Abstract
Background
Non-rhabdomyosarcoma soft tissue sarcomas (NRSTS) with initially unresected tumours represent a particular subset of patients with a poor outcome. Various international research groups pooled their data in a joint study in order to investigate prognostic variables and treatment modalities.
Methods
The study population consisted of 304 patients <21 years old treated between 1980 and 2005 using a multimodality therapeutic strategy.
Results
Synovial sarcoma and malignant peripheral nerve sheath tumour (MPNST) were the most frequent histotypes. Most patients received initial chemotherapy: major responses were recorded in 41% and minor in 16% of cases. Overall survival (OS) was 60.0% and 51.5% at 5 and 10 years, respectively, and it was significantly associated with patient's age, histological subtype, tumour site and size, quality of delayed surgical resection, radiotherapy administration and response to induction chemotherapy. MPNST associated to neurofibromatosis type 1 was the tumour type with the worst rate of response to chemotherapy and the worst outcome.
Conclusions
In unresected NRSTS patients, radiotherapy and delayed surgery are of crucial importance. Patients who respond to chemotherapy have better chance of survival. However, given the relatively poor prognosis, research on intensive multimodal treatment approaches and novel strategies is warranted.
Keywords: Non-rhabdomyosarcoma soft tissue sarcomas; Unresected sarcoma; Paediatric sarcoma; Synovial sarcoma, malignant peripheral nerve sheath tumour; Chemotherapy, response to chemotherapy; Radiotherapy; Surgery; Prognostic factors
1. Introduction
Non-rhabdomyosarcoma soft tissue sarcomas (NRSTS) are half of paediatric soft tissue sarcomas (STS) and represent a heterogeneous group of neoplasms with a different biology, clinical behaviour and response to treatment, many of which are tumours typical of adulthood.1
Like their adult counterparts, paediatric NRSTS seem to be relatively insensitive to chemotherapy, making local therapy (and surgery in particular) the unquestioned cornerstone of their treatment. Treatment strategies have changed a little in recent years, however, and multimodality treatments including systemic chemotherapy have increasingly been attempted for high-risk patients.2 A particular subset of NRSTS patients with unsatisfactory outcome is the so-called Intergroup Rhabdomyosarcoma Study (IRS)3 group III, namely cases with locally advanced and unresected tumours4,5: in these patients, chemotherapy is generally administered to shrink the tumour and make such cases amenable to conservative complete resections.6 The impact of such initial chemotherapy and of the different prognostic factors remains to be seen, however, and it is still not clear whether the clinical behaviour of paediatric NRSTS is the same as for adult STS of the same stage.7
Given the limited data on group III NRSTS cases,5,8–10 and the well-established collaboration among the international research groups treating children with STS, these same groups pooled their prospectively-collected data in a joint study so as to analyse a series large enough to enable a thorough assessment of the prognostic factors and response to chemotherapy in the different NRSTS histotypes.
2. Patients and methods
Analyses were performed after pooling the data from various groups,4,5,8,9 as shown in Table 1: a total of 304 cases were collected. Some of these patients had been already included in previous publications.4,5,8,9,11–14 Table 1 describes also the inclusion criteria of the study and the information extracted from the single institutional databases. In order to target a subgroup of NRSTS histotypes as specific and homogeneous as possible, the study included only synovial sarcoma and the so-called ‘adult-type’ NRSTS, according to the previously coined definition, currently used in the European Pediatric Soft Tissue Sarcoma Study Group (EpSSG)2,4,15 (Table 2).
Table 1.
Participating groups, inclusion criteria and information extracted from the single institutional databases.
| Participating groups |
| POG 8654, (1986–1993) – 20 pts |
| SJCRH, (1981–2004) – 21 pts |
| INT Milan, (1980–2005) – 43 pts |
| POG 9553, 1996–2000) – 21 pts |
| AIEOP-STSC, (1980–2005) – 63 ptsa |
| SIOP-MMT, (1980–2005) – 136 ptsb |
| Inclusion criteria |
| Study period: 1980–2005 |
| Patient's age: 0–21 years |
| Histological diagnosis: synovial sarcoma or adult-type NRSTS |
| IRS group III |
| No distant metastases |
| All tumour sites except for the viscera |
| No pre-treatment (apart from initial resection) |
| Data collected |
| Clinical findings |
| Gender |
| Age |
| Tumour site and size |
| Histological subtype and grade |
| T and N status |
| Treatments details |
| Initial surgical approach |
| Delayed surgery |
| Radiotherapy |
| Chemotherapy |
| Response to chemotherapy |
POG = Pediatric Oncology Group.
