Abstract
Purpose
To provide a radiation pneumonitis risk estimate and investigate the correlation of clinical and dosimetric factors in pediatric patients receiving chest irradiation.
Methods and Materials
A total of 122 patients diagnosed with sarcoma or Hodgkin lymphoma who received radiotherapy to the chest were evaluated for symptomatic radiation pneumonitis (Common Toxicity Criteria Grade 1 with respiratory symptom or higher grade). Pneumonitis data were collected from either prospective toxicity screenings as part of a clinical trial or through chart review. Dosimetric parameters including V10–V25, mean lung dose, binned lung dose, and tissue complication probability models were used, as well as clinical features to correlate with the development of pneumonitis.
Results
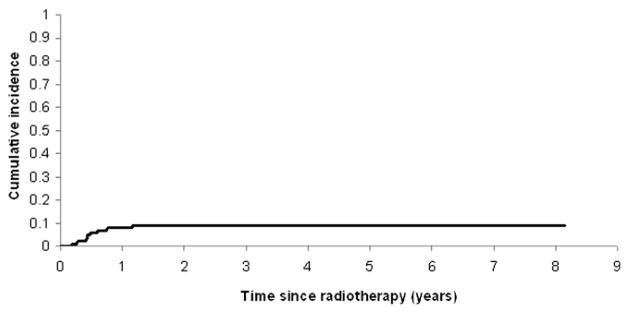
The 1- and 2-year cumulative incidence of symptomatic radiation pneumonitis for all patients was 8.2% and 9.1%, respectively. Nine patients experienced symptomatic Grade 1 toxicity, and 2 experienced Grade 2. From univariate analysis, chemotherapy containing bleomycin (χ2 test, p = 0.027) and V24 (logistic regression, p = 0.019) were the clinical and dosimetric factors that resulted in statistically significant differences in the occurrence of pneumonitis. The probability of pneumonitis increased more dramatically with increasing V24 in patients receiving bleomycin than in those who did not. Adult tissue complication models did not differentiate pediatric patients with radiation pneumonitis from those without.
Conclusions
The incidence of symptomatic radiation pneumonitis in pediatric patients is low and its severity mild. Parameters frequently used in adult radiation oncology provide some guidance as to risk, but pediatric patients warrant their own specific models for risk assessment, incorporating dosimetry and clinical factors.
Keywords: Radiation pneumonitis, Hodgkin lymphoma, Sarcoma, Pediatric radiotherapy
INTRODUCTION
Partial lung irradiation in pediatric radiation oncology is typically incidental to intrathoracic nodal or extrapulmonary solid tumor therapy. Therapy for Hodgkin lymphoma and pediatric sarcomas often includes irradiating a significant volume of lung, which could result in radiation pneumonitis (1, 2). For pediatric partial lung irradiation, radiation oncologists often rely on adult lung cancer data and empirical experience derived from pediatric whole-lung irradiation to assess the risk of developing acute and late pulmonary toxicity (3–6). There are very limited data available on radiation pneumonitis incidence and dosimetric planning guidelines for children treated in the three-dimensional (3D) conformal and intensity-modulated radiotherapy (IMRT) era. Lack of knowledge of dose–volume effects for pediatric lungs makes it difficult to predict the consequence of a lung dose–volume histogram (DVH) when exceeding the constraints set for adult lung cancer patients.
Pediatric patients frequently have very different side-effect profiles compared with adults when exposed to similar doses of radiation (7–9). We hypothesized that children and young adults receiving radiotherapy for Hodgkin lymphoma and sarcomas of the chest wall have a different incidence and risk factors of radiation pneumonitis from those for adults. We tested the effects of known dosimetric correlates learned from adult lung cancer experience, new dosimetric parameters, normal tissue complication probability (NTCP) models, and clinical variables on the incidence and development of symptomatic radiation pneumonitis in patients treated for Hodgkin lymphoma and sarcomas at St. Jude Children’s Research Hospital.
