Abstract
Radiation proctitis is a frequent complication of pelvic radiation for cancer. This condition can present acutely within several weeks of radiation, or chronically many months or years after radiation, leading to rectal bleeding and transfusion-dependent anemia. Various medical and endoscopic therapies have been described to treat this condition; however, some patients fail to respond to the current standard therapies. Here we present a case of refractory radiation proctitis, with suboptimal response to other therapies, treated successfully with a novel method, radiofrequency ablation.
Keywords: Radiation proctitis, radiofrequency ablation, gastrointestinal bleeding, proctopathy
Introduction
Radiation therapy (RT) for malignancies of the abdomen and pelvis can cause acute and chronic intestinal injury. The rectum and distal colon are vulnerable to radiation injury due to their exposure to radiation in patients with genitourinary or gastrointestinal cancers who undergo pelvic radiation. Radiation proctitis can present acutely within 6 weeks of the therapy initiation, with diarrhea, tenesmus, and bleeding, or re-emerge chronically, months or years after therapy, usually with frequent rectal bleeding often causing anemia [Babb, 1996]. Different medical and endoscopic modalities have been used to treat bleeding from chronic radiation proctitis (CRP). Argon plasma coagulation (APC) has emerged as the favorite endoscopic method to control the bleeding from CRP, but some patients do not respond to this therapy or develop complications, including strictures [Villavicencio et al. 2002]. Radiofrequency ablation (RFA) is a newer endoscopic technique that uses a catheter to deliver radiofrequency energy to ablate tissue. While RFA is commonly performed for the ablation of dysplastic Barrett’s esophagus, we present an interesting case of the use of RFA for the treatment of CRP with recurrent bleeding despite APC therapy.
Case report
An 81-year-old man with past history of prostate cancer, treated with RT 15 years ago, was admitted to the hospital with complaints of dizziness and rectal bleeding. He started having episodes of rectal bleeding 3 days prior to admission, 8–10 episodes per day, which he described as ‘fresh blood mixed with stool’, associated with dizziness. His past medical history was also significant for hypertension, coronary artery disease, anemia, hyperlipidemia, osteoporosis, and gastroesophageal reflux disease. He underwent a right hemicolectomy due to bleeding from colonic angioectasias 7 years ago. Following surgery, he did relatively well until 3 years ago when he started having intermittent rectal bleeding with multiple admissions to the hospital for symptomatic anemia. Multiple colonoscopies in the past had revealed changes consistent with radiation proctitis, which were treated with several sessions of APC to control the bleeding. His medications at home included iron and clopidogrel. There was a remote history of smoking, but no history of alcohol or drug abuse. On examination, he was in mild discomfort, with stable vital signs and no orthostatic changes. Physical examination was unremarkable, except for the presence of fresh blood on rectal examination. Initial blood tests revealed a hemoglobin of 5.4 mmol/l with a mean corpuscular volume of 93, white blood count of 6800/mm3, and platelets of 146,000/mm3. Coagulation parameters as well as a comprehensive metabolic panel were normal. Clopidogrel was held on admission. Two units of packed red blood cells (PRBC) were transfused, which brought his hemoglobin to 9.3 mmol/l. A colonoscopy performed the next day revealed a friable and edematous rectal mucosa with multiple angioectasias, consistent with radiation proctitis. APC was applied to the bleeding areas of the mucosa with an energy level of 40 W and gas flow of 1 l/min. The next day, the patient was discharged home with instructions to restart taking clopidogrel after 48 h. He remained asymptomatic for the next 5 months, until he presented to the emergency room with multiple episodes of rectal bleeding. His hemoglobin was 5.3 mmol/l, compared with a baseline of 6.2-6.8 mmol/l. He received two units of PRBC and underwent a flexible sigmoidoscopy, which again showed friable, edematous mucosa with multiple angioectasias, oozing blood (Figure 1). APC was applied to control the bleeding. Bleeding recurred the next day. A repeat sigmoidoscopy revealed scar tissue from previous therapy, with intervening friable, hemorrhagic mucosa and angioectasias. Considering the extent of the disease and multiple episodes of bleeding despite frequent APC therapies, a decision was made to use a different therapeutic modality. We elected to apply RFA, using a HALO90 electrode (BARRX Medical Inc., Sunnyvale, CA, USA), originally designed to ablate Barrett’s esophagus (Figures 2 and 3). Using this technique, all the bleeding spots were ablated by pressing the electrode against the angioectatic tissue and supplying two applications of 10 J/cm2 energy. Unlike the ablation technique used for the Barrett’s, the ablated tissue was not scraped after the initial application. Following the procedure, no further bleeding occurred. The patient was discharged home. He remained asymptomatic with a stable hemoglobin level. A repeat flexible sigmoidoscopy performed 1 month later showed a normal rectal mucosa with no bleeding or angioectasias (Figure 4). He has been asymptomatic during outpatient follow up, with no further need for blood transfusions.