SJCRH = St Jude Children's Research Hospital, Memphis, United States.
INT = Istituto Nazionale Tumori in Milan, Italy.
AIEOP-STSC = Associazione Italiana Ematologia Oncologia Pediatrica – Soft Tissue Sarcoma Committee.
SIOP-MMT = International Society of Pediatric Oncology Malignant Mesenchymal Tumour Group.
IRS = Intergroup Rhabdomyosarcoma Study.
13 from the RMS'79, 28 from the RMS'88 and 22 from the RMS'96 protocol.
17 from the RMS84, 35 from the MMT89 and 67 from the MMT95 study, plus 17 cases from the United Kingdom database.
Table 2.
Main patient and disease characteristics.
| N | % | |
|---|---|---|
| Total | 304 | – |
| Gender | ||
| Female | 151 | 49.7 |
| Male | 153 | 50.3 |
| Age, years | ||
| median (IQ range) | 11 (6–14 years) | |
| <10 | 116 | 38.2 |
| ≥10 | 188 | 61.8 |
| Histological subtype | ||
| Synovial sarcoma | 107 | 35.2 |
| MPNST | 71 | 23.4 |
| Adult-type fibrosarcoma | 10 | 3.3 |
| Alveolar soft part sarcoma | 10 | 3.3 |
| Leiomyosarcoma | 9 | 3.0 |
| Malignant haemangiopericytoma | 9 | 3.0 |
| Epithelioid sarcoma | 7 | 2.3 |
| MPH | 7 | 2.3 |
| Liposarcoma | 5 | 1.6 |
| Clear cell sarcoma | 5 | 1.6 |
| Angiosarcoma | 3 | 1.0 |
| Spindle cell NOS | 61 | 20.1 |
| Tumour grade | ||
| G1 | 7 | 2.3 |
| G2 | 23 | 7.6 |
| G3 | 81 | 26.6 |
| Unknown | 193 | 63.5 |
| Tumour site | ||
| Extremity | 113 | 37.2 |
| Head–neck | 89 | 29.3 |
| Trunk wall | 40 | 13.2 |
| Mediastinum, lung, pleura | 14 | 4.6 |
| Abdomen, pelvis, retroperitoneum | 48 | 15.8 |
| Tumour size, cm | ||
| Median (IQ range) | 8 (6–10) | |
| ≤5 | 65 | 21.4 |
| >5 | 239 | 78.6 |
| TNM | ||
| T1A | 35 | 11.5 |
| T1B | 57 | 18.8 |
| T2A | 30 | 9.9 |
| T2B | 182 | 59.9 |
| N0 | 276 | 90.8 |
| N1 | 28 | 9.2 |
IQ, interquartile; MPNST, malignant peripheral nerve sheath tumour; MPH, malignant fibrous histiocytoma; NOS, not otherwise specified; TNM, tumour, node, metastasis classification.
Since arrangements for central pathology review were already in place within each collaborative group, the histological diagnoses were not specifically re-reviewed for this analysis. Tumour grade was available for 111 cases (Table 2), and it was assigned according to the Pediatric Oncology Group (POG) system16 with the exception of the cases from the Istituto Nazionale Tumori Milan, recently reviewed for the purpose of another study according to the French Federation of Cancer Centers Sarcoma Group's (FNCLCC) system.17 Grading evaluation was not available for the majority of cases because it was not routinely assessed in the past in some of the groups involved in the current study, and because histological diagnosis was done in most cases on biopsy specimens, which were not adequate to permit a definition of tumour grade in many cases (whereas the specimens of the delayed surgery were not proper for grading evaluation because of the primary chemotherapy).
2.1. Treatment
Patients were treated using a multimodality therapeutic strategy. The first surgical approach was biopsy in 206 cases and attempted resection with macroscopic residual tumour in 67 (and not known in 31 cases). Most patients (295/304) received neo-adjuvant chemotherapy; the other 9 had radiotherapy ± delayed surgery.
