Abstract
Physical functioning is often impaired in adolescents with chronic pain, which has largely been demonstrated through subjective self-report measures. Actigraphy uses motion monitoring as an objective means for assessing one dimension of physical functioning, physical activity level. This study used subjective and objective measures to assess multiple dimensions of physical functioning in a clinical sample of adolescents with chronic pain (n = 78) and a comparison group of healthy adolescents (n = 59). Individual and pain characteristics were also examined as predictors of actigraphy variables within the chronic pain sample. Results indicated that adolescents with chronic pain demonstrate significant impairment in subjective measures of physical functioning and evidence lower levels of physical activity. Actigraphic measures of physical activity were moderately correlated with self-report measures of physical functioning. Individual characteristics, including adolescent age, sex, Body Mass Index percentile, were associated with physical activity levels among adolescents with chronic pain. Physical activity represents a distinct dimension of physical functioning. Assessing physical activity may provide additional description of physical functioning among adolescents with chronic pain, and may help identify targets for intervention in this population.
Perspective
This study demonstrates that adolescents with chronic pain have lower physical activity levels, as measured objectively via actigraphy, as well as poorer subjective reports of physical functioning, compared to healthy adolescents. Actigraphic measurement of physical activity provides objective source data about one dimension of physical functioning.
Keywords: Chronic pain, Adolescents, Physical functioning, Physical activity
Introduction
Impairments in physical functioning are common in adolescents with chronic pain, and many youth report withdrawal from physical activities such as team sports, gym, walking, and running.11, 14 Physical functioning is a multidimensional domain encompassing a number of constructs such as physical fitness, physical activity, functional capacity, and subjective disability, which are related, but distinct aspects of functioning. Physical functioning has been identified as an important outcome domain to assess in clinical trials of pain interventions.13 In addition to being an important clinical feature of chronic pain, participation in regular physical activity has broad long-term implications for adolescent health.22, 30 Most prior research examining physical functioning has relied on subjective report on measures of functional disability, activity limitations, and physical health-related quality of life.
Actigraphy offers an objective means for assessing physical activity, which may be useful to assess in adolescents with chronic pain for a number of reasons. First, as has been noted by Kashikar-Zuck and colleagues, there are a number of sources of bias (e.g., negative affect) that could potentially impact the reliability and accuracy of adolescent reports on self-report measures of physical functioning.10 Second, a better understanding of how subjective and objective measures of physical functioning relate is necessary, as changes in self-report measures are not necessarily reflective of changes in objectively measured physical function.27 Third, not all adolescents with chronic pain demonstrate reductions in physical activity10 and a better understanding of factors that are associated with lower and higher levels of physical activity may help identify youth who are at higher risk for negative health outcomes, as well as identify potential targets for intervention. Thus, actigraphic measures may provide useful additional information about physical functioning in this population of youth.
To our knowledge, only two previous studies have examined actigraphic measures of daytime physical activity in adolescents with chronic pain. One study examined a small sample (n = 20) with mixed chronic pain problems (headache, abdominal, or musculoskeletal pain) and showed lower levels of daytime physical activity compared to healthy adolescents.12 In this study, physical activity was higher in younger adolescents, and was associated with self-reports of activity limitations and depressive symptoms. In a larger sample of adolescents (n = 104) with juvenile primary fibromyalgia syndrome, higher levels of physical activity were observed in some adolescents, which was associated with significantly lower reports of pain intensity, depressive symptoms, and functional disability.10 The current study extends findings from these previous studies by examining physical functioning in a relatively large sample of adolescents with chronic pain (n = 78) and healthy adolescents (n = 59). Specifically, we address a gap in knowledge of measurement of physical function and physical activity. Inclusion of a healthy comparison sample will increase understanding of the degree of difficulty in physical functioning that is experienced by adolescents with chronic pain.
The first aim of the current study was to describe objectively measured physical activity (collected via actigraphy), and subjective measures of physical functioning in adolescents with a variety of chronic pain conditions compared to a comparison group of healthy adolescents. It was hypothesized that adolescents with chronic pain would demonstrate lower levels of physical activity and subjective physical functioning compared to adolescents without chronic pain. The second aim was to examine associations between actigraphic measures of daytime physical activity and subjective reports of physical functioning. It was hypothesized that physical activity and subjective measures of disability would emerge as distinct constructs. The final aim was to explore potential contributors to physical activity among adolescents with chronic pain, including age, sex, BMI and pain characteristics.
Materials and Methods
The Institutional Review Board at the academic medical center where the study was conducted approved this study. Written informed consent was obtained from parents, and written assent was obtained from adolescents prior to participation in this study.
Participants
Participants were 137 adolescents, ages 11–17, and their parents. The sample included 78 adolescents with chronic pain and 59 children in a healthy comparison group. Chronic pain participants were drawn from two convenience clinical studies. The majority of participants were female (71.5%). For both studies, adolescents with chronic pain were recruited through specialty care physician referral from a multidisciplinary pediatric pain clinic, pediatric neurology clinic, and pediatric gastroenterology clinic at an academic health center children’s hospital in the northwestern United States. Referring physicians provided study flyers to potentially eligible participants, posted flyers in their clinic areas, and provided lists of potentially eligible patients who were then mailed study flyers.
