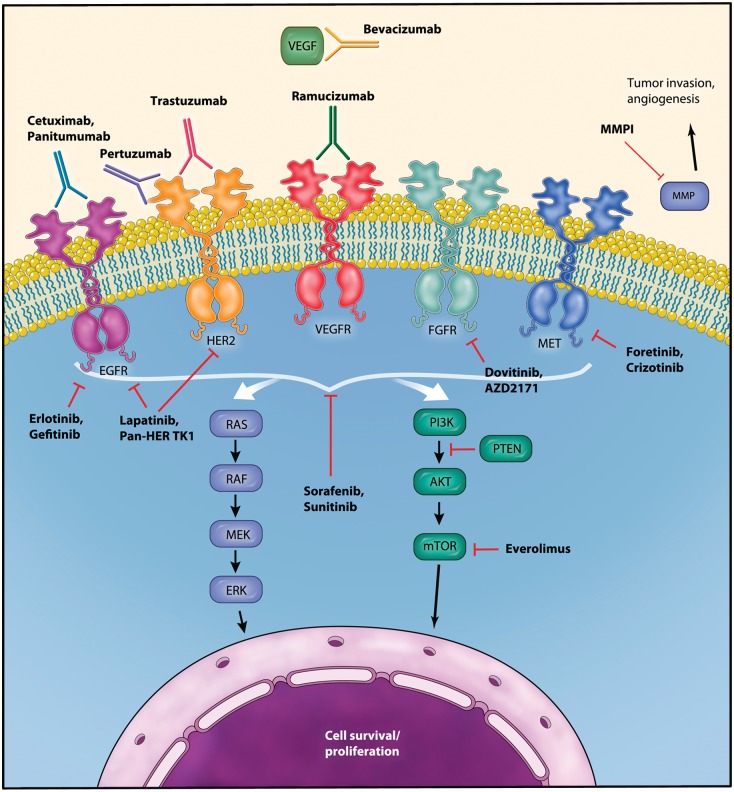
Figure 2.
Schematic diagram of signaling pathways in gastric cancer and targets for molecular therapy. EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; FGFR, fibroblast growth factor receptor; HER, human epidermal growth factor receptor; MMP, matrix metalloproteinase; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3 kinase; PTEN, phosphatase and tensin homolog; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor. (Illustration courtesy of Alessandro Baliani, Copyright © 2012.)
While EGFR overexpression is observed in 27–44% of all gastric cancer [Gamboa-Dominguez et al. 2004; Kim et al. 2008; Lieto et al. 2008; Matsubara et al. 2008], it is reported in 30–60% of proximal or esophageal adenocarcinoma [Al-Kasspooles et al. 1993; Yacoub et al. 1997; Wilkinson et al. 2004; Isinger-Ekstrand et al. 2010]. It was also observed that on EGFR inhibition with tyrosine kinase inhibitors (TKIs), response tended to occur in GEJ rather than gastric cancers [Dragovich et al. 2006; Rojo et al. 2006]. However, HER3 overexpression is more prevalent in diffuse gastric cancer (see later text), and little is known about HER4 in AGC.
In addition to HER2 and EGFR, amplification of the proto-oncogene MET by fluorescence in situ hybridization (FISH), although overall rare, occurs more frequently in GEJ (3%) compared with gastric tumors (0–2%), and more in intestinal than diffuse histology [Janjigian et al. 2011; Lee et al. 2011; Lennerz et al. 2011; Guo et al. 2012]. Increase in MET copy number was reported in up to 20% of all patients with gastric cancer [Lee et al. 2011], and MET mutation was also described [Lee et al. 2000]. Activation of receptor tyrosine kinase MET, whose ligand is hepatocyte growth factor, leads to proliferation and antiapoptotic signals [Migliore and Giordano, 2008].
HER2, EGFR and MET signaling are therefore of particular importance, especially in proximal nondiffuse tumors, and constitute logical targets for molecular therapy.
Targeting HER2
HER2 overexpression has been shown to predict response to trastuzumab, a humanized recombinant monoclonal antibody which selectively binds to the extracellular domain of HER2, thereby blocking its downstream signaling. The addition of trastuzumab to cisplatin plus capecitabine/fluorouracil was shown in the large phase III ToGA trial to significantly improve objective response rate (ORR) from 35% to 47% (p = 0.0017), progression free survival (PFS) from 5.5 to 6.7 months (p = 0.0002) and OS from 11.1 to 13.8 months (p = 0.0046) [Bang et al. 2010] in patients whose tumors were HER2 positive by FISH and 2–3+ by immunohistochemistry (IHC) in the first-line setting. Trastuzumab was well tolerated. The analysis of HER2 positivity in relationship to anatomical locations of primary tumors in the ToGA trial, in concordance with previous findings, showed a higher HER2-positive rate in GEJ than gastric cancers (33.2% and 20.9% respectively), and in the intestinal subtype than mixed and diffuse types (32.2%, 20.4% and 6.1% respectively) [Bang et al. 2009]. This observation again highlights disease heterogeneity in AGC and the potential role of incorporating histological and anatomical classifications into selecting patients for targeted therapy. Of note, as the level of HER2 overexpression may be associated with the magnitude of benefit from trastuzumab [Bang et al. 2009], its correlation with histological gastric cancer subtypes warrants further investigation.
HER2 dimerization inhibitor
Pertuzumab is a monoclonal antibody which binds to HER2 to prevent dimerization of HER2 with other HER receptors. In a HER2-positive, but not HER-negative, human gastric cancer xenograft model, pertuzumab in combination with trastuzumab showed significant antitumor activity compared with monotherapy. HER2-EGFR and HER2-HER3 heterodimerization and downstream signaling activation were inhibited [Yamashita-Kashima et al. 2011]. A phase II study is ongoing to investigate the combination of pertuzumab and trastuzumab in patients with HER2-positive AGC [ClinicalTrials.gov identifier: NCT01461057].
Targeting epidermal growth factor receptor
Unlike HER2 in HER2-positive gastric cancer, there are no established biomarkers to predict response to EGFR inhibitors. The predictive value of EGFR mutation, increased EGFR copy number, K-ras mutation status, and patients’ development of skin rash are controversial [Han et al. 2009; Zhang et al. 2009; Lordick et al. 2010].
Cetuximab is a recombinant human/mouse chimeric monoclonal antibody against EGFR. Many first-line phase II trials have evaluated cetuximab in combination with various chemotherapy regimens [Pinto et al. 2007, 2009; Woell et al. 2008; Han et al. 2009; Kanzler et al. 2009; Kim et al. 2009; Zhang et al. 2009; Lordick et al. 2010; Moehler et al. 2010] showing an ORR in the range of 40–60%, time to progression 5.5–8 months, and OS 9.5–16 months. However, preliminary results of a randomized phase II study showed no clinically significant benefit when cetuximab was added to docetaxel plus oxaliplatin [Richards et al. 2011]. A randomized phase III trial, EXPAND (Erbitux in Combination With Xeloda and Cisplatin in Advanced Esophago-gastric Cancer) [ClinicalTrials.gov identifier: NCT00678535] is ongoing to investigate first-line capecitabine and cisplatin with or without cetuximab. However, there are limited and conflicting data in the literature regarding the use of cetuximab-based therapy as salvage for pretreated patients with AGC [Stein et al. 2007; Tebbutt et al. 2008; Li et al. 2010].
In contrast to cetuximab, panitumumab is a fully humanized monoclonal antibody targeting EGFR. The REAL-3 trial aimed to explore the role of panitumumab in combination with epirubicin, oxaliplatin and capecitabine (EOC). Although in the phase II section of the study ORR of EOC plus panitumumab was promising at 52% [Chau et al. 2011], the phase III results showed a significantly inferior OS in the panitumumab-containing arm [8.8 months versus 11.3 months, hazard ratio (HR) 1.37, p = 0.013] [Wadell et al. 2012]. The negative results may be caused by the reduced dose intensity of chemotherapy in the panitumumab-containing arm, and by the recruitment of an unselected population. Similarly, the results of randomized phase II studies of other EGFR monoclonal antibodies, namely matuzumab and nimotuzumab, were both negative [Rao et al. 2010; Kim et al. 2011].