Chemotherapy was administered according to different regimens (most of them drawn from RMS protocols) as shown in Table 3. Overall, 291/295 patients received ifosfamide or cyclophosphamide, 162/295 received anthracyclines. Chemotherapy lasted from 6 to 10 courses: for all the study groups, chemotherapy was discontinued in the event of tumour progression. Response to chemotherapy was usually assessed after 3 cycles (after 2 in the POG 9553 study). In most studies, response was measured in terms of the radiologically-assessed reduction in the sum of the products of the perpendicular diameters (but based on volume reduction in the last cooperative Italian protocol). The definition of response differed, however, in the various studies. For the purpose of this analysis, we classified as a ‘major response’ all responses defined in the different studies as complete response (CR), partial response (PR) > 50% in terms of diameter, and PR > 2/3 in terms of volume; the term ‘minor response’ was used for PR < 50% in terms of diameter and PR > 1/3 but <2/3 in terms of volume; and ‘no response’ was used when the tumour remained stable in size or progressed (i.e. its size increased or new lesions were detected); response to chemotherapy was not assessed for cases receiving concomitant radiotherapy.
Table 3.
Response to neo-adjuvant chemotherapy.
| Major responses | Minor responses | No response | Not evaluable Not available | Not given chemotherapy | Total | |
|---|---|---|---|---|---|---|
| Synovial sarcoma | 38 (8 CR) | 18 | 39 | 9 | 3 | 107 |
| MPNST non-NFl | 16 (3 CR) | 9 | 16 | 2 | 1 | 44 |
| MPNST in NF1 | 2 | 23 | 2 | 27 | ||
| Alveolar soft part sarcoma | 3 (2 CR) | 1 | 5 | 1 | 10 | |
| Angiosarcoma | 2 | 1 | 3 | |||
| Clear cell sarcoma | 1 | 1 | 3 | 5 | ||
| Epithelioid sarcoma | 2 (1 CR) | – | 5 | 7 | ||
| Fibrosarcoma | 3 | 2 | 3 | 2 | 10 | |
| Haemangiopericytoma | 5 | 1 | 2 | 1 | 9 | |
| Leiomyosarcoma | 4 (2 CR) | – | 5 | 9 | ||
| Liposarcoma | 1 | 1 | 2 | 1 | 5 | |
| MPH | 4 (1 CR) | – | 2 | 1 | 7 | |
| Spindle cell NOS | 34 (8 CR) | 10 | 14 | 2 | 1 | 61 |
| Total | 115 (25 CR) | 44 | 119 | 17 | 9 | 304 |
MPNST, malignant peripheral nerve sheath tumour; NF1, neurofibromatosis type 1; MFH, malignant fibrous histiocytoma; NOS, not otherwise specified.
Chemotherapy administered
Vincristine, actinomycinD, cyclophosphamide (VAC) - 17 patients.
Vincristine, actinomycinD, cyclophosphamide, doxorubicin (VACA) - 41.
Vincristine, actinomycinD, cyclophosphamide, dacarbazine - 17.
Ifosfamide, vincristine, actinomycinD (IVA) - 100.
Vincristine, actinomycinD, ifosfamide, doxorubicin (VAIA) - 50.
Ifosfamide and doxorubicin (±vincristine) - 51.
Carboplatin, etoposide, vincristine, actinomycinD, ifosfamide, epirubicin (CEVAIE) - 26.
Carboplatin, etoposide - 4.
Carboplatin, etoposide, ifosfamide - 1.
In most cases, local treatment was planned after assessing response to chemotherapy; patients underwent delayed tumour resection where feasible. For the purpose of this analysis, delayed surgery was defined as ‘complete’ in case of surgical microscopically free margins, while the definition of ‘incomplete’ included both macroscopically and microscopically incomplete resections (not distinguished in most databases of the groups involved in the study).
Radiotherapy was generally delivered as external beam irradiation using megavoltage photon or electron beam energies (6 cases reportedly received brachytherapy).
2.2. Statistical analysis
Disease outcome was analysed in terms of overall survival (OS), event-free survival (EFS) and local relapse-free survival (LRFS). The time was defined as the interval from the date of diagnosis to the date of ‘event’, namely: (i) death due to any cause (OS); (ii) local progression/relapse, nodal or distant metastases, any joint occurrence of such events, other malignancy or death with no evidence of disease, whichever came first (EFS); (iii) local progression/relapse alone or associated with nodal and/or distant metastases, or death with no evidence of disease (LRFS).