Figure 1.
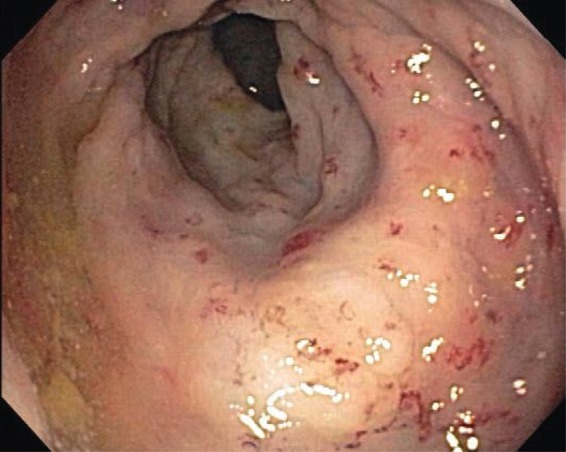
Edematous rectal mucosa with multiple angioectasias, characteristic of radiation proctitis.
Figure 2.

A HALO90 radiofrequency ablation catheter mounted on a scope.
Figure 3.
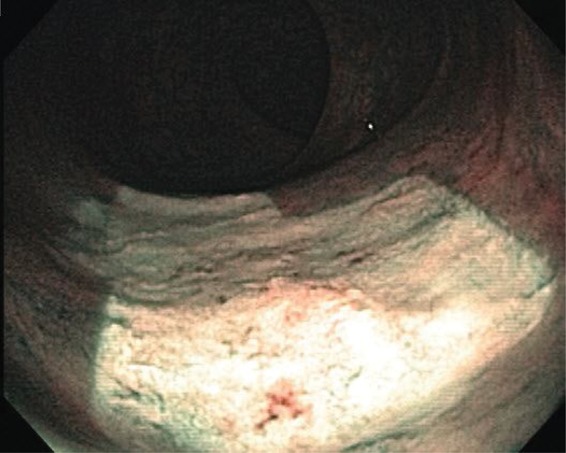
Rectal mucosa immediately after radiofrequency ablation.
Figure 4.
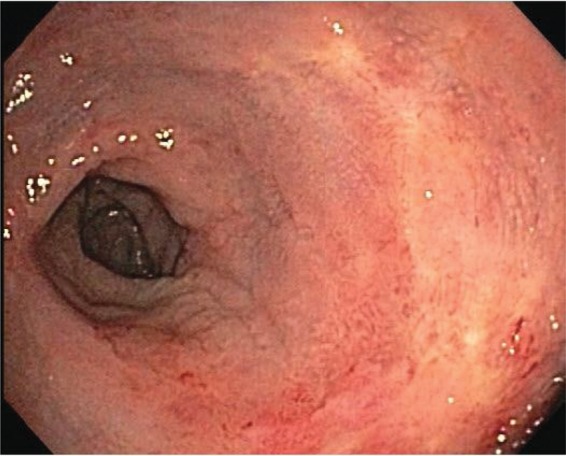
Follow-up sigmoidoscopy showing a healed rectal mucosa with minimal erythema.
Discussion
Gastrointestinal injury due to RT is categorized as acute or chronic. The incidence and severity of radiation-induced injury is determined by total radiation dose, treatment technique, and the presence or absence of systemic chemotherapy [Pilepich et al. 1987]. Patients with genitourinary cancers who undergo RT are at increased risk of developing lower gastrointestinal tract injury, especially proctitis. Acute radiation injury in the rectum occurs within 6 weeks of RT initiation and is manifested with diarrhea, urgency, tenesmus, and sometimes rectal bleeding [Ajlouni, 1999]. This is usually due to stem cell damage and decrease in the normal cell proliferation required for epithelial repair [Barnett et al. 2009]. Acute radiation proctitis is usually self limited, with resolution of symptoms within 2–3 months [Garg et al. 2006]. CRP has a delayed onset and occurs 1 year or later after exposure to RT [Henson, 2010]. CRP occurs in 5–20% of patients receiving pelvic radiation, depending on the radiation dose, and presence or absence of chemotherapy [Pilepich et al. 1987]. CRP results from the microvascular damage leading to ischemia, fibrosis, and neovascularization [Hasleton et al. 1985]. CRP presents with diarrhea, bleeding, rectal pain or urgency, and difficulty in defecation due to stricture formation. Diagnosis is based on endoscopy, which reveals mucosal edema, erythema, friability and telangiectasias. Many patients with CRP develop anemia due to continued blood loss from rectal angioectasias and can require multiple transfusions.