Inclusion criteria consisted of: (a) ages 11 to 17 years, (b) chronic idiopathic pain including headache, abdominal pain, or musculoskeletal pain present over the previous 3 months, and (c) pain occurs at least once per week. Healthy adolescents were recruited through advertisements and flyers in the community, including posting flyers in the children’s hospital lobby and waiting areas and advertisements on the health center’s research website. Inclusion criteria consisted of: (a) ages 11 to 17 years, (b) did not meet the criteria for chronic pain used in the chronic pain group. Participants in both groups were excluded if the child (a) had a serious comorbid chronic condition (e.g., diabetes, cancer), (b) was non-English speaking, or (c) had developmental delays or cognitive impairment. For the chronic pain group, 15.1% of youth screened were ineligible, and an additional 15.1% declined to participate. For the healthy group, 38.3% of youth screened were ineligible, and an additional 5.6% declined to participate. Two participants in the chronic pain group and one participant in the healthy group had missing data and were not included in analyses.
Procedures
After the initial screening by a member of the research team and enrollment, adolescents and parents completed retrospective questionnaire measures of pain characteristics, activity limitations, and physical health-related quality of life. Adolescents underwent 7–10 consecutive days of monitoring by actigraphy. Participants were provided with study materials during a routine outpatient visit or by mail. All participants were instructed to wear the wrist-mounted actigraphy device 24 hours per day during the monitoring period and to identify bedtime and wake time by pressing a button on the watch. The importance of wearing the device continuously was stressed to participants, and they were instructed to put the device back on immediately upon taking it off (e.g., to take a shower). Participants were asked to complete questionnaires independently at home at their convenience during the week that they wore the actigraphy device. Actigraphy devices and questionnaires were returned by business reply mail. Gift cards to local stores were given to the participants as compensation for their time.
Measures
Sociodemographics
Parents reported on their adolescent’s age, sex, ethnicity, and racial background, as well as on family income via a sociodemographic questionnaire.
Pain characteristics
Adolescents completed a retrospective pain questionnaire assessing usual pain intensity, frequency, and duration over the past month. Usual pain intensity was assessed using an 11-point numerical rating scale (NRS) with anchors of (0) no pain to (10) worst possible pain. Use of the NRS for measuring pain intensity has been well-validated in youth 25. Usual pain frequency was assessed using 7 response options ranging from (0) Not at all to (6) Daily. Usual pain duration was assessed using 4 response options ranging from (0) < 1 hour to (3) All day. Primary pain location was determined by referring physician’s pain diagnosis at the time of study screening and enrollment (headache, abdominal pain, or musculoskeletal pain).
Body Mass Index
Parents reported on adolescent height and weight, which was used to calculate body mass index (BMI) utilizing the formula BMI = Weight in kg/Height in m2. BMI percentile was calculated using exact adolescent age via the CDC’s Pediatric BMI online calculator.3 BMI percentile was used as the primary BMI variable in this study. Parent proxy report of child and adolescent height and weight has been demonstrated to provide a reliable estimate of BMI and weight status, with BMI calculated from parent report of height and weight being highly correlated (r = .92) with measured height and weight.6
Subjective Measures of Physical Functioning
Physical Health-related Quality of Life
Adolescents and their parents completed the Pediatric Quality of Life Inventory, Short Form (PedsQL), which has been found to be reliable and has been validated in a number of pediatric populations.24 Parent and adolescent report on the 5-item Physical Health subscale of the PedsQL was used in this study. Respondents are asked to rate how much of a problem they have had with physical activities in the past month on a 5-point scale ranging from (0) Never to (4) Almost Always. Items include “It is hard for me to walk more than one block” and “It is hard for me to lift something heavy”. Higher scores indicate better health-related quality of life.
Activity limitations
Activity limitations were assessed using parent and adolescent retrospective reports on the Child Activity Limitations Interview (CALI) 17, which has been found to be reliable and valid in pediatric chronic pain samples. Respondents are asked to rate “how difficult or bothersome doing these activities was due to pain” in the last four weeks. Examples of activities include “sports”, “housework or chores”, and “doing things with friends”. The primary score was derived from the sum difficulty ratings of the eight most difficult activities (from a list of 21 activities), which are obtained on a 5-point scale from (0) not very difficult to (4) extremely difficult, with total scores ranging from 0 to 32. Higher scores represent greater activity limitations.
Actigraphic Measures of Physical Activity
Physical activity was assessed objectively via ambulatory activity monitoring using the Actiwatch 64® device (Philips Respironics, Inc.). This small lightweight unit worn on the nondominant wrist provides continuous monitoring of the subject’s activity levels. Movement is sensed by an omni-directional mercury switch that is open when there is no movement and closed when movement is detected. Each time the switch closes, an activity count is generated. Activity counts were stored on the device in 1 min epochs, such that higher counts indicate more movement during each minute. Data were extracted using Respironics Actiware version 5.0 software. All data were obtained from daytime waking hours, accounting for sleep onset and offset during each 24 hour period of actigraphy data. Participants also completed a sleep log. Sleep onset and offset times were determined via event markers (button on the device pushed by participants at bedtime and wake time) and the sleep log. In the event that no clear sleep or wake event marker or sleep log data was available, sleep onset and offset time was determined based on 10 contiguous minutes below the sleep threshold set by the software.