EGFR-TKIs have shown modest activity, mainly in GEJ rather than more distal cancers. In a phase II trial of 70 patients, erlotinib monotherapy resulted in an ORR of 9% (including one complete response) in patients with GEJ cancer but none in the gastric cancer subgroup [Dragovich et al. 2006]. In contrast, gefitinib was associated with a lack of efficacy in patients with AGC mostly with distal gastric cancer [Doi et al. 2003].
Dual targeting HER2 and epidermal growth factor receptor
Lapatinib is a TKI with dual action against HER2 and EGFR. While phase II studies of lapatinib in patients with HER2-unselected AGC showed disappointing results [Iqbal et al. 2007; Hecht et al. 2008], two phase III trials targeting HER2-positive disease are currently ongoing. The Lapatinib Optimization Study in ErbB2 (HER2) Positive Gastric Cancer (LoGIC) is investigating first-line treatment with capecitabine and oxaliplatin with or without lapatinib [ClinicalTrials.gov identifier: NCT00680901]; Tykerb with taxol in Asian HrbB2+ gastric cancer (TYTAN) is investigating second-line paclitaxel with or without lapatinib in Asian patients [Satoh et al. 2010].
The combination of lapatinib and trastuzumab was shown in a preclinical study to be synergistic in inhibiting the cell growth of the HER2-amplified human upper gastrointestinal cancer cell lines [Wainberg et al. 2010]; these data provide a rationale for testing this interesting combination in early phase clinical trials.
Pan-HER inhibition
In HER2-positive gastric cancer in vitro and in vivo models, a few pan-HER TKIs have demonstrated activity against tumor growth and synergistic effects in combination with chemotherapeutic or molecular agents. These include BMS-599626 [Wong et al. 2006], HM781-36B [Nam et al. 2011] and PF00299804 [Nam et al. 2012]. A phase II study of PF00299804 monotherapy in patients with HER2-positive AGC is currently ongoing [ClinicalTrials.gov identifier: NCT01152853].
Role of downstream components of HER pathway
In gastric cancer, KRAS mutation was observed in 2–20% [Nanus et al. 1990; Hongyo et al. 1995; Lee et al. 1995, 2003; Hiyama et al. 2002; Kim et al. 2003] and BRAF in 0–2.7% [Kim et al. 2003; Lee et al. 2003]. These mutations lead to constitutively activated signaling proteins, and are linked to resistance to monoclonal antibodies targeting upstream receptors in colorectal cancer [Normanno et al. 2009; Bardelli and Siena, 2010]. In gastric cancer, the predictive ability of KRAS and BRAF has not been extensively studied, but small reports did not demonstrate such characteristics [Lordick et al. 2010; Park et al. 2010]. There is also little evidence whether these biomarkers are different across histological-anatomical subtypes of gastric cancer.
Targeting MET
Amplification of MET predicts response to MET inhibition in vitro [Smolen et al. 2006; Kataoka et al. 2011]. The interim results of a phase II study of foretinib (GSK1363089, GSK089, formerly XL880), a MET TKI, showed minimal activity in a MET-unselected AGC cohort while it was well tolerated with toxicities including liver function abnormalities, fatigue and venous thromboembolism [Jhawer et al. 2009]. Another MET inhibitor, crizotinib (PF02341066), achieved tumor shrinkage in two out of four patients with MET-amplified esophagogastric adenocarcinoma; in both the tumor was located at the GEJ [Lennerz et al. 2011].
Distal nondiffuse gastric cancer
Distal nondiffuse tumors are located between the body of the stomach and pylorus. On histopathology, chronic gastritis with a spectrum of intestinal metaplasia and dysplasia is present. Intestinal gastric cancer, especially that arising from the antrum, is often a consequence of chronic Helicobacter pylori infection [Peek and Blaser, 2002], which also occurs in diffuse gastric tumors and normal controls but at a significantly lower frequency [Asaka et al. 1994].
H. pylori infection promotes angiogenesis in gastric cancer, as reflected by greater tumor vascularity in patients with H. pylori-infected compared with H. pylori-eradicated gastric cancer [Sasaki et al. 2003]. Moreover, H. pylori activates nuclear factor κB [Sharma et al. 1998], and induces the expression of a number of angiogenic factors in gastric cancer cells, including vascular endothelial growth factor (VEGF), matrix metalloproteinase (MMP)-9, and interleukin 8 [Crabtree et al. 1994; Kitadai et al. 2003; Wu et al. 2005]. This in turn is concordant with the observation that intestinal with reference to diffuse subtype expresses significantly higher VEGF levels (mean VEGF staining intensity 2.2 versus 1.4), vessel counts (mean 37 versus 26) and VEGF receptor (VEGFR) expression (39% versus 15%), suggesting that the former is more dependent on angiogenesis [Takahashi et al. 1996].
Targeting vascular endothelial growth factor
Bevacizumab is a monoclonal antibody which binds to VEGF. In the phase III AVAGAST trial, patients with AGC were randomized to receive capecitabine and cisplatin with or without bevacizumab as first-line treatment. Capecitabine and bevacizumab or placebo were given until progression or unmanageable toxicity. Although the study did not meet its primary endpoint of OS, ORR was significantly better in the bevacizumab arm (46% versus 37%, p = 0.0315), and PFS improved from 5.3 to 6.7 months (HR 0.8, p = 0.0037) [Ohtsu et al. 2011]. The most commonly encountered grade 3–5 bevacizumab-related adverse events were thromboembolic events, hypertension, bleeding and proteinuria. Interestingly, patients in pan-America, but not those in Asia or Europe, derived survival benefit from the addition of bevacizumab. Although the results of correlative biomarker analyses are still pending, a recent unplanned exploratory analysis of AVAGAST showed that bevacizumab appeared to improve outcomes in non-Asian subjects with distal nondiffuse disease, as opposed to proximal nondiffuse cancer [Shah et al. 2012]. While awaiting prospective confirmation, this supports the particular role of angiogenesis and its targeted therapy in the distal nondiffuse subtype of AGC.
Targeting vascular endothelial growth factor receptor
Multitargeted tyrosine kinase inhibitors (MTIs) suppress angiogenesis by simultaneously targeting VEGFR and other signaling pathways. In AGC, MTIs are in phase I/II of clinical development. Sunitinib inhibits platelet-derived growth factor receptor (PDGFR), kit, RET and Flt3 together with VEGFR. In phase II studies, single agent sunitinib has limited activity as salvage therapy for patients with chemotherapy-refractory AGC [Moehler et al. 2009; Bang et al. 2011]. However, sorafenib, another inhibitor of multiple kinases including VEGFR-2, VEGFR-3 and PDGFR, has been evaluated in combination with chemotherapy in the first-line phase II setting [Sun et al. 2010]. The results showed ORR of 41%, PFS of 5.8 months and OS of 13.6 months, with significant toxicities. In pretreated patients, single agent sorafenib was studied in another phase II trial; preliminary analysis of 16 evaluable patients included one durable complete response and another protracted stable disease of over 19 months [Ilson et al. 2011]. Other MTIs under early-phase clinical investigation include telatinib [Alsina et al. 2011], axitinib [ClinicalTrials.gov identifier: NCT00842244] and apatinib [Li et al. 2011].
Ramucirumab (IMC-1121B), a fully human IgG1 monoclonal antibody targeting VEGFR-2, was evaluated in a phase I study of previously treated advanced solid tumors, and achieved responses in 4 out of 37 patients, including one with refractory gastric cancer [Spratlin et al. 2010]. It is currently in phase III investigation in combination with weekly paclitaxel in patients with AGC progressing after first-line chemotherapy [ClinicalTrials.gov identifier: NCT01170663].
Diffuse advanced gastric cancer
Diffuse gastric cancer is characterized by a diffuse pattern of infiltration and poorly differentiated signet ring cell type without apparent gastritis. In contrast to intestinal gastric cancer resulting from a multistep carcinogenic process, diffuse gastric cancer is believed to arise de novo and is associated with CDH1 downregulation [Becker et al. 1994; Carneiro et al. 2004].
CDH1 is a tumor suppressor gene that encodes E-cadherin, a cell adhesion protein that maintains cell polarity [Cavallaro and Christofori, 2004]. Downregulation of CDH1 plays a role in gastric tumorigenesis, invasion and metastases [Guilford et al. 1998; Perl et al. 1998]. Germline mutation in CDH1 is responsible in hereditary diffuse gastric cancer [Humar and Guilford, 2009], while somatic CDH1 mutations are found in over 50% of sporadic diffuse gastric cancers, much more often than in the intestinal subtype [Becker et al. 1994; Tamura et al. 1996]. Inactivation of the second CDH1 allele may be caused by mechanisms including loss of heterozygosity and DNA hypermethylation of the promoter CpG islands [Tamura et al. 2000; Liu et al. 2006]. In concordance, E-cadherin by IHC is expressed at a lower rate in diffuse (11%) compared with intestinal (31%) gastric cancer [Zhou et al. 2010].