METHODS AND MATERIALS
Patient characteristics
In this institutional review board–approved study, we reviewed medical records and analyzed treatment plans of 99 children with Hodgkin lymphoma treated on (n = 77) or according to (n = 22) a risk-adapted protocol between January 2000 and December 2007 and 23 children with bone or soft-tissue sarcomas enrolled in an institutional clinical trial between January 2003 and June 2007. All patients included in this study received external-beam radiotherapy with high-energy photon only (i.e., no additional electron treatment or brachytherapy). Hodgkin lymphoma patients received involved-field radiotherapy with (n = 20) or without (n = 79) concurrent low-dose whole-lung irradiation. Sarcoma patients were included in the study if their radiation fields for the primary treatment site encompassed part of the lungs. Sarcoma types include Ewing’s family of tumors (n = 9), soft-tissue sarcoma (n = 12), rhabdomyosarcoma (n = 1), and osteosarcoma (n = 1). Patients were followed clinically every 6 weeks until 90 days after radiotherapy, every 3 months for the first 2 years, and every 6 months for Years 3–5.
Treatment characteristics
Involved-field radiotherapy for Hodgkin lymphoma was administered with opposed anterior and posterior 6–15-MV photon fields after computed tomography simulation. We further created subfields for each beam angle by moving the jaw position to reduce dose heterogeneity in the superior–inferior direction. A total dose of 15 Gy or 25.5 Gy (1.5 Gy per fraction) was prescribed to Hodgkin lymphoma patients according to their risk stratification. Twenty of these patients received 8 Gy concurrently to uni- or bilateral lungs through partial transmission blocks for pulmonary involvement.
Radiotherapy for sarcoma was delivered using either the 3D conformal or IMRT techniques. Prescribed doses ranged from 41.4 Gy to 70.2 Gy (1.8 Gy per fraction) to the primary tumor. All patients included in the study had complete simulation imaging studies of the entire bilateral lungs. Treatment planning for all patients was performed using PLanUNC software (10), developed by the University of North Carolina at Chapel Hill. Tissue heterogeneity correction with a modified Batho method was performed in dose calculation.
Systemic therapy
For patients with Hodgkin lymphoma, radiotherapy was initiated approximately 2 weeks after completion of the last cycle of chemotherapy. Patients with favorable localized disease received four cycles of VAMP (vinblastine, doxorubicin, methotrexate, and prednisone) chemotherapy. Patients with intermediate-risk Hodgkin lymphoma received two alternating cycles of VAMP and COP (cyclophosphamide, vincristine, and prednisone) chemotherapy. Patients with unfavorable risk received 12 weeks of Stanford V chemotherapy (doxorubicin, vinblastine, nitrogen mustard, vincristine, bleomycin, etoposide, and prednisone). For sarcomas, chemotherapy drugs were administered either concurrently or before radiotherapy or both. Regimens varied with the tumor type. However, none of the sarcoma patients received bleomycin as part of their chemotherapy.
Radiation pneumonitis grading
Radiation pneumonitis data were collected from protocol databases and medical record review. We evaluated toxicities using a modified version of the National Cancer Institute Common Toxicity Criteria version 2.0. The following grading system was assigned to the radiation pneumonitis: Grade 0, no symptoms; Grade 1, persistent, mild, dry cough and/or radiographic changes; Grade 2, radiographic changes, severe cough, fever, and requiring administration of steroids; Grade 3, radiographic changes and requiring oxygen; and Grade 4, radiographic changes and requiring assisted ventilation.
Radiotherapy plan analysis
Total lung volumes, combining right and left lungs, of all patients in the study were contoured by the same person. Contoured lung volumes do not include intrathoracic lymph nodes in Hodgkin lymphoma patients and gross tumor volumes in sarcoma patients. We calculated differential and cumulative DVHs of the total lung volume without excluding the overlapping planning target volume. All disease groups were combined for the dosimetric analysis on the basis of the nonsignificance of disease as a predictor of pneumonitis, allowing for greater diversity in the dosimetry. Table 1 lists the specific DVH parameters we analyzed, which were obtained from the composite treatment plans on the basis of their potential correlation with radiation pneumonitis. They included simple parameters of volume of lung irradiated to at least a specific dose (e.g., V20), dose ranges (e.g., V20–30), and mean lung doses.
Table 1.