Treatment of CRP has been an ongoing challenge for physicians. Various medical, endoscopic and surgical therapies have been used to treat CRP. Very few randomized clinical trials are available to assess the efficacy of these modalities. Most recommendations are based on small case series, making it difficult to propose universal guidelines for treatment.
Proposed medical therapies for CRP include mesalamine and sulfasalazine, sucralfate, hyperbaric oxygen, estrogen with or without progesterone, metronidazole, short-chain fatty acids (SCFAs) enemas, and formalin.
The beneficial effects of mesalamine and sulfasalazine in the treatment of ulcerative proctitis have led to their use in CRP. Despite some anecdotal reports regarding the benefits of these medications [Goldstein et al. 1976], there is no convincing evidence to recommend them as an effective therapy for CRP [Baum et al. 1989]. A recent study on 23 patients with CRP showed a decrease in bleeding with a combination of oral and topical mesalamine [Kochhar et al. 1991]. A small, randomized clinical trial showed clinical and endoscopic improvement with a combination of oral sulfasalazine and rectal prednisolone [Seo et al. 2011].
Sucralfate has been known to have healing effects on damaged epithelium. Sucralfate enemas have been used to treat CRP with some success [Kochhar et al. 1991; Gul et al. 2002; Denton et al. 2002]. Both topical and oral sucralfate failed to provide effective prophylaxis against radiation injury in two randomized trials [O’Brien et al. 2002; Kneebone et al. 2004].
There are limited data on the use of antibiotics in CRP. One study on 60 patients with CRP showed improvement in symptoms by adding metronidazole to a regimen of mesalamine plus betamethasone enemas [Cavcić et al. 2000]. In a recent pilot study, a regimen of daily self-administered colonic irrigation with tap water and a 1-week period of oral antibiotics (ciprofloxacin and metronidazole) were used in 12 patients with hemorrhagic CRP [Sahakitrungruang et al. 2011]. There was a significant decrease in rectal bleeding accompanied by an improvement in bowel frequency and urgency, and diarrhea. Nevertheless, antibiotics cannot be recommended for the treatment of CRP due to lack of sufficient evidence and side effects, including the emergence of resistance.
At least one case report suggested a benefit from estrogen–progesterone combination therapy in CRP [Wurzer et al. 1998]. The exact mechanism of action of these hormones on bleeding telangiectasias is not clearly understood, but it has been attributed to a possible effect on blood coagulation [Daume, 1990], and restoration of the vascular endothelial integrity [Menefee et al. 1975].
Hyperbaric oxygen has been used in some centers [Dall’Era et al. 2006]. A randomized trial on 120 patients with CRP showed a significant clinical improvement with hyperbaric oxygen compared with a sham procedure [Clarke et al. 2008]. It has been proposed that hyperbaric oxygen may inhibit bacterial growth [Hill and Osterhout, 1972], promote angiogenesis, and inhibit toxin production [Craighead et al. 2011]. This method is not widely used due to its cost and limited availability.
SCFAs, especially butyrate, are derived from anaerobic bacterial metabolism, and are the preferred nutrients for colonocytes. SCFAs are implicated in the modulation of fluid and electrolyte transport, colonic motility, and mucosal blood flow and healing [Velázquez et al. 1997]. After 4 weeks of therapy with SCFA enemas some clinical benefit was shown in a case series of seven patients with radiation proctitis [Al-Sabbagh et al. 1996], but a randomized, placebo-controlled trial failed to show any benefit compared with placebo [Talley et al. 1997].