A descriptive analysis was based on the Kaplan-Meier method for estimating survival curves.18
Multivariable analyses were based on cause-specific hazards and consequently conducted using Cox regression models.19 The following covariates were considered: patient's age, histological subtype, tumour site and size, quality of surgery, response to induction chemotherapy and treatment with radiotherapy. To allow for the heterogeneity in the observed survival times for different study periods (1980-89, 1990–99, ≥2000) and different groups, we also included into the Cox model a shared frailty factor. Under various hypotheses about the distribution (gamma or Gaussian), and different methods for variance estimation, the frailty factor never reached statistical significance (p around 0.17). The results reported here are based on the model including the study covariates alone. To quantify the model's prognostic accuracy we calculated the c statistic,20 which was adjusted for possible over-optimism by means of a bootstrap.21
All the covariates were modeled within the Cox models as categorical by using dummy variables; the proportional hazard assumption was checked, relying on the graphical analysis of scaled Schoenfeld residuals.22 A multiple binary logistic model was used to test the association between RT administration and study period, collaborative group, patient's age, tumour site and size, type of surgery and histological subtype.
We considered two-sided P values below the conventional 5% threshold as significant. All the statistical analyses were performed using the SAS23 and R software.24
3. Results
Table 2 shows the characteristics of this series. Synovial sarcoma (SS) and MPNST accounted for 35% and 23% of the histotypes, respectively; 20% of cases were defined as not-otherwise-specified (NOS); 38% of MPNST patients had neurofibromatosis type 1 (NF1). In many cases, tumour arose from axial sites (29% head–neck; 16% abdomen, pelvis, retroperitoneum; 13% trunk; 5% mediastinum, lung, pleura), while limbs were the primary site in 37% of cases. Tumour size was larger than 5 cm in 79% of cases, and 9% had regional nodal involvement.
3.1. Response to neo-adjuvant chemotherapy
Chemotherapy was given to 295 patients, but response data were unavailable for 17 cases. Major responses were recorded in 41% and minor responses in 16% of cases (overall response rate 57%). Table 3 shows tumour response by histotype. Major and minor responses were respectively 40% and 19% for SS, 27% and 14% for MPNST, 58% and 17% for NRSTS NOS, and 42% and 12% for the remaining histotypes. When we analysed separately MPNST in NF1 and MPNST not associated to NF1, the overall response rates were 8% for the former and 60% for the latter (16 major and 9 minor responses out of 44 evaluable MPNST non-NF1 patients). Of the 119 patients not responding to chemotherapy, 37 had tumour progression at the time of their first assessment.
No statistically significant differences in response were observed according to the regimens adopted; major responses and minor responses were seen in 43% and 17% of cases treated with a regimen including both ifosfamide and doxorubicin (210 cases), and in 36% and 14% of those treated with other regimens, respectively (chi-square test, p = 0.342).
3.2. Local treatment
Delayed surgery was performed in 146 cases, while in 125 it was not; no surgical data were available for 33 patients (19 of whom received radiotherapy).
Surgery was defined as complete in 84 cases (10 amputations): 29/84 patients also had radiotherapy. Surgery was defined as incomplete in 51 cases and 34 of them also had radiotherapy. The status of the surgical margins was not specified in 11 cases (8 of whom had radiotherapy).
There was no significant association between response to chemotherapy and quality of delayed surgery (chi-square test, p = 0.954). Patients with a major response to chemotherapy were not more likely to have a complete resection than the others: surgery was complete in 30% of cases with a major response, in 44% of those with a minor response, and in 30% of those with no response to chemotherapy (37 of 124 patients failing to respond to chemotherapy had a delayed complete resection).
Radiotherapy was given to 162 patients (dose 36–70 Gy, median 54 Gy). In 72 cases, it was the only local treatment, in 71 it was associated with surgery (radiotherapy was given pre-operatively in 9 cases, postoperatively in 53, and not specified in 9). For 19 cases given radiotherapy, no data were available on whether or not surgery was performed. The median radiotherapy dose was 54 Gy for patients treated with radiotherapy alone or after incomplete resection, while it was 50 Gy in cases receiving radiotherapy associated with complete resection. Radiotherapy was given more frequently for head–neck tumours (p = 0.008), cases of SS (p = 0.008) and large tumours (p = 0.092); it was used more often in the more recent study periods (p = 0.011) and in patients who did not have delayed complete surgery (p = 0.002).