Dosimetric parameters and adult risk models studied
| Parameter | Definition |
|---|---|
| V10 | Percentage volume of total lung receiving a dose ≥10 Gy |
| V13 | Percentage volume of total lung receiving a dose ≥13 Gy |
| V20 | Percentage volume of total lung receiving a dose ≥20 Gy |
| V25 | Percentage volume of total lung receiving a dose ≥25 Gy |
| V0–10 | Percentage volume of total lung receiving a dose in the 0–<10 Gy dose bin |
| V10–20 | Percentage volume of total lung receiving a dose in the 10–<20 Gy dose bin |
| V20–30 | Percentage volume of total lung receiving a dose in the 20–<30 Gy dose bin |
| V30–40 | Percentage volume of total lung receiving a dose in the 30–<40 Gy dose bin |
| V40–50 | Percentage volume of total lung receiving a dose in the 40–<50 Gy dose bin |
| V50–60 | Percentage volume of total lung receiving a dose in the 50–<60 Gy dose bin |
| Mean lung dose | Average dose to the total lung |
| NTCP | NTCP model of Seppenwoolde et al. (11) with a TD50 of 30.8 Gy and a steepness parameter m of 0.37 |
| Radiation pneumonitis risk | A two-variable (mean lung dose and GTV location) model by Bradley et al. (12) to predict the probability of radiation pneumonitis |
Abbreviations: NTCP = normal tissue complication probability; TD50 = dose for complication of 50%; GTV = gross tumor volume.
To investigate whether models fitting adult data can be used for children, we chose a representative NTCP model developed by Seppenwoolde et al. (11). This mean lung dose–based model best fit 382 adult cancer patients’ data from two institutions to predict moderate to life-threatening radiation pneumonitis. We selected this model on the basis of its large number of patients studied and the variety of diseases included. We also tested another radiation pneumonitis risk model developed from a combined analysis of 324 adult lung cancer patients from Radiation Therapy Oncology Group trial 9311 and a Washington University dataset (12). This model incorporates mean lung dose and tumor location as two important variables. We applied this model to our pediatric sarcoma patients to verify whether irradiation to the more inferior parts of the lung is riskier, as adult data suggested.
Pulmonary function tests
Fifty-seven patients with Hodgkin lymphoma in the study had a baseline and at least two subsequent yearly pulmonary function studies that included diffusing capacity of carbon monoxide (percentage of predicted DLCO) corrected for individual hemoglobin concentrations. We studied the correlation between changes in DLCO in the first 2 years after therapy and the occurrence of symptomatic radiation pneumonitis. For each subject, a straight line was fitted to the three DLCO measurements to calculate the slope, which represents the magnitude of DLCO change per year.
Statistical analysis
Cumulative incidence of radiation pneumonitis was calculated with the Gray’s method (13) and plotted using SAS software (SAS Institute, Cary, NC). We used JMP software (SAS Institute) for other statistical analysis. Pearson’s χ2 test was performed to check the differences in frequencies of occurrence of radiation pneumonitis for selected clinical risk factors. Dosimetric parameters for patients with and without symptomatic radiation pneumonitis were compared using the t test. We also performed multivariate logistic regression to determine the relative importance of variables and assess the interaction effect.
RESULTS
Cumulative incidence of radiation pneumonitis
Patient and treatment characteristics are summarized in Table 2. The 1- and 2-year cumulative incidence of symptomatic radiation pneumonitis was 8.2% and 9.1%, respectively, for 122 pediatric patients (Fig. 1). Of 99 Hodgkin lymphoma patients, 9 developed Grade 1 radiation pneumonitis, and 1 developed Grade 2. Of 23 sarcoma patients, 1 developed Grade 2 radiation pneumonitis. No Grade 3 or greater pneumonitides were seen in either group.
Table 2.