Three activity scores were averaged from all daytime wake periods for analysis, including mean activity level, calculated as the mean number of activity counts per minute during each daytime wake period; peak activity level, calculated as the highest number of activity counts achieved in a single minute per daytime wake period; and sedentary activity, calculated as the number of minutes per day with activity counts ≤ 40 (immobile minutes with medium rest threshold in Actiware 5.0 software). Actigraphy has been used to measure physical activity in a number of pediatric populations,21 and the Actiwatch 64® has been validated with observation and pedometer readings.18 Any periods during daytime waking hours when the device was not worn (defined as 10 or more contiguous minutes of activity counts of 0) were excluded by hand and were not included in the scores. All participants had at least five days of ten or more hours of daytime data, and averages were calculated based on the number of days of actual data available per participant.
Statistical Analyses and Power Analyses
All analyses were performed using IBM SPSS 19.0 software. Descriptive statistics were calculated on sociodemographic, BMI, and pain characteristics for the sample, and t-test and χ2 procedures were used to examine group differences. Comparisons between healthy adolescents and adolescents with chronic pain were conducted using t-test and MANOVA procedures. Bivariate correlations were utilized to examine associations between actigraphic physical activity and subjective physical functioning within the entire sample. Within the sample of adolescents with chronic pain, multiple linear regressions were used to examine the multivariate associations of age, sex, BMI percentile, and pain characteristics with actigraphic measures of physical activity. Based on estimates of group differences observed in prior research comparing a mixed chronic pain condition sample of adolescents to a healthy sample12, analyses were conducted to determine the sample size needed to detect differences in actigraphic variables (peak and mean activity level). With an Alpha of .05, a sample size of 16 per group would be required to provide 81% power for mean activity level and 99% power for peak activity level.
Results
Sample sociodemographic, BMI, and pain characteristics
Table 1 summarizes the sample characteristics. Participants included 137 adolescents (39 male, 98 female) between the ages of 11 and 17 years, who were primarily Caucasian (86.9%) and middle to upper middle class as indicated by annual household income over $70,000 (70.8%). BMI was 22.22 in the sample on average, and ranged from 14.40 to 42.51. BMI percentile was 60.16 on average (range = 3–99), with 2.2% of the sample being in the underweight weight status category, 69.6% in the normal weight category, 14.8% in the overweight weight status category, and 13.3% in the obese weight status category.3 BMI percentile did not differ by child sex. Adolescents with chronic pain and healthy adolescents did not differ significantly on BMI, BMI percentile, or any sociodemographic variables, with the exception of adolescent racial background. There were more Caucasian adolescents in the chronic pain group (92.3%) than in the healthy group (79.7%; χ2(1) = 4.71, p < .05). The groups did not differ on ethnicity (Hispanic/Latino vs. non-Hispanic/Latino).
Table 1.
Sample characteristics by study group
|
Chronic Pain n=78 |
Healthy n=59 |
Total Sample n=137 |
|
|---|---|---|---|
|
| |||
| Characteristic | n (%) / M (SD) | n (%) / M (SD) | n (%) / M (SD) |
|
| |||
| Age in years | 15.04 (1.84) | 14.68 (1.76) | 14.88 (1.81) |
|
| |||
| Sex | |||
| Male | 22 (28.2 %) | 17 (28.8%) | 39 (28.5%) |
| Female | 56 (71.8 %) | 42 (71.2%) | 98 (71.5%) |
|
| |||
| Child Racial Background | |||
| Caucasian | 72 (92.3%) | 47 (79.7%) | 119 (86.9%) |
| African American | 0 (0.0%) | 7 (11.9%) | 7 (5.1%) |
| Amer. Indian/Alaskan Native | 2 (2.6%) | 1 (1.7%) | 3 (3.3%) |
| Asian | 1 (1.3%) | 1 (1.7%) | 2 (1.5%) |
| Other/biracial | 3 (5.1%) | 3 (3.8%) | 6 (4.4%) |
|
| |||
| Ethnicity | |||
| Hispanic/Latino | 4 (5.2%) | 6 (10.5%) | 10 (7.5%) |