Other molecular aberrations preferentially amplified or expressed in diffuse type gastric cancer include FGFR2 signaling and PI3K signaling; HER3 is also implicated. The FGFR2/K-sam gene, encoding the FGFR2 receptor protein, was shown to be amplified predominantly in signet ring cell stomach cancer cell lines and poorly differentiated human gastric cancer xenografts and surgical specimens [Hattori et al. 1990; Nakatani et al. 1990], although a minority of evidence did not confirm significant association between FGFR2 amplification and diffuse histology [Deng et al. 2012; Guo et al. 2012]. On IHC analysis, FGFR2 positivity was found in 20 out of 38 (53%) advanced diffuse type gastric cancer specimens but none of the intestinal type specimens [Hattori et al. 1996].
PI3K activity was higher in cell lines derived from signet ring cell gastric carcinoma than other adenocarcinomas, and was associated with the formation of poorly differentiated cancer in nude mice [Kobayashi et al. 1999, 2003]. In gastric cancer specimens unspecified for histology, PIK3CA, encoding the p110 catalytic subunit of PI3K, was reported to be amplified in 36% [Byun et al. 2003], PI3KCA activating mutation in 4–36% [Byun et al. 2003; Li et al. 2005], and phosphatase and tensin homolog loss in 20–36% [Kang et al. 2002; Byun et al. 2003]. Moreover, the PI3K–AKT–mTOR pathway is frequently activated in gastric cancer as suggested by the prevalent expression of phospho-AKT (29–86%) [Bellacosa et al. 2005; Murayama et al. 2009] and phospho-mTOR (47–64%) [Lang et al. 2007; Murayama et al. 2009; Yu et al. 2009]. In particular, the expression of phospho-mTOR, a negative prognosticator [Yu et al. 2009; An et al. 2010; Xu et al. 2010], is seen more frequently in diffuse (58–64%) than in intestinal (47–60%) gastric cancer samples [Lang et al. 2007; Feng et al. 2008], although in both subtypes it occurs in a significant proportion of tumors. Thus targeted treatment on this pathway should not be developed exclusively for the diffuse subtype.
PI3K is also activated by upstream HER3, which is selectively phosphorylated in undifferentiated gastric cancer cells [Kobayashi et al. 1999, 2003]. In addition, HER3 signaling is implicated in cell growth and survival in FGFR2-amplified gastric cancer cell lines [Kunii et al. 2008]. Concordantly, the rate of HER3 overexpression is higher in human gastric cancer specimens of the diffuse subtype compared with the intestinal subtype (26% versus 5%, p < 0.01) [Zhang et al. 2009].
Finally, expression of some MMPs are significantly more frequent in diffuse than in intestinal gastric cancer subtypes and contribute to tumor aggressiveness; for example, positivity of MMP-7 was reported in 62% and 32% in diffuse and intestinal tumors respectively [Kitoh et al. 2004], and MMP-1 in 90% and 70% respectively [Zhou et al. 2010]. In other studies evaluating various MMPs, the difference did not reach statistical significance [Gerstein et al. 2009; Kemik et al. 2011; Zhang et al. 2011]. With regard to tumor location, MMP expression was similar between proximal and distal tumors [Kitoh et al. 2004; Woolley et al. 2004]. MMPs are a family of zinc-dependent enzymes involved in degradation of extracellular matrix. They play a key role in metastasis, and represent potential targets for therapy, possibly more frequently in the diffuse subtype.
Targeting β catenin
The invasion suppressor signal of E-cadherin is mediated through binding β catenin [Wong and Gumbiner, 2003]. In normal cells, β catenin connects E-cadherin through α catenin to the actin cytoskeleton [Mareel et al. 1997], but when not bound to E-cadherin, it associates with T-cell factor (Tcf) to activate in the nucleus the transcription of genes implicated in tumor formation and progression [Mann et al. 1999; Tetsu and McCormick, 1999]. In gastric cancer cells with an activated β catenin/Tcf pathway, a recombinant adenovirus carrying a lethal gene under the control of a β catenin/Tcf-responsive promoter selectively inhibited cell growth in a time- and dose-dependent manner [Dvory-Sobol et al. 2007]. Such strategies are however still in the preclinical stage of development.
Targeting fibroblast growth factor receptor 2
Small molecule FGFR2 inhibitors such as PD173074 and AZD2171 inhibited FGFR2 phosphorylation and cell growth in FGFR2-amplified gastric cancer cell lines [Takeda et al. 2007; Kunii et al. 2008] and xenografts [Takeda et al. 2007]. Similarly, in a murine model of scirrhous gastric cancer (a unique phenotype of diffuse gastric cancer) but not nonscirrhous gastric cancer, Ki23057, another novel tyrosine kinase FGFR2 inhibitor, significantly decreased FGFR2 phosphorylation, inhibited tumor proliferation and demonstrated synergistic antitumor effects in combination with 5-fluorouracil [Nakamura et al. 2006; Yashiro et al. 2010]. More recently, dovitinib (TKI258) demonstrated growth inhibitory activity in FGFR2-amplified gastric cancer cell lines and xenografts [Deng et al. 2012]. To various extent, these agents inhibit other tyrosine kinase receptors in addition to FGFR2, including FGFR1, FGFR3, VEGFR-1, VEGFR-2, VEGFR-3, and PDGF-Rβ. However, monoclonal antibodies specific to FGFR2 have been shown to effectively inhibit tumor growth in FGFR2-overexpressing gastric cancer xenografts in mice [Zhao et al. 2010]. Ongoing phase II studies may clarify the role of dovitinib and AZD2171 in patients with FGFR-amplified AGC [ClinicalTrials.gov identifiers: NCT01576380 and NCT01457846].
Targeting PI3K–AKT–mTOR pathway
A number of PI3K inhibitors have demonstrated preclinical activity and are being investigated in phase I studies of solid tumors (reviewed by Markman and colleagues) [Markman et al. 2010]. As for gastric cancer, the PI3K inhibitor LY294002 inhibited growth of implanted tumors of human gastric carcinoma cells in nude mice, and reduced tumor expression of a number of angiogenic factors [Xing et al. 2009]. Similarly, the PI3K/mTOR inhibitor BEZ235 and PI3K inhibitor BKM120 were shown to have pro-apoptotic effects for human gastric and colon cancer cell lines [Moehler et al. 2012]. In another study, BEZ235 resulted in decreased cell viability of gastric cancer cell lines and dephosphorylation of downstream effector proteins in all xenograft models [Fuereder et al. 2011]. However, an in vivo antitumor effect was only observed in one of the xenografts, correlated with downregulation of the proliferation marker thymidine kinase 1 and reduced [(18)F]FLT uptake by animal positron emission tomography. Molecular mechanisms involved in the sensitivity to PI3K inhibitors are yet to be clarified to translate preclinical activity to clinical benefit, and to date, the development of PI3K inhibitors in AGC is still in the preclinical stage.
The PI3K–AKT–mTOR pathway can also be targeted at the mTOR level. In a scirrhous gastric cancer cell line and its related cell line with propensity for peritoneal metastases, the mTOR inhibitor everolimus demonstrated growth-inhibitory activity [Taguchi et al. 2011]. Similarly, mTOR inhibitors with 5-fluorouracil showed a synergistic antiproliferative effect in scirrhous but not in nonscirrhous gastric cancer cell lines [Matsuzaki et al. 2009]. In other preclinical studies not specific for diffuse histology, antitumor effects of everolimus alone or in combination with cytotoxic chemotherapy were also reported [Cejka et al. 2008; Lee et al. 2010; Xu et al. 2010]. Clinically, a phase II trial of everolimus as salvage therapy for patients with pretreated AGC showed a disease control rate of 55%, although no objective response was noted [Doi et al. 2010]. The median PFS and OS were 2.7 and 10.1 months respectively. Based on these provocative results, a phase III randomized multicenter trial is ongoing to compare everolimus plus best supportive care with placebo plus best supportive care in patients with progressive disease after one or two prior lines of chemotherapy [ClinicalTrials.gov identifier: NCT00879333].