Patient and treatment characteristics (n = 122)
| Parameter | Hodgkin lymphoma | Sarcoma |
|---|---|---|
| Gender | ||
| Male | 49 (49.5) | 16 (69.6) |
| Female | 50 (50.5) | 7 (30.4) |
| Race | ||
| White | 75 (75.8) | 15 (65.2) |
| Black | 23 (23.2) | 6 (26.1) |
| Other | 1 (1) | 2 (8.7) |
| Age at radiotherapy (y) | ||
| Median | 16.1 | 12.4 |
| Range | 4.8–22.3 | 2–22.7 |
| Follow-up time (months) | ||
| Median | 28.8 | 26.4 |
| Range | 2.4–97.2 | 6–56.4 |
| Disease stage | ||
| I | 2 (2) | N/A |
| II | 57 (58) | |
| III | 12 (12) | |
| IV | 28 (28) | |
| Prescription dose (Gy) | ||
| Median | 25.5 | 54 |
| Range | 15–25.5 | 41.4–70.2 |
| Induction chemotherapy | ||
| Yes, with bleomycin | 61 (61.6) | 0 |
| Yes, without bleomycin | 38 (38.4) | 19 (82.6) |
| No | 0 | 4 (17.4) |
| Concurrent chemotherapy | ||
| Yes | 0 | 17 (73.9) |
| No | 99 (100) | 6 (26.1) |
| EBRT method | ||
| AP/PA | 99 (100) | 0 |
| 3D-CRT | 0 | 13 (56.5) |
| IMRT | 0 | 10 (43.5) |
Abbreviations: N/A = not applicable; EBRT = external-beam radiotherapy; AP/PA = anteroposterior–posteroanterior; 3D-CRT = three-dimensional conformal radiotherapy; IMRT = intensity-modulated radiotherapy
Values are number (percentage) unless otherwise noted.
Fig. 1.
Cumulative incidence of symptomatic radiation pneumonitis in 122 pediatric patients with lung irradiation.
Clinical parameter analysis
There were no statistically significant differences in frequencies of occurrence of radiation pneumonitis for the following clinical factors: age at radiotherapy in binary (χ2 test, p = 0.514), gender (p = 0.930), race (p = 0.302), disease type (Hodgkin lymphoma vs. sarcoma) (p = 0.386), prior chemotherapy (p = 0.522), and concurrent chemotherapy (p = 0.627). However, the frequency of occurrence was significantly different comparing administration vs. non-administration of bleomycin-containing chemotherapy (p = 0.027). A higher cumulative incidence of radiation pneumonitis at 1 year (13.3% vs. 3.3%; Gray’s method, p = 0.028) was observed for the group of patients receiving chemotherapy containing bleomycin.
Dosimetric parameter analysis
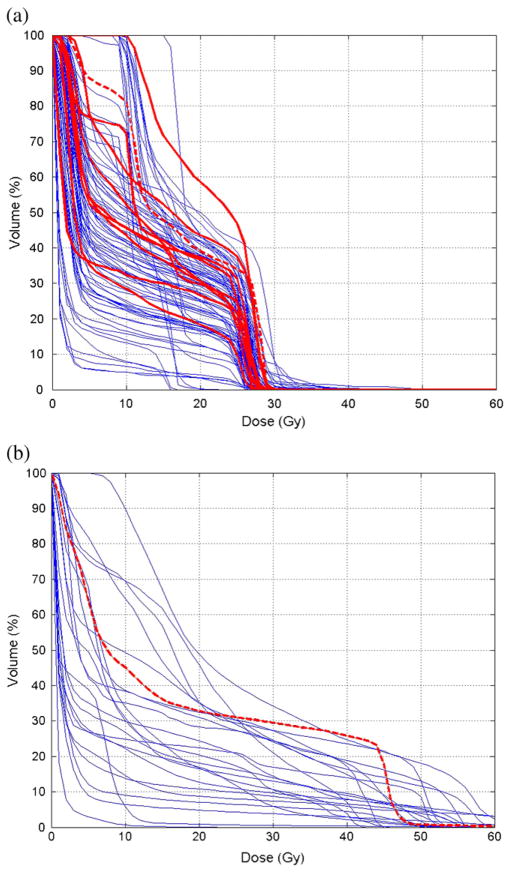
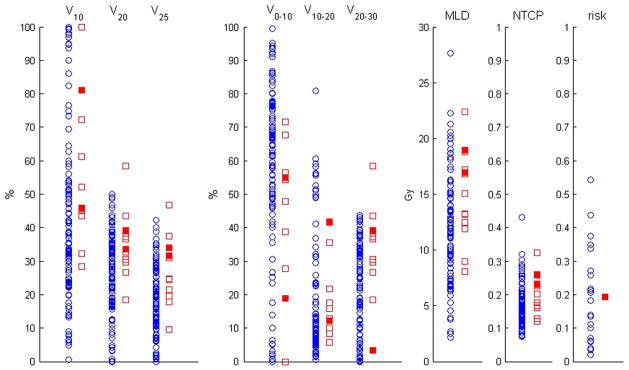
Figure 2 shows the cumulative DVH overlays of the total lung from radiotherapy plans of Hodgkin lymphoma and sarcoma patients. Figure 3 shows scatter plots of selected DVH parameters, calculated NTCP, and radiation pneumonitis risk using adult models. The NTCP values for 2 Grade 2 cases were higher than the average of the distribution but overlapped with those without symptoms. The adult radiation pneumonitis risk model that