| Non-Hispanic/Latino | 73 (94.8%) | 48 (85.7%) | 102 (89.5%) |
| Unknown | 0 (0.0%) | 2 (3.5%) | 2 (1.5%) |
|
| |||
| Family Income | |||
| < $29,000 | 7 (9.3%) | 4 (6.8%) | 11 (8.2%) |
| $30,000 – $49,000 | 9 (12.0%) | 3 (5.1%) | 12 (8.9%) |
| $50,000 – $69,000 | 11 (14.7%) | 3 (5.1%) | 14 (10.4%) |
| $70,000 – $99,000 | 14 (18.7%) | 4 (6.8%) | 18 (13.4%) |
| > $100,000 | 34 (45.3%) | 45 (76.3%) | 79 (58.9%) |
|
| |||
| Body Mass Index (BMI, kg/m2) | 22.43 (5.29) | 21.93 (5.36) | 22.22 (5.31) |
| BMI percentile | 59.71 (29.98) | 60.79 (27.01) | 60.16 (28.66) |
|
| |||
| Usual Pain Duration | |||
| < 1 hour | 5 (6.4%) | 33 (58.9%) | 38 (28.4%) |
| A few hours | 10 (12.8%) | 10 (17.9%) | 20 (14.9%) |
| About half the day | 15 (19.2%) | 6 (10.7%) | 21 (15.7%) |
| All day | 48 (61.5%) | 7 (12.5%) | 55 (41.0%) |
|
| |||
| Usual Pain Intensity (0–10 NRS) | 6.32 (1.78) | 3.21 (2.07) | |
Adolescents with chronic pain had primary pain problems of headache (35.9%), abdominal pain (33.3%), and musculoskeletal pain (e.g., back pain, limb pain; 30.8%). Most adolescents with chronic pain reported experiencing daily pain (75.6%) that was rated as moderate to severe in usual intensity (M = 6.32) and lasted all day (61.5%). Adolescents in the healthy comparison group reported experiencing mild usual pain intensity (M = 3.21) that occurred relatively infrequently (1–3 times per month or less) and was short in duration for the majority of the sample, lasting a few hours or less than one hour (76.8%). As expected, adolescents with chronic pain reported significantly higher usual pain intensity, t(135) = −9.43, p < .001, as well as more frequent pain, χ2(6) = 91.33, p < .001 and longer usual pain duration, χ 2(3) = 52.87, p < .001.
Subjective physical function and actigraphic physical activity: Group differences
As hypothesized, on the self-report instruments, adolescents with chronic pain and their parents reported significantly lower physical health-related quality of life and significantly higher activity limitations compared to healthy adolescents (see Table 2). These group differences represent large effect sizes. Additionally, the mean physical health-related quality of life among adolescents with chronic pain was in the very impaired range (< 60), indicating that adolescents with chronic pain and their parents perceive that these adolescents experience clinically relevant impairments in physical health-related quality of life.
Table 2.
MANOVA results comparing adolescents with chronic pain and healthy adolescents on measures of physical function
| Questionnaire Measuresa | Chronic Pain n=78 M (SD) |
Healthy n=59 M (SD) |
F | Partial eta squared |
|---|---|---|---|---|
|
| ||||
| PedsQL Physical Health: | ||||
| Adolescent report | 58.01 (26.19) | 87.07 (12.18) | 61.42*** | .31 |
| Parent report | 58.27 (27.06) | 93.53 (10.43) | 88.57*** | .40 |
|
| ||||
| CALI-21: | ||||
| Adolescent report | 18.55 (7.07) | 5.38 (5.09) | 145.18*** | .52 |
| Parent report | 19.05 (7.90) | 2.41 (3.88) | 217.92*** | .62 |
|
| ||||
| Actigraphic Measuresb | ||||
|
| ||||
| Mean activity levelc | 464.86 (150.65) | 517.76 (115.32) | 5.04* | .04 |
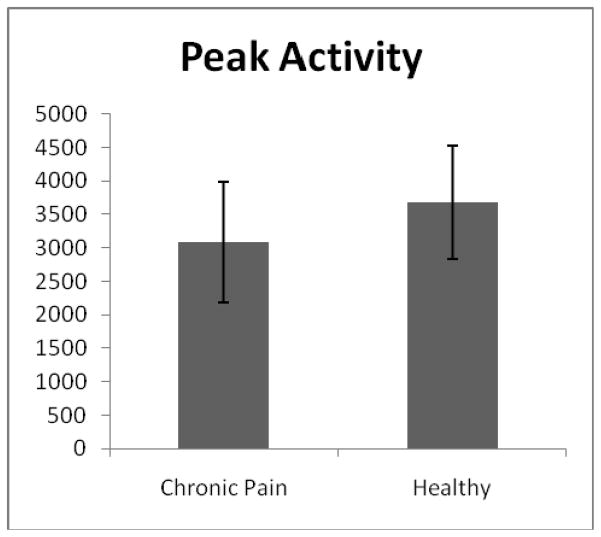
| Peak activity levelc | 3082.04 (895.88) | 3676.41 (845.90) | 15.51*** | .10 |
| Sedentary time in minutes | 68.57 (44.39) | 48.94 (26.22) | 9.12** | .06 |
Overall test of significance for MANOVA of questionnaire measures, F(4, 131) = 61.01, p < .001;
Overall test of significance for MANOVA of actigraphic measures, F(3, 133) = 7.11, p < .001
Measured in activity counts per one minute epoch
p < .05,
p < .01,
p < .001
Objectively measured daytime activity was significantly lower among adolescents with chronic pain compared to healthy adolescents, as hypothesized (see Table 2). Adolescents with chronic pain had lower mean activity (see Figure 1), achieved a lower peak activity level (see Figure 2), and were sedentary for more minutes per day compared to their healthy peers. It should be noted that the duration of the daytime wake hours did not differ between the two groups, t(135) = .19, p = .85. Actigraphic measurements of physical activity were similar to what has been observed in previous samples.12
Figure 1.