Formalin is a blood vessel chemical sclerosant, and has been used for the treatment of CRP for many years. It is highly irritating to the skin and normal mucosa, therefore it should be administered in a controlled fashion. Different methods have been described and it has been effective in 65–76% of patients [Haas et al. 2007; Parikh et al. 2003; De Parades et al. 2005]. Reported complications include anorectal pain, diarrhea, incontinence, colitis, rectal ulcerations, and stricture formation.
Overall, the efficacy of medical therapies is usually limited, mandating further endoscopic intervention. Bleeding and anemia requiring hospitalization and transfusion are the major complications of CRP. Endoscopic therapies are designed to control the bleeding, symptoms, and decrease the need for transfusions and hospitalizations. The currently available endoscopic modalities include contact probe thermal methods, laser therapy, i.e. neodymium-yttrium aluminum garnet (Nd:YAG) and potassium titanyl phosphate (KTP), cryoablation, and APC. These therapies have different success rates and complications.
The heater probe has a Teflon-coated tip that delivers standardized energy upon contact with the tissue. Bipolar electrocoagulation probes (BICAP) have two electrodes in the tip. The electric current passes from one electrode to another using the tissue as a vehicle for transmission, producing electrocoagulation. Both methods are useful in the setting of bleeding, providing cautery combined with coaptive coagulation of the bleeding vessel. Fuentes and colleagues reported eight cases of CRP, which were successfully treated with a heater probe [Fuentes et al. 1993]. One to four sessions of coagulation were performed with an intensity of 200–400 J per session. A good response was obtained in all patients, bleeding diminished or stopped completely, with improvement of blood counts and the need for transfusions. Both heater and bipolar probes were evaluated in a randomized prospective study involving 21 patients with successful cessation of rectal bleeding after 1–4 sessions of therapy, and a significant reduction in bleeding episodes [Jensen et al. 1997]. Both devices are widely available. One disadvantage is char formation at the tip of the electrode, mandating repeated cleaning. Also, the coagulation depth is variable and depends on the force of application, energy settings, and duration of treatment [Laine, 1991].
Nd:YAG laser is a noncontact method. It penetrates to a depth of 5 mm and is well absorbed by tissue [Wilson et al. 2006]. Three case series regarding the use of Nd:YAG laser in CRP involving a total of 19 patients reported an efficacy of 66–100% with no complications [Ventrucci et al. 2001; Barbatzas et al. 1996; Leuchter et al. 1982]. The possible complications of this modality include necrosis, fibrosis and stricture formation, bleeding, and fistulization. The use of Nd:YAG laser has declined over time due to the cost, possible complications, and availability of simpler, safer methods. A modification of this technique uses a KTP crystal to filter the laser and reduce its wavelength, and thus the depth of penetration [Pritikin et al. 1992]. In a report by Taylor and colleagues, a total of 23 patients with CRP were treated using this method, leading to a 65% symptomatic improvement [Taylor et al. 2000]. Two patients developed rectal ulcers.
Cryoablation is a noncontact technique that can ablate tissue using cold temperature. Currently, two different endoscopic catheters are available for cryoablation: the carbon dioxide system (Polar Wand, GI Supply, Camp Hill, PA, USA), and the liquid nitrogen system (Cryospray Ablation, CSA Medical, Baltimore, MD, USA). Kantsevoy and colleagues used this technique in seven patients with radiation proctitis and achieved a 100% bleeding cessation [Kantsevoy et al. 2003]. Shaib and colleagues reported a case of CRP, unresponsive to medical therapy that was treated with cryoablation using the liquid nitrogen spray, and achieved mucosal healing with resolution of symptoms [Shaib and Hou, 2008]. Hou and colleagues reported a series of 10 patients with hemorrhagic CRP who underwent a single endoscopic session of cryotherapy, with 80% symptomatic improvement, 70% endoscopic improvement, and 37% decrease in rectal telangiectasia density [Hou et al. 2011]. The use of this technique in CRP remains experimental. There are no studies comparing cryoablation with other endoscopic methods for the treatment of CRP. One of the risks of the procedure is overdistension of the bowel and perforation.