incorporates tumor location as a variable predicted >0.25 risk for 7 children without symptoms, whereas the patient with Grade 2 was predicted with a lower risk of 0.2. We computed the mean values for each parameter for patients with and without symptomatic radiation pneumonitis, as shown in Table 3. In our t test, V20, V25, and V20–30 were the dosimetric parameters that resulted in a significant difference (p < 0.05) between the means of two response groups. Further testing the VD in 1-Gy increments, we found V24 to have the lowest p value (0.014).
Fig. 2.
Dose–volume histogram overlays of the total lung from radiotherapy plans of 99 children with Hodgkin lymphoma (a) and 23 children with sarcoma (b). The bold curves are from the patients with symptomatic radiation pneumonitis. Among them, the dashed curves are from the ones with Grade 2 radiation pneumonitis.
Fig. 3.
Scatter plots of selected dose–volume histogram parameters for 122 Hodgkin lymphoma and sarcoma patients. Circles represent patients without symptomatic radiation pneumonitis. Squares represent patients with symptomatic radiation pneumonitis. Open squares represent Grade 1 pneumonitis and solid squares Grade 2. MLD = mean lung dose; NTCP = normal tissue complication probability; risk = radiation pneumonitis risk.
Table 3.
Comparison of patients with and without symptomatic radiation pneumonitis
| Parameter | Patients with pneumonitis (n = 11) | Patients without pneumonitis (n = 111) | p (t test) |
|---|---|---|---|
| V10 (%) | 55.2 | 42.8 | 0.120 |
| V13 (%) | 45.2 | 35.1 | 0.076 |
| V20 (%) | 34.9 | 25.7 | 0.023 |
| V25 (%) | 27.1 | 19.0 | 0.016 |
| V0–10 (%) | 44.8 | 57.2 | 0.098 |
| V10–20 (%) | 20.3 | 17.1 | 0.553 |
| V20–30 (%) | 32.2 | 22.4 | 0.023 |
| V30–40 (%) | 0.3 | 1.4 | 0.224 |
| V40–50 (%) | 2.3 | 1.1 | 0.313 |
| V50–60 (%) | 0.0 | 0.8 | 0.365 |
| Mean lung dose (Gy) | 14.4 | 11.9 | 0.094 |
| NTCP | 0.197 | 0.167 | 0.116 |
| Radiation pneumonitis risk (for sarcoma group only) | 0.193 | 0.192 | N/A* |
Abbreviation: NTCP = normal tissue complication probability; N/A = not applicable.
Values are means.
t test was not performed owing to there being only 1 radiation pneumonitis case among the sarcoma patients.
Twenty patients with Hodgkin lymphoma received 8-Gy uni- or bilateral whole-lung irradiation concurrently with their involved-field radiation. They had increased mean lung dose and V10–V25 compared with their counterparts receiving involved-field irradiation only. Despite this finding, delivery of whole-lung irradiation alone in this cohort did not predict for a greater incidence of pneumonitis (p = 0.307).
