In recent years, a new generation of cancer therapies has emerged, based on a growing understanding of the molecular events that contribute to malignant transformation and which therefore represent targets for selectively killing or inhibiting growth of malignant cells. In this context, there has been considerable interest in elucidating mechanisms that regulate the capacity for cell division in normal as well as transformed cells, and substantial attention has focused specifically on the role of telomeres as the “mitotic clock” that mediates replicative capacity (reviewed in ref. 1). Telomeres are complexes at the termini of linear eukaryotic chromosomes that play a critical role in maintaining chromosomal integrity. Mammalian telomeres consist of hexanucleotide TTAGGG repeats (with an average length of 5–15 kb in humans) and associated protein components. This DNA-complex functions to “cap” the chromosome ends that protect against events such as end-end fusion and assure that telomere ends are not recognized as DNA breaks that trigger repair, cell cycle arrest, or apoptosis. In the absence of compensatory mechanisms, telomeric DNA shortens with each cell division, reflecting incomplete synthesis of telomeric termini during chromosomal replication. When telomeres reach a critically short length, cells enter a state that is termed replicative senescence in which they are incapable of further division and may be susceptible to increased apoptotic cell death. This finite replicative capacity is a characteristic of most normal human somatic cells. In contrast, however, cancer cells, cells of the germ line, and some somatic cell populations escape or delay replicative senescence through expression of telomerase, a unique enzyme that is capable of synthesizing telomeric repeats (reviewed in ref. 1). Telomerase consists of an RNA component, telomerase RNA (TER), which includes the template for synthesis of telomere DNA, and a protein catalytic component, the telomerase reverse transcriptase (TERT), which has homology to viral reverse transcriptases and mediates RNA template-dependent synthesis of telomere DNA. These two components are both necessary and sufficient to mediate telomerase enzymatic activity in vitro, although a variety of additional molecules may play a role in regulating in vivo activity.
Low-level expression of mutant telomerase template RNA results in decreased tumor cell proliferation and increased cell death.
Studies of telomere function have attracted intense interest in recent years, in part reflecting the perceived importance of this topic to an understanding of basic cell biology. In addition, interest in this area has been driven by the demonstration that induced expression of telomerase can contribute to malignant transformation in vitro (2) and by the inferred possibility that manipulation of telomere function may have a role in therapeutic cancer intervention. The potential for medical targeting of telomeres is interestingly bidirectional. On the one hand, it has been suggested that some diseases or undesirable consequences of aging may be due to loss of telomere function and consequent compromise of cell function or capacity for cell division. Although a role of telomere dysfunction in human disease or aging has not been definitively established, this possibility has driven efforts designed to enhance or restore telomere function as a mode of treatment or disease prevention. In contrast to the possibility that some disorders may result from limitations in cell replicative capacity, cancer represents a striking example of the opposite problem, a disease marked by the absence of normal constraints on cell division. Cancer cells must maintain telomere function despite the tendency of telomeres to shorten progressively with cell division. The possibility that disruption of telomere function may be an effective approach to treatment of cancer has thus stimulated considerable investigation. In this issue of PNAS, Kim et al. (3) report the result of one approach to telomere-based inhibition of tumor growth. Previous work by the Blackburn laboratory had demonstrated that alterations in the sequence of the DNA repeats that make up telomeres can substantially affect telomere function in single cell organisms (4). In the present study, the authors apply this finding to human tumor cell lines and conclude that low-level expression of mutant telomerase template RNA results in decreased tumor cell proliferation and increased cell death, pointing to the possibility that this strategy may represent an approach to cancer treatment. These findings are of interest both as probes into the biology of telomere function and as a novel addition to the approaches being taken to develop telomere-based cancer intervention.
In recent years, a number of approaches have been taken to inhibiting growth of malignant cells by targeting their expression of telomerase (Table 1 and Fig. 1). One straightforward strategy has been the inhibition of telomerase activity in malignant cells. Inhibition of telomerase activity is expected to result in progressive telomere shortening during cell division. It therefore might be anticipated that there would be no initial consequence of telomerase inhibition on cell division, and that tumor cells would exhibit continued growth until telomere shortening reached a critical level at which cell division would be arrested and apoptosis increased. This pattern has indeed been demonstrated to be the outcome of telomerase inhibition by a variety of interventions such as treatment with antisense TER RNA or by transfection with dominant negative TERT (5–7).