APC has emerged as the favorite endoscopic modality for CRP. In this noncontact method, the laser current jumps from the tip of a monopolar electrode and conducted via an argon gas medium to the tissue. Coagulation depth depends on the generator power setting, flow rate of the argon gas, duration of application, and the distance of the probe tip to the target tissue, and ranges from 0.8 mm to 3.0 mm. The depth of penetration is automatically limited by desiccation of the tissue [Farin and Grund, 1994]. The noncontact nature of this therapy eliminates the problem with char formation, and allows treating a larger surface area in a shorter time. Multiple case reports and series have shown the efficacy of APC in the treatment of CRP [Rustagi and Mashimo, 2011; Swan et al. 2010; Dees et al. 2006]. The overall success rate of this method is above 80%, with 1–4 sessions required to control the bleeding. Although it has been hypothesized that the cauterized tissue breaks the circuit of APC current and minimizes the deeper injury, it is possible to create deep tissue penetration by prolonged and repeated applications [Farin and Grund, 1994]. Side effects of APC include colon distention, rectal pain, tenesmus, colonic explosion, perforation, ulceration, and stricture formation [Rustagi and Mashimo, 2011].
Despite the availability of these endoscopic modalities, the search for optimum treatment modality for CRP continues. All these methods are limited by their variable efficacy and side effects. Stricture formation is one of the complications of thermal therapy, and it has been reported with all these modalities, including APC [Rustagi and Mashimo, 2011].
RFA using the HALO® system, which uses a closely spaced array of electrodes to deliver radiofrequency energy, has emerged as the method of choice to ablate Barrett’s esophagus. This system induces a uniform thermal injury with a depth controlled by a generator, which can vary the power, density, and duration of the energy applied. There are two types of RFA catheters available: a circumferential HALO360 catheter and a smaller, endoscope-mounted catheter (the HALO90 ablation catheter) that can be used for more focal ablation [Akiyama and Triadafilopoulos, 2010]. Since RFA can provide a superficial ablation in a broader surface area, it is reasonable to consider it for the treatment of CRP. Zhou and colleagues have reported the use of RFA in three patients with CRP, two of which failed prior therapies with electrocautery and APC [Zhou et al. 2009]. In all cases, hemostasis was achieved in one or two sessions. No ulceration or stricture formation was noted up to 19 months after treatment. In this case series, authors utilized an endoscopic optical coherence tomography method to analyze the tissue structure pre- and post-treatment. This method uses the echo time delays of light waves to image the tissue. Using this method, the authors documented the squamous epithelialization of the treated mucosa and eradication of the ectatic vessels with RFA.
Recently, Nikfarjam and colleagues reported a case of refractory CRP with continued bleeding despite APC therapy, successfully treated with RFA using a HALO90 system over three sessions [Nikfarjam et al. 2010]. The patient was symptom free at 6 months follow up with minimal evidence of residual mucosal abnormalities.
These cases, together with the case presented in this article, demonstrate the utility of using RFA as an alternative treatment modality for CRP. RFA has some potential advantages over APC and electrocautery. First, unlike APC and other probe methods, it covers a broader surface area, making the treatment duration shorter for more extensive disease. In addition, pressing the probe against the tissue provides some coaptive coagulation before ablation. Moreover, the squamous re-epithelialization after RFA appears to minimize the bleeding and accelerate the healing with less potential for fibrosis and stricture formation.
Perhaps, the most important advantage of RFA is the superficial nature of the ablation (0.5–1 mm), compared with APC (less than 3 mm), and contact thermal methods (variable) [Smith et al. 2007]. In order to examine the depth of the injury induced by RFA in the colon and rectum, Trunzo and colleagues performed a study on 18 patients undergoing left hemicolectomy or proctocolectomy for different indications [Trunzo et al. 2011]. Focal RFA was performed in normal segments of the colon and rectum. After resection, a histological examination of the RFA segments was performed to assess the depth of injury, and its association with the number of applications and the energy density. Overall, considerable variation was noted in the depth of injury. The sites treated with more applications of RFA showed a deeper injury regardless of the energy used. Interestingly, there was an inverse relationship between the energy density and histologic depth. This was attributed to the desiccation of the tissue with higher energy on first application, and higher resistance to subsequent applications of RFA. The authors concluded that the deepest ablative effect of RFA in the colon and rectum was limited to the muscularis propria when no more than two ablations were applied in the same location.
In summary, RFA appears to be a feasible and useful method for the treatment of CRP. More experience is needed to assess the long-term effects of this therapy and optimal settings, and methods of administration. In the meantime, we propose that RFA should be considered as a therapeutic modality for CRP in patients who failed to respond to other endoscopic methods.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Rodney Eddi, Seton Hall University, School of Health and Medical Sciences – St. Michael’s Medical Center, Department of Medicine, Division of Gastroenterology, 111 Central Avenue, Newark, NJ 07102, USA.