Matrix metalloproteinase inhibition
Marimastat, an oral MMP inhibitor, demonstrated preclinical activity in xenograft models of human gastric cancer [Wada et al. 2003] and acceptable safety in a phase I study [Tierney et al. 1999]. Despite an improved 2-year OS from 3 to 9%, the phase III trial of marimastat did not meet its primary endpoint to show a significantly different OS (p = 0.07), and was complicated by poor tolerability with musculoskeletal inflammation and pain [Bramhall et al. 2002]. Similarly, other MMP inhibitors such as prinomastat are limited by their cytostatic rather than cytotoxic action, and unfavorable toxicity profiles [Zucker et al. 2000].
Conclusion
In this review, we have presented aberrant molecular pathways and their potential therapeutic targets in gastric cancer according to histological-anatomical classification (Table 2). Several pathways are involved predominantly in different subtypes: HER2-, EGFR- and possibly MET-dependent signaling in proximal nondiffuse cancers, angiogenesis and related pathways in distal nondiffuse cancers, and β catenin, FGFR2, PI3K and HER3 activity in diffuse gastric cancers. The increased recognition that distinct molecular aberrations prevail preferentially in different gastric cancer subtypes may help to explain the disappointing results of many published AGC trials in which study populations were heterogeneous regardless of clinicopathological characteristics of the primary tumor. Despite the associations, the occurrence of the pathways in the histological-anatomical subtypes described is not exclusive; in particular, both proximal and distal nondiffuse disease belong to the intestinal histology, thus evidence may overlap. Some may also occur as resistance mechanisms in other subtypes.
Table 2.
Targeted agents reaching clinical development, summarized according to the histological subtype in which the molecular target is most frequently present.
| Molecular target | Histology in which molecular target is more prevalent | Mechanism of action | Targeted agent | Phase of development |
|---|---|---|---|---|
| HER2 | Proximal nondiffuse | HER2 monoclonal antibody | Trastuzumab | III |
| HER2 dimerization inhibitor | Pertuzumab | II | ||
| HER2, EGFR TKI | Lapatinib | III | ||
| Pan-HER TKI | PF 00299804 | II | ||
| EGFR | Proximal nondiffuse | EGFR monoclonal antibody | Cetuximab, Panitumumab | III |
| EGFR TKI | Erlotinib, gefitinib | II | ||
| MET | Proximal nondiffuse | MET TKI | Foretinib, crizotinib | I–II |
| VEGF | Distal nondiffuse | VEGF monoclonal antibody | Bevacizumab | III |
| VEGFR | Distal nondiffuse | VEGFR2 monoclonal antibody | Ramucirumab | III |
| MTI | Sunitinib, sorafenib | II | ||
| FGFR | Diffuse | MTI | AZD2171, dovitinib | II |
| mTOR | Diffuse | mTOR inhibitor | Everolimus | III |
| MMP | Diffuse | MMP inhibitor | Marimastat, prinostat | III |
EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; MMP, matrix metalloproteinase; MTI, multitargeted tyrosine kinase inhibitor; mTOR, mammalian target of rapamycin; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
To date, knowledge on the prevalence, coexistence and carcinogenic role of molecular aberrancies with respect to gastric cancer subtypes is still preliminary. Data are mostly derived from preclinical studies or small patient series, rather than dedicated investigations. Other molecular pathways, such as Wnt, ubiquitin proteosome, and hedgehog pathways, are implicated in gastric cancer but have not been studied in correlation with histological or anatomical subtypes; these are out of the scope of this review.
In conclusion, while some molecular pathways are predominantly associated with particular histological and anatomical subtypes based on available evidence, further studies are necessary to confirm the suggestion that such classification may predict tumor biology. Currently, in clinical trial design and practical treatment decision making in AGC, the molecular aberrations to target are unclear in most cases. Although molecular classification is emerging, until more biomarkers are validated and molecular tools readily available, histological-anatomical classification may provide insights into tumor biology and thus facilitate selection of an enriched patient population for targeted agents in future studies and in the clinic.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Hilda Wong, Division of Hematology and Medical Oncology, Department of Medicine, Queen Mary Hospital, Hong Kong.
Thomas Yau, Division of Hematology and Medical Oncology, Department of Medicine, Room 405, Professorial Block, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong.
References
- Al-Kasspooles M., Moore J., Orringer M., Beer D. (1993) Amplification and over-expression of the EGFR and erbB-2 genes in human esophageal adenocarcinomas. Int J Cancer 54: 213–219 [DOI] [PubMed] [Google Scholar]
- Alsina M., Ko A., Garcia De Paredes M., Rivera F., Schwartzberg L., Fattaey A., et al. (2011) Clinical and pharmacodynamic (PD) results of TEL0805 trial: a phase II study of telatinib (TEL) in combination with capecitabine (X) and cisplatin (P) as first-line treatment in patients (pts) with advanced gastric or gastroesophageal junction (GEJ) cancer. J Clin Oncol 29(Suppl.): abstract 4122 [Google Scholar]
- An J., Kim K., Choi M., Noh J., Sohn T., Bae J., et al. (2010) Prognostic role of p-mTOR expression in cancer tissues and metastatic lymph nodes in pT2b gastric cancer. Int J Cancer 126: 2904–2913 [DOI] [PubMed] [Google Scholar]
- Asaka M., Kimura T., Kato M., Kudo M., Miki K., Ogoshi K., et al. (1994) Possible role of Helicobacter pylori infection in early gastric cancer development. Cancer 73: 2691–2694 [DOI] [PubMed] [Google Scholar]
- Bang Y., Chung H., Xu J., Lordick F., Sawaki A., Lipatov O., et al. (2009) Pathological features of advanced gastric cancer (GC): relationship to human epidermal growth factor receptor 2 (HER2) positivity in the global screening programme of the ToGA trial. J Clin Oncol 27(15 Suppl.): abstract 4556 [Google Scholar]
- Bang Y., Kang Y., Kang W., Boku N., Chung H., Chen J., et al. (2011) Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs 29: 1449–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang Y., Van Cutsem E., Feyereislova A., Chung H., Shen L., Sawaki A., et al. (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376: 687–697 (Epub 19 August 2010) [DOI] [PubMed] [Google Scholar]
- Bardelli A., Siena S. (2010) Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol 28: 1254–1261 [DOI] [PubMed] [Google Scholar]
- Becker K., Atkinson M., Reich U., Becker I., Nekarda H., Siewert J., et al. (1994) E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 54: 3845–3852 [PubMed] [Google Scholar]
- Bellacosa A., Kumar C., Di Cristofano A., Testa J. (2005) Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res 94, 29–86 [DOI] [PubMed] [Google Scholar]
- Blot W., Devesa S., Kneller R., Fraumeni J., Jr. (1991) Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 265: 1287–1289 [PubMed] [Google Scholar]
- Bramhall S., Hallissey M., Whiting J., Scholefield J., Tierney G., Stuart R., et al. (2002) Marimastat as maintenance therapy for patients with advanced gastric cancer: a randomised trial. Br J Cancer 86: 1864–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun D., Cho K., Ryu B., Lee M., Park J., Chae K., et al. (2003) Frequent monoallelic deletion of PTEN and its reciprocal association with PIK3CA amplification in gastric carcinoma. Int J Cancer 104: 318–327 [DOI] [PubMed] [Google Scholar]
- Carneiro F., Huntsman D., Smyrk T., Owen D., Seruca R., Pharoah P., et al. (2004) Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J Pathol 203: 681–687 [DOI] [PubMed] [Google Scholar]
- Cavallaro U., Christofori G. (2004) Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 4: 118–132 [DOI] [PubMed] [Google Scholar]
- Cejka D., Preusser M., Fuereder T., Sieghart W., Werzowa J., Strommer S., et al. (2008) mTOR inhibition sensitizes gastric cancer to alkylating chemotherapy in vivo. Anticancer Res 28: 3801–3808 [PubMed] [Google Scholar]