Table 1.
Telomerase-based targeting of cancer therapy
| Experimental approach | Characteristics of anticancer activity | Refs. |
|---|---|---|
| Immune elimination of telomerase-expressing cells | Eliminates cells that express TERT protein and that present TERT peptide in association with cell surface major histocompatibility molecules Effective only against TERT-expressing tumor cells Anticipated to affect TERT-expressing normal cells | 12–14 |
| Inhibition of telomerase enzymatic activity | Acts by producing progressive telomere shortening over the course of repeated cell division, hence delayed effects: tumor cell growth inhibition and cell death Effective only against telomerase-dependent tumor cells Anticipated to affect telomerase-dependent growth and function of normal somatic and germ-line cells | 5–7 |
| Expression of mutant telomerase RNA template | Presumably acts by disrupting telomere function after synthesis of mutant telomere repeats, leading to decreased cell growth and increased tumor cell death Presumably effective only against telomerase-expressing tumor cells Anticipated to affect telomerase-expressing normal cells | 3,15 |
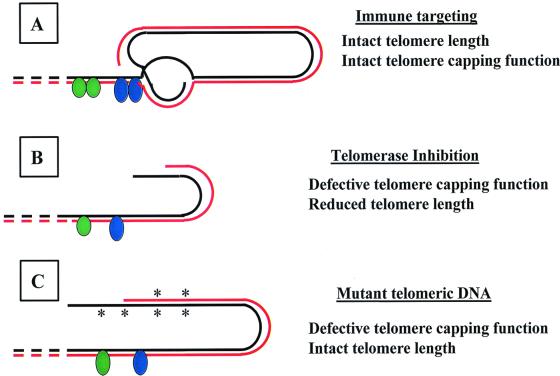
Figure 1.
Effect of alternative strategies for telomere-based targeting of cancer. Telomere structure is schematized consistent with the loop configuration described by Griffith et al. (20). Solid lines represent telomeric DNA and dashed lines represent subtelomeric DNA. The black line represents the 3′ terminus with single-strand overhang. Green and blue ovals are representative of telomere-associated proteins. (A) An immune response is induced against telomerase TERT peptides presented on the surface of TERT-expressing cells, eliminating these cells. The telomere complex and telomerase activity are not directly affected by this therapeutic intervention. (B) Inhibition of telomerase activity results in progressive telomere shortening due to the uncompensated consequences of chromosomal replication. Critical telomere shortening results in loss of telomere function, in turn leading to increased apoptosis and inhibition of cell replication. (C) Expression of mutant telomerase RNA template leads to synthesis of mutant telomere DNA, mutations denoted by *. Mutant telomeric DNA may fail to participate in DNA-protein or DNA-DNA interactions necessary for telomere capping function. The consequences may be similar or identical to those resulting from telomere shortening.
Also consistent with this delayed consequence of telomerase inhibition is the phenotype exhibited in mice rendered deficient in telomerase by genomic deletion of TER. Telomerase-deficient mice are initially phenotypically normal. However, successive generations of breeding result in progressive telomere shortening, eventually resulting in male and female sterility, reflecting germ cell senescence, as well as a variety of manifestations of somatic cell dysfunction (8). This model of telomerase-deficient mice also has allowed studies of the role of telomerase in tumorigenesis in vivo. Perhaps the most straightforward prediction in these mice was an expectation that tumor susceptibility and/or tumor growth would be limited as a result of telomere shortening uncompensated by telomerase. However, the effect of telomerase deficiency in this knockout model has been complex and is highly instructive of the considerations that require attention in targeting telomerase inhibition as a strategy for cancer treatment. It was observed that late-generation, telomerase-deficient mice have an increased, not decreased, incidence of spontaneous tumors (9). In interpreting this surprising finding, it was suggested that telomere shortening might predispose to chromosomal abnormalities that in turn may increase the risk of altered gene expression, leading to malignant transformation. Subsequent studies have analyzed the effect of telomerase deficiency in combination with other cancer-predisposing genetic alterations. A defect in either of the tumor suppressor genes, p53 or INK4a, leads to a marked increase in tumor incidence, as reflected in p53-deficient or INK4A-deficient mice. When double-deficient mice were constructed that lacked both TER and INK4A, it was found that tumor incidence in these mice was reduced relative to that in INK4A single-deficient mice (10). In this model, it is possible that a limitation of replicative capacity resulted from telomerase deficiency and in turn led to a reduction in the number of transformed INK4A-deficient cells that were capable of expanding to produce detectable tumors. However, in contrast to the reduction in tumor incidence observed in INK4A/TER double-deficient mice, mice that were deficient in both p53 and TER developed tumors at a rate greater than that observed in single-deficient p53 knockout mice (11). In interpreting this finding, it was suggested that in the absence of p53, chromosomal aberrations resulting from telomerase deficiency and telomere shortening accelerate development of tumors that otherwise would have been prevented by p53-dependent cell cycle arrest or apoptosis triggered by these chromosomal changes.