Means and standard deviations of mean activity counts
Figure 2.
Means and standard deviations of peak activity counts
Associations among actigraphic physical activity and subjective physical function
Overall, actigraphic measures of physical activity demonstrated low to moderate but significant associations with adolescent and parent subjective reports of physical function, with significant r values ranging from .17 to .34 (see Table 3). These associations were in the expected directions, such that lower activity levels measured via actigraphy were associated with poorer physical health related quality of life and more activity limitations as assessed via questionnaires. The actigraphy measure of peak activity level was most highly correlated with subjective physical function.
Table 3.
Bivariate correlations among actigraphy variables and questionnaire measures of physical functioning
| PedsQL Physical Health | CALI-21 | |||
|---|---|---|---|---|
| Actigraphy Variable: | Adolescent Report | Parent Report | Adolescent Report | Parent Report |
| Mean activity level | .21* | .24** | −.11 | −.18* |
| Peak activity level | .23** | .33** | −.27** | −.34** |
| Sedentary time | −.20* | −.17* | .23** | .23** |
p < .05,
p < .01,
p < .001
Associations between age, sex, BMI percentile, pain characteristics, and actigraphic measures of physical activity among adolescents with chronic pain
Multiple regressions predicting physical activity variables were conducted in the group of adolescents with chronic pain (n = 78). Preliminary analyses (bivariate correlations) examined adolescent age, sex, and BMI percentile as correlates of actigraphic measures. Each of these variables were significantly associated with one or more of the actigraphic measures at the p<.05 level. Thus, these individual characteristics were all retained for examination in regression models, and were entered in the first step of the regression models. Bivariate correlations between pain characteristics and actigraphy variables indicated that usual pain intensity and usual pain duration were also significantly associated with at least one actigraphic measure. Additionally, the presence of abdominal pain (vs. musculoskeletal or headache pain) was correlated with higher activity level. Thus, all pain characteristics, including pain location (abdominal pain vs. non-abdominal pain), usual pain duration, and usual pain intensity were also retained for examination in the regression and were entered in the second step of the models.
Results for the first step of the model (see Table 4) indicated that at entry, adolescent age made significant contributions to all actigraphy variables such that older age was associated with lower mean and peak activity, and higher sedentary time. At entry, adolescent sex was associated only with peak activity level; females achieved lower peak activity compared to males. At entry, BMI percentile contributed to mean and peak activity level such that adolescents with higher BMI percentile had lower activity. Higher BMI percentile was associated with more sedentary time at entry in the model.
Table 4.
Individual and pain factors associated with actigraphic variables among adolescents with chronic pain (n = 78)
| Mean Activity Level | Peak Activity Level | Sedentary Time | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| β at entry | β at final step | β at entry | β at final step | β at entry | β at final step | |
|
| ||||||
| Step One: | ||||||
| Age | −.31** | −.22* | −.32** | −.29** | .42*** | .41*** |
| Sexa | .12 | .13 | −.20* | −.19 | −.12 | −.12 |
| BMI percentile | −.32** | −.22* | −.33** | −.29** | .21* | .19 |
| Step Two: | ||||||
| Abdominal Painb | .16 | .09 | −.05 | |||
| Usual Pain Duration | −.20* | −.08 | .01 | |||
| Usual Pain Intensity | .09 | −.04 | .02 | |||
|
| ||||||
| Total R2 | .26*** | .25** | .22** | |||
Coded 0 = male, 1 = female;
Coded 0 = headache or musculoskeletal pain, 1 = abdominal pain
p < .05,
p < .01,
p < .001
Pain characteristics were entered in the second step of the model. After controlling for age, sex, and BMI percentile, pain characteristics made little additional contributions to actigraphic physical activity variables. Having abdominal pain was not associated with activity levels or sedentary time. Longer usual pain duration was associated with lower mean activity, but was not a significant predictor of peak activity or sedentary time. Usual pain intensity was not associated with any actigraphic variables. Overall, regression models accounted for relatively small proportions of the variance in actigraphic physical activity (Total R2 ranged from .22 to .26).
Discussion
Results from this study support the hypothesis that adolescents with chronic pain have poor subjective physical functioning and lower objective physical activity levels when compared to healthy adolescents. Parents and adolescents reported similar levels of subjective physical functioning, which is consistent with previous research demonstrating concordance between adolescent and parent reports of pain-related disability.2 This study also demonstrated similar magnitude of reduced physical activity among adolescents with chronic pain compared to a previous small comparison study using actigraphy.12 Thus, impairment in physical functioning appears to be a robust finding both across reporter and across measurement method. This study also provides additional support for the use of actigraphic measures of daytime activity as a measure of physical activity among adolescents with chronic pain.