Joseph R. DePasquale, Seton Hall University, School of Health and Medical Sciences – St. Michael’s Medical Center, Department of Medicine, Division of Gastroenterology, Newark, USA
References
- Ajlouni M. (1999) Radiation-induced proctitis. Curr Treat Options Gastroenterol 2: 20–26 [DOI] [PubMed] [Google Scholar]
- Akiyama J., Triadafilopoulos G. (2010) Endoscopic ablation therapy of Barrett’s esophagus. Minerva Gastroenterol Dietol 56: 405–420 [PubMed] [Google Scholar]
- Al-Sabbagh R., Sinicrope F., Sellin J., Shen Y., Roubein L. (1996) Evaluation of short-chain fatty acid enemas: treatment of radiation proctitis. Am J Gastroenterol 91: 1814–1816 [PubMed] [Google Scholar]
- Babb R. (1996) Radiation proctitis: a review. Am J Gastroenterol 91: 1309–1311 [PubMed] [Google Scholar]
- Barbatzas C., Spencer G., Thorpe S., Sargeant L., Bown S. (1996) Nd:YAG laser treatment for bleeding from radiation proctitis. Endoscopy 28: 497–500 [DOI] [PubMed] [Google Scholar]
- Barnett G., West C., Dunning A., Elliott R., Coles C., Pharoah P., et al. (2009) Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer 9: 134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum C., Biddle W., Miner P., Jr (1989) Failure of 5-aminosalicylic acid enemas to improve chronic radiation proctitis. Dig Dis Sci 34: 758–760 [DOI] [PubMed] [Google Scholar]
- Cavcić J., Turcić J., Martinac P., Jelincić Z., Zupancić B., Panijan-Pezerović R., et al. (2000) Metronidazole in the treatment of chronic radiation proctitis: clinical trial. Croat Med J 41: 314–318 [PubMed] [Google Scholar]
- Clarke R., Tenorio L., Hussey J., Toklu A., Cone D., Hinojosa J., et al. (2008) Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. Int J Radiat Oncol Biol Phys 72: 134–143 [DOI] [PubMed] [Google Scholar]
- Craighead P., Shea-Budgell M., Nation J., Esmail R., Evans A., Parliament M., et al. (2011) Hyperbaric oxygen therapy for late radiation tissue injury in gynecologic malignancies. Curr Oncol 18: 220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Era M., Hampson N., Hsi R., Madsen B., Corman J. (2006) Hyperbaric oxygen therapy for radiation induced proctopathy in men treated for prostate cancer. J Urol 176: 87–90 [DOI] [PubMed] [Google Scholar]
- Daume E. (1990) Influence of modern low-dose oral contraceptives on hemostasis. Adv Contracept 6(Suppl): 51–67 [PubMed] [Google Scholar]
- Dees J., Meijssen M., Kuipers E. (2006) Argon plasma coagulation for radiation proctitis. Scand J Gastroenterol Suppl 243: 175–178 [DOI] [PubMed] [Google Scholar]
- Denton A., Forbes A., Andreyev J., Maher E. (2002) Nonsurgical interventions for late radiation proctitis in patients who have received radical radiotherapy to the pelvis. Cochrane Database Syst Rev 1: CD003455 [DOI] [PubMed] [Google Scholar]
- De Parades V., Etienney I., Bauer P., Bourguignon J., Meary N., Mory B., et al. (2005) Formalin application in the treatment of chronic radiation-induced hemorrhagic proctitis – an effective but not risk-free procedure: a prospective study of 33 patients. Dis Colon Rectum 48: 1535–1541 [DOI] [PubMed] [Google Scholar]
- Farin G., Grund K. (1994) Technology of argon plasma coagulation with particular regard to endoscopic applications. Endosc Surg Allied Technol 2: 71–77 [PubMed] [Google Scholar]
- Fuentes D., Monserat R., Isern A., Salazar J., Bronstein M., Gumina C., et al. (1993) Colitis due to radiation: endoscopic management with heat probe. G E N 47: 165–167 [PubMed] [Google Scholar]
- Garg A., Mai W., McGary J., Grant W., 3rd, Butler E., Teh B. (2006) Radiation proctopathy in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys 66: 1294–1305 [DOI] [PubMed] [Google Scholar]