No local therapy was used as first-line treatment in 53 patients. Twenty-eight had early progression (some of them were irradiated as salvage therapy) and all died of their disease; the other 25 patients who did not receive local therapy had a major response to initial chemotherapy (and received systemic therapy as their only treatment): 11 had local recurrence (2 associated to metastases) and 9 died of disease.
3.3. Outcome
The first event was local failure in 109 patients (after 1–144 months), 42 having early progression during treatment and 67 local relapse; failure was local and distant in 23 patients (after 2–118 months); it was local and nodal in 1, only nodal in 1, and only with distant metastases in 28 (after 5–70 months).
With a median follow-up of 110 months (IQR 77–139 months), 163 patients were alive, 139 in first remission, 15 in second remission and 9 with disease; 136 died of disease progression, 5 of other causes (2 of second malignancies, 2 of toxicity, 1 of other causes). The 5- and 10-year EFS were 48.3% (95% confidence interval: 42.9–54.3%) and 44.0% (38.6–50.3%), respectively; the corresponding figures for OS were 60.0% (54.6–65.8%) and 51.5% (45.7–58.0%).
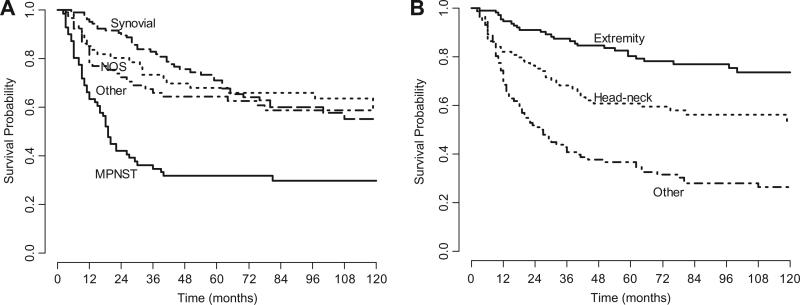
Fig. 1 shows the Kaplan-Meier OS curves according to histotype and tumour site. The worst survival was seen for patients with trunk, intra-abdominal and intra-thoracic tumour and for MPNST patients. In particular, only 3 of the 27 patients with MPNST–NF1 were alive at the time of the analysis, giving 5-year OS of 11.1% (3.8–32.3%), while 5-year OS in the MPNST non-NF1 group were 44.7% (32.1–62.3%). In univariate analysis, N-status did not have any impact on 5-year OS (60.3% for N0 and 56.3% for N1 cases); as for tumour grade, 5-year OS was 85.4% for G1, 56.5% for G2 and 55.0% for G3 cases (p = 0.21).
Fig. 1.
Kaplan-Meier overall survival curves according to histotype (panel A) and tumour site (panel B).
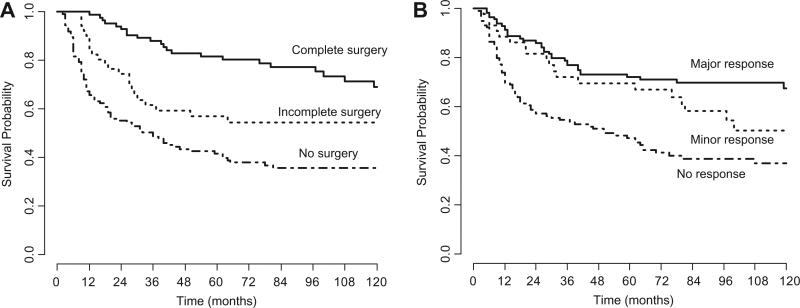
Survival was significantly better for patients who had a major response to chemotherapy and/or received a complete tumour resection (Fig. 2). Combining local treatment modalities, 5-year OS was 48.8% for cases receiving radiotherapy alone (72 cases), 85.1% in cases of complete resection (55), 74.8% in cases of complete resection plus radiotherapy (29), 35.3% in cases of incomplete resection (not followed by irradiation) (17), 68.7% in cases of incomplete resection plus radiotherapy (34), 31.8% for patients given no local treatment (53 cases) (p < 0.0001).
Fig. 2.
Kaplan-Meier overall survival curves according to quality of delayed surgery (panel A) and response to chemotherapy (panel B).