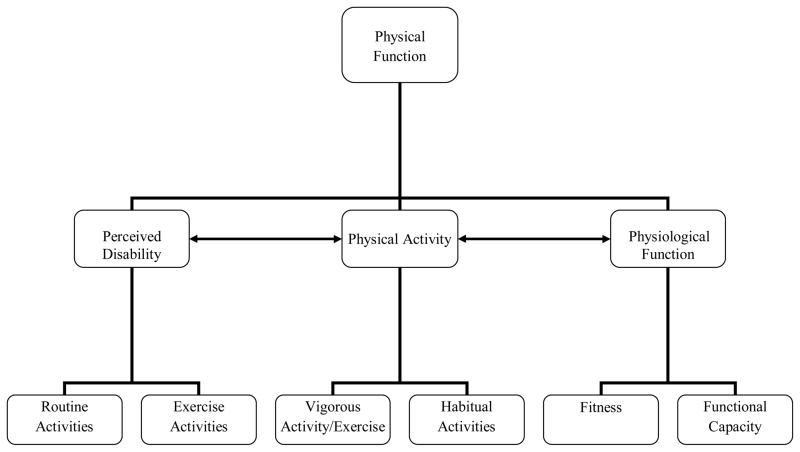
Physical functioning has been recommended for use as an outcome domain in pediatric chronic pain populations by the PedIMMPACT consensus group, which has emphasized that physical functioning is likely to include activities of everyday life 13. Measures recommended in the PedIMMPACT consensus statement included measures of subjective disability and subjective physical health-related quality of life. Physical functioning is a multidimensional domain, which includes an individual’s subjective perception of their physical function, including perceived difficulty performing everyday activities, but also includes actual participation in everyday activities, as well as additional dimensions such as vigorous physical activity participation and physical fitness. Results from the current study highlight physical activity as a dimension that is distinct from subjective disability. Recent measurement work has also demonstrated unique factors pertaining to vigorous and routine activity limitations in the FDI9 and the CALI-2116. In Figure 3, we present an organizational framework to conceptualize three broad dimensions within the overarching domain of physical functioning: perceived disability, physical activity, and physiological function. The framework shows the potential bidirectional associations between actual and perceived function, includes the important role of physiological factors impacted by general health, and distinguishes between vigorous activities such as sports and exercise, and more habitual or routine activities. Objective and subjective measurement tools may be used to capture the different dimensions outlined within this conceptual framework. In measuring physical function in pediatric populations, parent or other adult proxy-report of perceived disability and physical activity may supplement child self-report.
Figure 3.
A conceptual framework representing domains of physical function
As has been observed in previous studies, when associations between actigraphic measures of physical activity and subjective measures of functioning are significant, these are still relatively low correlations (e.g., .2–.3), indicating that objective measures of activity are capturing a distinct dimension or dimensions of functioning. In this study, we observed similarly small associations across reporters and with two self-report measures. Similar findings have been observed in adolescents with fibromyalgia; these data provide additional support for the importance of multi-method assessment when researching physical function.10 Assessing physical activity may provide additional useful information about an important dimension of physical functioning that has broad implications for adolescent health.
The current findings provide some support for distinctions between actigraphic measures of activity. Peak activity level or vigorous levels of activity seems to be most strongly associated with subjective disability across studies.10, 12 This may be due to the nature of this measurement, in that lower scores on actigraphic peak activity level may be an objective indicator of withdrawal from or non-participation in vigorous physical activities such as sports or physical education. Difficulty with such activities is specifically assessed on measures of subjective disability, such as the FDI,26 the CALI,17 and the PedsQL.24 Additionally, previous work suggests that participation in physical education or sports contributes more strongly to peak rather than mean activity levels; mean levels of physical activity may be capturing the habitual level of physical activity (see Figure 1), such as daily movement and active transportation habits28. Habitual activities may be less impacted by chronic pain for most youth, and thus less closely tied to subjective disability. Additionally, adolescent report on the CALI-21 was not significantly correlated with mean activity level (see Table 2). This measure asks respondents to report on the level of difficulty they experience participating in activities because of pain. It is possible that adolescent reports of activity limitations are more representative of internal perceptions of difficulty, whereas parent reports are based more on observations of adolescent behaviors which could be more highly correlated with actigraphy monitoring.
Different actigraphic measures may be more or less sensitive to change over time or to treatment effects, and specific measures may be more clinically relevant based on which aspects of functioning are being targeted (for instance, in treatment). Clinical researchers may want to choose actigraphic measures based on specific hypotheses about disability or behavior change, and to serve as complementary measures to self-report tools. For instance, investigators devising an exercise intervention to increase physical activity participation might examine peak activity measures to assess whether vigorous activity increases with treatment. In contrast, investigators examining risk for the development of pain-related disability might measure baseline mean activity to see whether lower habitual activity level increased risk for disability over time.
One of the major challenges with the current tools available for measuring disability in youth with chronic pain is that available measures are uni-dimensional and scored by summing or average all items, despite items representing a wide variety of activities. On the CALI-21 for instance, a total score of 18 may represent extreme difficulty attending school and doing things with friends for one child, but represent difficulty with sports, running, and physical education for another child. Thus, it is possible that with improvements in measurement tools and with use of factor scores to assess specific sub-domains of disability,16 more significant associations with actigraphic measures might emerge. Future research might utilize item-level analysis or other approaches to examine whether the content of particular self-report items or subscales is more strongly related to actigraphic measures.