- Goldstein F., Khoury J., Thornton J. (1976) Treatment of chronic radiation enteritis and colitis with salicylazosulfapyridine and systemic corticosteroids. A pilot study. Am J Gastroenterol 65: 201–208 [PubMed] [Google Scholar]
- Gul Y., Prasannan S., Jabar F., Shaker A., Moissinac K. (2002) Pharmacotherapy for chronic hemorrhagic radiation proctitis. World J Surg 26: 1499–1502 [DOI] [PubMed] [Google Scholar]
- Haas E., Bailey H., Farragher I. (2007) Application of 10 percent formalin for the treatment of radiation-induced hemorrhagic proctitis. Dis Colon Rectum 50: 213–217 [DOI] [PubMed] [Google Scholar]
- Hasleton P., Carr N., Schofield P. (1985) Vascular changes in radiation bowel disease. Histopathology 9: 517–534 [DOI] [PubMed] [Google Scholar]
- Henson C. (2010) Chronic radiation proctitis: issues surrounding delayed bowel dysfunction post-pelvic radiotherapy and an update on medical treatment. Therap Adv Gastroenterol 3: 359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill G., Osterhout S. (1972) Experimental effects of hyperbaric oxgen on selected clostridial species. I. In-vitro studies. J Infect Dis 125: 17–25 [DOI] [PubMed] [Google Scholar]
- Hou J., Abudayyeh S., Shaib Y. (2011) Treatment of chronic radiation proctitis with cryoablation. Gastrointest Endosc 73: 383–389 [DOI] [PubMed] [Google Scholar]
- Jensen D., Machicado G., Cheng S., Jensen M., Jutabha R. (1997) A randomized prospective study of endoscopic bipolar electrocoagulation and heater probe treatment of chronic rectal bleeding from radiation telangiectasia. Gastrointest Endosc 45: 20–25 [DOI] [PubMed] [Google Scholar]
- Kantsevoy S., Cruz-Correa M., Vaughn C., Jagannath S., Pasricha P., Kalloo A. (2003) Endoscopic cryotherapy for the treatment of bleeding mucosal vascular lesions of the GI tract: a pilot study. Gastrointest Endosc 57: 403–406 [DOI] [PubMed] [Google Scholar]
- Kneebone A., Mameghan H., Bolin T., Berry M., Turner S., Kearsley J., et al. (2004) Effect of oral sucralfate on late rectal injury associated with radiotherapy for prostate cancer: a double-blind, randomized trial. Int J Radiat Oncol Biol Phys 60: 1088–1097 [DOI] [PubMed] [Google Scholar]
- Kochhar R., Patel F., Dhar A., Sharma S., Ayyagari S., Aggarwal R., et al. (1991) Radiation-induced proctosigmoiditis. Prospective, randomized, double-blind controlled trial of oral sulfasalazine plus rectal steroids versus rectal sucralfate. Dig Dis Sci 36: 103–107 [DOI] [PubMed] [Google Scholar]
- Laine L. (1991) Determination of the optimal technique for bipolar electrocoagulation treatment. An experimental evaluation of the BICAP and Gold probes. Gastroenterology 100: 107–112 [DOI] [PubMed] [Google Scholar]
- Leuchter R., Petrilli E., Dwyer R., Hacker N., Castaldo T., Lagasse L., et al. (1982) Nd:YAG laser therapy of rectosigmoid bleeding due to radiation injury. Obstet Gynecol 59(Suppl 6): 65S–67S [PubMed] [Google Scholar]
- Menefee M., Flessa H., Glueck H., Hogg S. (1975) Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease). Arch Otolaryngol 101: 246–251 [DOI] [PubMed] [Google Scholar]
- Nikfarjam M., Faulx A., Laughinghouse M., Marks J. (2010) Feasibility of radiofrequency ablation for the treatment of chronic radiation proctitis. Surg Innov 17: 92–94 [DOI] [PubMed] [Google Scholar]
- O’Brien P., Franklin C., Poulsen M., Joseph D., Spry N., Denham J. (2002) Acute symptoms, not rectally administered sucralfate, predict for late radiation proctitis: longer term follow-up of a phase III trial – Trans-Tasman Radiation Oncology Group. Int J Radiat Oncol Biol Phys 54: 442–449 [DOI] [PubMed] [Google Scholar]
- Parikh S., Hughes C., Salvati E., Eisenstat T., Oliver G., Chinn B., et al. (2003) Treatment of hemorrhagic radiation proctitis with 4 percent formalin. Dis Colon Rectum 46: 596–600 [DOI] [PubMed] [Google Scholar]