Bivariate logistic regression analysis
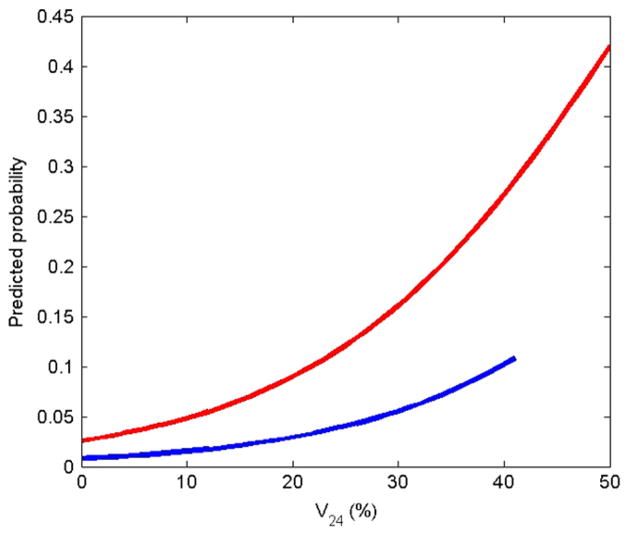
From univariate analysis, we identified the two most statistically significant variables: the administration of bleomycin-containing chemotherapy and the value of V24. However, they were found to be highly correlated (p < 0.001). This correlation is probably reflected in the loss of significance when the p value was calculated for the two variables, bleomycin-containing chemeotherapy (p = 0.159) and V24 (p = 0.074) in the bivariate logistic regression analysis. Odds ratios were 3.275 and 1.069, respectively. According to the regression model, the predicted probability of radiation pneumonitis increased more dramatically with increasing V24 in patients receiving chemotherapy containing bleomycin than in those without, as shown in Fig. 4.
Fig. 4.
Predicted probability of symptomatic radiation pneumonitis (Grade ≥1) based on bivariate logistic regression for patients who received chemotherapy containing bleomycin (upper curve) and those who did not (lower curve).
Radiation pneumonitis and DLCO changes
Seven patients with Hodgkin lymphoma who had sequential DLCO measurements developed radiation pneumonitis (6 with Grade 1 and 1 with Grade 2). Only 1 Grade 1 patient and the Grade 2 patient had a consistently downward trend in DLCO, from >110% of predicted DLCO at baseline to 73% and 82%, respectively, at Year 2. However, a significant decrease of ≥20% of predicted DLCO per year was also seen in 6 of the remaining 50 patients who had sequential DLCO measurements but no symptomatic radiation pneumonitis. Statistically, patients who developed symptomatic radiation pneumonitis and those without symptomatic radiation pneumonitis did not have different changes in DLCO in the first 2 years after therapy (p = 0.49).
DISCUSSION
Severe radiation pneumonitis did not occur in this population of patients receiving radiation to the chest for either Hodgkin lymphoma or sarcoma. The cumulative incidence of pneumonitis was 9.1% and was managed clinically, with only 2 of 122 children developing severe cough and fever, requiring administration of steroids.
Despite our favorable outcomes in the large cohort, fatal radiation pneumonitis cases in children have been reported historically (1) and recently (2), particularly in the context of sensitizing epirubicin-containing combination chemotherapy and whole-lung radiotherapy. Our experience seems to parallel the series reported by Oguz et al. (14), in which no acute or chronic respiratory symptoms developed over a median of 5 years in 23 pediatric patients receiving combined-modality therapy for Hodgkin lymphoma, including 24 Gy involved-field radiotherapy to the mediastinum. Approaches including more limited involved-field irradiation and the implementation of 3D conformal radiotherapy and IMRT have further reduced dose to the adjacent normal lung and could be responsible for the overall favorable outcomes.
We identified bleomycin-containing chemotherapy and the lung volume receiving a radiation dose ≥20–30 Gy as two statistically significant factors for developing radiation pneumonitis in our pediatric patient cohort. In the Stanford V chemotherapy regimen, bleomycin was administered at 5 U/m2 (no maximum dose) per dose on Day 1 of Weeks 2, 4, 6, 8, 10, and 12 of Stanford V chemotherapy. Unfortunately, the high correlation between the two above-mentioned variables prevents the relative importance from being determined in the bivariate regression analysis. However, the regression models plotted in Fig. 4 suggest that special attention during radiotherapy treatment planning should be paid to patients who received bleomycin as part of their multimodality therapy when their V24 is high.