- Chau I., Okines A., Gonzalez de Castro D., Saffery C., Barbachano Y., Wotherspoon A., et al. (2011) REAL3: a multicenter randomized phase II/III trial of epirubicin, oxaliplatin, and capecitabine (EOC) versus modified (m) EOC plus panitumumab (P) in advanced oesophagogastric (OG) cancer – response rate (RR), toxicity, and molecular analysis from phase II. J Clin Oncol 29(Suppl.): abstract 4131 [Google Scholar]
- Crabtree J., Wyatt J., Trejdosiewicz L., Peichl P., Nichols P., Ramsay N., et al. (1994) Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol 47: 61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew K., Neugut A. (2006) Epidemiology of gastric cancer. World J Gastroenterol 12: 354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng N., Goh L., Wang H., Das K., Tao J., Tan I., et al. (2012) A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 61: 673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran D., Johnson G. (2007) MAPKs: function, regulation, role in cancer and therapeutic targeting. Oncogene 26: 3097–3099 [DOI] [PubMed] [Google Scholar]
- Doi T., Koizumi W., Siena S., Cascinu S., Ohtsu A., Michael M., et al. (2003) Efficacy, tolerability and pharmacokinetics of gefitinib (ZD1839) in pretreated patients with metastatic gastric cancer. Proc Am Soc Clin Oncol 22: abstract 1036 [Google Scholar]
- Doi T., Muro K., Boku N., Yamada Y., Nishina T., Takiuchi H., et al. (2010) Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol 28: 1904–1910 [DOI] [PubMed] [Google Scholar]
- Dragovich T., McCoy S., Fenoglio-Preiser C., Wang J., Benedetti J., Baker A., et al. (2006) Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol 24: 4922–4927 [DOI] [PubMed] [Google Scholar]
- Dvory-Sobol H., Sagiv E., Liberman E., Kazanov D., Arber N. (2007) Suppression of gastric cancer cell growth by targeting the beta-catenin/T-cell factor pathway. Cancer 109: 188–197 [DOI] [PubMed] [Google Scholar]
- Feng W., Brown R., Trung C., Li W., Wang L., Khoury T., et al. (2008) Morphoproteomic profile of mTOR, Ras/Raf kinase/ERK, and NF-kappaB pathways in human gastric adenocarcinoma. Ann Clin Lab Sci 38: 195–209 [PubMed] [Google Scholar]
- Fuereder T., Wanek T., Pflegerl P., Jaeger-Lansky A., Hoeflmayer D., Strommer S., et al. (2011) Gastric cancer growth control by BEZ235 in vivo does not correlate with PI3K/mTOR target inhibition but with [18F]FLT uptake. Clin Cancer Res 17: 5322–5332 [DOI] [PubMed] [Google Scholar]
- Gamboa-Dominguez A., Dominguez-Fonseca C., Quintanilla-Martinez L., Reyes-Gutierrez E., Green D., Angeles-Angeles A., et al. (2004) Epidermal growth factor receptor expression correlates with poor survival in gastric adenocarcinoma from Mexican patients: a multivariate analysis using a standardized immunohistochemical detection system. Mod Pathol 17: 579–587 [DOI] [PubMed] [Google Scholar]
- Gerstein E., Sini L., Ryabov A., Dvorova E., Yurchenko A., Stilidi I., et al. (2009) Comparative enzyme immunoassay of matrix metalloproteinases-2, -7, -9 and their tissue inhibitor-2 in tumors and plasma of patients with gastric cancer. Bull Exp Biol Med 148: 899–902 [DOI] [PubMed] [Google Scholar]
- Gravalos C., Márquez A., García-Carbonero R., Rivera F., Colomer R., Sastre J., et al. (2007) Correlation between Her2/neu overexpression/amplification and clinicopathological parameters in advanced gastric cancer patients: a prospective study 2007. Presented at: Gastrointestinal Cancers Symposium 130: abstract 89. Available at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=45&abstractID=10315 (accessed 18 June 2012).
- Guilford P., Hopkins J., Harraway J., McLeod M., McLeod N., Harawira P., et al. (1998) E-cadherin germline mutations in familial gastric cancer. Nature 392: 402–405 [DOI] [PubMed] [Google Scholar]
- Guo T., Fan L., Ng W., Zhu Y., Ho M., Wan W., et al. (2012) Multidimensional identification of tissue biomarkers of gastric cancer. J Proteome Res 7 May (epub ahead of print). doi: 10.1021/pr300212g [DOI] [PubMed] [Google Scholar]
- Han S., Oh D., Im S., Park S., Lee K., Song H., et al. (2009) Phase II study and biomarker analysis of cetuximab combined with modified FOLFOX6 in advanced gastric cancer. Br J Cancer 100: 298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y., Itoh H., Uchino S., Hosokawa K., Ochiai A., Ino Y., et al. (1996) Immunohistochemical detection of K-sam protein in stomach cancer. Clin Cancer Res 2: 1373–1381 [PubMed] [Google Scholar]
- Hattori Y., Odagiri H., Nakatani H., Miyagawa K., Naito K., Sakamoto H., et al. (1990) K-sam, an amplified gene in stomach cancer, is a member of the heparin-binding growth factor receptor genes. Proc Natl Acad Sci U S A 87: 5983–5987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht J., Urba S., Koehler M., Ellis C., Gagnon R., Kemner A., et al. (2008) Lapatinib monotherapy in recurrent upper gastrointestinal malignancy: phase II efficacy and biomarker analyses. Presented at: 2008 Gastrointestinal Cancer Symposium: abstract 43. Available at: http://www.asco.org/ASCOv2/Meetings/Abstracts?vmview=abst_detail_view&confID=53&abstractID=10675 (accessed 18 June 2012).
- Hiyama T., Haruma K., Kitadai Y., Masuda H., Miyamoto M., Tanaka S., et al. (2002) K-ras mutation in helicobacter pylori-associated chronic gastritis in patients with and without gastric cancer. Int J Cancer 97: 562–566 [DOI] [PubMed] [Google Scholar]
- Hongyo T., Buzard G., Palli D., Weghorst C., Amorosi A., Galli M., et al. (1995) Mutations of the K-ras and p53 genes in gastric adenocarcinomas from a high-incidence region around Florence, Italy. Cancer Res 55: 2665–2672 [PubMed] [Google Scholar]
- Humar B., Guilford P. (2009) Hereditary diffuse gastric cancer: a manifestation of lost cell polarity. Cancer Sci 100: 1151–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilson D., Janjigian Y., Shah M., Kelsen D., Tang L., Campbell J., et al. (2011) Phase II trial of sorafenib in esophageal (E) and gastroesophageal junction (GEJ) cancer: response and protracted stable disease observed in adenocarcinoma. J Clin Oncol 29(Suppl.): abstract 4100 [Google Scholar]
- Iqbal S., Goldman H., Lenz H., Fenoglio-Preiser M., Blanke C. (2007) S0413: a phase II SWOG study of GW572016 (lapatinib) as first line therapy in patients with advanced or metastatic gastric cancer. J Clin Oncol 25(18 Suppl.): 4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isinger-Ekstrand A., Johansson J., Ohlsson M., Francis P., Staaf J., Jonsson M., et al. (2010) Genetic profiles of gastroesophageal cancer: combined analysis using expression array and tiling array – comparative genomic hybridization. Cancer Genet Cytogenet 200: 120–126 [DOI] [PubMed] [Google Scholar]
- Jaehne J., Urmacher C., Thaler H., Friedlander-Klar H., Cordon-Cardo C., Meyer H. (1992) Expression of Her2/neu oncogene product p185 in correlation to clinicopathological and prognostic factors of gastric carcinoma. J Cancer Res Clin Oncol 118: 474–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjigian Y., Tang L., Coit D., Kelsen D., Francone T., Weiser M., et al. (2011) MET expression and amplification in patients with localized gastric cancer. Cancer Epidemiol Biomarkers Prev 20: 1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhawer M., Kindler H., Wainberg Z., Ford J., Kunz P., Tang L., et al. (2009) Assessment of two dosing schedules of GSK1363089 (GSK089), a dual MET/VEGFR2 inhibitor, in metastatic gastric cancer (GC): interim results of a multicenter phase II study. J Clin Oncol 27(15 Suppl.): abstract 4502 [Google Scholar]
- Kang Y., Lee H., Kim W. (2002) Promoter methylation and silencing of PTEN in gastric carcinoma. Lab Invest 82: 285–291 [DOI] [PubMed] [Google Scholar]
- Kang Y., Ohtsu A., Van Cutsem E., Rha S., Sawaki A., Park S., et al. (2010) AVAGAST: a randomized, double-blind, placebo-controlled, phase III study of first-line capecitabine and cisplatin plus bevacizumab or placebo in patients with advanced gastric cancer (AGC). J Clin Oncol 28(7 Suppl.): abstract LBA4007 [DOI] [PubMed] [Google Scholar]
- Kanzler S., Trarbach T., Seufferlein T., Kubicka S., Lordick F., Geissler M., et al. German Arbeitsgemeinschaft Internistische O (2009) Cetuximab with irinotecan/folinic acid/5-FU as first-line treatment in advanced gastric cancer: a nonrandomized multicenter AIO phase II study. J Clin Oncol 27(15 Suppl.): abstract 4534 [Google Scholar]