Table 4 shows the results of the multivariable Cox model. OS was significantly associated with all the putative prognostic factors investigated. In particular, the quality of delayed surgical resection and the use of radiotherapy strongly influenced the outcome; patients who did not undergo surgery had a roughly four-fold higher risk of death (HR = 4.3) than patients who underwent complete surgery. The c statistic model reached a value as high as 0.80, dropping to 0.78 after adjusting for over-optimism.
Table 4.
Analysis of the effect of prognostic factors on overall survival using the multivariable Cox model.
| HR | CI | Pa | |
|---|---|---|---|
| Age, years | |||
| ≥10 versus <10 | 1.7 | (1.1,2.6) | 0.018 |
| Histologicalsubtype | |||
| MPNST versus other | 2.3 | (1.5,3.7) | <0.001 |
| Synovial sarcoma versus other | 0.7 | (0.4,1.2) | |
| Tumour site | |||
| Head–neck versus extremity | 2.0 | (1.1,3.7) | 0.001 |
| Other versus extremity | 2.7 | (1.6,4.5) | |
| Tumour size, cm | |||
| >5 versus ≤5 | 2.5 | (1.3,4.6) | 0.006 |
| Delayed surgery | |||
| Not performed versus complete | 4.3 | (2.4,7.5) | <0.001 |
| Not specified versus complete | 3.3 | (1.1,9.6) | |
| Incomplete versus complete | 2.2 | (1.1,4.3) | |
| Radiotherapy | |||
| Not performed versus performed | 1.8 | (1.2,2.8) | 0.003 |
| Response to chemotherapy | |||
| Minor versus major | 2.1 | (1.1,3.7) | <0.001 |
| None versus major | 3.2 | (1.9,5.1) |
HR, hazard ratio; CI, 95% confidence interval; HR is an estimate of the increase (if >1) or decrease (if <1) in the risk associated with a covariate category versus the reference category (assumed to carry a risk of one). The larger the HR, the stronger the association between the variable and mortality. Confidence intervals not including the value of one indicate a significant difference towards the reference category.
MPNST, malignant peripheral nerve sheath tumour.
Two-sided Wald test p value.
Excluding patients with NOS histotypes from the Cox model analysis did not substantially affect the results shown in Table 4 (Supplementary Table 5).
All the investigated variables but age influenced significantly LRFS at multivariables Cox model analysis. In particular, unfavourable factors were: head and neck or sites other than extremity, tumour size >5 cm, not performed or incomplete surgery, lack of radiotherapy, MPNST histology and lack of response to chemotherapy (Supplementary Table 6).
4. Discussion
This study combined the series described in previous reports4,5,8,9 on paediatric NRSTS patients with initially-unresected disease together with other cohorts from cooperative groups, and thus represents the largest reported series on NRSTS group III patients to date. These patients typically present with unfavourable clinical features, e.g. large tumour size, MPNST histology and axial location, and consequently have an unsatisfactory outcome despite intensive multimodality treatments.
Within this setting, heterogeneity is introduced by treatment strategies. In fact, group III includes patients who are unquestionably unresectable already at diagnosis as well as cases that are initially not resected because the physician – or the groupwise policy – has opted to administer neo-adjuvant chemotherapy first, not necessarily to shrink the tumour with a view to achieving a conservative complete resection, but to treat any micrometastases promptly. In other words, the definition of unresectable/unresected tumours may sometimes be arbitrary and depends on surgeons’ different level of expertise as well as on different treatment strategies. It is noteworthy that 37 patients in our series underwent delayed complete resection although no tumour shrinkage was achieved by initial chemotherapy.
Our study highlighted that the clinical variables influencing the outcome in advanced adult STS had much the same prognostic impact in childhood cases7: large tumours, MPNST histotypes14 and older patients25 had an unfavourable outcome, as well axial cases11 (head–neck cases faring better than those of the trunk, chest and abdomen). The clinical variables were interrelated, i.e. MPNST were often large, axial tumours.
Although chemotherapy has been used increasingly in recent years for high-risk STS (in adult patients too26–28), NRSTS are usually characterised by a dubious chemosensitivity. The analytical description of response to initial chemotherapy in our series is limited by differences in its definition and in the methods used to assess it in the different series, in addition to the different regimens adopted. Overall, a 41% response rate was recorded (in terms of CR and major PR), but the figure rose to 57% when minor responses were considered too. When NRSTS NOS were not considered, the response rates were 37% and 52%, respectively. As expected, the response rate was relatively high for SS11–13, and particularly low for MPNST associated to NF1. In our series, the response rates for MPNST not associated to NF1 and for the group of other NRSTS subtypes were not different from that recorded for SS. The limited number of cases for each subtype other than SS and MPNST hindered any further considerations for these rarer histotypes; however, major responses to chemotherapy were recorded also for these rarer tumour types.