- Pilepich M., Krall J., Sause W., Johnson R., Russ H., Hanks G., et al. (1987) Correlation of radiotherapeutic parameters and treatment related morbidity in carcinoma of the prostate – analysis of RTOG study 75–06. Int J Radiat Oncol Biol Phys 13: 351–357 [DOI] [PubMed] [Google Scholar]
- Pritikin J., Weinman D., Harmatz A., Young H. (1992) Endoscopic laser therapy in gastroenterology. West J Med 157: 48–54 [PMC free article] [PubMed] [Google Scholar]
- Rustagi T., Mashimo H. (2011) Endoscopic management of chronic radiation proctitis. World J Gastroenterol 17: 4554–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakitrungruang C., Thum-Umnuaysuk S., Patiwongpaisarn A., Atittharnsakul P., Rojanasakul A. (2011) A novel treatment for haemorrhagic radiation proctitis using colonic irrigation and oral antibiotic administration. Colorectal Dis 13: e79–e82 [DOI] [PubMed] [Google Scholar]
- Seo E., Kim T., Kim T., Joo H., Park J., Park S., et al. (2011) The efficacy of the combination therapy with oral and topical mesalazine for patients with the first episode of radiation proctitis. Dig Dis Sci 56: 2672–2677 [DOI] [PubMed] [Google Scholar]
- Shaib Y., Hou J. (2008) Complete endoscopic healing of radiation proctitis with low pressure cryoablation. Am J Gastroenterol 103(Suppl 1): S230 [Google Scholar]
- Smith C., Bejarano P., Melvin W., Patti M., Muthusamy R., Dunkin B. (2007) Endoscopic ablation of intestinal metaplasia containing high-grade dysplasia in esophagectomy patients using a balloon-based ablation system. Surg Endosc 21: 560–569 [DOI] [PubMed] [Google Scholar]
- Swan M., Moore G., Sievert W., Devonshire D. (2010) Efficacy and safety of single-session argon plasma coagulation in the management of chronic radiation proctitis. Gastrointest Endosc 72: 150–154 [DOI] [PubMed] [Google Scholar]
- Talley N., Chen F., King D., Jones M., Talley N. (1997) Short-chain fatty acids in the treatment of radiation proctitis: a randomized, double-blind, placebo-controlled, cross-over pilot trial. Dis Colon Rectum 40: 1046–1050 [DOI] [PubMed] [Google Scholar]
- Taylor J., Disario J., Bjorkman D. (2000) KTP laser therapy for bleeding from chronic radiation proctopathy. Gastrointest Endosc 52: 353–357 [DOI] [PubMed] [Google Scholar]
- Trunzo J., McGee M., Poulose B., Willis J., Ermlich B., Laughinghouse M., et al. (2011) A feasibility and dosimetric evaluation of endoscopic radiofrequency ablation for human colonic and rectal epithelium in a treat and resect trial. Surg Endosc 25: 491–496 [DOI] [PubMed] [Google Scholar]
- Velázquez O., Lederer H., Rombeau J. (1997) Butyrate and the colonocyte. Production, absorption, metabolism, and therapeutic implications. Adv Exp Med Biol 427: 123–134 [PubMed] [Google Scholar]
- Ventrucci M., Di Simone M., Giulietti P., De Luca G. (2001) Efficacy and safety of Nd:YAG laser for the treatment of bleeding from radiation proctocolitis. Dig Liver Dis 33: 230–233 [DOI] [PubMed] [Google Scholar]
- Villavicencio R., Rex D., Rahmani E. (2002) Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc 55: 70–74 [DOI] [PubMed] [Google Scholar]
- Wilson S., Rex D. (2006) Endoscopic treatment of chronic radiation proctopathy. Curr Opin Gastroenterol 22: 536–540 [DOI] [PubMed] [Google Scholar]
- Wurzer H., Schafhalter-Zoppoth I., Brandstätter G., Stranzl H. (1998) Hormonal therapy in chronic radiation colitis. Am J Gastroenterol 93: 2536–2538 [DOI] [PubMed] [Google Scholar]
- Zhou C., Adler D., Becker L., Chen Y., Tsai T., Figueiredo M., et al. (2009) Effective treatment of chronic radiation proctitis using radiofrequency ablation. Therap Adv Gastroenterol 2: 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]