- Kataoka Y., Mukohara T., Tomioka H., Funakoshi Y., Kiyota N., Fujiwara Y., et al. (2011) Foretinib (GSK1363089), a multi-kinase inhibitor of MET and VEGFRs, inhibits growth of gastric cancer cell lines by blocking inter-receptor tyrosine kinase networks. Invest New Drugs 8 June (epub ahead of print). doi: 10.1007/s10637-011-9699-0 [DOI] [PubMed] [Google Scholar]
- Kemik O., Kemik A., Sumer A., Dulger A., Adas M., Begenik H., et al. (2011) Levels of matrix metalloproteinase-1 and tissue inhibitors of metalloproteinase-1 in gastric cancer. World J Gastroenterol 17: 2109–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Lee J., Ryu M., Chang H., Kim T., Lim H., et al. (2009) A prospective phase II study of cetuximab in combination with XELOX (capecitabine and oxaliplatin) in patients with metastatic and/or recurrent advanced gastric cancer. Invest New Drugs 29: 366–373 [DOI] [PubMed] [Google Scholar]
- Kim I., Park J., Kang H., Shin Y., Park H., Park H., et al. (2003) Mutational analysis of BRAF and K-ras in gastric cancers: absence of BRAF mutations in gastric cancers. Hum Genet 114: 118–120 [DOI] [PubMed] [Google Scholar]
- Kim M., Lee H., Lee H., Jeon Y., Yang H., Kim W. (2008) EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology 52: 738–746 [DOI] [PubMed] [Google Scholar]
- Kim Y., Sasaki Y., Lee K., Rha S., Park S., Boku N., et al. (2011) Randomized phase II study of nimotuzumab, an anti-EGFR antibody, plus irinotecan in patients with 5-fluorouracil-based regimen-refractory advanced or recurrent gastric cancer in Korea and Japan: preliminary results. J Clin Oncol 29(Suppl. 4): abstract 87 [Google Scholar]
- Kitadai Y., Sasaki A., Ito M., Tanaka S., Oue N., Yasui W., et al. (2003) Helicobacter pylori infection influences expression of genes related to angiogenesis and invasion in human gastric carcinoma cells. Biochem Biophys Res Commun 311: 809–814 [DOI] [PubMed] [Google Scholar]
- Kitoh T., Yanai H., Saitoh Y., Nakamura Y., Matsubara Y., Kitoh H., et al. (2004) Increased expression of matrix metalloproteinase-7 in invasive early gastric cancer. J Gastroenterol 39: 434–440 [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Iwamatsu A., Shinohara-Kanda A., Ihara S., Fukui Y. (2003) Activation of ErbB3-PI3-kinase pathway is correlated with malignant phenotypes of adenocarcinomas. Oncogene 22: 1294–1301 [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Nagata S., Iwasaki T., Yanagihara K., Saitoh I., Karouji Y., et al. (1999) Dedifferentiation of adenocarcinomas by activation of phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A 96: 4874–4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen H., Wright B., Burt A., Quirke P., McNicol A., Dybdal N., et al. (2001) Overexpression of HER2/neu in solid tumours: an immunohistochemical survey. Histopathology 38: 96–104 [DOI] [PubMed] [Google Scholar]
- Kunii K., Davis L., Gorenstein J., Hatch H., Yashiro M., Di Bacco A., et al. (2008) FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res 68: 2340–2348 [DOI] [PubMed] [Google Scholar]
- Lang S., Gaumann A., Koehl G., Seidel U., Bataille F., Klein D., et al. (2007) Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int J Cancer 120: 1803–1810 [DOI] [PubMed] [Google Scholar]
- Lauren P. (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64: 31–49 [DOI] [PubMed] [Google Scholar]
- Lee J., Han S., Cho H., Jennings B., Gerrard B., Dean M., et al. (2000) A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene 19: 4947–4953 [DOI] [PubMed] [Google Scholar]
- Lee J., Seo J., Jun H., Ki C., Park S., Park Y., et al. (2011) Impact of MET amplification on gastric cancer: possible roles as a novel prognostic marker and a potential therapeutic target. Oncol Rep 25: 1517–1524 [DOI] [PubMed] [Google Scholar]
- Lee K., Hur H., Im S., Lee J., Kim H., Yoon Y., et al. (2010) RAD001 shows activity against gastric cancer cells and overcomes 5-FU resistance by downregulating thymidylate synthase. Cancer Lett 299: 22–28 [DOI] [PubMed] [Google Scholar]
- Lee K., Lee J., Suh C., Kim S., Kim S., Lee J., et al. (1995) Clinicopathologic significance of the K-ras gene codon 12 point mutation in stomach cancer. An analysis of 140 cases. Cancer 75: 2794–2801 [DOI] [PubMed] [Google Scholar]
- Lee S., Lee J., Soung Y., Kim H., Park W., Kim S., et al. (2003) BRAF and KRAS mutations in stomach cancer. Oncogene 22: 6942–6945 [DOI] [PubMed] [Google Scholar]
- Lennerz J., Kwak E., Ackerman A., Michael M., Fox S., Bergethon K., et al. (2011) MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol 29: 4803–4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Chong J, Lordick F, Kang YK, Park SR, Bang YJ, Sawaki A, et al. (2007) HER2 positivity in advanced gastric cancer is comparable to breast cancer. J Clin Oncol - ASCO Annual Meeting Proceedings; Part I, 25(18S): abstract 15057. Available at: http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&confID=47&abstractID=35399 [Google Scholar]
- Li J., Liu X., Wang B., Guo W., Yin J., Zhu S., et al. (2010) Phase II study of cetuximab in combination with modified FOLFIRI in patients with advanced gastric cancer who failed first-line chemotherapy (EFFI study). J Clin Oncol 28(7 Suppl.): abstract 4107 [Google Scholar]
- Li J., Qin S., Xu J., Guo W., Xiong J., Bai Y., et al. (2011) A randomized, double-blind, multicenter, phase II, three-arm, placebo-control study of apatinib as third-line treatment in patients with metastatic gastric carcinoma. J Clin Oncol 29(Suppl.): abstract 4019 [Google Scholar]
- Li V., Wong C., Chan T., Chan A., Zhao W., Chu K., et al. (2005) Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer 5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieto E., Ferraraccio F., Orditura M., Castellano P., Mura A., Pinto M., et al. (2008) Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol 15: 69–79 [DOI] [PubMed] [Google Scholar]
- Liu Y., Shen C., Wu H., Hsieh T., Chan D., Chen C., et al. (2006) Mechanisms inactivating the gene for E-cadherin in sporadic gastric carcinomas. World J Gastroenterol 12: 2168–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordick F., Luber B., Lorenzen S., Hegewisch-Becker S., Folprecht G., Woll E., et al. (2010) Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer 102: 500–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann B., Gelos M., Siedow A., Hanski M., Gratchev A., Ilyas M., et al. (1999) Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A 96: 1603–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareel M., Boterberg T., Noe V., Van Hoorde L., Vermeulen S., Bruyneel E., et al. (1997) E-cadherin/catenin/cytoskeleton complex: a regulator of cancer invasion. J Cell Physiol 173: 271–274 [DOI] [PubMed] [Google Scholar]
- Markman B., Atzori F., Perez-Garcia J., Tabernero J., Baselga J. (2010) Status of PI3K inhibition and biomarker development in cancer therapeutics. Ann Oncol 21: 683–691 [DOI] [PubMed] [Google Scholar]
- Matsubara J., Nishina T., Yamada Y., Moriwaki T., Shimoda T., Kajiwara T., et al. (2008) Impacts of excision repair cross-complementing gene 1 (ERCC1), dihydropyrimidine dehydrogenase, and epidermal growth factor receptor on the outcomes of patients with advanced gastric cancer. Br J Cancer 98: 832–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki T., Yashiro M., Kaizaki R., Yasuda K., Doi Y., Sawada T., et al. (2009) Synergistic antiproliferative effect of mTOR inhibitors in combination with 5-fluorouracil in scirrhous gastric cancer. Cancer Sci 100: 2402–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore C., Giordano S. (2008) Molecular cancer therapy: can our expectation be MET? Eur J Cancer 44: 641–651 [DOI] [PubMed] [Google Scholar]
- Moehler M., Hartmann J., Lordick F., Al-Batran S., Reimer T., Trarbach T., et al. (2009) Sunitinib in patients with chemo-refractory metastatic gastric cancer: preliminary results of an open-label, prospective nonrandomized multicenter AIO phase II trial. Presented at: 2009 Gastrointestinal Cancer Symposium: abstract 61. Available at: http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&confID=63&abstractID=10434 (accessed 18 June 2012).