Compared with adults with lung cancer, pediatric patients with Hodgkin lymphoma and sarcoma seem to have higher tolerance for partial lung irradiation and lower incidence of radiation pneumonitis. On the basis of a figure of radiation pneumonitis rate vs. mean lung dose published by Hernando and Marks (15, 16), which compared adult complication data from five institutions, the average Grade ≥2 pneumonitis rate was approximately 10% and 20% for a mean lung dose of 15 Gy and 20 Gy, respectively. Our Grade ≥2 pneumonitis rate was lower, 2.5% and 13.3%, correspondingly. Using an-other popular dosimetric metric, V20, Graham et al. (17) reported a 7% and 13% incidence of Grade ≥2 pneumonitis by 2 years for patients with a V20 in the range of 22–31% and 32–40%, respectively (17). Their pneumonitis incidence was considered to be lower than other published adult data (15, 18–21). However, our Grade ≥2 pneumonitis rate was even lower, 0 and 6.5% in the corresponding V20 volume ranges. There was no Grade ≥3 in our patient cohort for V20<50%. Such a low incidence has also been reported in one recent study of modern Hodgkin lymphoma treatment for adults (22).
In this study, we performed dosimetric analysis using the physical dose. Additional insight into the difference of children and adult lung sensitivity may be provided by analyzing biologic equivalent dose or normalized total dose (23, 24) that takes into account the dose fractionation scheme and normal tissue repair. This requires the α/β ratio for acute damage in pediatric lung to be estimated. Note that the number of fractions in this study ranged from 17 to 39, whereas adult lung cancer treatment is typically delivered in 30–40 fractions in a regular fractionation scheme. Therefore, for the same V20 it is potentially more biologically damaging to our pediatric patients. Given the fact that the pneumonitis incidence was actually lower, it suggests that the dose–volume effects are different for children with sarcoma and Hodgkin lymphoma compared with adult lung cancer patients.
Our study investigated the incidence of radiation pneumonitis and its clinical and dosimetric correlates. We have not addressed the risk of late pulmonary toxicity that can occur after chest irradiation (6, 14, 25). Our pulmonary function test data revealed a downward trend in the percentage of predicted DLCO for some patients after therapy. However, patients who developed symptomatic radiation pneumonitis and those without did not have statistically different changes in DLCO in the first 2 years after therapy. Longer follow-up will be necessary to determine whether modern, more limited, and more conformal radiotherapy approaches produce significantly less long-term toxicity. The importance of understanding the predictors of pneumonitis specific to the pediatric age group lies in the paucity of data guiding treating pediatric radiation oncologists. This limitation results in treatment plans that may significantly underdose tumor to avoid this possible treatment effect. Adult dosimetric parameters and models provide insight but do not truly elucidate patients at the highest risk for this complication. Pediatric-specific models of radiation pneumonitis are necessary to provide guidance in the therapy of these young patients.
Acknowledgments
Supported by funding from the Lance Armstrong Foundation and the American Lebanese Syrian Associated Charities
The authors thank Tina Davis for patient data management and Dr. Andrew Jackson for valuable discussions and suggestions.
Footnotes
Conflict of interest: none.