An alternative strategy for cancer treatment has been directed, not at inhibition of telomerase enzymatic activity, but rather at targeting immune recognition and destruction of cells that express telomerase. It has been suggested that telomerase activity in vivo is determined primarily by regulated expression of TERT and that telomerase-expressing tumor cells, but not telomerase-negative cells, express TERT protein. Immune responses, specifically cytotoxic T cell responses, have been generated against peptide sequences of the TERT protein, and it has been demonstrated that these cytotoxic T cells are capable of selectively lysing target cells that express TERT protein and therefore present recognizable TERT target peptide in the context of cell surface class I MHC molecules (12–14). In principle, this approach would result in rapid immune elimination of telomerase-expressing tumor cells without the lag involved in strategies that inhibit telomerase function and depend on gradual telomere shortening to inhibit tumor growth.
The experiments reported in this issue by Kim et al. (3) and the earlier work of Marusic et al. (15) represent a third approach to targeting of telomeres in cancer cells (3) (Table 1). The authors conclude that expression of mutant telomerase template RNA inhibits tumor cell proliferation by a mechanism that does not involve either telomere shortening or inhibition of telomerase activity. The fact that this effect is rapid in onset and is not delayed by a requirement for progressive telomere shortening secondary to cell division offers a clear theoretical advantage as an approach for treating cancer with immediate impact on tumor cells. The mechanism by which mutant RNA template exerts its effect is not addressed in the study reported here. Blackburn and colleagues, as well as others, have previously suggested that loss of telomere capping function can occur through alterations in any of several telomere components and can result in replicative senescence and apoptosis without shortening of telomeres (4, 16, 17). The findings reported here are consistent with such a model, in which incorporation of mutant sequences into telomeric DNA interferes with telomere function, possibly through altered DNA-protein or DNA-DNA interactions, and triggers inhibition of proliferation and induction of apoptosis.
Significant challenges now lie in the translation of these experimental approaches into in vivo treatment of tumors. Anti-TERT immunization has already been reported to be effective in inhibiting growth of transplantable tumors in mice (14). Methods for inhibiting telomerase activity in tumors in vivo are likely to focus on pharmacologic as well as genetic approaches. The introduction of mutant or otherwise altered telomeric DNA into tumors growing in vivo will present its own challenges. A potential limitation of any of these therapeutic approaches is their likely ineffectiveness in treating telomerase-negative tumors. Studies of large numbers of human tumors have indicated that although most tumors are telomerase positive, ≈10–15% of tumors do not express telomerase and appear to maintain telomere length through an alternative mechanism (18). It would be expected that such telomerase-negative tumors would not be influenced by interventions that inhibit telomerase activity. An immune approach targeting cells expressing TERT peptides would similarly be ineffective against TERT-nonexpressing telomerase-negative cells. Although not tested in the report by Kim et al. (3), antitumor effects of mutant RNA template presumably will require telomerase-dependent incorporation of mutant telomere sequences into telomere DNA and therefore also will be effective only against tumors with functional telomerase enzymatic activity.