Individual characteristics that impact physical activity in healthy adolescents also appear to contribute to physical activity in adolescents with chronic pain. Increasing age was associated with declines in activity and increases in sedentary time, as has been demonstrated in a number of studies.20 Female gender was related to lower peak physical activity, which is also commensurate with research showing greater declines in activity in females during adolescence.20 Higher BMI percentile was also associated with lower activity and more time spent in sedentary activity, which has been demonstrated in previous studies.23 While these associations were in the expected directions, lower levels of physical activity may be particularly problematic among adolescents with chronic pain given that pain in and of itself may limit activity and lead to weight gain, which can in turn increase discomfort and pain associated with activity.8 Greater attention might be paid to addressing modifiable risk factors in this population, particularly BMI percentile, as weight status has been noted to play an important role in chronic pain outcomes in prior research.7, 29 It may also be important to increase focus on exercise participation and overall physical activity in treatments for chronic pain, particularly for adolescents who have BMI percentiles in the overweight or obese ranges.
There are several limitations to this study that should be noted. First, although we utilized an objective measure of physical activity, there are several other dimensions of physical function that were not assessed in this study, including physical fitness or observed physical function. There are a number of laboratory-based measures that have been used in other studies to measure these dimensions, such as a timed walk and sit-to stand tasks,4 exercise tasks,19 or physiological measures of fitness.1 Inclusion of these types of measures might have strengthened our ability to capture the multidimensional nature of physical functioning. Additionally, the measure of peak physical activity that was utilized, the highest level of activity achieved in a single one minute epoch, does not capture sustained moderate or vigorous activity. Other accelerometer devices and software allow for examination of the amount of time spent at higher levels of activity, which might provide more accurate information about vigorous exercise participation. While actigraphic measures provide objective information about activity, they do not provide any information about the function or meaning of these activities for individuals. Information about the function or purpose of physical activity may be important for understanding behavior change, adherence, and other aspects of functioning.
It is also important to note that selection factors may contribute to the group differences we observed between chronic pain and healthy groups, as the chronic pain group was recruited through specialty care physicians. In addition to having chronic pain, these youth may have had more concerned parents than the healthy group, or had primary care providers who were more likely to refer them to specialty care for a variety of reasons; these unmeasured factors may have contributed to the observed group differences. Another important limitation to our study was that it was cross-sectional and we did not examine associations between pain and individual characteristics and physical functioning and activity over time. Longitudinal research in this area will provide important insights into predictors of activity. We are also unable to draw causal inferences about the role of pain in reducing activity levels. It is possible that adolescents with chronic pain evidence lower levels of physical activity compared to their peers prior to developing chronic pain. Thus, low activity levels may be a risk factor for chronic pain development and/or a correlate or symptom of chronic pain.
While one of the primary goals of psychological and interdisciplinary treatments for chronic pain in children and adolescents is to improve physical functioning, few studies have demonstrated changes in physical functioning following treatment, and most have utilized measures of subjective disability to assess physical function.5, 15 It is largely unknown whether treatments for pain can improve physical activity level. Researchers aiming to improve overall physical activity or physical fitness among adolescents with chronic pain will need to carefully consider the multidimensional nature of physical functioning and choose outcome measures appropriately. Measuring physical functioning in multiple methods and across dimensions will help us better understand the impact of chronic pain on children and adolescents, as well as identify appropriate targets for intervention and measure responses to treatment.
Acknowledgments
The authors wish to thank the adolescents and parents who participated in this study, and would like to thank Caitlin Murray for assistance with actigraphy scoring.
Footnotes
Disclosures
This research was supported by grants K23HD064705 (PI: Wilson) and R01HD05343 (PI: Palermo) from the National Institutes of Health/National Institute of Child Health and Human Development. The authors report no conflicts of interest.
References
- 1.Arvidsson D, Slinde F, Hulthen L. Free-living energy expenditure in children using multi-sensor activity monitors. Clin Nutr. 2009;28:305–12. doi: 10.1016/j.clnu.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Cohen LL, Vowles KE, Eccleston C. Adolescent chronic pain-related functioning: Concordance and discordance of mother-proxy and self-report ratings. European Journal of Pain. 2010;14:882–886. doi: 10.1016/j.ejpain.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. [Accessed October 18, 2010];BMI calculator for child and teen: English version. Available from: http://apps.nccd.cdc.gove/dnpabmi/calculator.aspx.