- Moehler M., Mueller A., Bachmann E., Schimanski C., Galle P. (2012) Selective PI3K inhibition by BKM120 and BEZ235 alone or in combination with chemotherapy in wild-type and mutated human gastrointestinal cancer cell lines. J Clin Oncol 30(Suppl. 4): abstract 522 [DOI] [PubMed] [Google Scholar]
- Moehler M., Mueller A., Trarbach T., Lordick F., Seufferlein T., Kubicka S., et al. (2010) Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol 22: 1358–1366 [DOI] [PubMed] [Google Scholar]
- Murayama T., Inokuchi M., Takagi Y., Yamada H., Kojima K., Kumagai J., et al. (2009) Relation between outcomes and localisation of p-mTOR expression in gastric cancer. Br J Cancer 100: 782–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Yashiro M., Matsuoka T., Tendo M., Shimizu T., Miwa A., et al. (2006) A novel molecular targeting compound as K-samII/FGF-R2 phosphorylation inhibitor, Ki23057, for Scirrhous gastric cancer. Gastroenterology 131: 1530–1541 [DOI] [PubMed] [Google Scholar]
- Nakatani H., Sakamoto H., Yoshida T., Yokota J., Tahara E., Sugimura T., et al. (1990) Isolation of an amplified DNA sequence in stomach cancer. Jpn J Cancer Res 81: 707–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam H., Ching K., Kan J., Kim H., Han S., Im S., et al. (2012) Evaluation of the antitumor effects and mechanisms of PF00299804, a pan-HER inhibitor, alone or in combination with chemotherapy or targeted agents in gastric cancer. Mol Cancer Ther 11: 439–451 [DOI] [PubMed] [Google Scholar]
- Nam H., Kim H., Yoon Y., Hur H., Song S., Kim M., et al. (2011) Antitumor activity of HM781-36B, an irreversible Pan-HER inhibitor, alone or in combination with cytotoxic chemotherapeutic agents in gastric cancer. Cancer Lett 302: 155–165 [DOI] [PubMed] [Google Scholar]
- Nanus D., Kelsen D., Mentle I., Altorki N., Albino A. (1990) Infrequent point mutations of ras oncogenes in gastric cancers. Gastroenterology 98: 955–960 [DOI] [PubMed] [Google Scholar]
- Normanno N., Tejpar S., Morgillo F., De Luca A., Van Cutsem E., Ciardiello F. (2009) Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol 6: 519–527 [DOI] [PubMed] [Google Scholar]
- Ohtsu A., Shah M., Van Cutsem E., Rha S., Sawaki A., Park S., et al. (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 29: 3968–3976 [DOI] [PubMed] [Google Scholar]
- Ooi C., Ivanova T., Wu J., Lee M., Tan I., Tao J., et al. (2009) Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet 5: e1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Kook M., Choi I., Kim C., Lee J., Cho S., et al. (2010) Predictive factors for the efficacy of cetuximab plus chemotherapy as salvage therapy in metastatic gastric cancer patients. Cancer Chemother Pharmacol 65: 579–587 [DOI] [PubMed] [Google Scholar]
- Peek R., Jr, Blaser M. (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2: 28–37 [DOI] [PubMed] [Google Scholar]
- Perl A., Wilgenbus P., Dahl U., Semb H., Christofori G. (1998) A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392: 190–193 [DOI] [PubMed] [Google Scholar]
- Pinto C., Di Fabio F., Barone C., Siena S., Falcone A., Cascinu S., et al. (2009) Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study). Br J Cancer 101: 1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto C., Di Fabio F., Siena S., Cascinu S., Rojas Llimpe F., Ceccarelli C., et al. (2007) Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study). Ann Oncol 18: 510–517 [DOI] [PubMed] [Google Scholar]
- Polkowski W., van Sandick J., Offerhaus G., ten Kate F., Mulder J., Obertop H., et al. (1999) Prognostic value of Lauren classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol 6: 290–297 [DOI] [PubMed] [Google Scholar]
- Rao S., Starling N., Cunningham D., Sumpter K., Gilligan D., Ruhstaller T., et al. (2010) Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: a randomised, multicentre open-label phase II study. Ann Oncol 21: 2213–2219 [DOI] [PubMed] [Google Scholar]
- Richards D., Kocs D., Spira A., McCollum A., Boehm K., Zhan F., et al. (2011) Results of docetaxel plus oxaliplatin (DOCOX) with or without cetuximab in patients with metastatic gastric and/or gastroesophageal junction adenocarcinoma: results of a randomized phase II study. J Clin Oncol 29(Suppl.): abstract 4015 [DOI] [PubMed] [Google Scholar]
- Rojo F., Tabernero J., Albanell J., Van Cutsem E., Ohtsu A., Doi T., et al. (2006) Pharmacodynamic studies of gefitinib in tumor biopsy specimens from patients with advanced gastric carcinoma. J Clin Oncol 24: 4309–4316 [DOI] [PubMed] [Google Scholar]
- Ross J., McKenna B. (2001) The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest 19: 554–568 [DOI] [PubMed] [Google Scholar]
- Sasaki A., Kitadai Y., Ito M., Sumii M., Tanaka S., Yoshihara M., et al. (2003) Helicobacter pylori infection influences tumor growth of human gastric carcinomas. Scand J Gastroenterol 38: 153–158 [DOI] [PubMed] [Google Scholar]
- Satoh T., Bang Y., Wang J., Xu J., Chung H., Yeh K., et al. (2010) Interim safety analysis from TYTAN: a phase III Asian study of lapatinib in combination with paclitaxel as second-line therapy in gastric cancer. J Clin Oncol 28(7 Suppl.): abstract 4057 [Google Scholar]
- Schlessinger J. (2004) Common and distinct elements in cellular signaling via EGF and FGF receptors. Science 306: 1506–1507 [DOI] [PubMed] [Google Scholar]
- Shah M., Khanin R., Tang L., Janjigian Y., Klimstra D., Gerdes H., et al. (2011) Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res 17: 2693–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M., Van Cutsem E., Kang Y., Dakhil S., Satoh T., Chin K., et al. (2012) Survival analysis according to disease subtype in AVAGAST: first-line capecitabine and cisplatin plus bevacizumab (bev) or placebo in patients (pts) with advanced gastric cancer. J Clin Oncol 30(Suppl. 4): abstract 5 [Google Scholar]
- Sharma S., Tummuru M., Blaser M., Kerr L. (1998) Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol 160: 2401–2407 [PubMed] [Google Scholar]
- Smolen G., Sordella R., Muir B., Mohapatra G., Barmettler A., Archibald H., et al. (2006) Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A 103: 2316–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratlin J., Cohen R., Eadens M., Gore L., Camidge D., Diab S., et al. (2010) Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 28: 780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Al-Batran S., Arnold D., Peinert S., Siewczynski R., Schmoll H. (2007) Cetuximab with irinotecan as salvage therapy in heavily pretreated patients with metastatic gastric cancer. Presented at: 2007 Gastrointestinal Cancers Symposium: abstract 47. Available at: http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&confID=45&abstractID=10567 (accessed 18 June 2012).