References
- 1.Littman P, Davis LW, Nash J, et al. The hazard of acute radiation pneumonitis in children receiving mediastinal radiation. Cancer. 1974;33:1520–1525. doi: 10.1002/1097-0142(197406)33:6<1520::aid-cncr2820330608>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Prestwich RJ, Picton SV, Glaser A, et al. Fatal pneumonitis in children with metastatic rhabdomyosarcoma following whole lung radiotherapy and sequential epirubicin. Pediatric Blood Cancer. 2007;48:586–590. doi: 10.1002/pbc.20660. [DOI] [PubMed] [Google Scholar]
- 3.Benoist MR, Lemerle J, Jean R, et al. Effects of pulmonary function of whole lung irradiation for Wilm’s tumor in children. Thorax. 1982;37:175–180. doi: 10.1136/thx.37.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith LM, Mendenhall NP, Cicale MJ, et al. Results of a prospective study evaluating the effects of mantle irradiation on pulmonary function. Int J Radiat Biol Phys. 1989;16:79–84. doi: 10.1016/0360-3016(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 5.Ellis ER, Marcus RB, Cicale MJ, et al. Pulmonary function tests after whole-lung irradiation and doxorubicin in patients with osteogenic sarcoma. J Clin Oncol. 1992;10:459–463. doi: 10.1200/JCO.1992.10.3.459. [DOI] [PubMed] [Google Scholar]
- 6.Weiner DJ, Maity A, Carlson CA, et al. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatric Blood Cancer. 2006;46:222–227. doi: 10.1002/pbc.20457. [DOI] [PubMed] [Google Scholar]
- 7.Krasin MJ, Xiong X, Wu S, et al. The effects of external beam irradiation on the growth of flat bones in children: Modeling a dose-volume effect. Int J Radiat Biol Phys. 2005;62:1458–1463. doi: 10.1016/j.ijrobp.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Merchant TE, Kiehna EN, Li C, et al. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int J Radiat Biol Phys. 2006;65:210–221. doi: 10.1016/j.ijrobp.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 9.Hua C, Bass JK, Khan R, et al. Hearing loss after radiotherapy for pediatric brain tumors: Effect of cochlear dose. Int J Radiat Biol Phys. 2008;72:892–899. doi: 10.1016/j.ijrobp.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 10.Sherouse GW, Chaney EL. The portable virtual simulator. Int J Radiat Biol Phys. 1991;21:475–482. doi: 10.1016/0360-3016(91)90799-a. [DOI] [PubMed] [Google Scholar]
- 11.Seppenwoolde Y, Lebesque JV, de Jaeger K, et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2003;55:724–735. doi: 10.1016/s0360-3016(02)03986-x. [DOI] [PubMed] [Google Scholar]
- 12.Bradley JD, Hope A, Naja IE, et al. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys. 2007;69:985–992. doi: 10.1016/j.ijrobp.2007.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1140–1154. [Google Scholar]
- 14.Oguz A, Tayfun T, Citak EC, et al. Long-term pulmonary function in survivors of childhood Hodgkin disease and non-Hodgkin lymphoma. Pediatric Blood Cancer. 2007;49:699–703. doi: 10.1002/pbc.21175. [DOI] [PubMed] [Google Scholar]
- 15.Hermando ML, Marks LB, Bentel GC, et al. Radiation induced pulmonary toxicity: A dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–659. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 16.Marks LB. Dosimetric predictors of radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2002;54:313–316. doi: 10.1016/s0360-3016(02)02928-0. [DOI] [PubMed] [Google Scholar]
- 17.Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small-cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45:323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 18.Oetzel D, Schraube P, Hensley F, et al. Estimation of pneumonitis risk in three-dimensional treatment planning using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 1995;33:455–460. doi: 10.1016/0360-3016(95)00009-N. [DOI] [PubMed] [Google Scholar]
- 19.Kwa SL, Lebesque JV, Theuws JC, et al. Radiation pneumonitis as a function of mean lung dose: An analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42:1–9. doi: 10.1016/s0360-3016(98)00196-5. [DOI] [PubMed] [Google Scholar]
- 20.Yorke ED, Jackson A, Rosenzweig KE, et al. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2002;54:329–339. doi: 10.1016/s0360-3016(02)02929-2. [DOI] [PubMed] [Google Scholar]
- 21.Tsujino K, Hirota S, Endo M, et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2003;55:110–115. doi: 10.1016/s0360-3016(02)03807-5. [DOI] [PubMed] [Google Scholar]
- 22.Koh ES, Sun A, Tran TH, et al. Clinical dose-volume histogram analysis in predicting radiation pneumonitis in Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2006;66:223–228. doi: 10.1016/j.ijrobp.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 23.Van Dyk J, Mah K, Keane TJ. Radiation-induced lung damage: Dose-time-fractionation considerations. Radiother Oncol. 1989;14:55–69. doi: 10.1016/0167-8140(89)90009-1. [DOI] [PubMed] [Google Scholar]
- 24.Lebesque JV, Keus RB. The simultaneous boost technique: The concept of relative normalized total dose. Radiother Oncol. 1991;22:45–55. doi: 10.1016/0167-8140(91)90068-r. [DOI] [PubMed] [Google Scholar]
- 25.Mertens AC, Yasul Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. Cancer. 2002;95:2431–2441. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]