Final outcome correlated with response to initial chemotherapy, i.e. patients achieving a good tumour shrinkage with chemotherapy had a better outcome. So, in cases when chemotherapy is effective, it can have a role in survival terms. This might support the use of more intensive chemotherapy or, better still, it should prompt efforts to explore new effective combined strategies and novel agents with alternative mechanisms of action.
Local treatment is crucial in group III NRSTS patients; in fact, local progression or relapse was the major cause of treatment failure in our series. The best outcome was recorded for patients who succeeded in undergoing delayed complete resection. The use of radiotherapy correlated with a better survival. Our data would suggest that radiotherapy improved survival after incomplete resection, but offered no benefit after complete surgery. These findings should be considered with caution, however, because there was a selection bias, in that patients receiving radiotherapy were a selected subset with unfavourable features. A weakness of our analysis lies, in fact, in that the motives for using radiotherapy or not were not homogeneous and they also differed over time in the various groups and different centers participating in the study.
In fact, in the present study the price to pay for the advantage of relying upon a large number of cases was a lack of homogeneous conditions among the different study settings. As an example, histological revision of the diagnoses (centrally reviewed at the time of the various studies) was unrealistic. However, given the large number of cases with a diagnosis of NRSTS NOS, the prognostic factor analysis was repeated after excluding them and no major differences came to light. Further limitations of our analysis are the different protocols used by the centers involved as regards various factors, e.g. type of chemotherapy regimen, criteria for assessing response to initial chemotherapy, indications for radiotherapy. However, the potential heterogeneity relating to the different groups involved, to the study and to the different study periods were investigated in the Cox model, showing no significant influence on survival.
In conclusion, the overall prognosis for these patients remains unfavourable, since only half of the cases are cured by current treatment approaches. Intensive multimodal treatments are recommended for these patients and every effort should be made to achieve a complete surgical resection. New therapeutic strategies capable of combining novel systemic therapies and local or loco-regional treatments29,30 are warranted, particularly for the NRSTS subtype, which is known to be less chemosensitive. In the near future, histology-driven target agents may hopefully enable the current limits of systemic therapies in NRSTS to be overcome.
Supplementary Material
Footnotes
Conflict of interest statement
None declared.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ejca.2010.11.013.
REFERENCES
- 1.Okcu MF, Hicks J, Merchant TE, et al. Nonrhabdomyosarcomatous soft tissue sarcomas. In: Pizzo PA, Poplack DC, editors. Principles and practice of pediatric oncology. 5th Edn. Lippincott Williams and Wilkins; Philadelphia: 2006. pp. 1033–73. [Google Scholar]
- 2.Ferrari A, Casanova M. New concepts for the treatment of pediatric non-rhabdomyosarcoma soft tissue sarcomas. Expert Rev Anticancer Ther. 2005;5:307–18. doi: 10.1586/14737140.5.2.307. [DOI] [PubMed] [Google Scholar]
- 3.Maurer HM, Beltangady M, Gehan EA, et al. The intergroup rhabdomyosarcoma study I: a final report. Cancer. 1988;61:209–20. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari A, Casanova M, Meazza C, et al. Adult-type soft tissue sarcomas in pediatric age: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol. 2005;23:4021–30. doi: 10.1200/JCO.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 5.Spunt SL, Ashley Hill D, Motosue AM, et al. Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhaddomyosarcoma soft tissue sarcoma. J Clin Oncol. 2002;20:3225–35. doi: 10.1200/JCO.2002.06.066. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari A. Role of chemotherapy in pediatric nonrhabdomyosarcoma soft-tissue sarcomas. Expert Rev Anticancer Ther. 2008;8:929–38. doi: 10.1586/14737140.8.6.929. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari A, Miceli R, Casanova M, et al. Adult-type soft tissue sarcomas in pediatric age: a nomogram-based prognostic comparison with adult sarcomas. Eur J Cancer. 