- 4.Eccleston C, Malleson PN, Clinch J, Connell H, Sourbut C. Chronic pain in adolescents: Evaluation of a programme of interdisciplinary cognitive behaviour therapy. Arch Dis Child. 2003;88:881–5. doi: 10.1136/adc.88.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eccleston C, Palermo T, Williams A, Lewandowski A, Morley S. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2009:Art. No.: CD007407. doi: 10.1002/14651858.CD003968.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106:52–58. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- 7.Hainsworth KR, Davies WH, Khan KA, Weisman SJ. Co-occurring chronic pain and obesity in children and adolescents: The impact on health-related quality of life. Clin J Pain. 2009;25:715–21. doi: 10.1097/AJP.0b013e3181a3b689. [DOI] [PubMed] [Google Scholar]
- 8.Hussey J, Gormley J, Bell C, Roche EF, Hoey H. Exercise tolerance and physical activity levels in children referred to a weight reduction clinic. Ir Med J. 2006;99:46–7. [PubMed] [Google Scholar]
- 9.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, Palermo TM, Wilson AC. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain. 2011 doi: 10.1016/j.pain.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashikar-Zuck S, Flowers SR, Verkamp E, Ting TV, Lynch-Jordan AM, Graham TB, Passo M, Schikler KN, Hashkes PJ, Spalding S, Banez G, Richards MM, Powers SW, Arnold LM, Lovell D. Actigraphy-based physical activity monitoring in adolescents with juvenile primary fibromyalgia syndrome. J Pain. 2010;11:885–93. doi: 10.1016/j.jpain.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clin J Pain. 2001;17:341–9. doi: 10.1097/00002508-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Long AC, Palermo TM, Manees AM. Brief report: Using actigraphy to compare physical activity levels in adolescents with chronic pain and healthy adolescents. J Pediatr Psychol. 2008;33:660–5. doi: 10.1093/jpepsy/jsm136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9:771–83. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: A critical review of the literature. J Dev Behav Pediatr. 2000;21:58–69. doi: 10.1097/00004703-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Palermo TM, Eccleston C, Lewandowski AS, Williams AC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: An updated meta-analytic review. Pain. 2010;148:387–397. doi: 10.1016/j.pain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palermo TM, Lewandowski AS, Long AC, Burant CJ. Validation of a self-report questionnaire version of the Child Activity Limitations Interview (CALI): The CALI-21. Pain. 2008;139:644–652. doi: 10.1016/j.pain.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palermo TM, Witherspoon D, Valenzuela D, Drotar D. Development and validation of the Child Activity Limitations Interview: A measure of pain-related functional impairment in school-age children and adolescents. Pain. 2004;109:461–70. doi: 10.1016/j.pain.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Puyau MR, Adolph AL, Vohra FA, Butte NF. Validation and calibration of physical activity monitors in children. Obes Res. 2002;10:150–7. doi: 10.1038/oby.2002.24. [DOI] [PubMed] [Google Scholar]
- 19.Reid GJ, McGrath PJ, Lang BA. Parent-child interactions among children with juvenile fibromyalgia, arthritis, and healthy controls. Pain. 2005;113:201–10. doi: 10.1016/j.pain.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Riddoch CJ, Bo Andersen L, Wedderkopp N, Harro M, Klasson-Heggebo L, Sardinha LB, Cooper AR, Ekelund U. Physical activity levels and patterns of 9- and 15-yr-old European children. Med Sci Sports Exerc. 2004;36:86–92. doi: 10.1249/01.MSS.0000106174.43932.92. [DOI] [PubMed] [Google Scholar]
- 21.Sirard JR, Pate RR. Physical activity assessment in children and adolescents. Sports Med. 2001;31:439–54. doi: 10.2165/00007256-200131060-00004. [DOI] [PubMed] [Google Scholar]
- 22.Swallen KC, Reither EN, Haas SA, Meier AM. Overweight, obesity, and health-related quality of life among adolescents: The National Longitudinal Study of Adolescent Health. Pediatrics. 2005;115:340–7. doi: 10.1542/peds.2004-0678. [DOI] [PubMed] [Google Scholar]
- 23.Treuth MS, Hou N, Young DR, Maynard LM. Accelerometry-measured activity or sedentary time and overweight in rural boys and girls. Obesity. 2005;13:1606–1614. doi: 10.1038/oby.2005.197. [DOI] [PubMed] [Google Scholar]
- 24.Varni JW, Seid M, Rode CA. The PedsQL: Measurement model for the Pediatric Quality of Life Inventory. Med Care. 1999;37:126–39. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 25.von Baeyer CL. Numerical rating scale for self-report of pain intensity in children and adolescents: Recent progress and further questions. Eur J Pain. 2009;13:1005–7. doi: 10.1016/j.ejpain.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Walker LS, Greene JW. The Functional Disability Inventory: Measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 27.Wiborg JF, Knoop H, Stulemeijer M, Prins JB, Bleijenberg G. How does cognitive behaviour therapy reduce fatigue in patients with chronic fatigue syndrome? The role of physical activity. Psychological Medicine First View. :1–7. doi: 10.1017/S0033291709992212. [DOI] [PubMed] [Google Scholar]
- 28.Wickel EE, Eisenmann JC. Contribution of youth sport to total daily physical activity among 6- to 12-yr-old boys. Med Sci Sports Exerc. 2007;39:1493–500. doi: 10.1249/mss.0b013e318093f56a. [DOI] [PubMed] [Google Scholar]
- 29.Wilson AC, Samuelson B, Palermo TM. Obesity in children and adolescents with chronic pain: Associations with pain and activity limitations. Clin J Pain. 2010;26:705–11. doi: 10.1097/AJP.0b013e3181e601fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittmeier KD, Mollard RC, Kriellaars DJ. Physical activity intensity and risk of overweight and adiposity in children. Obesity. 2008;16:415–20. doi: 10.1038/oby.2007.73. [DOI] [PubMed] [Google Scholar]