A critical feature of any approach to cancer treatment is the ability to selectively interfere with growth or viability of cancer cells while avoiding or minimizing toxicity to normal noncancer cells. A number of studies have indicated that telomerase is expressed by a variety of normal stem cells as well as differentiated somatic cells (19). The effect of telomerase inhibition on normal cells in vivo has been assessed in telomerase knockout mice, where deficiencies in functions including hematopoietic stem cell activity and wound healing were identified (8, 9). It is difficult to predict from such information how favorable a “therapeutic ratio” can be expected from telomerase inhibition. It is also unclear how much toxicity for normal cell function would result from cytotoxic T cell or other immune responses to normal cells expressing telomerase. The studies of Kim et al. (3) similarly do not indicate what level of toxicity might be expected as a result of mutant telomeric sequences incorporated in the DNA of telomerase-positive nontumor cells.
The several strategies that have now been described for treating cancer by targeting telomerase expression are likely to provide useful insights into the role of telomere function in cell survival and cell cycle regulation. Testing of the therapeutic potential of these strategies in treating tumors now awaits the translation of these approaches in relevant in vivo systems where both antitumor effectiveness and complicating side effects on normal cell function can be evaluated.
Footnotes
See companion article on page 7982.
References
- 1.McEachern M J, Krauskopf A, Blackburn E H. Annu Rev Genet. 2000;34:331–358. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- 2.Hahn W C, Counter C M, Lundberg A S, Beijersbergen R L, Brooks M W, Weinberg R A. Nature (London) 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 3.Kim M M, Rivera M A, Botchkina I L, Shalaby R, Thor A D, Blackburn E H. Proc Natl Acad Sci USA. 2001;98:7982–7987. doi: 10.1073/pnas.131211098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEachern M J, Blackburn E H. Nature (London) 1995;376:404–409. doi: 10.1038/376403a0. [DOI] [PubMed] [Google Scholar]
- 5.Kondo S, Kondo Y, Li G, Silverman R H, Cowell J K. Oncogene. 1998;16:3323–3330. doi: 10.1038/sj.onc.1201885. [DOI] [PubMed] [Google Scholar]
- 6.Herbert B, Pitts A E, Baker S I, Hamilton S E, Wright W E, Shay J W, Corey D R. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn W C, Stewart S A, Brooks M W, York S G, Eaton E, Kurachi A, Beijersbergen R L, Knoll J H, Meyerson M, Weinberg R A. Nat Med. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 8.Blasco M A, Lee H W, Hande M P, Samper E, Lansdorp P M, DePinho R A, Greider C W. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 9.Rudolph K L, Chang S, Lee H W, Blasco M, Gottlieb G J, Greider C, DePinho R A. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg R A, Chin L, Femino A, Lee K H, Gottlieb G J, Singer R H, Greider C W, DePinho R A. Cell. 1999;97:515–525. doi: 10.1016/s0092-8674(00)80761-8. [DOI] [PubMed] [Google Scholar]
- 11.Chin L, Artandi S E, Shen Q, Tam A, Lee S L, Gottlieb G J, Greider C W, DePinho R A. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 12.Vonderheide R H, Hahn W C, Schultze J L, Nadler L M. Immunity. 1999;10:673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 13.Minev B, Hipp J, Firat H, Schmidt J D, Langlade-Demoyen P, Zanetti M. Proc Natl Acad Sci USA. 2000;97:4796–4801. doi: 10.1073/pnas.070560797. . (First Published April 4, 2000, 10.1073/pnas.070560797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair S K, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski J S, Vieweg J, Gilboa E. Nat Med. 2000;6:1011–1017. doi: 10.1038/79519. [DOI] [PubMed] [Google Scholar]
- 15.Marusic L, Anton M, Tidy A, Wang P, Villeponteau B, Bacchetti S. Mol Cell Biol. 1997;17:6394–6401. doi: 10.1128/mcb.17.11.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J, Wang H, Bishop J M, Blackburn E H. Proc Natl Acad Sci USA. 1999;96:3723–3728. doi: 10.1073/pnas.96.7.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lange T. Science. 2001;2 92:1075–1076. doi: 10.1126/science.1061032. [DOI] [PubMed] [Google Scholar]
- 18.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 19.Weng N-P, Hodes R J. J Clin Immunol. 2000;20:257–267. doi: 10.1023/a:1017223602293. [DOI] [PubMed] [Google Scholar]
- 20.Griffith J D, Comeau L, Rosenfeld S, Stansel R M, Bianchi A, Moss H, de Lange T. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]