- Sun W., Powell M., O’Dwyer P., Catalano P., Ansari R., Benson A. (2010) Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol 28: 2947–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Kodera Y., Katanasaka Y., Yanagihara K., Tamura T., Koizumi F. (2011) Efficacy of RAD001 (everolimus) against advanced gastric cancer with peritoneal dissemination. Invest New Drugs 29: 1198–1205 [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Cleary K., Mai M., Kitadai Y., Bucana C., Ellis L. (1996) Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin Cancer Res 2: 1679–1684 [PubMed] [Google Scholar]
- Takeda M., Arao T., Yokote H., Komatsu T., Yanagihara K., Sasaki H., et al. (2007) AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin Cancer Res 13: 3051–3057 [DOI] [PubMed] [Google Scholar]
- Tamura G., Sakata K., Nishizuka S., Maesawa C., Suzuki Y., Iwaya T., et al. (1996) Inactivation of the E-cadherin gene in primary gastric carcinomas and gastric carcinoma cell lines Jpn J Cancer Res 87: 1153–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura G., Yin J., Wang S., Fleisher A., Zou T., Abraham J., et al. (2000) E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst 92: 569–573 [DOI] [PubMed] [Google Scholar]
- Tanner M., Hollmen M., Junttila T., Kapanen A., Tommola S., Soini Y., et al. (2005) Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 16: 273–278 [DOI] [PubMed] [Google Scholar]
- Tay S., Leong S., Yu K., Aggarwal A., Tan S., Lee C., et al. (2003) A combined comparative genomic hybridization and expression microarray analysis of gastric cancer reveals novel molecular subtypes. Cancer Res 63: 3309–3316 [PubMed] [Google Scholar]
- Tebbutt N., Sourjina T., Strickland A., Van Hazel G., Pavlakis N., Ganju V., et al. (2008) ATTAX2: docetaxel plus cetuximab as second-line treatment for docetaxel refractory oesophago-gastric cancer – final results of a multicentre phase II trial by the AGITG. J Clin Oncol 26(659 Suppl.): abstract 15554 [Google Scholar]
- Tetsu O., McCormick F. (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426 [DOI] [PubMed] [Google Scholar]
- Tierney G., Griffin N., Stuart R., Kasem H., Lynch K., Lury J. T., et al. (1999) A pilot study of the safety and effects of the matrix metalloproteinase inhibitor marimastat in gastric cancer. Eur J Cancer 35: 563–568 [DOI] [PubMed] [Google Scholar]
- Wada N., Otani Y., Kubota T., Kimata M., Minagawa A., Yoshimizu N., et al. (2003) Reduced angiogenesis in peritoneal dissemination of gastric cancer through gelatinase inhibition. Clin Exp Metastasis 20: 431–435 [DOI] [PubMed] [Google Scholar]
- Waddell T., Chau I., Barbachano Y., de Castro D., Wotherspoon A., Saffery C., et al. (2012) A randomized multicenter trial of epirubicin, oxaliplatin, and capecitabine (EOC) plus panitumumab in advanced esophagogastric cancer (REAL3). J Clin Oncol 30(Suppl.): LBA4000 [Google Scholar]
- Wainberg Z., Anghel A., Desai A., Ayala R., Luo T., Safran B., et al. (2010) Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res 16: 1509–1519 [DOI] [PubMed] [Google Scholar]
- Wilkinson N., Black J., Roukhadze E., Driscoll D., Smiley S., Hoshi H., et al. (2004) Epidermal growth factor receptor expression correlates with histologic grade in resected esophageal adenocarcinoma. J Gastrointest Surg 8: 448–453 [DOI] [PubMed] [Google Scholar]
- Woell E., Greil R., Eisterer W., Fridrik M., Grunberger B., Zabernigg A., et al. (2008) Oxaliplatin, irinotecan, and cetuximab in advanced gastric cancer. First efficacy results of a multicenter phase II trial (AGMT Gastric-2). J Clin Oncol 26(662 Suppl.): abstract 15587 [Google Scholar]
- Wong A., Gumbiner B. (2003) Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol 161: 1191–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H., Yau T. (2012) Targeted therapy in the management of advanced gastric cancer: are we making progress in the era of personalized medicine? Oncologist 17: 346–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T., Lee F., Yu C., Luo F., Oppenheimer S., Zhang H., et al. (2006) Preclinical antitumor activity of BMS-599626, a pan-HER kinase inhibitor that inhibits HER1/HER2 homodimer and heterodimer signaling. Clin Cancer Res 12: 6186–6193 [DOI] [PubMed] [Google Scholar]
- Woolley P., Reitz J., Dharbhamulla A. (2004) Expression of matrix metalloproteinases-2, -3, and -9, cathepsin D and urokinase plasminogen activator in normal tissue, stroma and tumor tissue of proximal and distal stomach. Presented at: 2004 American Society of Clinical Oncology – Gastrointestinal Cancers Symposium: abstract 47 Available at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=27&abstractID=510, January, San Francisco, CA, USA [Google Scholar]
- Wu C., Wang C., Tseng C., Chen H., Wu M., Lin J., et al. (2005) Helicobacter pylori promote gastric cancer cells invasion through a NF-kappaB and COX-2-mediated pathway. World J Gastroenterol 11: 3197–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Nie Y., Guo C., Chen Y., Ding J., Fan D. (2009) Molecular basis of therapeutic approaches to gastric cancer. J Gastroenterol Hepatol 24: 37–41 [DOI] [PubMed] [Google Scholar]
- Xing C., Zhu B., Fan X., Liu H., Hou X., Zhao K., et al. (2009) Effects of LY294002 on the invasiveness of human gastric cancer in vivo in nude mice. World J Gastroenterol 15: 5044–5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Geng Q., Tian Y., Cai M., Fang X., Zhan Y., et al. (2010) Activated mammalian target of rapamycin is a potential therapeutic target in gastric cancer. BMC Cancer 10: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub L., Goldman H., Odze R. (1997) Transforming growth factor-alpha, epidermal growth factor receptor, and MiB-1 expression in Barrett’s-associated neoplasia: correlation with prognosis. Mod Pathol 10: 105–112 [PubMed] [Google Scholar]
- Yamashita-Kashima Y., Iijima S., Yorozu K., Furugaki K., Kurasawa M., Ohta M., et al. (2011) Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res 17: 5060–5070 [DOI] [PubMed] [Google Scholar]
- Yano T., Doi T., Ohtsu A., Boku N., Hashizume K., Nakanishi M., et al. (2006) Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep 15: 65–71 [PubMed] [Google Scholar]
- Yashiro M., Shinto O., Nakamura K., Tendo M., Matsuoka T., Matsuzaki T., et al. (2010) Synergistic antitumor effects of FGFR2 inhibitor with 5-fluorouracil on scirrhous gastric carcinoma. Int J Cancer 126: 1004–1016 [DOI] [PubMed] [Google Scholar]
- Yin M., Hu Z., Tan D., Ajani J., Wei Q. (2009) Molecular epidemiology of genetic susceptibility to gastric cancer: focus on single nucleotide polymorphisms in gastric carcinogenesis. Am J Transl Res 1: 44–54 [PMC free article] [PubMed] [Google Scholar]
- Yu G., Wang J., Chen Y., Wang X., Pan J., Li G., et al. (2009) Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of Chinese patients with gastric cancer. Clin Cancer Res 15: 1821–1829 [DOI] [PubMed] [Google Scholar]
- Zhang M., Zhu G., Gao H., Zhao S., Xue Y. (2011) Expression of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in gastric adenocarcinoma. J Surg Oncol 103: 243–247 [DOI] [PubMed] [Google Scholar]
- Zhang X., Xu J., Shen L., Wang J., Liang J., Xu N., et al. (2009) A phase II study of cetuximab with cisplatin and capecitabine as first-line treatment in advanced gastric cancer. Presented at: 2009 Gastrointestinal Cancer Symposium: abstract LBA39. Available at: http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&confID=63&abstractID=10721 (accessed 18 June 2012).
- Zhang X., Yang Y., Xu D., Qu J., Guo M., Gong Y., et al. (2009) Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J Surg 33: 2112–2118 [DOI] [PubMed] [Google Scholar]
- Zhao W., Wang L., Park H., Chhim S., Tanphanich M., Yashiro M., et al. (2010) Monoclonal antibodies to fibroblast growth factor receptor 2 effectively inhibit growth of gastric tumor xenografts. Clin Cancer Res 16: 5750–5758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Li G., Wu J., Zhang Z., Wu Z., Fan P., et al. (2010) Clinicopathological significance of E-cadherin, VEGF, and MMPs in gastric cancer. Tumour Biol 31: 549–558 [DOI] [PubMed] [Google Scholar]
- Zucker S., Cao J., Chen W. (2000) Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene 19: 6642–6650 [DOI] [PubMed] [Google Scholar]