2007;43:2691–7. doi: 10.1016/j.ejca.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Pratt CB, Maurer HM, Gieser P, et al. Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group Study. Med Pediatr Oncol. 1998;30:201–9. doi: 10.1002/(sici)1096-911x(199804)30:4<201::aid-mpo1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Pappo AS, Devidas M, Jenkins J, et al. Phase II trial of neoadjuvant vincristine, ifosfamide, and doxorubicin with granulocyte colony-stimulating factor support in children and adolescents with advanced-stage nonrhabdomyosarcomatous soft tissue sarcomas: a Pediatric Oncology Group Study. J Clin Oncol. 2005;23:4031–8. doi: 10.1200/JCO.2005.03.209. [DOI] [PubMed] [Google Scholar]
- 10.Nathan PC, Tsokos M, Long L, et al. Adjuvant chemotherapy for the treatment of advanced pediatric nonrhabdomyosarcoma soft tissue sarcoma: the National Cancer Institute experience. Pediatr Blood Cancer. 2005;44:449–54. doi: 10.1002/pbc.20262. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari A, Bisogno G, Alaggio R, et al. Synovial sarcoma of children and adolescents: the prognostic role of axial sites. Eur J Cancer. 2008;44:1202–9. doi: 10.1016/j.ejca.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Okcu MF, Munsell M, Treuner J, et al. Synovial sarcoma of childhood and adolescence. a multicenter, multivariate analysis of outcome. J Clin Oncol. 2003;21:1602–11. doi: 10.1200/JCO.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101:627–34. doi: 10.1002/cncr.20386. [DOI] [PubMed] [Google Scholar]
- 14.Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German Soft Tissue Sarcoma Cooperative Group. J Clin Oncol. 2005;23:8422–30. doi: 10.1200/JCO.2005.01.4886. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari A, Casanova M. Specification on the definition of adult-type soft tissue sarcoma. J Clin Oncol. 2006;24(24):4042–3. doi: 10.1200/JCO.2006.07.1415. [DOI] [PubMed] [Google Scholar]
- 16.Parham DM, Webber BL, Jenkins JJ, et al. Nonrhabdomyosarcomatous soft tissue sarcomas of childhood: formulation of a simplified system for grading. Mod Pathol. 1995;8:705–10. [PubMed] [Google Scholar]
- 17.Trojani M, Contesso G, Coindre JM, et al. Soft tissue sarcomas of adults: study of pathological prognostic variables and definitions of a histopathological grading system. Int J Cancer. 1984;33:37–42. doi: 10.1002/ijc.2910330108. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL. Meier P: non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 19.Cox DR. Regression models and life tables (with discussion). J Royal Stat Soc, Ser B. 1972;34:187–220. [Google Scholar]
- 20.Harrell FE, Lee KL, Califf R, Pryor DB, Rosati RA. Regression modeling strategies for improved prognostic prediction. Stat Med. 1984;3:143–52. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 21.Efron B, Tibshirani RJ. An introduction to the bootstrap. Chapman and Hall; New York: 1993. [Google Scholar]
- 22.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–41. [Google Scholar]
- 23.SAS Institute Inc . SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. Cary, NC, USA: [Google Scholar]
- 24.R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2006. [Feb 8th 2010]. ISBN 3 900051-07-0. http://www.r-project.org/ [Google Scholar]
- 25.Hayes-Jordan AA, Spunt SL, Poquette CA, et al. Nonrhabdomyosarcoma soft tissue sarcomas in children: is age at diagnosis an important variable? J Pediatr Surg. 2000;35:948–54. doi: 10.1053/jpsu.2000.6934. [DOI] [PubMed] [Google Scholar]
- 26.Grobmyer SR, Maki RG, Demetri GD, et al. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol. 2004;15:1667–72. doi: 10.1093/annonc/mdh431. [DOI] [PubMed] [Google Scholar]
- 27.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117–27. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 28.Cormier JN, Langstein HN, Pisters PW. Preoperative therapy for soft tissue sarcoma. Cancer Treat Res. 2004;120:43–63. doi: 10.1007/1-4020-7856-0_3. [DOI] [PubMed] [Google Scholar]
- 29.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft tissue sarcoma of the limbs: a randomized trial. Lancet. 2002;359:2235–41. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 30.Rossi CR, Mocellin S, Pilati P, et al. Hyperthermic isolated perfusion with low-dose tumor necrosis factor alpha and doxorubicin for the treatment of limb-threatening soft tissue sarcomas. Ann Surg Oncol. 2005;12:398–405. doi: 10.1245/ASO.2005.12.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.